Abstract
Objective:
To better understand the impact of a microscopically positive margin (R1) on patterns of disease recurrence and survival after pancreaticoduodenectomy (PD) for pancreatic adenocarcinoma.
Summary Background Data:
A positive resection margin after PD is considered to be a poor prognostic factor, and some have proposed that an R1 margin may be a biologic predictor of more aggressive disease. The natural history of patients treated with contemporary multimodality therapy who underwent a positive margin PD has not been described.
Methods:
We analyzed our experience from 1990 to 2004, which included the prospective use of a standardized system for pathologic analysis of all PD specimens. All patients who underwent PD met objective computed tomographic criteria for resection. Standard pathologic evaluation of the PD specimen included permanent section analysis of the final bile duct, pancreatic, and superior mesenteric artery (SMA) margins. First recurrences (all sites) were defined as local, regional, or distant. Survival and follow-up were calculated from the date of initial histologic diagnosis to the dates of first recurrence or death and last contact, respectively.
Results:
PD was performed on 360 consecutive patients with pancreatic adenocarcinoma. Minimum follow-up was 12 months (median, 51.9 months). The resection margins were negative (R0) in 300 patients (83.3%) and positive (R1) in 60 (16.7%); no patients had macroscopically positive (R2) margins. By multivariate analysis (MVA), high mean operative blood loss and large tumor size were independent predictors of an R1 resection. Patients who underwent an R1 resection had a median overall survival of 21.5 months compared with 27.8 months in patients who underwent an R0 resection. After controlling for other variables on MVA, resection status did not independently affect survival. By MVA, only lymph node metastases, major perioperative complications, and blood loss adversely affected survival.
Conclusions:
There was no statistically significant difference in patient survival or recurrence based on R status. However, this series is unique in the incorporation of a standardized surgical technique for the SMA dissection, the prospective use of a reproducible system for pathologic evaluation of resection margins, the absence of R2 resections, and the frequent use of multimodality therapy.
Pancreaticoduodenectomy for pancreatic adenocarcinoma was performed in 360 consecutive patients. A standardized system for the pathologic analysis of all surgical specimens was used. The presence of a microscopically positive margin of resection did not affect the pattern of first recurrence and, on multivariate analysis, did not impact survival duration.
The completeness of resection resulting from pancreaticoduodenectomy (PD) for pancreatic adenocarcinoma, while not part of the TNM staging system, has powerful prognostic significance for recurrence and survival.1–3 Numerous studies have reported that a positive margin of resection was an independent predictor of poor long-term survival following PD for pancreatic adenocarcinoma.1–11 However, most of these studies did not describe the system or technique used for the pathologic evaluation of surgical specimens and, therefore, margin analysis, and did not distinguish margins that were grossly positive from those that were microscopically positive. Three categories are used as descriptors of the presence or absence of residual tumor following surgical resection: an R0 resection is defined as a grossly complete resection with microscopically negative margins; an R1 resection is defined as a grossly complete resection with microscopically positive margins; and an R2 resection is defined as a grossly incomplete resection.12
As defined by the American Joint Committee on Cancer (AJCC), the soft tissue containing autonomic nerves adjacent to the right lateral border of the proximal 3 to 4 cm of the superior mesenteric artery (SMA) has been referred to as the retroperitoneal, mesenteric, or uncinate margin.13 Although we have previously termed this margin the retroperitoneal margin, a more anatomically precise description is the “SMA margin.” While the pancreatic and bile duct transection margins can be reresected if intraoperative frozen section analysis determines that they are positive, the SMA margin cannot be reexcised, as surgeons do not typically resect the SMA when performing PD. Therefore, the SMA margin is the margin most commonly positive following PD.14,15
With careful patient selection, state-of-the-art imaging, and proper surgical technique, R2 resections can be largely avoided. Such is not the case for R1 resections which may occur in some patients, even when the surgeon removes all tissue to the right of the SMA, due to the propensity for pancreatic adenocarcinoma to extend along the perineural autonomic plexus surrounding the SMA, beyond the extent of palpable or visible tumor. The natural history of patients who have a microscopically positive margin following a grossly complete resection (R1) and who are treated with contemporary multimodality therapy (chemotherapy and external-beam radiation therapy) has not been described. Therefore, we reviewed our institutional experience by analyzing data from prospectively evaluated surgical specimens from patients who underwent PD for pancreatic adenocarcinoma to further understand the impact of a microscopically positive margin (R1) on patterns of disease recurrence and survival.
METHODS
After approval by the Institutional Review Board, data on all patients who underwent PD for periampullary malignancies at the University of Texas M.D. Anderson Cancer Center between July 31, 1990, and July 31, 2004, were retrieved from a prospective pancreatic tumor database. This analysis was limited to patients who underwent PD for adenocarcinoma of pancreatic origin; those with invasive intraductal papillary mucinous neoplasms and mucinous cystadenocarcinomas were excluded. Patients who underwent pancreatic resections other than PD (eg, distal pancreatectomy or total pancreatectomy) were also excluded.
Preoperative evaluations included physical examination, routine laboratory testing, chest radiography, and contrast-enhanced, multislice computed tomography (CT). To be considered for PD, patients were required to have radiographic evidence of resectable or borderline resectable disease as defined by CT imaging. Resectable pancreatic cancer (stage I or II) was defined as: 1) the absence of extrapancreatic disease; 2) no evidence of tumor extension to the SMA or celiac axis, as defined by the presence of a tissue plane between the tumor and these arterial structures; and 3) a patent superior mesenteric-portal vein (SMPV) confluence.16 The third criterion is based on the assumption that resection and reconstruction of the superior mesenteric vein (SMV), portal vein (PV), or SMPV confluence could be performed when necessary.17 Over the past 3 to 4 years, we have gained experience in the operative management of borderline resectable disease (subcategory of stage III), which is defined as tumors that exhibit the following: encasement of a short segment of the hepatic artery, without evidence of tumor extension to the celiac axis, that is amenable to resection and reconstruction; abutment of the SMA involving ≤180° of the circumference of the artery; or short-segment occlusion of the SMV, PV, or SMPV confluence that is amenable to vascular reconstruction because of a normal SMV below and normal PV above the area of tumor involvement. Patients with borderline resectable disease received preoperative chemotherapy and chemoradiation prior to PD.18
PD was performed in a standard fashion, as previously described.14 The most oncologically important part of the operation was the final step involving division of the pancreas and completion of the mesenteric and retroperitoneal dissection by removing all soft tissue to the right of the adventitia of the SMA, ie, the SMA margin.14 Tangential or segmental resection of the SMV, PV, or SMPV confluence was performed when the operating surgeon could not separate the pancreatic head and/or the uncinate process from the SMPV confluence without leaving gross tumor on the vein or risking a venotomy.17
Since July 1990, a standardized system for the pathologic evaluation of PD specimens has been used at our institution.19 This system enabled prospective evaluation of the status of the SMA margin of resection. The technique for assessment of SMA margin status was the same regardless of whether or not vascular resection was performed. Early in our institutional experience, the SMA margin was evaluated by microscopic examination of an en face section. Beginning in January 2000, the SMA margin has been evaluated according to the AJCC Cancer Staging Manual (6th edition) guidelines, as illustrated in Figure 1. 13 The SMA margin, posterior to the groove of the SMPV confluence, was inked and submitted in its entirety for microscopic examination on permanent sections by sectioning the specimen perpendicular to the inked margin. The pancreatic transection margin and the common bile/hepatic duct transection margins were evaluated by examining a complete en face section of each margin. At the discretion of the surgeon, these margins were often evaluated using frozen-section analysis, and if positive, additional tissue was resected. Final margins were recorded as negative (R0) or positive (R1) for tumor. A margin was designated “R0” if no tumor cells were identified at all of the resection margins. A margin was designated “R1” if tumor cells were present at the inked SMA margin or any of the en face sections from the aforementioned margins. SMA margins interpreted as suspicious for carcinoma (n = 2) were also considered positive (R1) for the purpose of this analysis. Pancreatic transection margins with focal high-grade dysplasia (PanIN 3) without invasive carcinoma were considered negative for the purpose of this analysis.
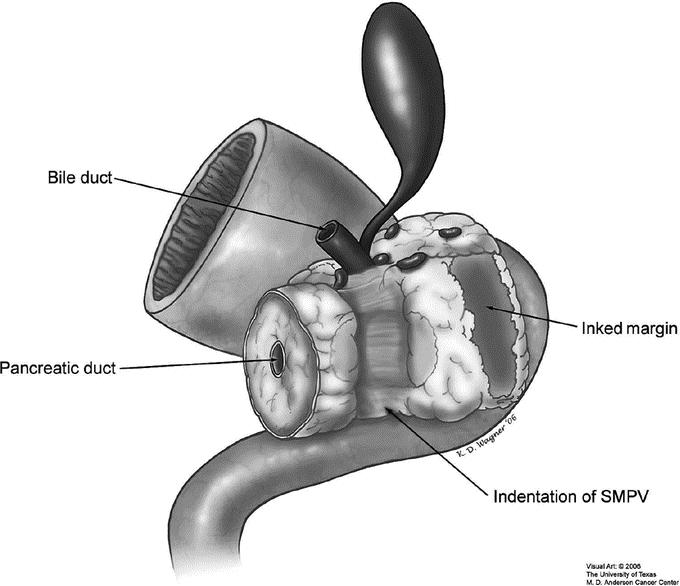
FIGURE 1. Illustration of a pancreaticoduodenectomy specimen demonstrating how the superior mesenteric artery margin should be inked at the time of permanent section pathologic examination. This margin cannot be retrospectively evaluated if the margin was not inked for identification at the time of gross inspection. SMPV, superior mesenteric-portal vein.
Patient age was recorded at the time of histologic diagnosis. Operative blood loss (in mL) was recorded from the anesthesia record. Major postoperative complications were defined as previously described and included perioperative death (within the first 30 days following surgery or during the original hospital stay if longer than 30 days); need for reoperation; clinically evident pancreaticojejunal anastomotic leak (as defined by drain amylase >2.5 times the upper limit of normal and fever or leukocytosis); intra-abdominal hemorrhage; intra-abdominal fluid collection (sterile collection or abscess) requiring intervention; cardiac arrhythmia; myocardial infarction or sudden cardiac death; pulmonary complications, including pneumonia; gastrointestinal bleeding; and sepsis syndrome.20 Of note, in the contemporary experience at our institution, the pancreatic anastomosis was rarely drained; therefore, only a clinically evident pancreatic anastomotic leak, significant enough to require percutaneous drainage, would be reported. Hospital stay was calculated by considering the day of surgery as day 1; the day of discharge was not counted as a hospital day.
Preoperative and/or postoperative chemotherapy and/or chemoradiation included either protocol-based or off-protocol treatment. Radiation therapy was delivered using either 50.4 Gy in 28 fractions or 30 Gy in 10 fractions. Concomitant chemotherapy included 5-fluorouracil, paclitaxel, gemcitabine, or capecitabine.21–23 Chemotherapy given before or after chemoradiation consisted of gemcitabine alone or in combination.
Patients were followed for a minimum of 12 months postoperatively or until death (if sooner). However, 8 patients, including 2 international patients, were lost to follow-up within the 12 months following surgery but were alive at last contact. Routine follow-up consisted of physical examination, laboratory studies, and CT imaging at 3- to 4-month intervals for the first 2 years postoperatively, at 6 month intervals for years 3 through 5, and then at yearly intervals. The first site or sites of disease recurrence were classified as local, regional, or distant. Local recurrence was defined as recurrence in the region of the pancreatic bed and root of mesentery. Regional recurrence was defined as recurrence in the soft tissues or lymph nodes beyond the pancreatic bed or within the peritoneal cavity (including ascites and/or wound implants). Distant recurrence was defined as recurrence in the liver, lungs, or other distant organs. Radiographic findings consistent with recurrent disease were considered adequate proof of recurrence; tissue confirmation was rarely obtained. Only first sites of recurrence were documented.
χ2 tests were used to compare categorical variables. Independent t tests and Mann-Whitney tests were used to evaluate continuous variables.
The primary outcome of interest was overall survival (OS). The secondary endpoint was pattern of recurrence. OS and follow-up were calculated from the time of initial cytologic or histologic diagnosis to date of death or last contact. The Kaplan-Meier method was used to estimate OS.24 The log-rank test was used to compare differences in time to event distributions for covariates of interest. Cox proportional hazards regression was performed to test the effects of potential prognostic factors on OS. Covariates included age, gender, tumor size, lymph node (N) status, need for reoperative PD, presence of a major complication, amount of operative blood loss (per 100 mL), SMA margin status, use of adjuvant therapy (preoperative and/or postoperative), and need for major vascular resection. Logistic regression was performed to examine the impact of age, sex, vascular resection, tumor size, lymph node status, and preoperative therapy on margin status. Covariates were tested in a univariate manner. Variables that were significant on univariate analysis at P < 0.10 were included in multivariate analyses.
A P value of less than 0.05 was considered statistically significant. All data analyses were performed with SPSS version 12.0 software (SPSS, Inc., Chicago, IL).
RESULTS
Patient Characteristics
PD for adenocarcinoma of pancreatic origin was performed in 360 (49.7%) of 724 patients who underwent PD for a variety of histologic diagnoses during the period of this study. There were no R2 resections recorded in the medical records, pathology reports, or operative dictations, a function of the objective CT criteria used at our institution to define a potentially resectable pancreatic neoplasm and the multidisciplinary approach to treatment decisions, including surgery. Margins were histologically positive (R1) in 60 (16.7%) and negative (R0) in 300 (83.3%) of the 360 patients. R1 resections occurred in 40 (21%) of 191 patients prior to January 1, 2000 (at which time the system for SMA margin analysis changed; refer to Methods) and in 20 (12%) of 169 after January 1, 2000. The SMA margin was positive in 53 (88.3%) of 60 patients with R1 resections; the remaining 7 patients (11.7%) had isolated positive pancreatic transection margins. Among the 53 patients with positive SMA margins, 5 patients also had other positive margins: 3 positive pancreatic transection margins, 1 positive bile duct transection margin, and 1 positive pancreatic and bile duct transection margins. Tumor size data were not available in 32 patients either because size could not be accurately assessed due to the treatment effect induced by preoperative chemotherapy and/or chemoradiation or because the data was not recorded.
Predictors of R1 Resection
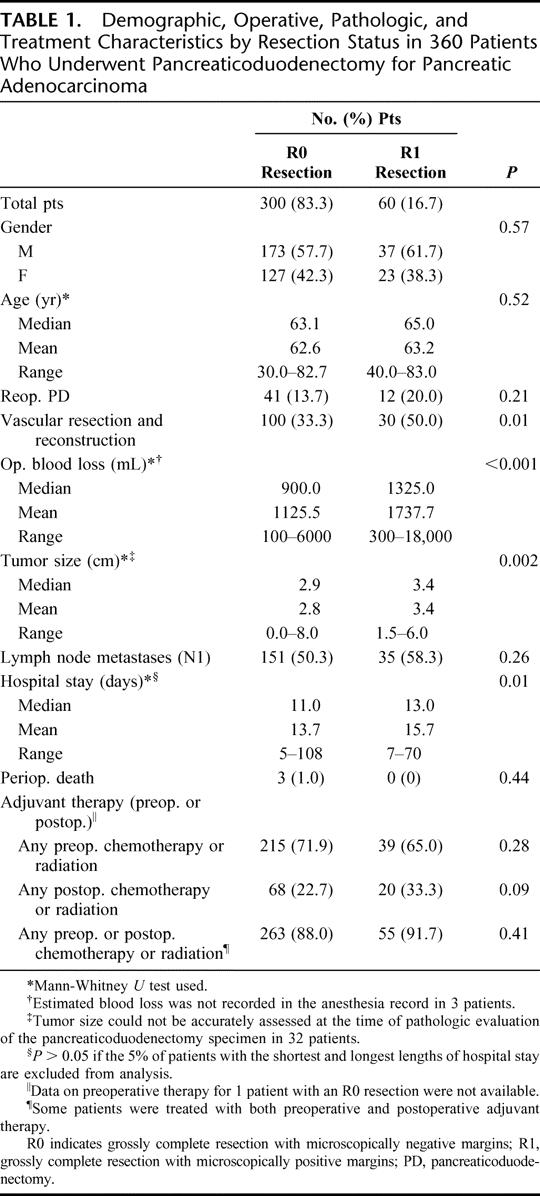
Univariate analysis of demographic, operative, pathologic, and treatment characteristics of the 2 resection groups is shown in Table 1. Patients who underwent vascular resection and reconstruction were more likely to have had an R1 resection. Patients who had an R1 resection had greater mean operative blood loss, larger median tumor size, and a longer mean hospital stay (Table 1).
TABLE 1. Demographic, Operative, Pathologic, and Treatment Characteristics by Resection Status in 360 Patients Who Underwent Pancreaticoduodenectomy for Pancreatic Adenocarcinoma
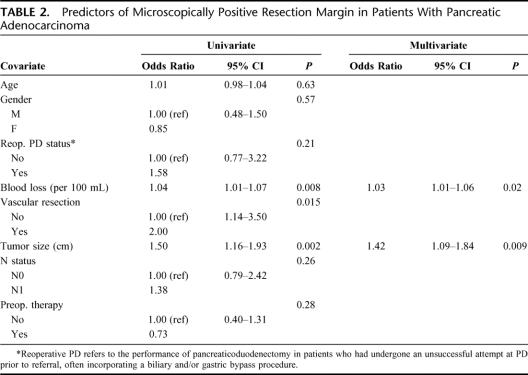
Logistic regression was used to determine which covariates (other than length of stay) were significantly associated with an R1 resection (Table 2). Operative blood loss (per 100 mL), vascular resection, and tumor size were statistically significant by univariate analyses. When adjusting for these factors in a backward stepwise fashion, only operative blood loss (odds ratio [OR] = 1.03; 95% confidence interval [CI], 1.01–1.06) and tumor size (OR = 1.42; 95% CI, 1.09–1.84) were associated with an R1 resection.
TABLE 2. Predictors of Microscopically Positive Resection Margin in Patients With Pancreatic Adenocarcinoma
Overall Survival
Univariate analysis of factors influencing median OS in this study is shown in Table 3. The median OS for the 360 patients was 25.1 months. Log-rank tests were used to compare Kaplan-Meier survival curves for each prognostic factor of interest. Lymph node metastases, R1 resection, and major perioperative complications were associated with decreased OS. Median OS was 21.6 months in 186 patients with lymph node metastases (N1), compared with 31.9 months for 174 patients with node-negative (N0) disease (P = 0.002). Median OS was 21.5 months in 60 patients after an R1 resection, compared with 27.8 months in 300 patients after an R0 resection (P = 0.026). Kaplan-Meier survival curves comparing R status are shown in Figure 2. Median OS was 21.6 months in 93 patients with major perioperative complications, compared with 28.4 months in 263 patients without major perioperative complications (P = 0.013).
TABLE 3. Median Survival in 360 Patients Who Underwent Pancreaticoduodenectomy for Pancreatic Adenocarcinoma
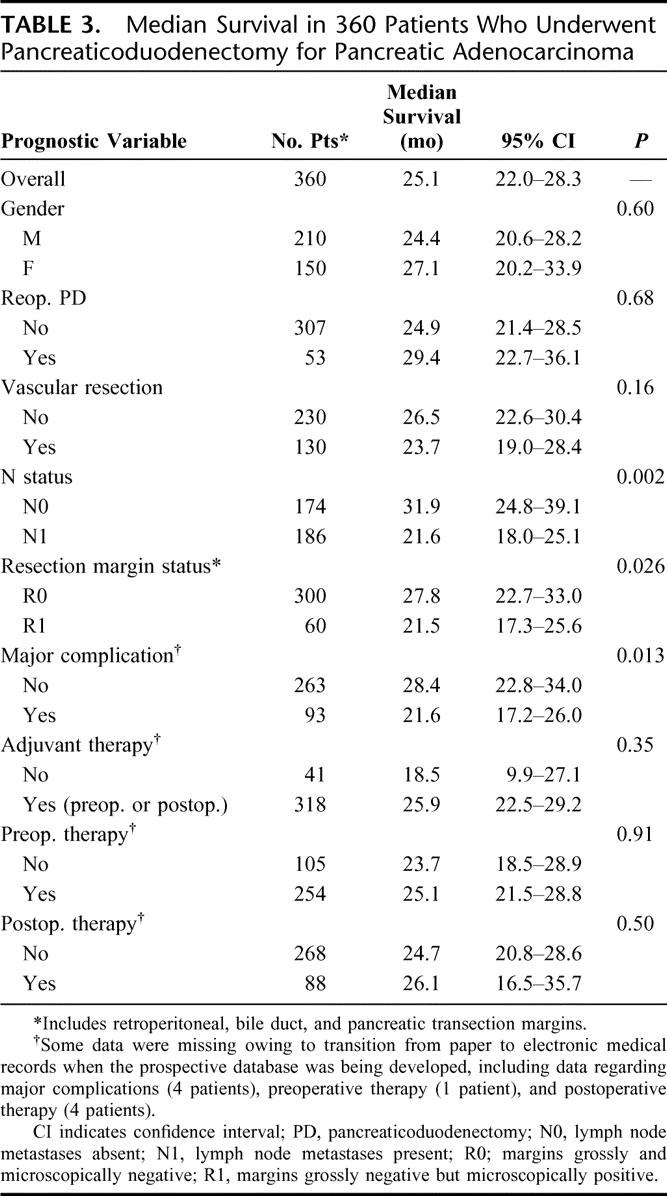
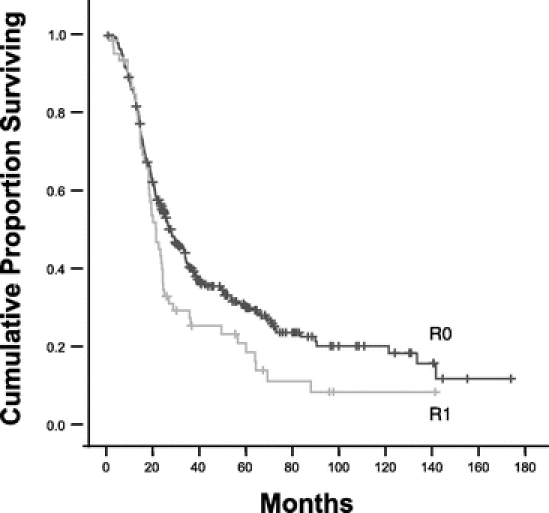
FIGURE 2. Kaplan-Meier survival curves for patients with pancreatic adenocarcinoma who underwent pancreaticoduodenectomy resulting in an R0 or an R1 resection. Median survival for 300 patients undergoing R0 resection was 27.8 months. Median survival for 60 patients undergoing R1 resection was 21.5 months (P = 0.027 on univariate analysis). On multivariate analysis, an R1 resection did not independently affect survival.
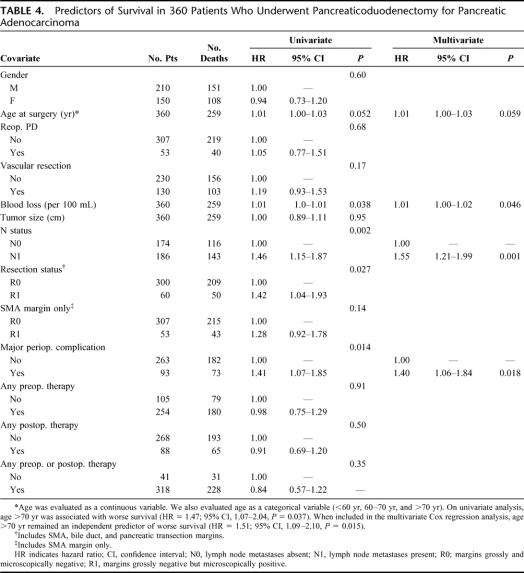
Table 4 lists the covariates that were included in separate univariate Cox proportional hazards regression analyses. Covariates that affected OS at the P < 0.10 level of significance were included in a multivariate Cox proportional hazards model. After adjusting for these variables in a backwards stepwise fashion, only the presence of lymph node metastases (hazard ratio [HR] = 1.55; 95% CI, 1.21–1.99), major perioperative complications (HR = 1.40; 95% CI, 1.06–1.84), and operative blood loss (HR = 1.01; 95% CI, 1.00–1.02) adversely affected OS. Although R status influenced OS on univariate analysis (HR = 1.42; 95% CI, 1.04–1.93), an R1 resection did not independently affect survival after controlling for all other variables.
TABLE 4. Predictors of Survival in 360 Patients Who Underwent Pancreaticoduodenectomy for Pancreatic Adenocarcinoma
To determine the impact of a positive SMA margin on OS, we reanalyzed our data by categorizing as R1 only those patients with a positive SMA margin (n = 53, 14.7%) and as R0 all other patients, regardless of pancreatic or bile duct transection margins (n = 307, 85.3%). On univariate analysis, R1 resection was not statistically significant (HR = 1.28; 95% CI, 0.92–1.78, P = 0.14). The multivariate analysis included the following variables, which were significant by univariate approach: age, nodal status, major complications, and blood loss. Again, nodal status, major complications, and blood loss remained significant in the final model.
Impact of Resection Status on Disease Recurrence
Median follow-up for censored patients was 51.9 months (mean, 59.5 months; range, 7.5–173.8 months) and for all patients, including those who had died, was 24.0 months (mean, 34.7 months; range, 0.4–173.8 months). Recurrent disease was identified in 41 (68.3%) of 60 patients who underwent an R1 resection and in 199 (66.3%) of 300 patients who underwent an R0 resection (Table 5). Some patients developed first recurrence at more than one site. Resection margin status did not affect the pattern of first recurrence; the proportion of patients with local, regional, or distant sites of first recurrence was similar in the R0 and R1 resection groups. Disease status at last follow-up also appears in Table 5.
TABLE 5. Recurrent Disease and Disease Status

DISCUSSION
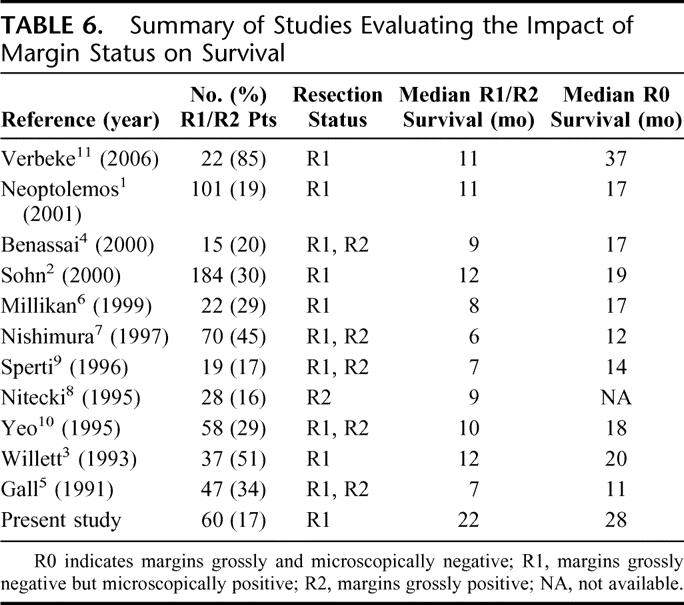
It is a generally accepted principle of oncology that a positive surgical margin after resection of a solid tumor is a poor prognostic factor. Much of the data supporting this principle in pancreatic cancer have been based on studies that did not sufficiently differentiate between R1 and R2 resections (Table 6). This is because few authors have had access to margin data obtained prospectively using a standardized system for pathologic analysis of the PD specimen. Even in studies in which exclusion of R2 resections was intended, it is unclear which margins were reported and how they were analyzed. The distinction between R1 and R2 resections is difficult to determine accurately for several reasons. First, R status cannot be recorded retrospectively unless the surgical margins were inked appropriately and the operative note described the presence or absence of a complete resection. Second, there is no consensus opinion on how the PD specimen should be pathologically evaluated; therefore, when reviewing a pathology report, it is often difficult to understand which margin has been examined. We have used the system recommended by the AJCC Cancer Staging Manual, which evaluates the soft tissue and perineural fibers adjacent to the right lateral border of the SMA. Most would agree that this margin is the most likely location (although not the only one) for tumor extension, which may result in an incomplete resection. Third, the pathologist at the time of gross and microscopic inspection cannot differentiate a grossly positive from a microscopically positive margin. Unless the surgeon documented whether all gross tumor was or was not removed, the only way to determine the likelihood of a grossly incomplete resection is to examine the preoperative CT scan; if tumor is encasing the SMA or celiac axis, it is very likely that a positive margin described in the pathology report reflects a grossly incomplete (R2) resection. Fourth, the operative note rarely contains information regarding the completeness of resection, and if it does, it represents a subjective, nonreproducible assessment; therein lies the rationale for utilizing an objective, reproducible CT-based system for defining resectability based on the preoperative assessment of critical tumor-vessel relationships.
TABLE 6. Summary of Studies Evaluating the Impact of Margin Status on Survival
In examining our data set of surgical specimens evaluated prospectively using a standardized system for pathologic analysis of the PD specimen, we found that resection status did not significantly affect disease recurrence and survival. The strength of the data set reported herein includes the use of objective radiographic criteria for preoperative patient selection as an objective way to minimize the potential for an R2 resection, a standardized surgical technique designed to minimize R1 resections, and the prospective pathologic evaluation of surgical specimens to accurately determine resection status.
The majority of the R1 resections in this report (88.3%) were due to a positive SMA margin. In this article, we introduce the term “SMA margin” to describe the perivascular soft tissue, primarily perineural and mesenteric tissue, adjacent to (and posterior to) the right lateral border of the proximal SMA. The term previously used by us and commonly used by others to describe this area is the “retroperitoneal margin.” However, this term is somewhat imprecise, as the retroperitoneal margin may also refer to the tissue anterior to the inferior vena cava and aorta and posterior to the pancreatic head and uncinate process. Similarly, some have used the term “uncinate margin” to describe the margin of resection along the entire proximal SMA; this also is somewhat anatomically incorrect. Therefore, we propose that the margin of resection along the posterolateral border of the proximal SMA be termed the “SMA margin,” a term more anatomically precise than mesenteric, uncinate, or retroperitoneal. As described in Methods, the technique for assessment of the SMA margin changed in January 2000 from an en face section of the margin (usually taken by the surgeon) to a system of inking the margin and microscopic examination of sections taken perpendicular to the inked margin (performed by the pathologist). It is possible that the older method of evaluating the SMA margin was responsible for the higher rate of margin positivity (prior to the year 2000) in comparison to the current method. However, surgeon experience with respect to patient selection and surgical technique may also have affected this difference in margin positivity over time. One could argue that other soft tissue margins of resection such as the anterior and posterior pancreatic margins should also be evaluated.11 While this may be true, a system for PD specimen analysis more complicated than currently recommended in the AJCC Cancer Staging Manual is unlikely to be used in the United States where most pathologists have limited experience with PD specimens.
By univariate analysis, R1 resections were associated with larger tumors, greater mean operative blood loss, longer hospital stays, and the need for vascular resection and reconstruction. However, multivariate analysis of all variables that may influence margin status found that tumor size and mean operative blood loss were the only covariates that affected margin status. This is consistent with the findings of a previous manuscript from our group analyzing our experience with vascular resection in a subset of the current study population.17 The present study differs from that report in terms of sample size and the examination of all resection margins (including bile duct and pancreatic transection margins), rather than only the SMA margin. In both reports, larger tumor size was associated with an increased risk of a positive SMA margin. It is reasonable to assume that larger tumor size was associated with an increased likelihood of tumor extension into the SMA margin simply on the basis of anatomic considerations. In addition, a larger tumor may necessitate a technically more difficult operation, potentially leading to increased blood loss. Importantly, tumor size was recorded at the time of gross pathologic evaluation of the PD specimen. Although preoperative chemotherapy and chemoradiation may have reduced the median tumor size to some degree (as assessed following resection), the effect should have been comparable in the R0 and the R1 groups (Table 1) because approximately two thirds of patients in both groups received preoperative therapy.
In the present analysis, neoadjuvant therapy was not a statistically significant predictor of margin status; an R1 resection occurred in 39 (15.4%) of 254 patients who received some form of preoperative therapy and in 21 (20.0%) of 105 patients who went straight to surgery (P = 0.28). If the analysis is limited to positive SMA margins, and the 7 patients with isolated positive pancreatic transection margins are considered R0, the numbers change to 33 (13.0%) of 254 patients who received preoperative therapy and 20 (19.0%) of 105 patients who did not (P = 0.17). While not significant, these results were likely affected by some degree of selection bias. For example, patients who did not receive preoperative therapy may have had more favorable tumors (by multidisciplinary CT review) for immediate resection and been thought to be at lowest risk for an R1 resection. Our data do not allow further analysis of this concern.
An R1 resection did not affect the pattern of first recurrence. Specifically, the presence of a positive margin did not result in a greater number of local or regional recurrences. The dominant pattern of failure, irrespective of margin status, was distant disease. An R1 resection was also not an independent predictor of poor survival by multivariate analysis. To what degree these findings were influenced by preoperative staging (accurate assessment of resectability), operative technique (completeness of the SMA dissection), and the use of multimodality therapy cannot be accurately quantified. These results do not support the conclusion of the ESPAC-1 trial that tumors with positive resection margins represent a biologically more aggressive cancer, independent of patient selection, surgical technique, and the use of adjuvant chemotherapy and/or chemoradiation.1
In addition to careful patient selection for surgery, we used a standardized operative technique that emphasizes the removal of all mesenteric and perineural tissue from the right lateral border of the SMA with clear visual identification of this vessel.14 Because the tumor-SMA interface may measure only a few millimeters, it is reasonable to assume that if the surgeon simply palpates the SMA, without exposing it, and then divides the tissue to the right of the SMA with a series of clamps or a stapling device, the risk of a positive margin would be increased. The critical importance of intraoperative attention to the SMA has probably not been adequately emphasized in the education of surgeons and in the surgical literature, despite the oncologic importance associated with this part of the operation. For example, it has been our anecdotal experience that the majority of our surgical fellows who have completed general surgical residency have not seen the adventitia of the SMA when assisting with a PD as a resident. Importantly, even when a complete SMA dissection is performed, with no palpable or visible evidence of tumor, a microscopically positive margin of resection may occur due to the infiltrative nature of pancreatic adenocarcinoma. The incidence of R1 resections in the current study was 17%. To what extent this number was affected by variables associated with a specialty center (preoperative imaging, experienced surgeons, preoperative therapy) cannot be accurately determined. However, one would assume that the frequency of positive margins would be higher when this operation is performed in a multi-institutional setting.
Our data provide valuable information for the conduct of clinical trials that involve PD. Such trials must distinguish between grossly complete (R0/R1) resections and grossly incomplete (R2) resections owing to the impact of an R2 resection on survival, as suggested by previous authors.1–10 This distinction cannot be made accurately by retrospective review of operative notes, which may or may not contain subjective assessments of the completeness of resection, unless the participating surgeons have all adopted a standard operative technique. Differentiating an R1 from an R2 resection is a complicated and difficult challenge and requires the use of accurate pretreatment staging using high-quality cross-sectional imaging, an objective definition of resectable disease (using such imaging) combined with a standard surgical technique, and a well-defined system for the pathologic analysis of the PD specimen. It has not yet been demonstrated that these criteria can be met in a multi-institutional setting, which is a major impediment to the conduct of cooperative group trials for localized, potentially resectable pancreatic cancer.
Our current system for reporting the final resection status requires that the surgeon wait to finalize the operative note until after the pathology report has been completed. The pathologist cannot, in general, distinguish an R1 from an R2 resection; such information has to be accurately recorded by the surgeon. For example, assuming both the surgeon and pathologist are experienced, if the surgeon appreciates that the tumor extends to the left of (medial to) the SMA and is therefore not completely removed at the time of surgery, a histologically positive SMA margin should result in an R2 designation on the final operative dictation. In contrast, if the surgeon performs a grossly complete resection and the SMA margin is histologically positive when accurately identified, inked, and examined by the pathologist, then the operative dictation should reflect the performance of an R1 resection. Our data suggest that almost one in 5 patients will have a microscopically positive margin following PD for pancreatic adenocarcinoma. This number clearly may change as more effective therapies are developed for this disease involving more innovative treatment schema that apply systemic and/or locoregional therapies prior to surgery.
ACKNOWLEDGMENTS
The authors thank Dr. Henry Gomez for data acquisition and analysis, Drs. Chusilp Charnsangavej and Eric Tamm for diagnostic imaging, Dr. Karen Cleary for histopathology, and Drs. James Abbruzzese and Gauri Varadhachary for the medical oncology management of many of these patients.
Footnotes
Supported by the Lockton Fund for Pancreatic Cancer Research, the Hamill Foundation, the Various Donor Fund at the University of Texas M. D. Anderson Cancer Center, and NIH 1 P20 CA101936-01 (SPORE in Pancreatic Cancer).
Presented in part at the 45th Annual Meeting of the Pancreas Club, New Orleans, Louisiana, May 15–19, 2004.
Reprints: Douglas B. Evans, MD, Department of Surgical Oncology, Box 444, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030. E-mail: devans@mdanderson.org.
REFERENCES
- 1.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. [DOI] [PubMed] [Google Scholar]
- 3.Willett CG, Lewandrowski K, Warshaw AL, et al. Resection margins in carcinoma of the head of the pancreas: implications for radiation therapy. Ann Surg. 1993;217:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benassai G, Mastrorilli M, Quarto G, et al. Survival after pancreaticoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Chir Ital. 2000;52:263–270. [PubMed] [Google Scholar]
- 5.Gall FP, Kessler H, Hermanek P. Surgical treatment of ductal pancreatic carcinoma. Eur J Surg Oncol. 1991;17:173–181. [PubMed] [Google Scholar]
- 6.Millikan KW, Deziel DJ, Silverstein JC, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg. 1999;65:618–623; discussion 623–624. [PubMed]
- 7.Nishimura Y, Hosotani R, Shibamoto Y, et al. External and intraoperative radiotherapy for resectable and unresectable pancreatic cancer: analysis of survival rates and complications. Int J Radiat Oncol Biol Phys. 1997;39:39–49. [DOI] [PubMed] [Google Scholar]
- 8.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas: is it really improving? Ann Surg. 1995;221:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperti C, Pasquali C, Piccoli A, et al. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg. 1996;83:625–631. [DOI] [PubMed] [Google Scholar]
- 10.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg. 1995;221:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verbeke CS, Leitch D, Menon KV, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. [DOI] [PubMed] [Google Scholar]
- 12.General information on cancer staging and end-results reporting. In: Greene FL, Page DL, Fleming ID, et al, eds. AJCC Cancer Staging Manual. Chicago: Springer, 2002:1–16. [Google Scholar]
- 13.Exocrine pancreas. In: Greene FL, Page DL, Fleming ID, et al, eds. AJCC Cancer Staging Manual. Chicago: Springer, 2002:157–164. [Google Scholar]
- 14.Yen T, Abdalla E, Pisters PWT, et al. Pancreaticoduodenectomy. In: von Hoff DD, Evans DB, Hruban RH, eds. Pancreatic Cancer. Sudbury, MA: Jones and Bartlett, 2005. [Google Scholar]
- 15.Scoggins CR, Lee JE, Evans DB. Pancreaticoduodenectomy with en bloc vascular resection and reconstruction for localized carcinoma of the pancreas. In: von Hoff DD, Evans DB, Hruban RH, eds. Pancreatic Cancer. Sudbury, MA: Jones and Bartlett, 2005:321–334. [Google Scholar]
- 16.Tamm EP, Silverman PM, Charnsangavej C, et al. Diagnosis, staging, and surveillance of pancreatic cancer. AJR Am J Roentgenol. 2003;180:1311–1323. [DOI] [PubMed] [Google Scholar]
- 17.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–950. [DOI] [PubMed] [Google Scholar]
- 18.Varadhachary GR, Tamm EP, Crane C, et al. Borderline resectable pancreatic cancer. Curr Treat Options Gastroenterol. 2005;8:377–384. [DOI] [PubMed] [Google Scholar]
- 19.Staley CA, Cleary KR, Abbruzzese JL, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12:373–380. [DOI] [PubMed] [Google Scholar]
- 20.Pisters PW, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–132. [DOI] [PubMed] [Google Scholar]
- 22.Pisters PWT, Wolff RA, Janjan NA, et al. Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: toxicities, histologic response rates, event-free outcome. J Clin Oncol 2002;20:2537–2544. [DOI] [PubMed] [Google Scholar]
- 23.Wolff RA, Evans DB, Crane C, et al. Initial results of preoperative gemcitabine (GEM)-based chemoradiation for resectable pancreatic adenocarcinoma. 38th Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, vol. 21, 2002:130a.
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;185:1457–1481. [Google Scholar]