Abstract
Summary Background Data:
Chronic portal obstruction can lead to formation of portal cavernoma (PC). Half of all patients with PC will develop cholestasis, termed portal biliopathy, and some will progress to symptomatic biliary obstruction. Because of the high hemorrhage risk associated with biliary surgery in patients with PC, the optimal therapeutic strategy is controversial.
Methods:
Retrospective review of a single hepatobiliary center experience, including 64 patients with PC identified 19 patients with concurrent symptomatic biliary obstruction. Ten patients underwent initial treatment with a retroperitoneal splenorenal anastomosis. For the remaining 9 patients, portal biliopathy was managed without portosystemic shunting (PSS). Outcomes, including symptom relief, the number of biliary interventions, and survivals, were studied in these 2 groups.
Results:
Within 3 months of PSS, 7 of 10 patients (70%) experienced a reduction in biliary obstructive symptoms. Five of these 10 patients subsequently underwent uncomplicated biliary bypass, and none has recurred with biliary symptoms or required biliary intervention with a mean follow-up of 8.2 years. For patients without PSS, repeated percutaneous and endobiliary procedures were required to relieve biliary symptoms. Four of the 9 patients with persistent PC required surgical intrahepatic biliary bypass, which was technically more challenging. With a mean follow-up of 8 years, 1 of these 9 patients died of severe cholangitis, 1 remained jaundiced, and 7 were asymptomatic.
Conclusions:
This study, which represents the largest published experience with the surgical treatment of patients with symptomatic portal biliopathy, indicates that retroperitoneal splenorenal anastomosis improves outcomes and should be the initial treatment of choice.
The association of portal cavernoma and symptomatic biliary obstruction is a rare situation where the extrahepatic portal hypertension is the cause of biliary compression and the obstacle of its treatment. An initial portal decompression by retroperitoneal splenorenal anastomosis could solve the problem with or without a secondary biliodigestive bypass.
A portal cavernoma (PC) or a cavernous transformation of the portal vein is a concentrated vascular system composed of dilated periportal veins within the hepatic pedicle.1 This venous abnormality develops from chronic obstruction of the distal portal vein in a compensatory effort to maintain hepatofugal flow. The magnitude of PC is related to the extent of portal thrombosis. Typically, the livers of patients with PC are otherwise normal.
In the absence of abdominal surgery, where PC may lead to significant intraoperative hemorrhage, this abnormality is generally asymptomatic.2,3 However, within the hepatic pedicle dilatation of veins around and inside the bile duct wall can impact on the adjacent biliary ducts.4 Indeed, the majority of patients with PC will develop morphologic biliary abnormalities, termed “portal biliopathy.”5 Portal biliopathy may lead to asymptomatic cholestasis in half of patients; and more rarely, it can cause symptomatic biliary obstruction.6
The development of symptomatic portal biliopathy from PC poses a difficult therapeutic problem, as the PC is frequently a significant obstacle to treatment of the biliary obstruction. Furthermore, there are very few reports in the literature describing the treatment of portal biliopathy and even fewer with long-term outcome analyses. The aim of this study, therefore, was to report our therapeutic strategies and long-term results following the treatment of 19 patients with PC complicated by symptomatic biliary obstruction.
MATERIALS AND METHODS
Inclusion Criteria
From 1980 to 2000, 64 patients with the diagnosis of PC in the absence of malignancy were hospitalized at the Hepato-Biliary Center in Paul Brousse Hospital. Nineteen (29%) of these 64 patients presented with signs and symptoms of biliary obstruction (jaundice and/or cholangitis), and comprise the cohort analyzed here.
Biochemical Evaluation
Jaundice with hyperbilirubinemia (defined as a concentration of total bilirubin >30 μmol/L) and cholestasis (defined as a concentration of γ-glutamyl transferase >50 IU/L) was confirmed in all patients. Cholangitis, defined as the clinical picture of jaundice with abdominal pain and temperature >38.5°C and/or the presence of pathogenic microorganisms in cultured bile, was present in 7 (36%) patients.
Diagnostic Evaluation
To analyze biliary and portal abnormalities, an admission transparietal liver echography was performed in all cases. Doppler echography was used to diagnose PC and to assess the extent of portal thrombosis. When the PC was not obscuring the extrahepatic bile ducts, echography was also used to characterize the portal biliopathy. To further assess portal biliopathy, percutaneous cholangiography was performed in 11 (58%) of 19 patients. Further evaluation of the portal venous system was made with angiography in 13 (68%) of 19 patients (Fig. 1). To assess the presence and grade of esophageal varices according to the criteria of Paquet,7 an upper gastrointestinal endoscopy was performed in 10 (52%) of 19 patients.
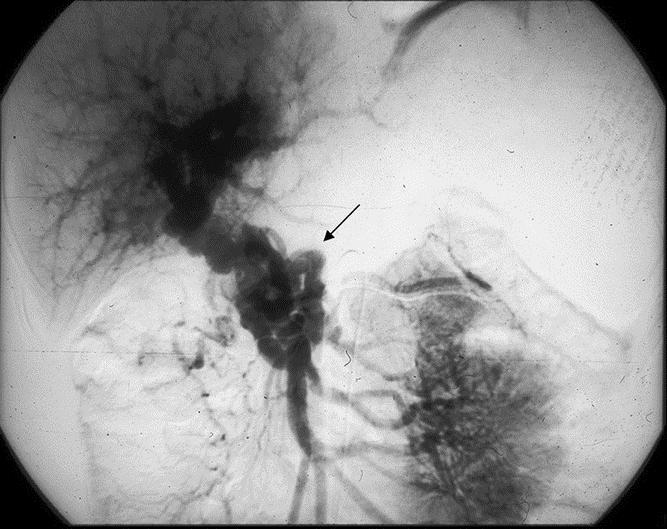
FIGURE 1. Portal flow through portal cavernoma after injection of contrast in the superior mesenteric artery: typical appearance of portal cavernoma within the hepatic pedicle (arrow).
Therapeutic Strategy
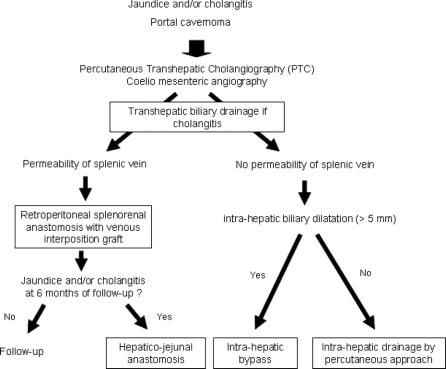
In patients with PC complicated by biliary obstruction, the primary goal is to urgently address life-threatening cholangitis, if present. When possible, however, we prefer percutaneous decompression to surgical decompression for 2 reasons. First, attempts at biliary-enteric bypass in the setting of PC are associated with a high risk of hemorrhage. Second, mechanical biliary obstruction may extend high into the porta hepatis and even into the liver, making an adequate surgical biliary diversion difficult to achieve. Given these issues, we prefer to treat the PC with portal decompression prior to definitively addressing the biliary obstruction. In some patients, this maneuver may, in itself, relieve the biliary obstruction. In those patients with persistent biliary obstruction, a surgical bypass is made safer by the previous portal decompression. Hence, treatment of patients with symptomatic biliary obstruction can be approached in 3 steps:
Initial transhepatic biliary drainage and antibiotic therapy to treat cholangitis, if present;
Surgical portosystemic anastomosis to collapse the PC;
Biliodigestive anastomosis (hepatico-jejunal anastomosis with Roux-en-Y) in patients who remain symptomatic and/or develop intrahepatic calculi despite portal decompression.
All eligible patients presenting to our institution with symptomatic portal biliopathy were evaluated for treatment with this strategy. The approach was possible in 10 (52%) of the 19 patients. For the remaining 9 (47%) patients, however, portal decompression with portosystemic anastomosis was not feasible due to the extent of portal thrombosis or other patient-related factors. For this group of patients, the treatment involved 1 or 2 phases: biliary drainage associated with or without an intrahepatic biliary-enteric anastomosis.
RESULTS
Definition of Groups and Demographic Data
For data analysis, we defined 2 groups of patients based on our ability to decompress the PC prior to surgical treatment of the biliary obstruction; respectively, the portosystemic shunt group (PSS) (eg, patient 1 to patient 10) and the no portosystemic shunt group (NPSS) (eg, patients 11 to 19) (Table 1). Decompression of PC was not attempted in the NPSS group patients due to extensive thrombosis of the portal circulation in 4 patients (eg, patients 11, 12, 14, and 16), effective biliary drainage by the endoscopic approach before referral in 3 patients (eg, patients 13, 15, and 17), and initial inability to confirm the diagnosis of PC prior to exploration in 2 patients (eg, patients 18 and 19). For these 2 patients, preoperative angiographic assessment was unavailable, explaining the absence of surgical PC decompression prior to surgical biliary bypass.
TABLE 1. Characteristics of Patients
Timing of PC Diagnosis
In the PSS group patients, PC was discovered in 5 patients during pre-referral laparotomy (Table 1). For the other patients in the PSS group, PC was known before biliary obstruction occurred in 3 patients (eg, patients 1, 2, and 3) and discovered during exploration for biliary symptoms in 2 patients (eg, patients 4 and 7). For 2 patients in the NPSS group, PC was diagnosed during laparotomy: 1 patient urgently explored for massive hemobilia after endoscopic introduction of a plastic stent (eg, patient 18), and 1 patient explored for a presumed diagnosis of malignant biliary stricture (eg, patient 19). This second patient had initially received pre-referral treatment with an endobiliary stent and 6 months of systemic chemotherapy. For the other patients in the NPSS group, PC was known before biliary obstruction in 6 patients (eg, patients 11, 12, 14, 15, 16, and 17) and discovered during exploration of biliary symptoms in 3 patients (eg, patients 13, 18 and 19).
Results of Radiologic Assessment of the Biliary Obstruction
In 4 of the 10 PSS patients, surrounding PC limited sonographic evaluation of the extrahepatic bile ducts. In 5 patients, extrahepatic biliary dilatation was found on echography. Finally, 1 patient had intrahepatic bile duct dilatation but no extrahepatic dilatation was identified. Intrahepatic calculi were found in 3 patients (eg, patients 3, 6, and 9).
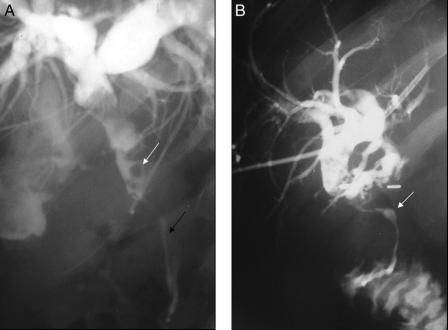
Transhepatic cholangiography was performed in 9 of these 10 patients (Fig. 2) These examinations provided precise morphologic descriptions of each patient's biliary abnormalities, which were consistently located in superior or middle part of the extrahepatic bile duct. Although some patients had multiple abnormalities, 3 separate types of abnormalities were observed: discreet short-segment bile duct stenosis, smooth long-segment filling defect, and acute angulation of the bile duct (Table 2). In addition, 3 patients with pre-referral attempts at biliary-enteric bypass were diagnosed with intrahepatic calculi during cholangiography.
FIGURE 2. Percutaneous transhepatic cholangiography: features of symptomatic biliary obstruction in portal cavernoma. A, Tight and irregular stenosis of common bile duct (black arrow) with “fingerprint sign” (white arrow). B, Tight stenosis of the common bile duct by portal cavernoma (white arrow) proximal to an ineffective choledochojejunal anastomosis.
TABLE 2. Results of Cholangiography in Group PSS
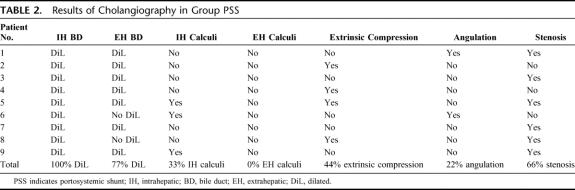
In 2 NPSS group patients, the extrahepatic bile duct was obscured by PC. Echo in the other patients showed extrahepatic bile duct dilatation. At cholangiography, intrahepatic calculi were found in 4 patients (eg, patients 11, 14, 16, and 19) and extrahepatic calculi in 4 patients (eg, patients 11, 12, 13, and 17).
Initial Management Group PSS
Biliary Drainage
Because of initial severe sepsis from cholangitis, percutaneous transhepatic biliary drainage with extraction of intrahepatic calculi was performed in 1 patient (eg, patient 9).
Portosystemic Shunt
A splenorenal anastomosis with internal jugular interposition graft was performed in the 10 patients via a retroperitoneal approach. For this procedure, the patient is placed in the right lateral decubitus position with the left neck included in the operative field. An oblique incision is made in 10th intercostal space toward the left flank with a phrenotomy. The spleen is mobilized anteriorly and the kidney retracted caudally. The renal hilum is identified and the superior side of left renal vein is dissected free for a length of 3 cm. The splenic vein is controlled at the inferior surface of the pancreatic tail. Both veins are punctured with a small needle connected to manometry to confirm a pressure gradient in excess of 10 mm of mercury. The proximal splenic vein is separated from the pancreatic tail by serial ligature of collateral veins with 6–0 Prolene. The internal jugular vein is harvested for a length of 5 cm in a standard fashion noting the direction of flow. The left renal vein is laterally clamped to perform an end-to-side anastomosis with running 6–0 Prolene suture to the distal part of the venous graft. The splenic vein is then laterally clamped and the proximal part of the venous graft is sutured to it using the same technique. After clamp removal, the pressure gradient is once again measured to confirm a reduction of at least 50%. After hemostasis is confirmed, a closed suction drain is left in the retroperitoneal space and the wound is closed.
Mortality following these procedures was nil. The postoperative morbidity rate was 27%, including 1 chylus fistula and 2 early thromboses of the splenorenal anastomosis. These thromboses were diagnosed by postoperative Doppler echography, which were systematically performed on a daily basis from postoperative day 1 to 5. In each of these cases, the thrombosis was successfully treated by anticoagulant treatment and selective angiography-guided pneumatic dilatation. When present prior to PSS, biliary drains were removed before discharge. The mean length of hospitalization for this group was 20 ± 12 days.
Treatments in Group NPSS
Biliary Drainage (n = 5 of 9)
Before referral, endoscopic biliary drainage had been performed in 3 patients (eg, patients 13, 18, and 19). An isolated extraction of calculi was performed via a sphincterotomy in one of these patients (eg, patient 13) and a plastic endobiliary stent was introduced in the other 2 patients (eg, patient 18 and 19). After referral, we performed percutaneous biliary drainage because of cholangitis in 4 patients (eg, patients 12, 15, 17, and 18) and because of severe jaundice with pruritus in 2 patients (eg, patients 13 and 19).
Biliodigestive Anastomosis (n = 4 of 9)
Intrahepatic biliodigestive anastomosis to the left liver (n = 3) or to the right liver (n = 1) was performed in 4 of 9 patients in the NPSS group (eg, patient 11, 12, 14 and 16), all of whom presented with intrahepatic biliary dilatation. In 1 patient, a previously placed transhepatic biliary catheter was closed for 3 weeks prior to operation, to allow the intrahepatic bile ducts to dilatate (eg, patient 12).
Short-term Results in Group PSS (n = 10)
Clinical
All patients were examined at 3 months following portosystemic shunt. There was a distinct improvement in patient symptoms with resolution of jaundice and/or cholangitis in 7 (70%) of 10 patients. Mild jaundice persisted in 1 of 10 patients (eg, patient 1) (bilirubin = 38 μmol/L) and 2 of 10 patients (eg, patients 4 and 9) experienced an increase in jaundice, although neither patient developed cholangitis.
Biologic
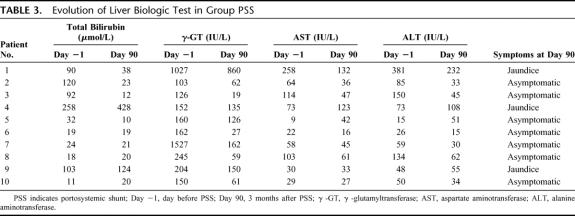
Results of liver biologic tests drawn before and 3 months after portosystemic shunt (Table 3) were compared. This analysis determined that there were no significant changes in these parameters between these 2 time periods. However, there was a trend toward improvement in liver function tests in patients treated by PSS.
TABLE 3. Evolution of Liver Biologic Test in Group PSS
Upper Gastrointestinal Endoscopy
Results of upper gastrointestinal endoscopy have been compared before and 3 months after portosystemic shunt in 5 patients. In 1 patient, a reduction in the grade of esophageal varices from grade II to grade I was observed (eg, patient 10). There was no modification in the grade of varices in the 4 other patients, all of whom initially presented with grade I esophageal varices (eg, patients 1, 2, 6, and 7).
Echography
Results of echography performed before and 3 months after portosystemic shunt in 9 patients were also compared. Except for 1 patient, who had normal echography (eg, patient 3), there was persistence of intrahepatic bile duct dilatation despite portosystemic shunt. However, contrary to initial echography where PC obscured the extrahepatic biliary ducts in some patients, extrahepatic bile ducts were visible in all patients treated with PSS.
Angiography
Results of mesenteric angiography with portal venography were compared before and 3 months after portosystemic shunt in 5 patients. The splenorenal anastomosis was patent in all each of these cases. These examinations determined that PC flow was unchanged in 1 patient (eg, patient 2) and diminished in the 4 other patients (eg, patients 4, 5, 6, and 8).
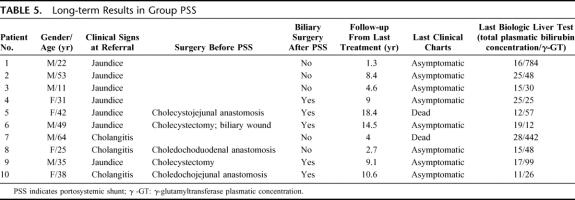
Long-term Results in Group PSS
Between 4 and 30 months after PSS, half of the patients (5 of 10) required a biliodigestive anastomosis (Table 4) due to persistent jaundice in 1 patient and recurrent cholangitis and intrahepatic calculi in 4 patients. Except for 1 of these 5 patients (eg, patient 4), all patients who required biliary surgery after PSS had a history of attempted biliary bypass prior to referral.
TABLE 4. Patients of Group PSS With Biliodigestive Anastomosis Secondarily
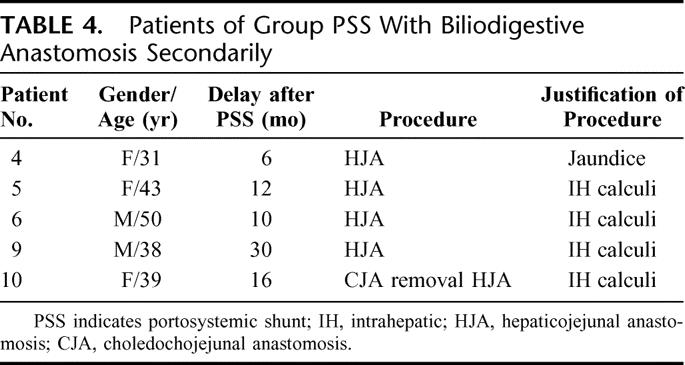
Before biliodigestive anastomosis, each patient underwent transhepatic cholangiography. These examinations revealed that PSS had produced little change in the character of the biliary obstructions for each of these patients. For biliary bypass, a side-to-side hepaticojejunal anastomosis on the biliary convergence with Roux-en-Y jejunal loop was performed. There was no mortality following these procedures; however, 1 patient developed a postoperative biliary fistula that spontaneously resolved.
In the PSS group, the mean follow-up was 8.2 ± 5.3 years after portosystemic shunt. The long-term outcomes in this group are summarized in Table 5. One patient (eg, patient 7) was lost to follow-up 1 year after portosystemic shunt. At this date, he had cholestasis without jaundice. He died 4 years after portosystemic shunt with the diagnosis of decompensated alcoholic cirrhosis in another hospital. Another patient (eg, patient 5) lived for 19 years following PSS without biliary symptoms, eventually dying of a perforated duodenal ulcer.
TABLE 5. Long-term Results in Group PSS
Biochemically, cholestasis persisted in 2 of the 5 patients treated with only a portosystemic shunt. As well, 2 of the 5 patients secondarily operated to perform biliodigestive bypass experienced persistent mild cholestasis.
Results in Group NPSS
For patients in the NPSS group, the mean follow-up interval after their initial percutaneous and/or surgical biliary drainage procedure was 8.3 ± 0.4 years.
Five of these 9 patients have been exclusively treated with biliary drainage. One of these patients has been successfully treated by endoscopic sphincterotomy alone and was subsequently lost to follow-up (eg, patient 13). For the 2 other patients initially managed by endoscopic drainage, this approach failed (eg, patients 18 and 19). One of these patients was jaundiced at the last follow-up (eg, patient 18) and the other (eg, patient 19) patient required repeated percutaneous transhepatic cholangioscopies to extract calculi. The 2 other patients (eg, patients 15 and 17) have been successfully treated by repeated transhepatic cholangioscopies and prolonged internal-external biliary drainage.
Four of the NPSS group patients were treated with intrahepatic biliodigestive anastomosis (eg, patients 11, 12, 14, and 16). Postoperative courses were simple. Although these procedures were associated with an initial reduction in symptoms, repeated percutaneous biliary cholangioscopies via the afferent bowel loop were needed to extract symptomatic intrahepatic calculi in all 4 of these patients. Biliary obstructive symptoms reappeared between 7 months and 40 months after intrahepatic biliodigestive anastomosis. At long-term follow-up 1 patient (eg, patient 11) had died 8.6 years after surgical bypass due to severe cholangitis and hemobilia. The other patients were alive and asymptomatic with a follow-up range between 8 and 9 years.
DISCUSSION
Symptomatic portal biliopathy can raise a difficult problem, where the PC is both the cause of biliary obstruction and the obstacle to its safe surgical treatment. Our preferred therapeutic strategy aimed to primarily decompress the PC with a retroperitoneal PSS. Using this approach, we were able to achieve immediate relief of biliary obstruction in the majority of patients. Furthermore, this strategy allowed the safe performance of a subsequent biliodigestive bypass without mortality. In patients who could not be primarily treated with portal decompression, an intrahepatic biliodigestive bypass, more easily performed when the intrahepatic bile ducts are dilatated, or a prolonged percutaneous external-internal biliary drainage was chosen (Fig. 3). Given that this group required multiple subsequent cholangioscopies to extract residual intrahepatic calculi, we feel this treatment is inferior to PSS.
FIGURE 3. Decision algorithm for treatment of symptomatic portal biliopathy.
Biliary morphologic abnormalities are commonly identified in patients with PC and are referred to as “portal biliopathy.”5 This “portal biliopathy” was initially described with endoscopic retrograde cholangiography in patients with PC.5 Subsequent studies suggest that the prevalence of these abnormalities in patients with PC can range from 80% to 93%.8–11 Recently, a prospective imaging analysis of PC by magnetic resonance (MR) cholangiography coupled with MR portography found biliary abnormalities in 92% of 25 consecutive patients with PC.6,11 As observed in our patients, these biliary morphologic abnormalities include stenoses and angulations associated with wall-filling defects related to compression of the biliary tree by the lumen of PC veins and were predominant in the suprapancreatic part of the common bile duct.6,11 The reported incidence of clinical cholestasis in PC, as ascertained by an elevated serum γ-glutamyl transferase, ranges between 40% and 85% in adult series6,9,10 but appears to be much less common in children (5%).8,12
The rate of symptomatic biliary obstruction in “portal biliopathy” is difficult to ascertain because various reports have defined “symptoms” differently. While some studies have included acute biliary pain as a potential symptom, other studies, including our study, only included patients with persistent jaundice or cholangitis. Considering only the 5 series reporting more than 20 patients with portal biliopathy and after exclusion of patients with acute abdominal pain as their only symptom, the incidence of symptomatic portal biliopathy ranges between 5% and 18% of patients.6,8–11 Again, obstructive jaundice was less common in children with portal vein obstruction (5%).8,12 The repeated finding of a higher frequency of jaundice in adults with PC is probably related to the duration of time with PC and, perhaps, is linked to the density of PC.
Despite the apparent association between time with PC and incidence of symptomatic portal biliopathy, the pathophysiology of “portal biliopathy” has not been clearly elucidated. This biliary disease could be related to the dilatation of paracholedochal veins of Petren13 that run closely, but separately, from the bile duct and/or because of the dilatation of the epicholedochal plexus of Saint14 that runs over the bile duct wall. Although an ischemic hypothesis has been proposed as the cause of portal biliopathy,15 given the reversal of obstructive symptoms seen after PC decompression in our patients, this is not likely to be the mechanism.
Management of patients with PC complicated by biliary obstruction is difficult for 2 reasons. First is the rarity of symptomatic portal biliopathy, with only 32 cases reported in the literature since the first description in 1944.2,3,5,6,9,10,12,15–22 Second, the clinical and radiologic presentation can be similar to cholangiocarcinoma22,23 or sclerosing cholangitis.8 These diagnostic issues may lead to major errors in the management of this disease. The principal error is to initially attempt a surgical approach to the bile duct within a hepatic pedicle affected by PC. Dissection of the hepatic pedicle in PC can lead to massive hemorrhage,2,17,19 and even to patient death.3,5 Furthermore, the success of an initial attempt at biliodigestive anastomosis can be may be limited by the presence of intrahepatic portions of the PC. Anastomoses placed below the level of biliary obstruction (patients 8 and 10) are likely to be nonfunctional.6
The literature describes several modes of treatment of patients with PC complicated by symptomatic portal biliopathy. Ten cases of endoscopic treatment with introduction of plastic stent have been reported to date in the literature.6,17,19,21,24 Although none of these procedures caused clinically significant hemobilia, 4 cases were complicated by nonlethal cholangitis, cholecystitis, or pancreatitis.24 In addition, an unexpected massive papillary hemorrhage from bile duct varices has previously been described after endoscopic sphincterotomy in a patient with PC.25 The duration of internal drainage reported for patients treated endoscopically ranged from 2 days to 3 years. In the largest series (6 patients), cholestasis persisted in 4 patients and a secondary surgical treatment of the PC was required in 4 patients.24 Only 5 cases in the literature have documented successful treatment of symptomatic portal biliopathy with only the insertion of a plastic stent, with a minimal follow-up of 2 years.6,19,24 In our series, 3 patients were treated only with a plastic endobiliary stent, but in each case this was introduced by the percutaneous transhepatic approach. In 1 case, this drainage was complicated by a massive hemobilia requiring urgent laparotomy to perform a packing of the hepatic pedicle and the jaundice remained.
To our knowledge, utilization of metallic endobiliary stents for this benign indication has not been reported. In general, metallic stents appear to lack utility in this situation because of a 5-year patency rate inferior to 25%26 and the relative difficulty in removal, if biliary surgery becomes necessary.27
The first case of portosystemic anastomosis for portal biliopathy was published by Choudhuri et al in 1988.28 They performed a splenectomy and proximal splenorenal shunt in a patient who initially developed PC following an episode of endoscopic retrograde cholangiography induced cholangitis and represented with hematemesis secondary to extrahepatic portal hypertension. The splenorenal shunt successfully treated both the upper gastrointestinal hemorrhage and the jaundice. To date, 12 adults and 9 children treated for portal biliopathy with portosystemic anastomosis have been reported in the literature.5,12,18,20,24,28 In children, a mesocaval shunt with jugular vein interposition was the most frequent approach.12 In adults, only a splenorenal shunt via the transperitoneal approach has been described. Of the 10 adult patients with sufficient follow-up, 8 have been successfully treated with portal decompression alone and only 2 have required a secondary biliodigestive bypass.5,24 Our outcomes with 10 previously unreported cases of portal decompression as the primary treatment of patients with symptomatic portal biliopathy confirm the efficacy of this strategy. Interestingly, despite the clinical improvements observed in patients treated only by portal decompression, morphologic abnormalities, notably those identified by echography, tended to persist as occurs in asymptomatic patients with portal cavernoma.6,11
Our report also includes the first description of a retroperitoneal approach to perform a splenorenal shunt for this indication.29 The principal benefit of this approach is that the formation of peritoneal adhesions that could complicate a potentially necessary subsequent biliodigestive bypass is avoided. Furthermore, the retroperitoneal approach may be less hazardous previously operated patients, which is a common occurrence. For example, 4 of the 10 patients treated by portosystemic shunt in our series had pre-referral major biliary surgery.
When a portosystemic anastomosis was not feasible because of extensive portal thrombosis, an intrahepatic biliodigestive bypass was performed, avoiding the hemorrhagic risks associated with dissection of the hepatic pedicle containing undecompressed PC. Although the patients treated with this approach (also previously never reported for this indication) required repeated percutaneous biliary cholangioscopies to extract calculi that could not be endoscopically extracted because of bile duct varices, all experienced long-term survivals.
CONCLUSION
Pretreatment recognition of PC in patients with biliary obstruction is necessary to avoid potentially catastrophic surgical complications. When feasible, the retroperitoneal splenorenal anastomosis is the preferred initial treatment of symptomatic portal biliopathy, as it may alleviate the biliary obstruction without the need for bilioenteric bypass. And, in those patients whose symptoms are not relieved by PSS, this approach makes performance of a secondary biliodigestive bypass safer. When a PSS is not feasible, an intrahepatic biliodigestive anastomosis seems more appropriate than only repeated endoscopic procedures.
Footnotes
Reprints: Daniel Azoulay, MD, PhD, 12 avenue Paul Vaillant Couturier, 94804 Villejuif Cedex, France. E-mail: daniel.azoulay@pbr.aphp.fr.
REFERENCES
- 1.Gibson J, Richards R. Cavernous transformation of the portal vein. J Pathol Bact. 1955;70:81–95. [DOI] [PubMed] [Google Scholar]
- 2.Fraser J, Brown AK. A clinical syndrome associated with a rare anomaly of the vena portae system. Surg Gynecol Obstet. 1944;78:520–524. [Google Scholar]
- 3.Hymes JL, Haicken BN, Schein CJ. Varices of the common bile duct as a surgical hazard. Am Surg. 1977;43:686–688. [PubMed] [Google Scholar]
- 4.Hunt AH. Compression of the common bile-duct by an enlarging collateral vein in a case of portal hypertension. Br J Surg. 1965;52:636–637. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary A, Dhar P, Karin SK, et al. Bile duct obstruction due to portal biliopathy in extrahepatic portal hypertension: surgical management. Br J Surg. 1998;85:326–329. [DOI] [PubMed] [Google Scholar]
- 6.Condat B, Vilgrain V, Asselah T, et al. Portal cavernoma-associated cholangiopathy: a clinical and MR cholangiography coupled with MR portography imaging study. Hepatology. 2003;37:1302–1308. [DOI] [PubMed] [Google Scholar]
- 7.Paquet KJ. Prophylactic endoscopic sclerosing treatment of the esophageal wall in varices: a prospective controlled randomized trial. Endoscopy. 1982;14:4–5. [DOI] [PubMed] [Google Scholar]
- 8.Dilawari JB, Chawla YK. Pseudosclerosing cholangitis in extrahepatic portal venous obstruction. Gut. 1992;33:272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khuroo MS, Yattoo GN, Zargar SA, et al. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807–813. [PubMed] [Google Scholar]
- 10.Malkan GH, Bhatia SJ, Bashir K, et al. Cholangiopathy associated with portal hypertension: diagnostic evaluation and clinical implications. Gastrointest Endosc. 1999;49:344–348. [DOI] [PubMed] [Google Scholar]
- 11.Nagi B, Kochhar R, Bhasin D, et al. Cholangiopathy in extrahepatic portal venous obstruction: radiological appearances. Acta Radiol. 2000;41:612–615. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier-Villars M, Franchi S, Gauthier F, et al. Cholestasis in children with portal vein obstruction. J Pediatr. 2005;146:568–573. [DOI] [PubMed] [Google Scholar]
- 13.Petren T. Die extrahepatischen gallenweqsveness and in repathologish anatomischa Bedeutung. Verb Anat Ges. 1932;41:138–143. [Google Scholar]
- 14.Saint JA. The epicholedochal venous plexus and its importance as a means of identifying the common bile duct during operations on extrahepatic biliary tract. Br J Surg. 1961;48:89–98. [DOI] [PubMed] [Google Scholar]
- 15.Dhiman RK, Puri P, Chawla Y, et al. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischemic? Gastrointest Endosc. 1999;50:646–652. [DOI] [PubMed] [Google Scholar]
- 16.Meredith HC, Vujic I, Schabel SI, et al. Obstructive jaundice caused by cavernous transformation of the portal vein. Br J Radiol. 1978;51:1011–1012. [DOI] [PubMed] [Google Scholar]
- 17.Mackenzie RL, Tubbs HR, Laws JW, et al. Obstructive jaundice and portal vein calcification. Br J Radiol. 1978;51:953–925. [DOI] [PubMed] [Google Scholar]
- 18.Bejanin H, Baumann R, Choury A, et al. Portal cavernoma compressing the bile duct: apropos of three cases. Gastroenterol Clin Biol. 1993;17:134–138. [PubMed] [Google Scholar]
- 19.Thervet L, Faulques B, Pissas A, et al. Endoscopic management of obstructive jaundice due to portal cavernoma. Endoscopy. 1993;25:423–425. [DOI] [PubMed] [Google Scholar]
- 20.Arotcarena R, Roulot D, Aubert A, et al. Successful treatment by mesocaval anastomosis of a common-bile-duct compression by choledochal varices [Letter]. J Hepatol. 1995;23:629–630. [DOI] [PubMed] [Google Scholar]
- 21.Beltrami M, Fornaciari G, Conigliaro R, et al. Biliary obstruction caused by portal cavernoma in a patient with laterality sequence. J Hepatol. 1997;26:1427–1428. [DOI] [PubMed] [Google Scholar]
- 22.Leclerc JC, Cannard L, Debelle L, et al. MRI of portal cavernoma with biliary involvement. J Radiol. 2002;83:341–349. [PubMed] [Google Scholar]
- 23.Bayraktar Y, Balkanci F, Ozenc A, et al. The ‘pseudo-cholangiocarcinoma sign’ in patients with cavernous transformation of the portal vein and its effect on the serum alkaline phosphatase and bilirubin levels. Am J Gastroenterol. 1995;90:2015–2019. [PubMed] [Google Scholar]
- 24.Dumortier J, Vaillant E, Boillot O, et al. Diagnosis and treatment of biliary obstruction caused by portal cavernoma. Endoscopy. 2003;35:446–450. [DOI] [PubMed] [Google Scholar]
- 25.Tighe M, Jacobson I. Bleeding from bile duct varices: an unexpected hazard during therapeutic ERCP. Gastrointest Endosc. 1996;43:250–252. [DOI] [PubMed] [Google Scholar]
- 26.Lopez RR Jr, Cosenza CA, Lois J, et al. Long-term results of metallic stents for benign biliary strictures. Arch Surg. 2001;136:664–669. [DOI] [PubMed] [Google Scholar]
- 27.Vibert E, Farges O, Regimbeau JM, et al. Benign hilar biliary strictures stented with metallic stents can be resected by using an oncologic approach. Surgery. 2005;137:506–510. [DOI] [PubMed] [Google Scholar]
- 28.Choudhuri G, Tandon RK, Nundy S, et al. Common bile duct obstruction by portal cavernoma. Dig Dis Sci. 1988;33:1626–1628. [DOI] [PubMed] [Google Scholar]
- 29.Rigau J, Teres J, Visa J, et al. Long term follow-up of 100 patients with portal hypertension treated by a modified splenorenal shunt. Br J Surg. 1986;73:708–711. [DOI] [PubMed] [Google Scholar]