Abstract
Objective:
To evaluate the indications, perioperative, and long-term outcomes of a large cohort of patients who underwent middle pancreatectomy (MP).
Summary Background Data:
MP is a parenchyma-sparing technique aimed to reduce the risk of postoperative exocrine and endocrine insufficiency. Reported outcomes after MP are conflicting.
Methods:
Patients who underwent MP between 1990 and 2005 at the Massachusetts General Hospital and at the University of Verona were identified. The outcomes after MP were compared with a control group that underwent extended left pancreatectomy (ELP) for neoplasms in the mid pancreas.
Results:
A total of 100 patients underwent MP. The most common indications were neuroendocrine neoplasms, serous cystadenoma, and branch-duct IPMNs. Comparison with 45 ELP showed that intraoperative blood loss and transfusions were significantly higher for ELP. The 2 groups showed no differences in overall morbidity, abdominal complications, overall pancreatic fistula, and grade B/C pancreatic fistula rate (17% in MP and 13% in ELP), but the mean hospital-stay was longer for MP patients (P = 0.005). Mortality was zero. In the MP group, 5 patients affected by IPMNs had positive resection margins and 3 had recurrence. After a median follow-up of 54 months, incidence of new endocrine and exocrine insufficiency were significantly higher in the ELP group (4% vs. 38%, P = 0.0001 and 5% vs. 15.6%, P = 0.039, respectively).
Conclusions:
MP is a safe and effective procedure for treatment of benign and low-grade malignant neoplasms of the mid pancreas and is associated with a low risk of development of exocrine and endocrine insufficiency. MP should be avoided in patients affected by main-duct IPMN.
We evaluated indications, perioperative, and long-term outcomes of 100 patients who underwent middle pancreatectomy (MP) at 2 high-volume centers. The outcomes after MP were compared with a control group that underwent extended left pancreatectomy (ELP) for neoplasms in the mid pancreas. The incidence of new endocrine and exocrine insufficiency was significantly higher in the ELP group compared with MP one, with a 9-fold increase of new-onset diabetes (38% vs. 4%, P = 0.0001) and a 3-fold increase in exocrine insufficiency (15.6% vs. 5%, P = 0.039).
In recent years, there has been a marked increase of incidentally discovered cystic and endocrine neoplasms of the pancreas.1,2 Although the natural history of these lesions is unclear, the malignant potential of some of them is well known and this has led to more pancreatic resections.1–3
In this setting, neoplasms in the neck or in the proximal body of the pancreas represent a challenge for surgeons and are usually treated by either an extended right or, most often, an extended left pancreatectomy. These extended resections performed for a benign or borderline neoplasms result in removal of normal pancreatic tissue, increasing the risk of loss of exocrine and endocrine function.4–6 While enucleation is an option in some of these lesions, it should be avoided in neoplasms embedded deep in the substance of the gland, in those measuring more than 2 cm, and when neoplasms are not clearly benign and margins cannot be compromised.7,8
Middle pancreatectomy (MP), also known as central or medial pancreatectomy, has been proposed as an alternative technique in these cases, preserving pancreatic parenchyma and reducing the risk of exocrine and endocrine insufficiency.4,7–11 Since 1984, when Dagradi and Serio performed the first MP with an oncologic indication,12 nearly 200 cases have been reported,5,9 with only one collective series describing more than 50 patients.13 Because of the small number of cases and the short follow-up of most of these patients, both oncologic and functional long-term results of MP have not been clearly defined.
The primary endpoint of this study is to describe a large series of MP from 2 high-volume centers for pancreatic surgery, analyzing indications, operative morbidity, and long-term results regarding tumor recurrence and exocrine and endocrine function. To better evaluate the latter, we compared these outcomes with a control group that underwent extended left pancreatectomy (ELP) during the same time period.
PATIENTS AND METHODS
After obtaining Institutional review board approval, prospective databases of patients who underwent pancreatic surgery at the Departments of Surgery of the University of Verona (UV) and of the Massachusetts General Hospital (MGH) were queried to identify patients who have had a MP between January 1990 and December 2005.
In the study period, 2951 patients (1310 from UV and 1641 from MGH) underwent pancreatic resection at both Institutions; of these, 100 (2.9%) underwent MP (17 males, 83 females; mean age, 52 years, median, 54 years; range, 14–77 years), and constitute the study cohort. Sixty-two resections were performed at UV and 38 at MGH.
Demographic characteristics, past medical history, clinical presentation, preoperative work-up, and intraoperative and pathology data were collected; tumors were classified according to the WHO classification of the exocrine and endocrine pancreatic neoplasms.
Perioperative mortality, defined as in-hospital or 30-day death, and postoperative complications were evaluated. The pancreatic fistula definition was retrospectively assessed according to the International Study Group on Pancreatic Fistula recommendations.14
As a part of ongoing clinical trials, a few patients received prophylactic administration of somatostatin analogues15,16; since these trails were randomized, placebo-controlled, and double-blinded, we don't know in which patients somatostatin analogues were given; therefore, this variable was not considered in the statistical analysis. No somatostatin analogue was administrated in the remaining patients.
Follow-up was based on clinical, radiologic, and laboratory assessments. Specific aims of long-term follow-up were to evaluate tumor recurrence and long-term endocrine and exocrine function. For this latter purpose, patients underwent clinical and laboratory evaluation every 6 to 12 months. According to the World Health Organization recommendations,17 fasting glucose blood level was used as the reference test for the diagnosis of new onset diabetes and oral glucose tolerance test was performed in doubtful cases. In patients with preoperative disease, glycemia and HbA(1c) levels were monitored. Worsening diabetes was defined as deterioration in the metabolic control of previously diagnosed diabetes, requiring modification of the medical treatment. No specific exocrine function tests were performed. New onset of exocrine insufficiency was defined as steatorrhea and weight loss requiring pancreatic enzymes supplementation.
To better evaluate perioperative and long-term functional outcomes, we compared patients who underwent MP with a group of 45 patients (20 male, 25 female; mean age, 59 years; median, 60 years; range, 26–82 years) who underwent ELP for neoplasms located in the neck or in the proximal body of the gland during the same time period. Twenty-two of these patients were treated at UV and 23 at MGH. In every single case of ELP, the resection was performed to the right of the superior mesenteric vein with ligature of the gastroduodenal artery, encompassing up to 80% of the gland.
Surgical Technique
Only patients who underwent MP were considered. Reviewing our databases, we identified some cases of resections of the proximal body-neck of the pancreas associated with a partial resection of the head, but these patients were not included in the present study. MP was carried out according to previously described techniques.4,12 Reconstruction was accomplished with a retrocolic, end-to-side, mucosa-to-mucosa, Roux-en-Y pancreaticojejunostomy or with a pancreaticogastrostomy.
A small stent (a 5-Fr pediatric feeding tube or equivalent) was placed in the main pancreatic duct while performing pancreaticoenteric anastomosis. While at UV the stent was removed after mucosal suturing, at MGH it was often left in place for anastomotic stenting and drainage.4,7
Statistical Analysis
Results are presented as mean ± SD. Normally distributed continuous variables were compared using a two-sample Student t test; the Mann-Whitney U test was used for non-normally distributed variables. Categorical variables were compared using a Pearson χ2 test and Fisher exact test when cell counts were <5. A P value of <0.05 was considered statistically significant.
RESULTS
Clinical Characteristics
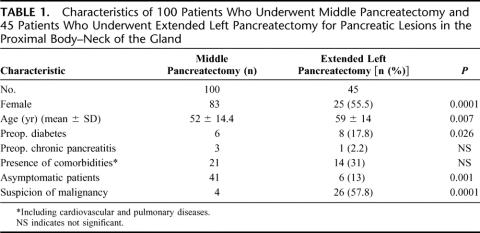
Table 1 shows the clinical characteristics of the 2 groups (MP and ELP). In the MP group, there was a preponderance of women (83% vs. 55.5%, P = 0.0001), of asymptomatic (41% vs. 13%, P = 0.001), and younger (52 ± 14.4 vs. 59 ± 14, P = 0.007) patients, and of smaller lesions (26.5 ± 14.6 vs. 46.2 ± 27, P = 0.0001). ELP patients had a higher incidence of preoperative diabetes (17.8% vs. 6%, P = 0.026). The 2 groups were well matched for the presence of chronic pancreatitis and comorbidities. Preoperative suspicion of malignancy was higher in ELP patients (57.8% vs. 4%, P = 0.0001).
TABLE 1. Characteristics of 100 Patients Who Underwent Middle Pancreatectomy and 45 Patients Who Underwent Extended Left Pancreatectomy for Pancreatic Lesions in the Proximal Body–Neck of the Gland
Surgical Resections and Intraoperative Data
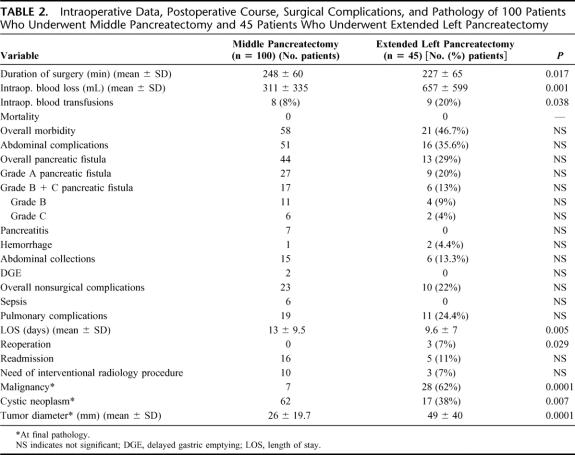
In the MP group, a stent was left in place in 28 patients (28%). The reconstruction was carried on with a pancreaticojejunostomy (PJ) in 95 cases (95%) and with a pancreaticogastrostomy (PG) in the remaining five. No anastomoses were performed in the ELP group. Although MP required a longer operative time (248 ± 60 minutes vs. 227 ± 65 minutes), the mean operative blood loss (657 ± 599 mL vs. 311 ± 335 mL) and the percentage of patients needing blood transfusions (20% vs. 8%) was significantly higher in the ELP group (Table 2).
TABLE 2. Intraoperative Data, Postoperative Course, Surgical Complications, and Pathology of 100 Patients Who Underwent Middle Pancreatectomy and 45 Patients Who Underwent Extended Left Pancreatectomy
Postoperative Course and Complications: MP Versus ELP
Table 2 shows postoperative data and complications. Mortality was zero in both groups. Although the rates of overall morbidity, abdominal complications, overall, grade A, B, C and grade B + C (clinically significant) pancreatic fistula, pancreatitis, and sepsis were consistently higher in the MP group, none of the differences was statistically significant. No patients required reoperation in the MP group, while 3 (7%) patients in the ELP group underwent surgical reexploration for bleeding, bowel obstruction, and intra-abdominal abscess, respectively (P = 0.029). No differences were found between the 2 groups regarding abdominal collections, nonsurgical complications, readmission rate, and need for interventional radiology procedures.
The mean length of stay (LOS) was 13 ± 9.5 days in the MP group and 9.6 ± 7 in the ELP (P = 0.005). In the MP group, the mean LOS before January 2001 (42 patients) was 15.6 ± 11 days, and after January 2001 (58 patients) 11.3 ± 8 days (P = 0.006).
Pancreatic Fistula in the MP Group
The incidence of overall pancreatic fistula in the MP group was 44%, but the rate of clinically significant (grade B + C) fistula, as defined by the International Study Group on Pancreatic Fistula,14 was only 17% (11 grade B and 6 grade C fistula). The mean LOS was 9.9 ± 5 days in patients without pancreatic fistula, 10.6 ± 3.5 in patients with grade A fistula, and 28 ± 13 in those with grade B/C fistula (P = 0.0001). The incidence of abdominal collections was 9% in patients without fistula, 11% in those with grade A fistula, and 41% in grade B/C group (P = 0.004). The type of reconstruction (PG vs. PJ) and the presence of stent did not affect the rate of any complication.
Postoperative Course in MP Group: UV Versus MGH
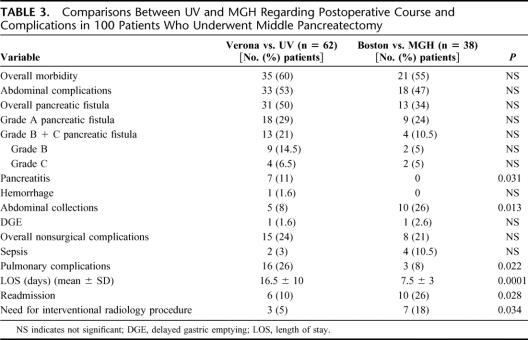
Postoperative complications for patients operated at UV and MGH are shown in Table 3. There was a higher incidence of pancreatitis (11% vs. 0%) and of pulmonary complications (26% vs. 8%) at UV. The LOS was significantly higher at UV (median of 12.5 vs. 7 days). At UV the mean LOS before January 2001 (29 patients) was 19 ± 11 days (median, 14 days) compared with 14 ± 9 days (median, 11 days) after January 2001 (P = 0.006); at MGH the LOS was more likely the same along these 2 periods (mean LOS before January 2001: 8 ± 2 days, 13 patients; mean LOS after January 2001: 7 ± 3 days, 25 patients). By contrast, the rate of abdominal collections (26% vs. 8%), readmission (26% vs. 10%), and need for interventional radiology procedures (18% vs. 5%) were significantly higher at MGH
TABLE 3. Comparisons Between UV and MGH Regarding Postoperative Course and Complications in 100 Patients Who Underwent Middle Pancreatectomy
Surgical Pathology
Seven patients (7%) in the MP group and 28 (62%) in the ELP had a malignant tumor (P = 0.0001). Because of this difference, no comparison between the 2 groups was done as regard to positive margins and recurrence rate. Patients in MP group had more likely cystic (62% vs. 38%) and smaller (26 ± 19.7 mm vs. 49 ± 40 mm) neoplasms than ELP patients.
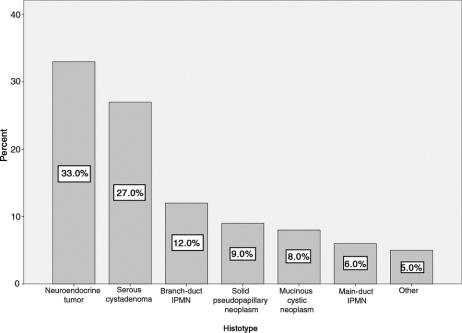
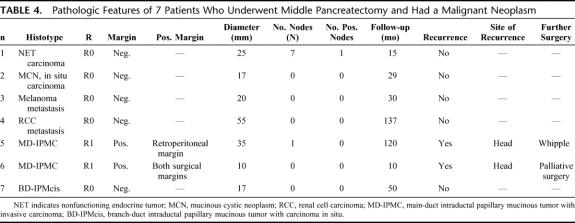
Figure 1 shows the histopathologic diagnosis of the 100 patients who underwent MP. The 2 most common indications for MP were neuroendocrine neoplasms (33%) and serous cystadenoma (27%). Positive resection margins were found in 5 patients (5%). All of them had intraductal papillary mucinous neoplasms (IPMNs), main-duct IPMN in 4 cases (1 adenoma, 1 borderline, and 2 invasive carcinoma) and branch-duct IPMN adenoma in 1 case. Table 4 shows the pathologic features of 7 MP patients with malignant neoplasms at final pathology.
FIGURE 1. Pathologic diagnosis of 100 patients who underwent MP (Other: pancreatic metastasis from renal cell carcinoma, 1; pancreatic metastasis from melanoma, 1; pancreatic true cyst, 2; pancreatic amartoma, 1).
TABLE 4. Pathologic Features of 7 Patients Who Underwent Middle Pancreatectomy and Had a Malignant Neoplasm
Long-term Functional Follow-up
Follow-up was complete and updated at July 2006 in 96% of patients in the MP group and 91% of patients in the ELP group. However, all the patients lost at follow-up had one clinical evaluation performed at least after 20 months from surgery.
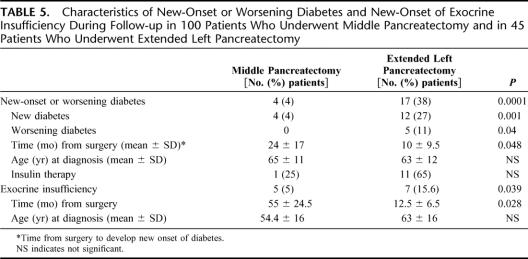
Table 5 shows the long-term functional follow-up in the 2 groups. The median follow-up was 54 months (mean, 62 ± 43 months) for the MP group and 23 months (mean, 24 ± 16 months) for the ELP group. Overall, 4 (4%) patients in the MP group and 17 (38%) in the ELP developed new onset or worsening diabetes (P = 0.0001). In the MP group, 6 patients had preoperative diabetes, which did not worsen after surgery, while 4 patients developed new onset of diabetes; of these, only one required insulin administration. In the ELP group 12 (27%), patients developed new onset of diabetes, requiring insulin administration in 7 cases; moreover, other 5 (11%) patients had preoperative diabetes that worsened after surgery: 1 patient with mild diabetes treated initially with diet alone needed oral drugs, while 4 patients who were treated with oral drugs required insulin. Five (5%) patients in the MP group and 7 (15.6%) in the ELP group developed new onset of exocrine insufficiency (P = 0.039).
TABLE 5. Characteristics of New-Onset or Worsening Diabetes and New-Onset of Exocrine Insufficiency During Follow-up in 100 Patients Who Underwent Middle Pancreatectomy and in 45 Patients Who Underwent Extended Left Pancreatectomy
The mean time from surgery to development of new onset of both exocrine and endocrine insufficiency was significantly longer in the MP group than in ELP (endocrine insufficiency: 24 ± 17 months in the MP group vs. 10 ± 9.5 in ELP; exocrine insufficiency: 55 ± 24.5 months in the MP group vs. 12.5 ± 6.5 in ELP). Of the 4 patients in the MP group who developed diabetes, only 1 patient required insulin; whereas in the ELP group, 11 of 18 did (Table 5).
Recurrences in the MP Group
Three (3%) patients developed a neoplastic recurrence. The first 2 patients had a main-duct IPMN with invasive carcinoma and positive resection margins (Table 4). They had a recurrence in the head of the pancreas 67 and 9 months after MP, respectively. The first underwent a Whipple, and she is still alive and without evidence of disease after 53 months. The second patient underwent only surgical palliation and died of disease. The third patient had a 2.3-cm nonfunctioning endocrine tumor with uncertain biologic behavior and negative resection margins. He developed a “recurrence” (new or second lesion) in the tail after 43 months. He underwent a left pancreatectomy and now, 15 months after the second operation, is alive and without evidence of further disease.
DISCUSSION
The history of surgical resection of the pancreas began in 1898, when in Italy Alessandro Codivilla performed an “en block” resection of the head of the pancreas and of the duodenum for a periampullary cancer.18 He was followed by Kausch, who performed in 1909 the first pancreaticoduodenectomy in 2 stages,19 by Gordon-Taylor, who described the first successful left pancreatectomy for a primary pancreatic neoplasm in 1927,18,20 and by Whipple, who in 1935 described the operation that nowadays carries his name.21 Since then, pancreatic resections were performed with increasing frequency, although associated with high morbidity and mortality. In the 1980s and 1990s, markedly improved surgical outcomes and long-term survival following pancreatic resections were reported from high-volume centers, to the point that these operations are now considered safe.22,23 Simultaneously, increased use of cross-sectional imaging has led to incidental diagnosis of pancreatic lesions in asymptomatic patients.1 This in turn has led to more resections performed for benign or low-grade malignant tumors1–3,22 and has driven the use of parenchyma-sparing techniques, such as enucleation and middle pancreatectomy (MP), with the goal of reducing the incidence of exocrine and endocrine impairment.6
The first MP with 2 pancreaticoenteric anastomoses was described by Guillemin and Bessot in 1957 in a patient with chronic pancreatitis.24 Letton and Wilson in 1959 reported on another MP, after traumatic transection of the pancreas, describing suture of the proximal stump and pancreatojejunostomy of the distal one.25 The first MP for a neoplasm was done by Dagradi and Serio, who performed one in 1984 to remove a benign insulinoma.12 Many case reports and small series have been reported since then, but the validity of MP has not been clearly demonstrated. Most of the series are heterogeneous, describe a high incidence of postoperative complications and short or no follow up, and therefore lack information regarding functional long-term results or tumor recurrence. Moreover, comparisons between MP and other techniques are limited.26,27
We report here the first study to compare perioperative and long-term functional results of a large cohort of patients who undergoing MP with a group undergoing ELP, which is the surgical procedure that would have been performed instead of MP for tumors located in the pancreatic neck or proximal body. Indeed, a “standard” left pancreatectomy or pancreaticoduodenectomy cannot be performed to treat these lesions and an extended resection is needed; at our institutions, we perform an ELP since it is safer and less complex than an extended pancreaticoduodenectomy. To overcome the differences between MP and ELP groups, we would have had to carry out a prospective randomized trial, but this would not be considered feasible from an ethical point of view.
One of the major deterrents to the widespread acceptance of MP among pancreatic surgeons is the high rate of complications, and above all that of pancreatic fistula.9,10,28 Although a recent review by Roggin et al10 showed that in 207 patients from 16 different series the overall morbidity was 33% and the reported fistula rate was 22%, indeed in many series the fistula rate after MP is greater than 30%.8–10,13,29 Our data show that following MP the incidence of postoperative surgical complications, and in particular pancreatic fistula is quite high (Table 2). However, the great majority of the leakage observed is grade A fistula, namely, transient fistula without any clinical impact, and the rate of clinically significant pancreatic fistula (grade B and C) was no different between MP and ELP (17% vs. 13%, respectively; P = NS).
We also found no difference between the MP and ELP groups with regard to intra-abdominal collections and need for radiologic intervention, nonsurgical complications, and readmission but did find a higher rate of reoperation (7%) in the ELP group while all the complications after MP were managed nonoperatively (P = 0.029). In our experience, ELP is actually associated with a rate of postoperative complications very close to that reported after MP.
At final pathologic examination after MP, 7 patients had a malignant tumor and 5 had positive resection margins. Three patients with a malignancy and all patients with positive resection margins had IPMNs. Two of the patients with positive margins had malignant main-duct IPMNs that recurred; the other 3 patients had benign IPMNs and have not developed recurrence but continued to be closely followed. We think that in IPMNs, MP should be indicated only for small, benign branch duct lesions of the mid pancreas and should be avoided in main-duct neoplasms, not only because of the likelihood of positive margins, but also because they are associated with malignancy in 70% of the cases.30,31 Regarding other diagnoses, we found only one recurrence in a patient with endocrine neoplasm with uncertain behavior and negative resection margins 43 months after MP. In this case, it is probable that the patient developed a new endocrine tumor rather than a recurrence. Of note is that none of the other 32 patients who underwent MP for a neuroendocrine neoplasm had a recurrence, and, given that our mean follow-up is 5 years, we think that small (<3 cm) neuroendocrine neoplasms in the mid pancreas are a very good indication for this operation.
The main goal of MP is the preservation of endocrine and exocrine function. Many authors have stressed the good long-term functional results after MP,4,7–13 suggesting that not only the preservation of pancreatic parenchyma but also of the duodenum may play a key role in maintaining pancreatic exocrine and endocrine function.32,33 In this retrospective study, we evaluated endocrine function by determining if there was new onset or worsening of diabetes according to the WHO diagnostic criteria for diabetes,17 and exocrine function by assessing if the patients had clinical manifestation of exocrine insufficiency and were taking enzymes. By these criteria, the incidence of both new endocrine and exocrine insufficiency was significantly higher in the ELP group compared with MP one, with a 9-fold increase of new onset diabetes (38% vs. 4%, P = 0.0001) and a 3-fold increase in exocrine insufficiency (15.6% vs. 5%, P = 0.039). The low frequency of endocrine and exocrine insufficiency found by us is consistent with that found in Roggin's review,10 and by Falconi et al.34 Furthermore, the time from surgical intervention needed to develop both endocrine and exocrine insufficiency was significantly longer in the MP group (Table 5), and there was no statistically significant difference between the 2 groups with respect to age, indicating that there are no factors other than the removal of healthy pancreatic tissue accounting for this difference.
In our series, patients in the MP group are younger, with smaller lesions, and with a significantly higher rate of benign tumors than the ELP group. This reflects strict selection criteria taken into account while planning a MP, which make this procedure uncommon even in referral centers. Tumors must be located in the proximal body or neck of the pancreas, not be amenable to enucleation, and be either benign or with low-grade malignancy.4,7–11 The operation is also well suited for single, small pancreatic metastasis.13 Our data show that MP can be appropriately considered not only for young patients but also in the elderly, since the rate of complications between MP and ELP is similar, and long-term functional outcomes are significantly better after MP, even in those patients already affected by diabetes. Indeed, while in the ELP group, 5 of 8 patients with preoperative diabetes showed worsening of the disease; in the MP group, all diabetic patients (n = 6) had stable disease after a median follow-up of 54 months.
Interesting observations emerge after analyzing the postoperative course and complications in the 2 institutions. Even if not statistically significant, the rate of pancreatic fistula was higher at UV than at MGH, and the postoperative LOS was longer in the Italian institution (16.5 ± 10 days vs. 7.5 ± 3; P = 0.0001), whereas the rate of abdominal collections, readmission, and the need for interventional radiology procedures were significantly higher in the American patients. This likely reflects not only differences in the health policy between the 2 hospitals (and the 2 countries) but also different drain-management: the majority of MGH patients were promptly discharged without drain after a median of 7 days and “late” leakages were not identified as pancreatic fistula but manifested as intra-abdominal collections with subsequent readmission and drainage by interventional radiology. Perhaps a more conservative drain management delaying drain removal is preferable in patients who undergo MP.
CONCLUSION
The very good long-term functional outcome after MP suggests that this is an alternative and effective procedure for the treatment of benign or low-grade malignant neoplasms as well as for small pancreatic metastases located in the midportion of the pancreas. Our data show that this operation is not appropriate for main-duct IPMNs, given the high rate of positive margins and recurrences observed in these cases. The rate of clinical significant complications after MP did not significantly differ from those reported for ELP and did not affect long-term functional results. Careful patient selection and performance of the operation in specialized centers35 are of paramount importance in this setting.
Footnotes
Supported by the International Hepato-Pancreato-Biliary Association and by the Fondazione Italiana Malattie Pancreas (to S.C.).
Reprints: Carlos Fernández-del Castillo, MD, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Wang Ambulatory Care Center 336, 15 Parkman Street, Boston, MA 02114. E-mail: cfernandez@partners.org.
REFERENCES
- 1.Fernandez-del Castillo C, Targarona J, Thayer SP, et al. Incidental pancreatic cyst: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plockinger U, Wiedenmann B. Neuroendocrine tumors of the gastro-entero-pancreatic system: the role of early diagnosis, genetic testing and preventive surgery. Dig Dis. 2002;20:49–60. [DOI] [PubMed] [Google Scholar]
- 3.Walsh RM, Vogt DP, Henderson JM, et al. Natural history of indeterminate pancreatic cysts. Surgery. 2005;138:665–670 discussion 670–671. [DOI] [PubMed]
- 4.Warshaw AL, Rattner DW, Fernandez-del Castillo C, et al. Middle segment pancreatectomy. Arch Surg. 1998;133:327–331. [DOI] [PubMed] [Google Scholar]
- 5.Kahl S, Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004;18:947–955. [DOI] [PubMed] [Google Scholar]
- 6.Aranha GV, Shoup M. Nonstandard pancreatic resections for unusual lesions. Am J Surg. 2005;189:223–228. [DOI] [PubMed] [Google Scholar]
- 7.Iacono C, Bortolasi L, Serio G. Is there a place for central pancreatectomy in pancreatic surgery? J Gastrointest Surg. 1998;2:509–517. [DOI] [PubMed] [Google Scholar]
- 8.Sperti C, Pasquali C, Ferronato A, et al. Median pancreatectomy for tumours of the neck and body of the pancreas. J Am Coll Surg. 2000;190:711–716. [DOI] [PubMed] [Google Scholar]
- 9.Christein JD, Smoot RL, Farnell MB. Central pancreatectomy: a technique for the resection of pancreatic neck lesions. Arch Surg. 2006;141:293–299. [DOI] [PubMed] [Google Scholar]
- 10.Roggin KK, Rudloff U, Blumgart LH, et al. Central pancreatectomy revisited. J Gastrointest Surg. 2006;10:804–812. [DOI] [PubMed] [Google Scholar]
- 11.Rotman N, Sastre B, Fagniez P. Median pancreatectomy for tumours of the neck of the pancreas. Surgery. 1993;113:532–535. [PubMed] [Google Scholar]
- 12.Dagradi A, Serio G. Pancreatectomia intermedia. In: Enciclopedia Medica Italiana: Pancreas, vol. 11. Florence: Scientifiche; 1984:850:–851.
- 13.Sauvanet A, Partensky C, Sastre B, et al. Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the French Pancreas Club. Surgery. 2002;132:836–843. [DOI] [PubMed] [Google Scholar]
- 14.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 15.Pancreatic Surgery Group. Sarr MG, The potent somatostatin analogue vapreotide does not decrease pancreas-specific complications after elective pancreatectomy: a prospective, multicenter, double-blinded, randomized, placebo-controlled trial. J Am Coll Surg. 2003;196:556–564. [DOI] [PubMed] [Google Scholar]
- 16.Pederzoli P, Bassi C, Falconi M, et al. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Italian Study Group. Br J Surg. 1994;81:265–269. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications: 1. Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 18.Howard JM, Hess W. History of the Pancreas: Mystery of a Hidden Organ. New York: Kluver Academic/Plenum Publisher; 2002. [Google Scholar]
- 19.Kausch W. Das carcinoma der papilla duodeni und seine radikale Entfeinung. Beitr Z Clin Chir. 1912;78:439–486. [Google Scholar]
- 20.Gordon-Taylor G. The radical surgery of cancer of the pancreas. Ann Surg. 1934;100:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whipple AO, Parson WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg. 1935;102:763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balcom JH 4th, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. [DOI] [PubMed] [Google Scholar]
- 23.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257 discussion 257–260. [DOI] [PMC free article] [PubMed]
- 24.Guillemin P, Bessot M. Pancreatite chronique calcifiante chez un tuberculeux renal: pancreatojejunostomie seion une technique originale. Mem Acad Chir Paris. 1957;83:869–871. [PubMed] [Google Scholar]
- 25.Letton AH, Wilson JP. Traumatic severance of pancreas treated by Roux-Y anastomosis. Surg Gynecol Obstet. 1959;109:473–478. [PubMed] [Google Scholar]
- 26.Falconi M, Mantovani W, Frigerio I, et al. Intermediate resection and distal pancreatectomy for benign neoplasms of the pancreas: comparison of postoperative complications and costs. Chir Ital. 2001;53:467–474. [PubMed] [Google Scholar]
- 27.Yamaguchi K, Yokohata K, Ohkido M, et al. Which is less invasive: distal pancreatectomy or segmental resection? Int Surg. 2000;85:297–302. [PubMed] [Google Scholar]
- 28.Hines OJ, Reber HA. Median pancreatectomy: do the risks justify the effort? J Am Coll Surg. 2000;190:715–716. [DOI] [PubMed] [Google Scholar]
- 29.Efron DT, Lillemoe KD, Cameron JL, et al. Central pancreatectomy with pancreaticogastrostomy for benign pancreatic pathology. J Gastrointest Surg. 2004;8:532–538. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. [DOI] [PubMed] [Google Scholar]
- 31.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685 discussion 685–687. [DOI] [PMC free article] [PubMed]
- 32.Buchler MW, Friess H, Muller MW, et al. Randomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis. Am J Surg. 1995;169:65–69 discussion 69–70. [DOI] [PubMed]
- 33.Yasuda H, Takada T, Toyota N, et al. Limited pancreatectomy: significance of postoperative maintenance of pancreatic exocrine function. J Hepatobiliary Pancreat Surg. 2000;7:466–472. [DOI] [PubMed] [Google Scholar]
- 34.Falconi M, Mantovani W, Crippa S, et al. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. In press. [DOI] [PubMed]
- 35.Bassi C, Falconi M, Salvia R, et al. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg. 2001;18:453–457. [DOI] [PubMed] [Google Scholar]