Concepts regarding the eukaryotic plasma membrane have been evolving in light of growing evidence that it is segregated into distinct lateral domains known as lipid rafts. These sterol- and sphingolipid-rich raft domains are thought to play important roles in dynamic processes, including protein sorting, cell polarity, and signal transduction. Because of this, it has been very intriguing that sterol-rich domains (SRDs) that are much larger than lipid rafts have been identified in the plasma membranes of fungi. The larger SRDs can be readily observed in cells stained with filipin, a naturally fluorescent sterol-binding antibiotic. These large SRDs are located at sites of active morphogenesis and are regulated in a developmental or cell-cycle-regulated manner. For example, SRDs were detected at the tips of mating projections in Saccharomyces cerevisiae and hyphae in Candida albicans, but not in budding cells. In the fission yeast Schizosaccharomyces pombe, an SRD forms in a cell-cycle regulated manner in the medial zone of the rod-shaped cells at the future site of cell division. SRDs have also been reported at sites of polarized morphogenesis in Cryptococcus neoformans and Aspergillus nidulans. These domains may be formed by the clustering of lipid rafts and may therefore also function as organizing centers in the membrane. In this review, we assess recent progress in the analysis of lipid rafts and large SRDs in fungi. Comparisons with other plasma membrane domains, including eisosomes and septin boundary domains, are made to examine how these specialized regions work together to contribute to polarized morphogenesis and cytokinesis.
LIPID RAFT ORGANIZATION AND FUNCTION IN FUNGI
Lipid rafts, liquid-ordered domains, and detergent-resistant membranes (DRMs)—what's the skinny on these fatty regions?
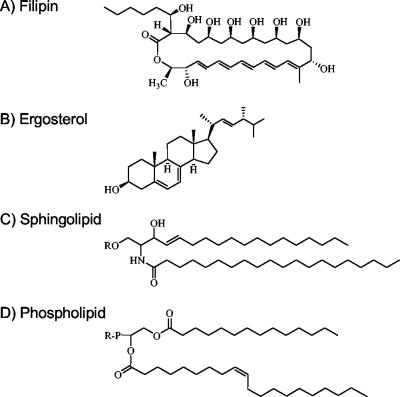
Plasma membranes differ from most subcellular membranes because they contain a high fraction of sterols and sphingolipids in addition to the glycerophospholipids. Studies on model membranes indicate that sterols and sphingolipids can cluster into liquid-ordered domains instead of forming homogeneous mixtures with glycerophospholipids (58). The underlying basis for this is that sterols are composed of a four-ring structure and an aliphatic tail that can pack together in a favorable manner with sphingolipids, which are comprised of ceramide and long saturated acyl chains (57) (Fig. 1). The lipid acyl chains pack tightly with the rigid sterols to create a compacted zone of condensed bilayer that has been termed the liquid-ordered state (10, 60). Glycerophospholipids with unsaturated side chains do not pack in this manner and are excluded from the raft domains.
FIG. 1.
Comparison of filipin and plasma membrane lipid structures. (A) Structure of filipin, a polyene antibiotic that binds sterols. (B) Structure of ergosterol, which contains a four-ring structure common to other sterols, including cholesterol. (C) Sphingolipids are based on a ceramide and contain saturated acyl chains that are variable in length (26 carbon atoms are shown) attached to the sphingosine base. The R group is phosphocholine in the case of sphingomyelin or sugar(s) in the case of the glycosphingolipids. (D) Glycerophospholipids are based on diacylglycerol and typically carry acyl chains of 16 to 18 carbon atoms. One of the acyl chains contains a cis double bond, which prevents close packing with saturated acyl chains present in lipid rafts.
The possibility that these liquid-ordered domains may occur in vivo, where they would preferentially associate with certain types of proteins (e.g., acylated and glycosylphosphatidylinositol [GPI]-anchored proteins), gave rise to the idea that these protein-lipid domains would form lipid rafts in the plasma membrane (11, 71). Because of their unique lipid composition, the liquid-ordered domains are more resistant to extraction with nonionic detergents, which, as described below, is why these domains are sometimes referred to as DRMs. Although the terms lipid raft, liquid-ordered domain, and DRM are intended to represent similar concepts, they have distinct implications and should not be used interchangeably (50).
The pioneering studies on lipid rafts and their biological roles have been primarily carried out in mammalian cells (10, 11, 23, 38, 60). However, the general properties of plasma membrane organization and lipid raft function appear to be similar in fungi. For example, on a molar basis, the yeast plasma membrane lipid content is similar to that of mammalian cells in that it is about 30 to 40% sterols, 10 to 20% sphingomyelin, and typically a small fraction of glycosphingolipids (60, 76). The primary sterol found in fungi is ergosterol, which is a better raft former than cholesterol (97). In addition to lateral organization into lipid rafts, the inner and outer leaflets of the plasma membrane also contain distinct lipid contents. Raft-forming sphingolipids are found primarily in the outer leaflet, and some glycerophospholipids, like phosphatidylserine, are primarily present in the inner leaflet (32). Asymmetry is maintained by active transport, which is why phosphatidylserine is seen only on the outer leaflet in mammalian cells undergoing apoptosis.
DRMs.
DRM analysis was one of the first indicators that raft domains could form in cellular membranes (12). In mammalian cells, plasma membrane proteins differ in their abilities to associate with DRMs, which is inferred to represent differential abilities to partition into rafts in vivo. Analysis of DRMs in S. cerevisiae has confirmed that they are enriched in ergosterol and sphingolipids, as expected (4, 5). In contrast to mammalian cells, it appears that most yeast integral plasma membrane proteins primarily partition with the DRM fraction (46, 53). Only three yeast plasma membrane proteins have been reported to partition primarily in the detergent-soluble fraction: Tre1 (Ypl176c), Gap1, and Hxt1 (5, 54). However, a recent study found that Gap1 and Hxt1 were in fact primarily present in DRMs when these proteins were localized to the plasma membrane, but they dissociate from DRMs during endocytic trafficking (46). It is not clear why there are discrepancies, given that control experiments in these studies indicated that the relevant proteins appeared to be present in the plasma membrane. Possibly, it has to do with differences in yeast strains or growth conditions, since the trafficking of Gap1 and Hxt1 is regulated by nutritional conditions. Alternatively, it could be due to differences in DRM extraction conditions, as discussed below. Although DRM analysis has not identified differences in the abilities of plasma membrane proteins to partition into lipid rafts in fungi the way it has in mammalian cells, it has played a very significant role in other areas, such as defining the stages of protein trafficking to the plasma membrane that will be described below.
DRM analysis has provided important new insights into the role of lipid rafts in biological membranes, but it has also been controversial, in part because of different experimental methods and interpretation of data. For example, some investigators consider a protein to be DRM associated if it is insoluble after detergent extraction at 4°C without doing further controls. Since many proteins, such as components of the cytoskeleton, are insoluble after extraction with nonionic detergents, it is more reliable to float the DRMs on a density gradient before concluding whether a protein is in the DRM fraction. Even so, caution must be used, because other proteins may stick to the DRMs nonspecifically. In addition, the components of DRMs should be insoluble at 4°C but soluble at warmer temperatures. Another potential source of variability is that it is important to extract DRMs using the same ratio of protein to detergent in each sample (66). Also, different results can be obtained using different nonionic detergents. Most studies use 1% Triton X-100 to determine detergent insolubility, but other nonionic detergents, such as CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, will also work (77). Perhaps these experimental differences or other variables explain why the plasma membrane ATPase Pma1 was initially reported to be absent from DRMs in S. cerevisiae (44) but was subsequently found to be almost exclusively in DRMs in S. cerevisiae and C. albicans (4, 37, 55). Similar conflicting results for Gap1 and Hxt1 are described above. Another concern regarding the analysis of DRMs is that even if two different proteins are found in the DRM fraction, this does not necessarily imply that they were present in the same subset of lipid rafts in vivo (50).
Lipid raft assembly in fungi.
Studies on yeast DRMs indicate that lipid rafts begin to assemble in the endoplasmic reticulum (ER) (4, 46). This differs from mammalian cells, where the association of proteins with DRMs takes place in the Golgi (11, 12). Ergosterol is synthesized in the ER and is present along the secretory pathway and in the plasma membrane (101). Interestingly, transport of ergosterol can occur by a process that is independent of the standard secretory pathway, because an S. cerevisiae sec18ts mutant does not affect ergosterol transport (56). Sphingolipid synthesis begins in the ER and continues in the Golgi, and then the sphingolipids are sent to the plasma membrane in secretory vesicles (27). Sterols and sphingolipids are also internalized during endocytosis and are present in endocytic vesicles. However, these raft constituents are presumably recycled back to the plasma membrane, as they are not found in the vacuole (68). Proteins such as the OSH gene-encoded oxysterol binding proteins also contribute to proper sterol distribution in cells (8). Other factors that mediate proper sterol distribution have been identified through the study of human genetic diseases that cause abnormal accumulation of sterols (52, 56).
Lipid raft functions.
One presumed function of lipid rafts is to act as organizing centers by preferentially associating with specific proteins. Lipid-modified proteins, such as GPI-anchored proteins on the extracellular side and acylated proteins on the intracellular side, are thought to partition into rafts due to the favorable packing of their saturated acyl chains. It is not clear what targets transmembrane proteins into rafts. In yeast, lipid rafts have been implicated in protein sorting, secretion, endocytosis, and cell polarity (6, 91). Although it is difficult to obtain direct evidence for lipid rafts in these processes, ergosterol and sphingolipids are clearly involved. For example, transport of the GPI-anchored Gas1 protein from the ER to the Golgi is dependent on sphingolipid synthesis (36, 84). Also, a mutant form of Pma1 that failed to associate with DRMs was defective in trafficking, suggesting that proper association with lipid rafts is important for trafficking (3, 31). Analysis of the amino acid permease Gap1 during exocytic and endocytic trafficking showed that DRM association correlated with plasma membrane localization (46). Ergosterol and sphingolipid synthesis are also important for endocytosis. Analysis of ergΔ mutants that accumulate different sterol intermediates showed that sterols function in the early stages of endocytosis and also in the later stages of vesicle trafficking (34, 59).
Why are lipid rafts so controversial?
The small size of lipid rafts and consequent difficulties in visualizing them in vivo has made it necessary to rely on indirect methods to define their structure and function. Differences in how these indirect approaches are carried out and how they are interpreted has created controversy (60). In addition to the problems with the analysis of DRMs described above, other methods used to examine lipid rafts have also led to disagreements. For example, the depletion of cholesterol from membranes using methyl-β-cyclodextrin has been used in many studies to infer the role of lipid rafts. However, cholesterol depletion is also expected to cause other effects, such as changes in bilayer properties and permeability (60). New approaches that are being developed will therefore play an important role in better defining the structure and function of these domains in the future (38). One new approach that is less controversial is single-molecule tracking, which maps the lateral mobility of single proteins and lipids in the plasma membrane. This approach has revealed that raft constituents become trapped in transient confinement zones that have properties that are consistent with those of lipid rafts (15, 18, 45). Other promising new approaches for studying rafts include atomic force microscopy (16) and mass spectrometry (43).
SRDs IN FUNGI
Recent studies have identified SRDs in fungi that are much greater in size than lipid rafts are assumed to be. SRDs appear to range from about 3 to 15 μm2, depending on the organism and location. In contrast, recent estimates suggest that lipid rafts are about 75 nm2 (23). So far, SRDs are unique to fungi and have not been reported to be present in mammalian cells. These large fungal SRDs are of interest for their potential roles in membrane organization, and they may therefore also have special significance for the presentation of virulence factors and as targets for antifungal therapy. The SRDs have been primarily visualized by staining cells with filipin, which is a polyene antibiotic that forms a complex with free 3-β-hydroxy sterols (Fig. 1). Filipin was named for its identification as a compound produced by an actinomycete, Streptomyces filipinensis, found in soil from the Philippines (96). Filipin is naturally fluorescent with maximum excitation (360 nm) and emission (480 nm) properties that allow it to be detected using typical microscope filter sets used for DAPI (4′,6′-diamidino-2-phenylindole) staining (21). It is a common histochemical stain for cholesterol and is used in the diagnosis of altered sterol storage caused by diseases such as type C Niemann-Pick disease. However, filipin can be tricky to work with; it is not stable in solution and should be freshly dissolved before use, it photobleaches rapidly, and cells should be visualized quickly after being stained, as it is toxic and can deform membranes. Earlier studies took advantage of the ability of filipin to promote deformation of sterol-containing membranes in order to compare the sterol compositions of membranes by electron microscopy (65, 88). More recent studies have analyzed filipin by fluorescence microscopy, since this can be carried out more quickly and is not subject to other limitations of the membrane deformation approach. Nonetheless, additional methods need to be developed to confirm sterol localization, such as new fluorescent probes or acyl-green fluorescent protein (GFP) (100), because filipin has the potential to cause deleterious effects on cell viability and membrane structure.
Little is known about how these large SRDs are formed or their functions in vivo. In fact, the mechanism of formation may be distinct in different organisms. Another question that also needs to be addressed is quantifying the degree of sterol enrichment observed by filipin staining of these domains. Since the overall plasma membrane is about 30 to 40% ergosterol, this presumably limits the enrichment that can occur in the SRDs. Therefore, the key observations regarding the identification of large SRDs in different fungi are summarized below to compare their properties. The sites of filipin staining in different fungi are also summarized in Table 1, and some examples are shown in Fig. 2. Models for the formation of SRDs and their potential roles in cell polarity and pathogenesis are then described.
TABLE 1.
Comparison of sites of sterol-rich domains in different fungi
| Parameter | Cell type
|
|||||
|---|---|---|---|---|---|---|
| S. cerevisiae buds | C. neoformans buds |
C. albicans
|
A. nidulans hyphae | S. pombe rods | ||
| Buds | Hyphae | |||||
| Morphogenesis | Absent | Present | Absent | Present | Present | Present |
| Site | Bud tips | Hyphal tips | Hyphal tips | Ends of rods | ||
| Septae | Absent | Present | Absent | Present | Present | Present |
| Pheromone-induced cells | Tips of mating projections | Tips of mating projections | Not reported | Not reported | Not reported | Tips of mating projections |
| Reference | 5 | 62 | 55 | 55 | 67 | 92 |
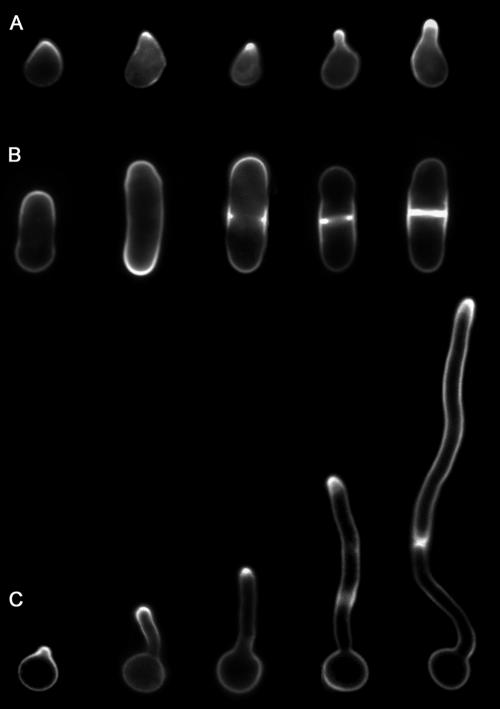
FIG. 2.
Examples of filipin staining. (A) S. cerevisiae cells induced with α-factor. (B) S. pombe cells grown to log phase. (C) C. albicans induced to form hyphae with serum. The cells were stained with filipin and then viewed by fluorescence microscopy as previously described (5, 55, 92).
S. cerevisiae.
In the budding yeast S. cerevisiae, filipin strongly stained the tips of cells induced with mating pheromone (5, 69). An example of this is shown in Fig. 2. Filipin staining was not detected in vegetative cells, which polarize their growth into the new bud. The tips of pheromone-induced cells undergo highly polarized morphogenesis to form the conjugation tube that connects cells during mating. Disruption of sterol or sphingolipid production resulted in less polarized filipin staining. Other controls indicated that filipin staining is specific for sterols in S. cerevisiae, such as the decreased filipin staining of erg9Δ and erg6Δ mutants, which are defective in forming ergosterol (8, 52). Thus, it was proposed that this SRD at the tips of pheromone-induced cells plays an important role in cell polarization and proper localization of components needed for cell and membrane fusion during mating (5). Consistent with this, several proteins that localize to the tips of the pheromone-induced projections were present in DRMs.
The significance of the filipin staining at the tips of pheromone-induced cells was called into question for several reasons (90, 91). One is that this staining pattern was not detected in formaldehyde-fixed cells. However, it is possible that there could have been movement of the lipids postfixation (90). Another criticism was that uptake of the endocytosis tracer dye FM4-64 was perturbed in budding cells treated with filipin, and it accumulated in the same region of a nascent bud as filipin. It is also not clear whether the SRD plays a role in localizing proteins to the tip, since some proteins achieve this localization through endocytic recycling and other proteins may be anchored by cytoskeletal interactions (69, 90). However, additional evidence for a polarized lipid domain at the tips of pheromone-induced cells was obtained by staining the cells with Laurdan, which is an environmentally sensitive dye that shifts its emission peak depending on whether it is in a liquid-ordered or -disordered lipid domain (69). The results demonstrated that the membrane was more ordered and condensed at the tips of the pheromone-induced cells than at the opposite sides. Similar results were observed in a strain that is deficient in endocytosis (end4Δ) but not in a strain with a defect in sphingolipid synthesis (lcb1-100) (69).
C. neoformans.
The budding yeast C. neoformans is a member of the Basidiomycota division of the fungal kingdom, which makes it evolutionarily quite distant from the members of the Ascomycotina division that includes S. cerevisiae and the other fungi that are discussed below (39). Filipin showed intense staining of the leading edges of mating projections in C. neoformans, similar to what was observed in S. cerevisiae (62). In contrast to S. cerevisiae, filipin also stained the actively growing sites at bud tips and at sites of septation in C. neoformans. Staining at bud tips is so far unique to C. neoformans, but as described below, staining at septation sites was also observed in the fission yeast S. pombe (92), in the filamentous fungus A. nidulans (67), and in C. albicans hyphae (55). It has been proposed that lipid rafts may serve to concentrate virulence factors and regulate their release from C. neoformans, which is a human pathogen (80).
C. albicans.
The multimorphic fungus C. albicans is capable of growing in a variety of morphologies, including buds, pseudohyphae (chains of elongated cells), and hyphae (long filamentous chains of cells). Strong staining with filipin was detected at the tip of hyphal growth and was not seen in buds or pseudohyphae (55). Examples of filipin staining are shown in Fig. 2. In cells switching from budding to hyphal growth, strong filipin staining was observed at the earliest stage of hyphal growth (germ tube formation) and was maintained at the leading edge in mature hyphae. This filipin staining was observed only at the actively growing hyphal tips and not at inactive tips after cells switched to a new site of hyphal morphogenesis. Filipin stained the plasma membrane and was not associated with the subapical cluster of vesicles known as the Spitzenkörper (17). The formation of this SRD was prevented by treating cells with latrunculin A, indicating a role for actin (55). Inhibitors of ergosterol (ketoconazole) or sphingolipid (myriocin) synthesis diminished the formation of the SRD and also caused defects in polarized hyphal growth (55). Thus, the SRD may play a role in proper localization of cell polarity components that help restrict growth to the narrow zone at the tip of the hypha. In addition, as discussed below, the SRD at the hyphal tip may be important for proper presentation of virulence factors, many of which are GPI anchored (61, 72, 83). Proteomic comparison identified more than 40 hyphal DRM proteins that were not detected in buds, some of which are candidates for associating with SRDs (2).
Filipin staining in C. albicans hyphae was also more intense at sites of septum formation, although this staining was not as strong as that seen at hyphal tips (55). This contrasts with septal sites in C. albicans buds or pseudohyphae, which did not show increased filipin staining. Prior to cytokinesis in hyphae, filipin staining appeared on the side of the septin ring toward the hyphal tip. At later stages after cytokinesis, filipin staining was detectable on both sides of the septum. It is not clear why filipin staining was detectable only at septation sites in hyphae and not in buds. Perhaps the septin ring captures sterol-rich membrane at the hyphal tip, since septin localization studies indicate that the ring initially forms at the tip (82, 94). Alternatively, as discussed below, there may be underlying similarities with the sterol-rich ring that forms at sites of septation in the fission yeast S. pombe (92). Either way, this distinct aspect of septum formation in hyphae is interesting in that it correlates with other differences in hyphal septation sites. For example, the hyphal septation site does not separate after cytokinesis and thereby shields β-glucans at this site from being recognized by macrophages (28, 35).
A. nidulans.
The filamentous fungus A. nidulans shows enriched filipin staining at both hyphal tips and sites of septation (67). The polarization of sterols to the hyphal tip correlated with the integrity of the actin cytoskeleton; a mesA mutant in which actin cables were not localized to hyphal tips showed depolarized filipin staining, including increased staining in subapical regions. Interestingly, staining at the septation sites was not affected in the mesA mutant. Similarly, the localization of the SepA formin was also rapidly lost from the hyphal tip in a mesA mutant, but SepA was still present at septation sites and septum formation was normal. Thus, the SRDs at hyphal tips and septation sites are regulated differently.
Subsequent studies demonstrated that addition of HSAF (heat-stable antifungal factor) (99) caused rapid loss of filipin staining at the hyphal tip (49). The cells treated with HSAF still grew, but the hyphal tips swelled, and numerous branches formed at apical and subapical sites. A barA mutant was identified that was resistant to HSAF, and at 42°C, it showed a phenotype similar to that of HSAF treatment, including defects in forming polarized SRDs and defects in polarized hyphal growth (49). The barA gene was found to encode a ceramide synthase that is involved in sphingolipid production. Reduced expression of a distinct second ceramide synthase, lagA, also caused defects in polarized hyphal growth, but the morphological defects were still exacerbated by HSAF. It was concluded that A. nidulans contains two distinct types of ceramide synthases, with LagA producing the bulk of the ceramide and BarA producing a specialized pool that is targeted by HSAF.
S. pombe.
SRDs have been observed at three sites associated with morphogenesis in the fission yeast S. pombe: at the ends of the rod-shaped cells, in a medial zone where cells undergo cytokinesis, and at the tips of mating projections (70, 92). Examples of this are shown in Fig. 2. The SRDs have been primarily studied by filipin staining but were also found to be enriched in the lipid raft marker protein acyl-GFP (87). The SRD in the medial region of the cell has been studied most extensively, because it forms in a cell-cycle-regulated manner as the cells prepare to undergo cytokinesis at this site. Temporal studies demonstrated that the cytokinetic actin ring forms first, then the SRD forms at the corresponding site in the plasma membrane around the medial zone, and then the actin ring contracts. Although the SRD colocalizes with the cytokinetic ring, actin and microtubules were not required to form the medial sterol-rich ring. Takeda et al. (87) further dissected the genetic requirements and found that several other genes related to the function of actin, septins, or septum formation were not required to form the medial SRD. However, a functional secretory pathway was needed, as brefeldin A blocked its formation (92).
Subsequent studies identified two proteins that were needed to form SRDs in S. pombe. One is the cdc15 protein, which is a member of the PCH family that is involved in cytokinesis (87). cdc15 mutant cells failed to form a medial ring and instead frequently formed spirals of SRDs in the plasma membrane. Overproduction of cdc15 resulted in abnormal SRDs that appeared as patches and spirals in the plasma membrane. Since these mutant phenotypes cannot be attributed to the role of cdc15 in actin organization, this suggests that cdc15 may be directly involved in promoting the SRD. Consistent with this, Cdc15-GFP localized to the medial zone prior to the formation of the SRD in an actin-independent manner, and the cdc15 protein was present in DRMs. Interestingly, cdc15 interacts with the cdc12 formin, similar to the interaction of A. nidulans MesA with the SepA formin (67, 87).
cdc15 also interacts with myo1, which is the other protein identified as playing a role in forming the medial-zone SRD (86). The myo1 protein localizes to the SRD and fractionates with DRMs, and its overproduction induces ectopic SRDs. Significantly, a myo1 mutant did not form SRDs. In particular, SRD formation required the myo1 TH1 domain, which contains a putative phospholipid-binding site that could potentially act as a membrane-organizing center (86). The myo1 mutation lacking the TH1 domain that was used in these studies did not block endocytosis, further indicating that myo1 may act directly to promote SRDs and not just indirectly through membrane-trafficking effects. Mutants lacking the TH1 domain were viable but exhibited partial defects in F-actin organization, septation, polarized growth, and viability under stress conditions.
These studies demonstrate that the cdc15 and myo1 proteins play key roles in the formation of the SRD, perhaps by binding to acidic phospholipids and promoting membrane organization. Although this helps to define the mechanisms of SRD formation, the biological role of SRDs in S. pombe is still not clear. It was initially assumed that the SRDs would provide a framework to facilitate interaction between proteins involved in the organization of processes such as cell polarity, cell wall synthesis, targeted membrane addition, and cytokinesis and septum formation (91, 92). However, the analysis of cdc15 and myo1 mutants indicates that the SRDs are not needed for viability, polarized growth, or cell division site placement (86, 87). Further study should provide insights into the links between actin assembly, endocytosis, and SRDs in the plasma membrane. In this regard, it is interesting that in addition to binding myo1, cdc15 also binds to both the ARP2/3 complex and the formin cdc12, thus binding to both forms of actin nucleation involved in forming the cytokinetic ring (13). In mammalian cells, lipid rafts are thought to be platforms for membrane-linked actin polymerization stimulated by ARP2/3 (73).
MODELS FOR THE FORMATION OF SRDs
The composition of SRDs is thought to be similar to that of lipid rafts, suggesting that a good starting point for developing models for the formation of SRDs involves the clustering of lipid rafts (5). In mammalian cells, clustering of lipid rafts is thought to be mediated by proteins that become cross-linked during processes such as virus budding and the activation of B-cell and T-cell receptors (1, 22, 78, 85). In an analogous fashion, protein-mediated clustering of lipid rafts could occur in S. pombe, since as described above, the myo1 protein that is needed to form SRDs binds to cdc15 and other proteins that could form a scaffold for stabilizing the SRD (86). The cdc15 protein also binds to other proteins, including the ARP2/3 complex and the cdc12 formin, which could contribute too.
It is not clear if myo1 is involved in forming SRDs in other organisms, since the SRD at the medial zone in S. pombe forms in a cell-cycle-regulated manner that is independent of actin, whereas in other fungi the SRDs are present constitutively or are formed in a developmentally regulated manner in which actin is required for proper morphogenesis. However, there are potential similarities, since C. albicans mutants with defects in myosin I (Myo5) or the actin-related protein Sla2 did not display SRDs, although these results are complicated by the fact that the mutants are also defective in forming hyphae (64). There are also similarities in the mechanisms of SRD formation in S. pombe and A. nidulans, in which the MesA protein is needed both to form SRDs at hyphal tips and for proper localization of the formin SepA (67).
Another model for clustering lipid rafts into larger SRDs involves GPI-anchored proteins. Since GPI-anchored proteins preferentially associate with lipid rafts (11), it has been suggested that the induction of GPI-anchored proteins during mating in S. cerevisiae and hyphal growth in C. albicans could promote clustering of lipid rafts (55). The GPI-anchored agglutinins that mediate intercellular adhesion are highly induced during mating in S. cerevisiae (51). Similarly, GPI-anchored adhesin proteins, such as Hwp1 and Als1, are also highly induced during hyphal growth in C. albicans (25). A common feature of the agglutinins and adhesins is that their GPI anchors are ultimately cleaved off, and they become covalently linked to the cell wall. Thus, the older sites of deposition of these proteins would no longer be able to cluster lipid rafts. Only the recently synthesized GPI-anchored proteins that are deposited at the site of polarized growth would contribute to the formation of an SRD at the hyphal tip. Further studies will be required to determine the mechanisms of SRD formation.
POTENTIAL ROLES OF SRDs IN VIRULENCE
The SRDs in pathogenic fungi are likely to play a role in pathogenesis by mediating the presentation of virulence factors and by influencing the biophysical properties of the plasma membrane. C. albicans produces many GPI-anchored virulence factors that are induced along with the SRD during hyphal growth. These include the members of the adhesin protein family (e.g., Hwp1 and Als1), which mediate adhesion to host cells and biofilm formation, and also the secreted aspartyl protease family, which is needed for virulence (i.e., Sap9 and Sap10) (9, 40, 63, 75, 83). Other GPI-anchored proteins that contribute to virulence include Eap1, Dfg5, and Phr1, which are involved in epithelial adhesion, cell wall biogenesis, and proper hyphal growth (48, 74, 81). Bioinformatics approaches have also predicted other likely GPI-anchored proteins whose functions are not yet known (47). In addition, acylated proteins on the intracellular side are also likely to be present in the SRD, such as the Ras1 protein that is needed for signaling hyphal growth and for virulence (24). Similarly, it has been suggested that special membrane domains in C. neoformans regulate membrane localization and release of the virulence factors phospholipase B1 and superoxide dismutase (80).
The SRDs are also expected to have distinct biophysical properties, which could contribute to virulence. For example, tight packing of lipid acyl chains with the sterols creates condensed zones that cannot be deformed to allow movement of small molecules across the membrane. This could be important at sites of polarized growth, such as hyphal tips, to provide additional strength while these regions are undergoing rapid cell wall remodeling. It is also possible that specialized membrane domains could influence signaling that occurs via lipids and their derivatives, such as farnesol, diacylglycerol, and eicosanoids in pathogenic fungi (79).
IMPLICATIONS OF SRDs FOR ANTIFUNGAL THERAPY
The identification and analysis of SRDs provides new insights into the actions of the most commonly used antifungal drugs, amphotericin and fluconazole, which target ergosterol (29). Amphotericin binds to ergosterol and forms pores in membranes. Fluconazole blocks ergosterol synthesis by inhibiting lanosterol 14-α-demethylase (Erg11). However, these types of drugs have additional effects on cells by altering morphogenesis and interfering with the formation of SRDs (55). Thus, in addition to their previously defined roles, these antifungal agents may also decrease pathogenicity by altering the presentation of virulence factors, as described above. Furthermore, defects in morphogenesis caused by these drugs may weaken the ability of pathogens such as C. albicans to grow invasively or form biofilms. Even subtle defects in morphogenesis can influence pathogenicity by causing abnormal cell wall synthesis that exposes β-glucans and allows them to be recognized by the immune system (95).
Several possibilities exist for developing new drugs that target lipid rafts and SRDs. For example, amphotericin analogs could be linked to inhibitor molecules in order to target novel therapeutic agents to neutralize virulence functions at hyphal tips. It may also be possible to identify inhibitors that disrupt SRDs. An example of this is the disruption of the SRDs in A. nidulans by the HSAF dihydromaltophilin, which prevents stabilization of the axis of polarized hyphal morphogenesis (49, 67). This agent does not affect S. cerevisiae, which does not encode a homolog of the BarA protein. Therefore, similarly selective agents could have value for human fungal pathogens.
RELATIONSHIP OF SRDs TO OTHER PLASMA MEMBRANE DOMAINS IN FUNGI
Many eukaryotic cells contain other types of plasma membrane domains that play important roles in plasma membrane organization and function, such as those defined by tight junctions in epithelial cells. In the case of fungi, studies on S. cerevisiae have identified at least two other types of plasma membrane domains, septin barriers and eisosomes. Septin barrier domains are formed by septin proteins during budding and cytokinesis. Eisosomes are static sites of endocytosis that appear as punctate structures distributed across the plasma membrane. These specialized types of plasma membrane domains in S. cerevisiae are described below to compare their functions with those of SRDs.
Septin barrier domains at the bud neck and the cytokinetic ring.
The septin proteins were identified in S. cerevisiae as peripheral membrane proteins that form a ring on the inner surface of the plasma membrane at the bud neck (20, 30). The septin ring acts as a scaffold to recruit proteins needed for cytokinesis and cell septation. Septins were subsequently shown to mediate a barrier function between the daughter and mother cells that blocks diffusion of integral membrane proteins, actin patches, exocyst and polarisome components, and Lte1, which is the guanine nucleotide exchange factor for the TEM1 GTPase that promotes exit from mitosis (7, 14, 89). Later in the cell cycle, the septin ring splits, and the two rings act as barriers to restrict proteins involved in cytokinesis and septum formation to the neck of the bud (19). A more diffuse type of septin ring also forms at the neck of pheromone-induced mating projections (26, 41, 42), but it has not been reported whether this ring plays a role in forming the SRDs at these sites. Interestingly, SRDs form at sites of septin rings in rod-shaped S. pombe and in hyphae of C. albicans and A. nidulans (55, 67, 92). In C. albicans, the SRD at the septation site was initially present on the side toward the actively growing hyphal tip, suggesting perhaps that septins can act as a barrier to prevent diffusion of lipids. In S. pombe, septin mutants did not affect the formation of the SRD, indicating that they are not necessary for its formation or localization in fission yeast (87). Thus, further work will be needed to determine the relationship between the septins and SRDs.
Eisosomes.
S. cerevisiae plasma membrane proteins are present in distinct lateral subdomains, which indicates another form of plasma membrane organization (33, 53, 54). One domain has been termed the eisosome, which refers to large immobile complexes in the plasma membrane that are sites of endocytosis (93). The eisosome proteins are present in a punctate pattern that appears as dots distributed throughout the plasma membrane. Three proteins were identified in eisosomes, Pil1, Lsp1, and Sur7. Two of them, Pil1 and Lsp1, are closely related cytoplasmic proteins that function in endocytosis. Sur7 is a transmembrane protein that influences sphingolipid levels, which are also important for endocytosis (98). Additional studies have shown that Fur4 (H+ symporter), Can1 (arginine permease), and Tat2 (tryptophan permease) colocalize with Sur7 in punctate spots (33, 53). These punctate spots, which correspond with eisosomes, are thought to represent a distinct lateral domain, since Can1 and Pma1 were found in nonoverlapping regions of the plasma membrane (54). Both Can1 and Pma1 are present in DRMs, so it was suggested that these proteins occupy distinct lipid raft domains. Interestingly, filipin staining was stronger at sites of Sur7-GFP localization, and this staining pattern was dependent on the Pil1 protein that is needed for eisosome formation (33). However, it is not known if there is any relationship between eisosomes and SRDs.
CONCLUDING COMMENTS AND FUTURE QUESTIONS
The discovery of SRDs in fungi is intriguing because of their potential to play important roles in plasma membrane organization, structure, and function. However, further work is needed to determine the specific function of these domains in vivo. At present, it is not clear if they act as organizing centers for proteins, as lipid domains with special biophysical properties, or both. Further progress in studying these domains will greatly benefit from the development of new approaches for studying membrane domains, such as those being developed for the analysis of lipid rafts. It will also be interesting to determine whether SRDs play similar roles in different fungi in view of the differences in the formation of SRDs at sites of septation in S. pombe versus the sites of polarized apical growth in S. cerevisiae mating projections and at hyphal tips in C. albicans and A. nidulans. Other significant avenues of study for these SRDs are to determine their roles in presenting virulence factors during fungal pathogenesis and as possible targets for the development of new antifungal therapies. The discovery of these SRDs in a broad range of evolutionarily diverse fungi also suggests that it is likely similar domains will be found in other organisms.
Acknowledgments
We thank Deborah Brown for helpful comments on the manuscript and for many valuable research discussions.
Research on C. albicans in our laboratory has been supported by a grant from the National Institutes of Health (RO1 AI47837). L.M.D. was supported in part by NIH training grant T32CAO9176.
Footnotes
Published ahead of print on 16 March 2007.
REFERENCES
- 1.Aloia, R. C., F. C. Jensen, C. C. Curtain, P. W. Mobley, and L. M. Gordon. 1988. Lipid composition and fluidity of the human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 85:900-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, F. J., and J. B. Konopka. 2006. Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans. Mol. Biol. Cell 18:965-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnat, M., A. Chang, and K. Simons. 2001. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell 12:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagnat, M., S. Keranen, A. Shevchenko, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagnat, M., and K. Simons. 2002. Cell surface polarization during yeast mating. Proc. Natl. Acad. Sci. USA 99:14183-14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnat, M., and K. Simons. 2002. Lipid rafts in protein sorting and cell polarity in budding yeast Saccharomyces cerevisiae. Biol. Chem. 383:1475-1480. [DOI] [PubMed] [Google Scholar]
- 7.Barral, Y., V. Mermall, M. S. Mooseker, and M. Snyder. 2000. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5:841-851. [DOI] [PubMed] [Google Scholar]
- 8.Beh, C. T., and J. Rine. 2004. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J. Cell Sci. 117:2983-2996. [DOI] [PubMed] [Google Scholar]
- 9.Blankenship, J. R., and A. P. Mitchell. 2006. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9:588-594. [DOI] [PubMed] [Google Scholar]
- 10.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 11.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. [DOI] [PubMed] [Google Scholar]
- 12.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 13.Carnahan, R. H., and K. L. Gould. 2003. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J. Cell Biol. 162:851-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillon, G. A., N. R. Adames, C. H. Rosello, H. S. Seidel, M. S. Longtine, J. A. Cooper, and R. A. Heil-Chapdelaine. 2003. Septins have a dual role in controlling mitotic exit in budding yeast. Curr. Biol. 13:654-658. [DOI] [PubMed] [Google Scholar]
- 15.Chen, Y., B. Yang, and K. Jacobson. 2004. Transient confinement zones: a type of lipid raft? Lipids 39:1115-1119. [DOI] [PubMed] [Google Scholar]
- 16.Connell, S. D., and D. A. Smith. 2006. The atomic force microscope as a tool for studying phase separation in lipid membranes. Mol. Membr. Biol. 23:17-28. [DOI] [PubMed] [Google Scholar]
- 17.Crampin, H., K. Finley, M. Gerami-Nejad, H. Court, C. Gale, J. Berman, and P. Sudbery. 2005. Candida albicans hyphae have a Spitzenkorper that is distinct from the polarisome found in yeast and pseudohyphae. J. Cell Sci. 118:2935-2947. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich, C., B. Yang, T. Fujiwara, A. Kusumi, and K. Jacobson. 2002. Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys. J. 82:274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobbelaere, J., and Y. Barral. 2004. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science 305:393-396. [DOI] [PubMed] [Google Scholar]
- 20.Douglas, L. M., F. J. Alvarez, C. McCreary, and J. B. Konopka. 2005. Septin function in yeast model systems and pathogenic fungi. Eukaryot. Cell 4:1503-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drabikowski, W., E. Lagwinska, and M. G. Sarzala. 1973. Filipin as a fluorescent probe for the location of cholesterol in the membranes of fragmented sarcoplasmic reticulum. Biochim. Biophys. Acta 291:61-70. [DOI] [PubMed] [Google Scholar]
- 22.Dykstra, M., A. Cherukuri, H. W. Sohn, S. J. Tzeng, and S. K. Pierce. 2003. Location is everything: lipid rafts and immune cell signaling. Annu. Rev. Immunol. 21:457-481. [DOI] [PubMed] [Google Scholar]
- 23.Edidin, M. 2003. The state of lipid rafts: from model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 32:257-283. [DOI] [PubMed] [Google Scholar]
- 24.Feng, Q., E. Summers, B. Guo, and G. Fink. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filler, S. G. 2006. Candida-host cell receptor-ligand interactions. Curr. Opin. Microbiol. 9:333-339. [DOI] [PubMed] [Google Scholar]
- 26.Ford, S. K., and J. R. Pringle. 1991. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC11 gene product and the timing of events at the budding site. Dev. Genet. 12:281-292. [DOI] [PubMed] [Google Scholar]
- 27.Futerman, A. H., and H. Riezman. 2005. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 15:312-318. [DOI] [PubMed] [Google Scholar]
- 28.Gantner, B. N., R. M. Simmons, and D. M. Underhill. 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24:1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladfelter, A. S. 2006. Control of filamentous fungal cell shape by septins and formins. Nat. Rev. Microbiol. 4:223-229. [DOI] [PubMed] [Google Scholar]
- 31.Gong, X., and A. Chang. 2001. A mutant plasma membrane ATPase, Pma1-10, is defective in stability at the yeast cell surface. Proc. Natl. Acad. Sci. USA 98:9104-9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham, T. R. 2004. Flippases and vesicle-mediated protein transport. Trends Cell Biol. 14:670-677. [DOI] [PubMed] [Google Scholar]
- 33.Grossmann, G., M. Opekarova, J. Malinsky, I. Weig-Meckl, and W. Tanner. 2007. Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J. 26:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heese-Peck, A., H. Pichler, B. Zanolari, R. Watanabe, G. Daum, and H. Riezman. 2002. Multiple functions of sterols in yeast endocytosis. Mol. Biol. Cell 13:2664-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinsbroek, S. E., G. D. Brown, and S. Gordon. 2005. Dectin-1 escape by fungal dimorphism. Trends Immunol. 26:352-354. [DOI] [PubMed] [Google Scholar]
- 36.Horvath, A., C. Sutterlin, U. Manning-Krieg, N. R. Movva, and H. Riezman. 1994. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J. 13:3687-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Insenser, M., C. Nombela, G. Molero, and C. Gil. 2006. Proteomic analysis of detergent-resistant membranes from Candida albicans. Proteomics 6(Suppl. 1):S74-S81. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson, K., O. G. Mouritsen, and R. G. Anderson. 2007. Lipid rafts: at a crossroad between cell biology and physics. Nat. Cell Biol. 9:7-14. [DOI] [PubMed] [Google Scholar]
- 39.James, T. Y., F. Kauff, C. L. Schoch, P. B. Matheny, V. Hofstetter, C. J. Cox, G. Celio, C. Gueidan, E. Fraker, J. Miadlikowska, H. T. Lumbsch, A. Rauhut, V. Reeb, A. E. Arnold, A. Amtoft, J. E. Stajich, K. Hosaka, G. H. Sung, D. Johnson, B. O'Rourke, M. Crockett, M. Binder, J. M. Curtis, J. C. Slot, Z. Wang, A. W. Wilson, A. Schussler, J. E. Longcore, K. O'Donnell, S. Mozley-Standridge, D. Porter, P. M. Letcher, M. J. Powell, J. W. Taylor, M. M. White, G. W. Griffith, D. R. Davies, R. A. Humber, J. B. Morton, J. Sugiyama, A. Y. Rossman, J. D. Rogers, D. H. Pfister, D. Hewitt, K. Hansen, S. Hambleton, R. A. Shoemaker, J. Kohlmeyer, B. Volkmann-Kohlmeyer, R. A. Spotts, M. Serdani, P. W. Crous, K. W. Hughes, K. Matsuura, E. Langer, G. Langer, W. A. Untereiner, R. Lucking, B. Budel, D. M. Geiser, A. Aptroot, P. Diederich, I. Schmitt, M. Schultz, R. Yahr, D. S. Hibbett, F. Lutzoni, D. J. McLaughlin, J. W. Spatafora, and R. Vilgalys. 2006. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818-822. [DOI] [PubMed] [Google Scholar]
- 40.Kapteyn, J. C., H. Van Den Ende, and F. M. Klis. 1999. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta 1426:373-383. [DOI] [PubMed] [Google Scholar]
- 41.Kim, H. B., B. K. Haarer, and J. R. Pringle. 1991. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J. Cell Biol. 112:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konopka, J. B., C. DeMattei, and C. Davis. 1995. AFR1 promotes polarized apical morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 15:723-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraft, M. L., P. K. Weber, M. L. Longo, I. D. Hutcheon, and S. G. Boxer. 2006. Phase separation of lipid membranes analyzed with high-resolution secondary ion mass spectrometry. Science 313:1948-1951. [DOI] [PubMed] [Google Scholar]
- 44.Kubler, E., H. G. Dohlman, and M. P. Lisanti. 1996. Identification of Triton X-100 insoluble membrane domains in the yeast Saccharomyces cerevisiae. Lipid requirements for targeting of heterotrimeric G-protein subunits. J. Biol. Chem. 271:32975-32980. [DOI] [PubMed] [Google Scholar]
- 45.Kusumi, A., C. Nakada, K. Ritchie, K. Murase, K. Suzuki, H. Murakoshi, R. S. Kasai, J. Kondo, and T. Fujiwara. 2005. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 34:351-378. [DOI] [PubMed] [Google Scholar]
- 46.Lauwers, E., and B. Andre. 2006. Association of yeast transporters with detergent-resistant membranes correlates with their cell-surface location. Traffic 7:1045-1059. [DOI] [PubMed] [Google Scholar]
- 47.Lee, S. A., S. Wormsley, S. Kamoun, A. F. Lee, K. Joiner, and B. Wong. 2003. An analysis of the Candida albicans genome database for soluble secreted proteins using computer-based prediction algorithms. Yeast 20:595-610. [DOI] [PubMed] [Google Scholar]
- 48.Li, F., and S. P. Palecek. 2003. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot. Cell 2:1266-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li, S., L. Du, G. Yuen, and S. D. Harris. 2006. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 17:1218-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lichtenberg, D., F. M. Goni, and H. Heerklotz. 2005. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 30:430-436. [DOI] [PubMed] [Google Scholar]
- 51.Lipke, P. N., and J. Kurjan. 1992. Sexual agglutination in budding yeasts: structure, function, and regulation of adhesion glycoproteins. Microbiol. Rev. 56:180-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malathi, K., K. Higaki, A. H. Tinkelenberg, D. A. Balderes, D. Almanzar-Paramio, L. J. Wilcox, N. Erdeniz, F. Redican, M. Padamsee, Y. Liu, S. Khan, F. Alcantara, E. D. Carstea, J. A. Morris, and S. L. Sturley. 2004. Mutagenesis of the putative sterol-sensing domain of yeast Niemann Pick C-related protein reveals a primordial role in subcellular sphingolipid distribution. J. Cell Biol. 164:547-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malinska, K., J. Malinsky, M. Opekarova, and W. Tanner. 2004. Distribution of Can1p into stable domains reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells. J. Cell Sci. 117:6031-6041. [DOI] [PubMed] [Google Scholar]
- 54.Malinska, K., J. Malinsky, M. Opekarova, and W. Tanner. 2003. Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol. Biol. Cell 14:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin, S. W., and J. B. Konopka. 2004. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot. Cell 3:675-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maxfield, F. R., and A. K. Menon. 2006. Intracellular sterol transport and distribution. Curr. Opin. Cell Biol. 18:379-385. [DOI] [PubMed] [Google Scholar]
- 57.Megha, O. B., and E. London. 2006. Cholesterol precursors stabilize ordinary and ceramide-rich ordered lipid domains (lipid rafts) to different degrees. Implications for the Bloch hypothesis and sterol biosynthesis disorders. J. Biol. Chem. 281:21903-21913. [DOI] [PubMed] [Google Scholar]
- 58.Mouritsen, O. G., and K. Jorgensen. 1997. Small-scale lipid-membrane structure: simulation versus experiment. Curr. Opin. Struct. Biol. 7:518-527. [DOI] [PubMed] [Google Scholar]
- 59.Munn, A. L., A. Heese-Peck, B. J. Stevenson, H. Pichler, and H. Riezman. 1999. Specific sterols required for the internalization step of endocytosis in yeast. Mol. Biol. Cell 10:3943-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munro, S. 2003. Lipid rafts: elusive or illusive? Cell 115:377-388. [DOI] [PubMed] [Google Scholar]
- 61.Naglik, J. R., S. J. Challacombe, and B. Hube. 2003. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 67:400-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nichols, C. B., J. A. Fraser, and J. Heitman. 2004. PAK kinases Ste20 and Pak1 govern cell polarity at different stages of mating in Cryptococcus neoformans. Mol. Biol. Cell 15:4476-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nobile, C. J., D. R. Andes, J. E. Nett, F. J. Smith, F. Yue, Q. T. Phan, J. E. Edwards, S. G. Filler, and A. P. Mitchell. 2006. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oberholzer, U., A. Nantel, J. Berman, and M. Whiteway. 2006. Transcript profiles of Candida albicans cortical actin patch mutants reflect their cellular defects: contribution of the Hog1p and Mkc1p signaling pathways. Eukaryot. Cell 5:1252-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orci, L., R. Montesano, P. Meda, F. Malaisse-Lagae, D. Brown, A. Perrelet, and P. Vassalli. 1981. Heterogeneous distribution of filipin-cholesterol complexes across the cisternae of the Golgi apparatus. Proc. Natl. Acad. Sci. USA 78:293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ostermeyer, A. G., B. T. Beckrich, K. A. Ivarson, K. E. Grove, and D. A. Brown. 1999. Glycosphingolipids are not essential for formation of detergent-resistant membrane rafts in melanoma cells. Methyl-beta-cyclodextrin does not affect cell surface transport of a GPI-anchored protein. J. Biol. Chem. 274:34459-34466. [DOI] [PubMed] [Google Scholar]
- 67.Pearson, C. L., K. Xu, K. E. Sharpless, and S. D. Harris. 2004. MesA, a novel fungal protein required for the stabilization of polarity axes in Aspergillus nidulans. Mol. Biol. Cell 15:3658-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pichler, H., and H. Riezman. 2004. Where sterols are required for endocytosis. Biochim. Biophys. Acta 1666:51-61. [DOI] [PubMed] [Google Scholar]
- 69.Proszynski, T. J., R. Klemm, M. Bagnat, K. Gaus, and K. Simons. 2006. Plasma membrane polarization during mating in yeast cells. J. Cell Biol. 173:861-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajagopalan, S., V. Wachtler, and M. Balasubramanian. 2003. Cytokinesis in fission yeast: a story of rings, rafts and walls. Trends Genet. 19:403-408. [DOI] [PubMed] [Google Scholar]
- 71.Rajendran, L., and K. Simons. 2005. Lipid rafts and membrane dynamics. J. Cell Sci. 118:1099-1102. [DOI] [PubMed] [Google Scholar]
- 72.Richard, M., S. Ibata-Ombetta, F. Dromer, F. Bordon-Pallier, T. Jouault, and C. Gaillardin. 2002. Complete glycosylphosphatidylinositol anchors are required in Candida albicans for full morphogenesis, virulence and resistance to macrophages. Mol. Microbiol. 44:841-853. [DOI] [PubMed] [Google Scholar]
- 73.Rozelle, A. L., L. M. Machesky, M. Yamamoto, M. H. Driessens, R. H. Insall, M. G. Roth, K. Luby-Phelps, G. Marriott, A. Hall, and H. L. Yin. 2000. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr. Biol. 10:311-320. [DOI] [PubMed] [Google Scholar]
- 74.Saporito-Irwin, S. M., C. E. Birse, P. S. Sypherd, and W. A. Fonzi. 1995. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol. Cell. Biol. 15:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schaller, M., M. Bein, H. C. Korting, S. Baur, G. Hamm, M. Monod, S. Beinhauer, and B. Hube. 2003. The secreted aspartyl proteinases Sap1 and Sap2 cause tissue damage in an in vitro model of vaginal candidiasis based on reconstituted human vaginal epithelium. Infect. Immun. 71:3227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schneiter, R., B. Brugger, R. Sandhoff, G. Zellnig, A. Leber, M. Lampl, K. Athenstaedt, C. Hrastnik, S. Eder, G. Daum, F. Paltauf, F. T. Wieland, and S. D. Kohlwein. 1999. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 146:741-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schuck, S., M. Honsho, K. Ekroos, A. Shevchenko, and K. Simons. 2003. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA 100:5795-5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sedwick, C. E., and A. Altman. 2002. Ordered just so: lipid rafts and lymphocyte function. Sci. STKE 2002:RE2. [DOI] [PubMed] [Google Scholar]
- 79.Shea, J. M., and M. Del Poeta. 2006. Lipid signaling in pathogenic fungi. Curr. Opin. Microbiol. 9:352-358. [DOI] [PubMed] [Google Scholar]
- 80.Siafakas, A. R., L. C. Wright, T. C. Sorrell, and J. T. Djordjevic. 2006. Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot. Cell 5:488-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spreghini, E., D. A. Davis, R. Subaran, M. Kim, and A. P. Mitchell. 2003. Roles of Candida albicans Dfg5p and Dcw1p cell surface proteins in growth and hypha formation. Eukaryot. Cell 2:746-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sudbery, P. E. 2001. The germ tubes of Candida albicans hyphae and pseudohyphae show different patterns of septin ring localization. Mol. Microbiol. 41:19-31. [DOI] [PubMed] [Google Scholar]
- 83.Sundstrom, P. 2002. Adhesion in Candida spp. Cell. Microbiol. 4:461-469. [DOI] [PubMed] [Google Scholar]
- 84.Sutterlin, C., T. L. Doering, F. Schimmoller, S. Schroder, and H. Riezman. 1997. Specific requirements for the ER to Golgi transport of GPI-anchored proteins in yeast. J. Cell Sci. 110:2703-2714. [DOI] [PubMed] [Google Scholar]
- 85.Takeda, M., G. P. Leser, C. J. Russell, and R. A. Lamb. 2003. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc. Natl. Acad. Sci. USA 100:14610-14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeda, T., and F. Chang. 2005. Role of fission yeast myosin I in organization of sterol-rich membrane domains. Curr. Biol. 15:1331-1336. [DOI] [PubMed] [Google Scholar]
- 87.Takeda, T., T. Kawate, and F. Chang. 2004. Organization of a sterol-rich membrane domain by cdc15p during cytokinesis in fission yeast. Nat. Cell Biol. 6:1142-1144. [DOI] [PubMed] [Google Scholar]
- 88.Takeo, K. 1985. A correlation between mode of growth and regional ultrastructure of the plasma membrane of Schizosaccharomyces pombe as revealed by freeze-fracturing before and after filipin treatment. J. Gen. Microbiol. 131:309-316. [DOI] [PubMed] [Google Scholar]
- 89.Takizawa, P. A., J. L. DeRisi, J. E. Wilhelm, and R. D. Vale. 2000. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290:341-344. [DOI] [PubMed] [Google Scholar]
- 90.Valdez-Taubas, J., and H. R. Pelham. 2003. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr. Biol. 13:1636-1640. [DOI] [PubMed] [Google Scholar]
- 91.Wachtler, V., and M. K. Balasubramanian. 2006. Yeast lipid rafts?—an emerging view. Trends Cell Biol. 16:1-4. [DOI] [PubMed] [Google Scholar]
- 92.Wachtler, V., S. Rajagopalan, and M. K. Balasubramanian. 2003. Sterol-rich plasma membrane domains in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 116:867-874. [DOI] [PubMed] [Google Scholar]
- 93.Walther, T. C., J. H. Brickner, P. S. Aguilar, S. Bernales, C. Pantoja, and P. Walter. 2006. Eisosomes mark static sites of endocytosis. Nature 439:998-1003. [DOI] [PubMed] [Google Scholar]
- 94.Warenda, A. J., and J. B. Konopka. 2002. Septin function in Candida albicans morphogenesis. Mol. Biol. Cell 13:2732-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wheeler, R. T., and G. R. Fink. 2006. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Whitfield, G., T. Brock, A. Ammann, D. Gottlieb, and H. Carter. 1955. Filipin, an antifungal antibiotic: isolation and properties. J. Am. Chem. Soc. 77:4799-4801. [Google Scholar]
- 97.Xu, X., R. Bittman, G. Duportail, D. Heissler, C. Vilcheze, and E. London. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). J. Biol. Chem. 276:33540-33546. [DOI] [PubMed] [Google Scholar]
- 98.Young, M. E., T. S. Karpova, B. Brugger, D. M. Moschenross, G. K. Wang, R. Schneiter, F. T. Wieland, and J. A. Cooper. 2002. The Sur7p family defines novel cortical domains in Saccharomyces cerevisiae, affects sphingolipid metabolism, and is involved in sporulation. Mol. Cell. Biol. 22:927-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu, F., K. Zaleta-Rivera, X. Zhu, J. Huffman, J. C. Millet, S. D. Harris, G. Yuen, X. C. Li, and L. Du. 2007. Structure and biosynthesis of heat stable antifungal factor (HSAF), a broad spectrum antimycotic with a novel mode of action. Antimicrob. Agents Chemother. 51:64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zacharias, D. A., J. D. Violin, A. C. Newton, and R. Y. Tsien. 2002. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296:913-916. [DOI] [PubMed] [Google Scholar]
- 101.Zinser, E., F. Paltauf, and G. Daum. 1993. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 175:2853-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]