Abstract
Patients with extensive subcortical cerebrovascular disease may have impaired memory, often despite the absence of medial temporal or diencephalic strokes. In this group, episodic memory failure may arise from frontal lobe dysfunction based on disruption of frontosubcortical loops caused by lacunae. We tested this idea by studying cognitively impaired subcortical stroke (CIS) patients and Alzheimer’s disease (AD) patients with [18F]-fluorodeoxyglucose positron emission tomography using a continuous verbal memory task during the period of tracer uptake. Patients were matched on severity of cognitive impairment and overall memory task performance. As hypothesized, we found a double dissociation in the relations between metabolism and memory in these groups, such that memory in CIS (but not in AD) correlates with prefrontal lobe metabolism, whereas in AD (but not in CIS), memory correlates with left hippocampal and temporal lobe metabolism. Analysis of memory subscores showed that CIS patients made more errors on short-delay trials, which is consistent with working memory failure. It seems that different pathogenic mechanisms underlie episodic memory failure in subcortical cerebrovascular disease and AD.
It is well recognized that patients with Alzheimer’s disease (AD) suffer from early and profound memory failure and that this is probably related to severe pathological involvement of the medial temporal lobe structures involved in episodic memory function. This mechanism of memory failure is supported in part by data from neuroimaging showing atrophy of medial temporal structures in AD,1,2 which is related to memory function.3-6 In addition to AD, however, patients with memory loss may suffer from cerebrovascular disease. These patients are sometimes diagnosed as having ischemic vascular dementia (IVD). Although the precise nature and frequency of cerebrovascular disease as a cause of dementia are contentious,7-9 there is little doubt that it frequently coexists with AD at the time of postmortem examination10,11 and may occur as the sole cause of dementia.12,13 The contributions of cere-brovascular pathological characteristics to cognitive loss are particularly uncertain when the pathological characteristics are manifested only in subcortical structures.
Severe persistent failure of long-term memory, as is common in IVD and AD, is classically related to lim-bic or diencephalic lesions. IVD patients frequently have no apparent strokes in these regions but are nonetheless amnestic.10,14-16 Studies suggest that the application of current diagnostic criteria for IVD17,18 generally excludes patients with AD alone and results in groups of patients with disease consisting of pure IVD and mixed AD/IVD. Thus, AD clearly contributes to the memory loss in many cases but may not be the only cause of memory loss in this population.
There are currently several competing models of how subcortical vascular pathological changes might lead to dementia, all of which emphasize diffuse cortical dysfunction as a general basis for cognitive loss.7,19-21 The strategic lesion model proposes that even a single stroke may cause dementia by destroying critical subcortical nuclei, with consequent widespread cortical dysfunction,22 although cumulative effect models propose that an aggregation of strokes may subtract a critical volume of brain parenchyma or may synergistically disrupt functional circuits.23 The exact mechanism of memory loss in subcortical IVD is unclear, however.
One explanation for memory loss in IVD patients with primarily subcortical cerebrovascular disease (“subcortical IVD”) invokes impairment of functional systems involving the frontal lobes. Recent studies, especially those that have comprised reasonably homoge-Association neous groups of vascular dementia patients, have generally found a consistent pattern of cognitive impairment in IVD that has been labeled “subcortical-frontal” and differs from the characteristic pattern of cognitive impairments in AD. Several studies have compared clinically diagnosed groups of patients with these two dementias and have found evidence of greater impairment of executive function24 and verbal fluency25,26 in IVD patients. Studies examining memory function in IVD patients have found overall better function than in comparably demented AD patients but have also found that retrieval, an aspect of memory performance with a large executive function component, is more impaired in IVD patients.26
Although the functional imaging literature shows that component processes of the episodic memory system are frequently widely distributed,27,28 it is also clear that lesions of prefrontal cortex and medial temporal structures result in significantly different types of memory deficits. Although the classic amnestic disorder associated with medial temporal lesions is not seen in patients with focal frontal lesions, these patients have impairments in organizing to-be-remembered material, in temporal ordering of memories, and in contextual memory.29-34 Frontal structures play a critical role in the regulation of working memory as well,35 the failure of which can contribute to episodic memory impairment. Because IVD and AD patients differ in the degree of dysfunction in frontal and temporal structures, one might predict that they would also differ in the degree to which memory impairment is related to the functioning of these different structures. If frontal dysfunction predominates in IVD, one might predict that memory failure would primarily correlate with the extent of physiological dysfunction in prefrontal cortex. Conversely, one might predict that in AD patients, in whom temporal lobe dysfunction predominates, memory failure would correlate primarily with temporal lobe dysfunction. A secondary prediction is that the pattern of memory failure would differ between patients in these 2 groups. This hypothesis does not necessitate entirely distinct neuropathological substrates for AD and IVD, because it is clear that AD pathological changes may occur in patients labeled as having “IVD.” Rather, the presence of substantial subcortical cerebrovascular disease may modify the expression of AD pathological changes so that the behavioral features more closely resemble those seen in subcortical frontal syndromes.
The purpose of this study was to test this model of memory failure in IVD by attempting to show a specific link between subcortical stroke, cortical glucose metabolism, and memory function. We performed high-resolution [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) studies using a verbal memory task during the period of tracer uptake and then looked for relations between metabolic activity and memory task performance. Our hypothesis was that different brain regions would be related to memory ability in the two conditions—prefrontal cortex in subcortical vascular disease and temporal lobes in AD.
Patients and Methods
Subjects were 15 patients with subcortical (but not cortical) stroke and cognitive impairment (cognitively impaired stroke [CIS] patients) and 15 patients with AD recruited through the University of California at Davis Alzheimer’s Disease Center and neurology clinics. The inclusion criteria for CIS subjects were cognitive impairment, one or more infarcts outside the cerebellum, and no cortical infarcts. As actually enrolled, all CIS subjects had multiple lacunae which were distributed bilaterally throughout the white matter and basal ganglia. CIS cases were otherwise unselected with regard to lesion location (eg, no special attempt was made to obtain cases with caudate or thalamic strokes). The AD subjects were selected so as to match the CIS subjects on global severity of cognitive impairment as assessed by the Mini-Mental State Examination (MMSE).36
Evaluations of both groups followed the same protocol and were all conducted at the Alzheimer’s Disease Center. For both groups, the evaluation consisted of a general medical history and physical examination, neurological examination, laboratory evaluation of serum chemistry, blood count, and evaluation of vitamin B12 level and thyroid functions. Comprehensive neuropsychological testing was performed on all subjects to assess general level of cognitive function, language, visuospatial ability, executive function, memory, and attention. In addition, all subjects had a magnetic resonance imaging (MRI) scan (on either a 1.5- or 0.5-T system) performed as part of the study protocol. Data from most of these stroke patients have been reported previously in a comparison of metabolic activity between stroke and control patients.37
Subjects were excluded if they were younger than the age of 55 years, were non-English language speaking, had severe dementia (Clinical Dementia Rating Scale38 score of >2), had a history of alcohol or substance abuse within 5 years of the onset of cognitive change, had any history of head trauma with loss of consciousness, had other significant neurological or psychiatric disorders, were on medications that affected cognitive function, or had serious unstable medical illness. In addition, subjects were excluded if the study MRI scan showed evidence of cortical infarction or structural brain disease other than cerebral atrophy, lacunar infarction, or white matter changes.
After the clinical evaluation, all subjects were diagnosed at case conference. AD patients met NINCDS-ADRDA39 criteria for probable AD. Potential AD subjects were excluded from the AD group if there was evidence of stroke by history or neurological examination, or if the MRI scan showed stroke. White matter signal hyperintensity was present to a variable degree in the AD group. The CIS group was defined only by radiologically ascertained subcortical lacunae and the presence of dementia (8 cases) or cognitive impairment (7 cases).
Lacunae, defined as small completed infarcts in basal ganglia, capsular, and thalamic regions 3 to 15 mm in diameter, were identified by a single neuroradiologist (masked to clinical diagnosis) according to the following criteria. All lacunae were hyperintense to surrounding tissue on proton density–weighted images. Because approximately 3% of lacunae are cavitated with signal characteristics identical to those of perivascular spaces (ie, low signal on T1-weighted images, signal isointense to surrounding tissue on proton density–weighted images, and signal equal to that of cerebrospinal fluid on T2-weighted images),40,41 additional location and size criteria were employed. Such lesions at the level of the anterior commissure were termed perivascular spaces. Such lesions outside that region were defined as cavitated lacunae if they were 3 mm in diameter or greater and as perivascular spaces if they were less than 3 mm in diameter. Areas of white matter hyperintensity not meeting these criteria were defined and rated as abnormal white matter. None of the AD patients had MRI findings meeting the criteria for lacunae.
Cognitive impairment was defined on the basis of detailed neuropsychological testing and careful functional histories, including informant report. These patients had mild neuro-psychological test abnormalities, always including memory, but did not meet the criteria for dementia, because the deficits were circumscribed or there was no significant functional impairment. No effort was made to exclude patients with a clinical diagnoses of “possible AD” from the CIS group (3 such cases were included).
CIS patients all had multiple supratentorial lacunae (mean number = 5.6); 1 patient had two lacunae (a large thalamic lacuna and a small white matter lacuna), 6 had three, 3 had four to six, and 5 had seven or more. The lacunae were widely distributed throughout the white matter, basal ganglia, and thalamus, without any hemispherical predominance to the distribution for the group as a whole. In all but 1 case, either the caudate, the thalamus, or both had lacunae, and there were six lacunae identified in the remaining case.
White matter abnormalities were present to varying degrees in both groups but were much more severe in CIS patients. Periventricular and deep white matter abnormalities were separately rated on four-point scales that have previously been demonstrated to be reliable.26 For periventricular changes, 0 = none, 1 = caps or pencil-thin rims, 2 = smooth halo, and 3 = irregular and extending within white matter. For deep white matter, 0 = none, 1 = punctate foci, 2 = early confluent areas, and 3 = large confluent areas. AD patients scored significantly lower than CIS patients on both scales (periventricular scale means: 0.75 vs 2.40; deep white matter scale means: 0.58 vs 2.20; p < 0.0001 in each case). None of the AD cases had ratings of 3 on either scale, and only 2 had ratings of 2, indicating that in nearly every case, the white matter abnormalities of the AD cases were either mild or absent.
In addition, memory task data from 15 healthy elderly control subjects are reported as a point of reference for the patients’ performance. These subjects were recruited through advertising and word of mouth, underwent all evaluations performed on patients, and passed all exclusion criteria. All control subjects were free of complaints of memory loss and performed normally on neuropsychological testing.
Demographic characteristics of all 3 groups are displayed in Table 1. One-way ANOVAs showed that the 3 groups differ significantly on level of education (F[2,42] = 12.39, p < 0.0001) and MMSE score (F[2,42] = 12.43, p < 0.0001). Post hoc analyses using Tukey’s HSD test showed that the educational level of the CIS group was lower than that of the AD and control groups, which did not differ from one another, and that both patient groups scored lower on the MMSE than controls. A two-group ANOVA comparing CIS and AD patients on MMSE scores was not significant (F[1,28] = 0.44).
Table 1.
Subject Characteristicsa
| Age
|
Years of Education
|
MMSE
|
||||||
|---|---|---|---|---|---|---|---|---|
| Group | N | Mean | SD | Mean | SD | Sex (M/F) | Mean | SD |
| CIS | 15 | 72.7 | 9.0 | 10.5 | 3.8 | 12/3 | 24.1 | 4.9 |
| AD | 15 | 72.8 | 9.8 | 14.2 | 2.4 | 7/8 | 23.3 | 3.0 |
| Control | 15 | 72.5 | 8.2 | 15.7 | 2.4 | 5/10 | 29.2 | 1.3 |
See text for between-group differences.
MMSE = Mini-Mental State Examination; CIS = cognitively impaired stroke; AD = Alzheimer’s disease.
Memory Task
All subjects performed a continuous verbal recognition memory task (CVRMT) during the tracer uptake phase of the PET scan. In the several minutes before injection, subjects were provided with instructions on the CVRMT and given a period of brief practice to ensure that performance requirements were understood. The task began 10 to 20 seconds before the injection and lasted for 20 minutes at least. During the task, subjects sat at a video display terminal and read words presented one at a time on the screen. Subjects were instructed to identify each word as “old” or “new” depending on whether or not it had been presented previously. Responses were recorded by a research assistant, who also prompted subjects as needed to maintain involvement in the task. Target words reappeared after their initial presentation after either 0, 1, 3, 7, or 15 intervening words. Words appeared roughly 4 seconds apart; thus, a 15-word lag corresponds to a delay of approximately 1 minute. Fifteen targets appeared at each delay, with the exception of 30 targets that appeared with 0 intervening words so as to make the instructions easier to remember. The same pseudorandom sequence of target words and lag intervals was used for all subjects.
Performance was analyzed using the variable Pr, a measure of response accuracy that estimates true recognition by adjusting the hit rate for false alarms. Pr is defined as hit rate minus false alarm rate when both rates are calculated so as to avoid infinite or zero values.42 Pr asymptotes at 1 and −1, and higher scores indicate better performance. To make Pr normally distributed, an arctation transformation was performed. All significance tests were run using the transformed scores, but raw values are reported here.
PET Imaging
PET studies were performed on a CTI/Siemens ECAT EXACT HR PET scanner (Knoxville, TN) using the glucose metabolic tracer FDG within 3 months of clinical evaluation and MRI. Characteristics of the scanner (resolution of 3.6 mm full width at half maximum (FWHM) at center to 4.5 mm tangentially and 7.4 mm radially, with an axial resolution of 4–6.7 mm) have been published.43
Each subject was injected with approximately 10 mCi of FDG. PET scanning commenced approximately 40 minutes after tracer injection. The patient was positioned so that the field of view (15 cm) encompassed the entire brain. Emission data were acquired in a two-dimensional mode for 40 minutes, with a count rate of approximately 60,000 events per second and dead time of 1%. After this, a transmission image was obtained in 20 minutes of imaging using a rotating 68Ge source consisting of three rods of approximately 2 mCi per rod. At the end of the experiment, a known standard of FDG was counted in the PET scanner. After the PET study, the data were reconstructed using standard two-dimensional filtered backprojection techniques (voxel size: 2.4 × 2.4 × 3.1 mm; volume size: 128 × 128 × 47 voxels).
PET Data Analysis
Metabolic activity was quantitated by defining regions of interest (ROIs) and normalizing atrophy-corrected regional activity to atrophy-corrected whole-brain counts. The regions were outlined using procedures developed locally for the analysis of whole-brain PET data sets, which are described in detail elsewhere.44 The approach uses a T1-weighted three-dimensional MRI data set for anatomical region specification and analysis of the PET data. Volumetric MRI and PET data are precisely coregistered with automatic techniques developed by Woods and colleagues.45 All subsequent region drawing is accomplished using only the MRI data, which are resliced to any orientation chosen to define the anatomy best for the structure(s) being outlined. On this resliced data set, a series, or stack, of two-dimensional ROIs are outlined on a set of parallel planes so as to define a structure. This process is facilitated by the use of a three-dimensional cursor that permits the simultaneous visualization of two surface-rendered images (left and right hemispheres) and all three orthogonal planes of section. The cursor actively drawing the ROI is seen reflected in the orthogonal planes and is projected onto the cortical surface. This procedure, combined with the ability to “mark” sulci and gyri on the surface-rendered images and view the cross sections of these markings on the drawing plane, permits precise and accurate visualization of cortical anatomy for region drawing. Once the two-dimensional ROIs are constructed, their surfaces are tiled together within a closed triangular mesh polyhedral surface model defining a three-dimensional region, or volume of interest (VOI). The surface model defining the VOI is then projected within the coordinate system of the original PET data and used to calculate the activity within a three-dimensional volume of the PET data.
The VOI count values are adjusted for the effects of partial volume caused by cerebral atrophy using the segmented MRI data set as prior information in the manner developed by Meltzer and co-workers.46 The proportion of each VOI containing brain and cerebrospinal fluid is determined using an MRI data set that has been convolved with the point spread function of the PET tomograph in the x-y plane and the slice profile in the z axis. This proportion is applied to the calculated metabolic rate to correct the VOI for atrophy using a bilevel correction (brain vs nonbrain).
All VOIs were drawn on the three-dimensional T1-weighted data acquired as described previously by either of two operators masked to patient classification. VOIs were drawn using detailed rules to guide the region boundaries. Interoperator reliability of region drawing in our laboratory is high, with differences in regional cerebral metabolism rate for glucose (rCMRglc) in regions drawn by different operators averaging under 5%.44 The regions included the dorso-lateral frontal cortex (DLFC), orbitofrontal cortex (OFC), middle temporal gyrus (MTG), inferior parietal lobe, occipital cortex, and hippocampus. The hippocampus included the subiculum, Ammon’s horn, and associated white matter tracts. Whole-brain counts were determined by drawing a VOI that encompassed the whole brain, including both cerebral hemispheres, the posterior fossa, and subcortical structures, and then applying the calculations outlined above, including atrophy correction. Thus, the PET data were analyzed in the form of count ratios, with atrophy-corrected VOI counts normalized to atrophy-corrected whole-brain counts.
Results
CVRMT data from the patient groups are presented relative to those of control subjects in Table 2 to show the level of memory impairment in CIS and AD. One-way ANOVA produced a significant effect for group on Pr (F[2,43] = 11.45; p < 0.0001), and post hoc testing using Tukey’s HSD test showed that the 2 patient groups differed from control subjects but did not differ from each other. There was also a significant group effect for hit rate (F[2,43] = 7.22; p < 0.01). In order to distinguish performance on trials that primarily assessed attention or working memory from performance on trials demanding secondary memory processes, separate hit rates were calculated for trials offset from the target by 0 or 1 intervening item (hit rate–immediate) and trials offset from the target by 7 or 15 words (hit rate–delayed). Groups differed on both of these variables (hit rate–immediate: F[2,43] = 4.66; p = 0.015; hit rate–delayed: F[2,43] = 5.28; p < 0.01). Post hoc analyses showed that the CIS group was lower than the other 2 groups on hit rate–immediate and that both patient groups were lower than control subjects on hit rate–delayed. The group effect for the false alarm rate was significant (F[2,43] = 5.89; p < 0.01), and post hoc tests showed that rates for the AD group were higher than for control subjects.
Table 2.
Group Means on Recognition Memory Scoresa
| Group | Hit Rate | Hit Rate (immediate) | Hit Rate (delayed) | False Alarm Rate | Pr |
|---|---|---|---|---|---|
| CIS | 0.70 (0.27) | 0.80 (0.26) | 0.67 (0.29) | 0.13 (0.18) | 0.57 (0.34) |
| AD | 0.82 (0.14) | 0.93 (0.09) | 0.68 (0.23) | 0.26 (0.26) | 0.56 (0.20) |
| Control | 0.94 (0.05) | 0.97 (0.02) | 0.90 (0.08) | 0.04 (0.05) | 0.90 (0.07) |
Standard deviations are given in parentheses.
See text for between-group differences.
CIS = cognitively impaired stroke; AD = Alzheimer’s disease.
To test the hypotheses regarding patterns of association between metabolic activity and CVRMT performance, we performed a series of multiple regressions in which a CVRMT variable was regressed on education and the activity count ratio for each ROI. Education was included as a term, because the groups differed on educational level and univariate analyses suggested that education was related to Pr. Separate regressions were run within each group on each dependent variable for each ROI. Alpha levels were not corrected for multiple comparisons, because the core analyses (eg, regression of Pr on DLFC in the CIS group, Pr on hippocampus in the AD group) were small in number and guided by a priori hypotheses; hence, correction was not appropriate. The remaining analyses (regressions with other regions) were done to test the specificity of our findings, and alpha correction was not an issue as demonstrated below.
As can be seen in Table 3, different patterns of significance emerged for the AD and stroke groups. Pr was significantly associated with left MTG and left hippocampus activity for AD but not for CIS. Conversely, Pr was associated with left DLFC and right OFC activity for CIS but not for AD. Effect plots for the four significant effects (Figs 1 and 2) show that the effects are not simply carried by a small number of outlying cases. There were trends toward significance (p < 0.10) for AD in the regressions of Pr on right hippocampus and left OFC. For CIS, there were trends toward significance in the regressions of Pr on right DLFC and left OFC. Between-group comparisons of the activity ratios in the regions that were significantly associated with memory function did not reveal any significant effects, except in right OFC, where activity for the CIS group was significantly lower than that for the AD group (p < 0.05).
Table 3.
R2 (standardized β for the region of interest term) from Significant Regressions of the Memory Measure Pr on Positron Emission Tomography Count Ratios and Education
| LDLF | ROF | LMTG | LH | |
|---|---|---|---|---|
| AD | 0.11 (−0.22) | 0.15 (0.31) | 0.67 (0.78)a | 0.36 (0.61)c |
| CIS | 0.47 (0.68)b | 0.41 (0.63)c | 0.19 (0.41) | 0.10 (0.30) |
p < 0.001.
p < 0.01.
p > 0.05.
LDLF = left dorsolateral frontal cortex; ROF = right orbital frontal cortex; LMTG = left middle temporal gyrus; LH = left hippocampus; AD = Alzheimer’s disease; CIS = cognitively impaired stroke.
Fig 1.
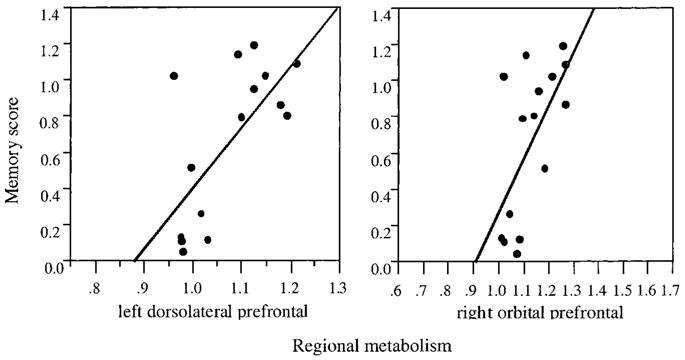
Effect plots of significant relations between metabolism and memory function in cognitively impaired stroke. Left dorsolateral and right orbital prefrontal count ratios are shown in relation to memory performance. As detailed in Table 3, neither region was significant in Alzheimer’s disease.
Fig 2.
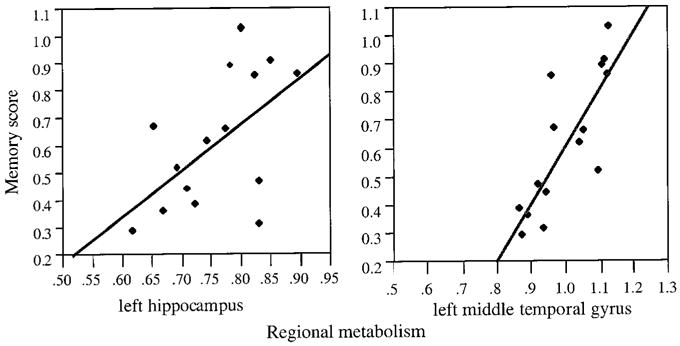
Effect plots of significant relations between metabolism and memory function in Alzheimer’s disease. Left hippocampal and left middle temporal gyrus count ratios are shown in relation to memory performance. As detailed in Table 3, neither region was significant in cognitively impaired stroke.
Pr is affected by both short-delay trials, which are dependent on immediate memory processes, and long-delay trials, which are dependent on delayed memory processes. Also, the groups differed in their hit rate (a component of Pr) for short-delay trials. We therefore did follow-up analyses to see whether short- and longer delayed responses were differentially related to metabolism. Separate Prs were calculated for immediate memory trials by using the hit rate for trials with 0 and 1 intervening words (Pr–immediate) and for delayed memory trials by using items with 7 and 15 intervening words (Pr–delayed). Results for both measures in the CIS group were similar to those obtained for the overall Pr. Pr–immediate was significantly related to both left and right DLFC (p < 0.05 in both cases) and showed a trend toward significance with right OFC (p < 0.10). Pr–delayed was significantly related to left DLFC and right OFC (p < 0.05 in both cases). For the AD group, the results for Pr–delayed were highly similar to those for the overall Pr, because the only significant PET terms were for the left hippocampus and left MTG. The results for the AD group on Pr–immediate were somewhat different; left OFC (p < 0.04) and left MTG (p < 0.01) were the only significant terms. Prs in hippocampal regions did not approach significance.
Discussion
The major finding is a double dissociation in the relations between memory dysfunction and regional glucose metabolic activity in AD and CIS. As hypothesized, lower metabolism in the prefrontal cortex was associated with episodic memory impairment in CIS but not in AD, although lower metabolism in the left hippocampus and left MTG was associated with memory impairment in AD but not in CIS. Relations between prefrontal metabolism and memory were present for CIS on both immediate- and delayed-memory trials, although the relation of left hippocampus to memory in AD appeared only on long-delay trials and in overall performance. Both groups were equivalently impaired in terms of overall memory and general cognitive function. Therefore, the group differences in the relations between metabolism and memory are unlikely to reflect simple differences in task difficulty or performance levels; it is more likely that they reflect differences in the mechanisms of memory failure in these two disorders.
The continuous recognition task has both working memory and episodic memory components, and it is likely that these components were differentially impaired in AD and CIS. In many respects, the task is essentially the same as a continuous performance test (CPT), which also demands selection of sequential stimuli that are separated by a few seconds but in which the target is constant. The CPT is used to measure sustained attention and working memory.47 Pure amnestic patients and even mildly demented AD patients generally do well on the CPT,48,49 whereas patients with frontal lobe damage are typically deficient.50 The recognition memory test alters the CPT by making each stimulus a potential target and by establishing a significant delay before some target presentations, clearly adding requirements for encoding, storage, and retrieval memory to the task. Nevertheless, the working memory component certainly remains important, because electroencephalographic studies have demonstrated prominent frontal activations in subjects performing continuous recognition memory tasks.51 Thus, although both CIS and AD patients showed equal overall performance deficits, their deficits need not reflect failures in the same underlying cognitive processes.
It is likely that failures of working memory or attentional processes were especially important in CIS patients. Although patients with focal frontal lesions do not typically show major deficits of episodic memory, various aspects of episodic memory have been linked to the frontal lobes. Frontal dysfunction in these patients could produce impairments on this particular memory task via various attentional, working memory, or executive function failures. Failures of vigilance (such as missing stimulus presentations), failures to maintain stimuli in working memory, and failures to switch responses appropriately (perseveration) could all lead to errors on this test. Each of these cognitive failures would be expected to produce errors on short-delay trials, and, indeed, the CIS patients had distinctly poor performance on short-delay trials. Impairment on delayed trials would follow, because the initial performance was poor, thus resulting in deficient encoding. In this way, working memory deficits could impair both short- and long-delay trials. The strong similarities in the patterns of findings for short- and long-delay trials in CIS is consistent with this account. Impaired performance on long delay trials could also be made worse by a loss of contextual or temporal ordering information, which are characteristics of memory failure associated with frontal lesions.52
The idea that working memory or attentional failures are important in CIS is supported by the literature on sustained attention in frontal lesion and vascular dementia patients. On CPTs, focal frontal lesion patients show slowed reaction times and make more errors than do control subjects.50,53 The error rate in these patients remains constant or even increases throughout extended testing sessions such as the one used here. Similar findings have been reported in IVD as well. Importantly, the degree of abnormality observed in IVD patients on the CPT was significantly greater than that found in comparably demented AD patients.54 Thus, previous work using a task that is quite similar to the one used here has revealed impairments in attention and information processing in both focal frontal lesion and IVD patients.
The CVRMT performance of the CIS subjects is also consistent with studies of cognitive deficits in IVD, which have suggested predominant frontal systems failure in IVD.55 When contrasted to AD groups, IVD groups generally show less episodic memory dysfunction and more executive dysfunction at an equal overall level of severity.25,26,56 When memory is examined in more detail, IVD subjects display learning and recall deficiencies, with comparatively good recognition, which is a pattern consistent with organizational and retrieval impairments (frontal functions) as opposed to storage (temporal lobe) difficulties.26
The CIS patients had multiple lacunae, often with extensive white matter disease, and these pathological findings might be predicted to produce predominant frontal dysfunction. A prior study that included most of these subjects found both generalized and prefrontal abnormalities of cerebral metabolic rate for glucose (CMRglc) relative to controls.37 As Ishii and coworkers57 initially noted, strokes associated with small-vessel ischemia tend to occur in the frontal white matter, basal ganglia, and thalamus. Subcortical lesions in these structures may affect cognition and behavior by disrupting prefrontal-striatal-thalamocortical circuits. Three segregated but parallel corticobasal-thalamocortical loops that separately regulate aspects of cognitive, social, and volitional behavior have been increasingly well delineated.58-60 Thus, distributed and varied lesions may result in a common predominant pattern of functional impairment, manifested as attentional and working memory failure in this case.
The selective association between hippocampal glucose metabolism and episodic memory in AD suggests that hippocampal dysfunction was a particularly important determinant of memory dysfunction in this group. Medial temporal lobe dysfunction has been associated with failure to form lasting new memories but not particularly with attentional deficits. Consistent with this, we found the left hippocampus to be correlated with delayed trial performance but not immediate trial performance, which further strengthens the argument that failures of storage mechanisms were especially important for AD. This is not surprising, considering what is known about the neuropathological characteristics of AD. Neuronal loss and the accumulation of neuritic plaques and neurofibrillary tangles in the hippocampus are characteristic early changes.61 Degeneration of efferent and afferent projections to the hippocampus seem to disconnect the hippocampus from cortical input,62 further reducing its function. The hippocampus is smaller in subjects with AD than in age-matched controls,1,2 medial temporal atrophy correlates with memory performance in AD,3-6 some PET activation studies demonstrate correlations in normal subjects between various aspects of memory and hippocampal activity (including recognition memory), and there are reports of direct correlations between hippocampal metabolism and memory performance in AD.3,4 Although the prefrontal cortex is clearly involved at a neuro-pathological and functional level, the extent of involvement is distinctly less than that of the mesial temporal lobe, especially in mild cases such as those we studied here.
The performance of the AD group on the CVRMT is consistent with greater medial temporal dysfunction and lesser frontal lobe dysfunction. On short-delay trials, where episodic memory demands are lowest but working memory is required, AD performance was not significantly different from normal (and was related to OFC metabolism), whereas on the long-delayed trials, which can only be accomplished with episodic memory, performance was markedly impaired.
It is important to note that although these findings support a distinction between greater prefrontal and working memory dysfunction in CIS and greater medial temporal and consolidation/storage dysfunction in AD, there is likely to be considerable overlap between these disorders in both the anatomical pathological changes and impairments of component memory processes that each causes. The difficulties in distinguishing these disorders clinically is well recognized. In addition, even if the distinctions in anatomical pathological findings were complete, overlaps in the functional anatomy of episodic memory processes are such that lesions in different regions may still affect a similar component memory process. For example, recent functional MRI and PET activation studies suggest that episodic encoding activates both the prefrontal cortex and temporal lobe and, often, other regions.27,63-65 Further, isolated activations of medial temporal lobe structures in relation to memory processes have generally not been found.66,67 Thus, especially in these two disorders with diffuse and overlapping pathological findings, it is certain that there are also shared features to their memory failures. Nonetheless, it seems that the differential patterns of pathological characteristics found in AD and CIS do produce distinguishable consequences with regard to memory function.
Methodological issues and limitations regarding our conclusions should be noted. The sample is small, and this may limit how generalizable the results are, particularly in vascular dementia, which is quite heterogeneous. These patients, however, were unselected with regard to lesion location (except to exclude cortical strokes) or memory pattern, and it is of interest that the results emerged as clearly as they do in this varied group. More important, perhaps, is how generalizable the results might be to similar patients at different levels of severity. Both the AD and CIS groups were mildly impaired. In more severe cases, where greater similarity of pathological involvement is likely, it is also likely that between-group differences in metabolic and memory associations would be less apparent. The PET scans were performed as long as 3 months after the MRI scans. Thus, we cannot exclude the possibility that some of the AD patients suffered silent infarcts during this interval or that some of the CIS patients suffered additional infarcts before PET. Nevertheless, it should be noted that no patient had clinical symptoms of stroke during this interval. We cannot exclude the likelihood that some of the CIS patients have coexisting AD, nor can we be certain that none of the AD patients had small strokes that were not observed on MRI. Indeed, it is likely that the pathological findings in these groups would overlap. The likelihood of overlap is less important than the observation that the groups clearly differed extensively in the degree of subcortical vascular disease. The CIS group was defined by the presence of lacunae, but more importantly, our CIS patients generally had a large number of lacunae that nearly always involved cognitively relevant nuclei and were accompanied by white matter abnormalities distinctly more severe than those seen in our AD sample. The extent of subcortical pathological changes in CIS makes it less likely that this finding was “incidental,” whereas it seems to be minor in most of the AD cases. It is also true that the behavioral task is multi-faceted and was administered over a fairly long period of tracer uptake, which means that numerous cognitive operations influenced the tracer distribution. The strength of the activation is demonstrated by the fact that all the relations between PET and this verbal memory task were stronger on the left side.
In summary, a double dissociation in the brain regions associated with recognition memory impairment in stroke patients and AD patients provides a direct demonstration of a distinction between IVD and AD that has been hypothesized previously. In mildly impaired patients, those with subcortical stroke have dysfunction of the prefrontal cortex that likely affects episodic memory via impairments in working memory and executive function. In mild AD, the role of frontal dysfunction is distinctly less significant, and memory dysfunction is related to failure of storage mechanisms based on mesial temporal dysfunction. These differing anatomical substrates give rise to subtle but significant differences in memory performance.
Acknowledgments
This work was supported in part by National Institute on Aging grants AG10129 and AG12435. It was also supported by the California Department of Health Services Alzheimer’s Disease Program and the Northern California Veterans Affairs System of Clinics.
We thank David Norman for reading the MRI scans and Ken Paller for helpful comments on an earlier draft of this paper.
References
- 1.Seab JP, Jagust WJ, Wong STS, et al. Quantitative NMR measurements of hippocampal atrophy in Alzheimer’s disease. J Magn Res Med. 1988;8:200–208. doi: 10.1002/mrm.1910080210. [DOI] [PubMed] [Google Scholar]
- 2.Jack CR, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 3.DeCarli C, Grady CL, Clark CM, et al. Comparison of positron emission tomography, cognition, and brain volume in Alzheimer’s disease with and without severe abnormalities of white matter. J Neurol Neurosurg Psychiatry. 1996;60:158–167. doi: 10.1136/jnnp.60.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desgranges B, Baron J-C, de la Sayette V, et al. The neural substrates of memory systems impairment in Alzheimer’s disease: a PET study of resting brain glucose utilization. Brain. 1998;121:611–631. doi: 10.1093/brain/121.4.611. [DOI] [PubMed] [Google Scholar]
- 5.Heun R, Mazanek M, Atzor KR, et al. Amygdala-hippocampal atrophy and memory performance in dementia of Alzheimer type. Dement Geriatr Cogn Disord. 1997;8:329–336. doi: 10.1159/000106651. [DOI] [PubMed] [Google Scholar]
- 6.Mori E, Yoneda Y, Yamashita H, et al. Medial temporal structures relate to memory impairment in Alzheimer’s disease: an MRI volumetric study. J Neurol Neurosurg Psychiatry. 1997;63:214–221. doi: 10.1136/jnnp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chui H. Rethinking vascular dementia: moving from myth to mechanism. In: Growdon JH, Rossor M, editors. The dementias. Boston: Butterworth-Heinemann; 1998. pp. 2–40. [Google Scholar]
- 8.O’Brien MD. Vascular dementia is underdiagnosed. Arch Neurol. 1988;45:797–798. doi: 10.1001/archneur.1988.00520310115025. [DOI] [PubMed] [Google Scholar]
- 9.Brust JCM. Vascular dementia is overdiagnosed. Arch Neurol. 1988;45:799–801. doi: 10.1001/archneur.1988.00520310117026. [DOI] [PubMed] [Google Scholar]
- 10.Lim A, Tsuang D, Kukull W, et al. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc. 1999;47:564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 11.Nolan KA, Lino MM, Seligmann AW, Blass JP. Absence of vascular dementia in an autopsy series from a dementia clinic. J Am Geriatr Soc. 1998;46:597–604. doi: 10.1111/j.1532-5415.1998.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 12.Pantoni L, Garcia JH, Brown GG. Vascular pathology in three cases of progressive cognitive deterioration. J Neurol Sci. 1996;135:131–139. doi: 10.1016/0022-510x(95)00273-5. [DOI] [PubMed] [Google Scholar]
- 13.Hulette C, Nochlin D, McKeel D, et al. Clinical-neuropathologic findings in multi-infarct dementia: a report of six autopsied cases. Neurology. 1997;48:668–672. doi: 10.1212/wnl.48.3.668. [DOI] [PubMed] [Google Scholar]
- 14.Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocor-tical disconnection syndrome? Neurology. 1992;42:1966–1979. doi: 10.1212/wnl.42.10.1966. [DOI] [PubMed] [Google Scholar]
- 15.Tatemichi TK, Desmond DW, Paik M, et al. Clinical determinants of dementia related to stroke. Ann Neurol. 1993;33:568–575. doi: 10.1002/ana.410330603. [DOI] [PubMed] [Google Scholar]
- 16.Esiri N, Wilcock G, Morris J. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neu-rosurg Psychiatry. 1997;63:749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 18.Chui HC, Victoroff JI, Margolin D, et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s disease diagnostic and treatment centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 19.Gorelick P. Status of risk factors for dementia associated with stroke. Stroke. 1997;28:135–142. doi: 10.1161/01.str.28.2.459. [DOI] [PubMed] [Google Scholar]
- 20.Erkinjuntti T. Types of multi-infarct dementia. Acta Neurol Scand. 1987;75:391–399. doi: 10.1111/j.1600-0404.1987.tb05467.x. [DOI] [PubMed] [Google Scholar]
- 21.Tatemichi TK. How acute brain failure becomes chronic: a view of the mechanisms of dementia related to stroke. Neurology. 1990;40:1652–1659. doi: 10.1212/wnl.40.11.1652. [DOI] [PubMed] [Google Scholar]
- 22.Tatemichi TK, Desmond DW, Prohovnik I. Strategic infarcts in vascular dementia: a clinical and brain imaging experience. Arzneimittelforschung. 1995;45:371–385. [PubMed] [Google Scholar]
- 23.Wolfe N, Babikian VL, Linn RT, et al. Are multiple cerebral infarcts synergistic? Arch Neurol. 1994;51:211–215. doi: 10.1001/archneur.1994.00540140129022. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe N, Linn R, Babikian VL, et al. Frontal systems impairment following multiple lacunar infarcts. Arch Neurol. 1990;47:129–132. doi: 10.1001/archneur.1990.00530020025010. [DOI] [PubMed] [Google Scholar]
- 25.Starkstein SE, Sabe L, Vazquez S, et al. Neuropsychological, psychiatric, and cerebral blood flow findings in vascular dementia and Alzheimer’s disease. Stroke. 1996;27:408–414. doi: 10.1161/01.str.27.3.408. [DOI] [PubMed] [Google Scholar]
- 26.Lafosse JM, Reed BR, Mungas D, et al. Fluency and memory differences between ischemic vascular dementia and Alzheimer’s disease. Neuropsychology. 1997;11:514–522. doi: 10.1037//0894-4105.11.4.514. [DOI] [PubMed] [Google Scholar]
- 27.Grady CL, McIntosh AR, Rajah MN, Craik FIM. Neural correlates of the episodic encoding of pictures and words. Proc Natl Acad Sci USA. 1998;95:2703–2708. doi: 10.1073/pnas.95.5.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiggs CL, Weisberg J, Martin A. Neural correlates of semantic and episodic memory retrieval. Neuropsychologia. 1999;37:103–118. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 29.Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychol Bull. 1984;95:3–28. [PubMed] [Google Scholar]
- 30.Shimamura AP, Jurica PJ, Mangels JA, Gershberg FB. Susceptibility to memory interference effects following frontal lobe damage: findings from tests of paired-associate learning. J Cogn Neurosci. 1995;7:144–152. doi: 10.1162/jocn.1995.7.2.144. [DOI] [PubMed] [Google Scholar]
- 31.Kopelman MD, Stanhope N. Recall and recognition memory in patients with focal frontal, temporal lobe, and diencephalic lesions. Neuropsychologia. 1998;36:785–796. doi: 10.1016/s0028-3932(97)00167-x. [DOI] [PubMed] [Google Scholar]
- 32.Schacter DL. Memory, amnesia, and frontal lobe dysfunction. Psychobiology. 1987;15:21–36. [Google Scholar]
- 33.Bondi MW, Kaszniak AW, Bayles KA, Vance KT. Contributions of frontal system dysfunction to memory and perceptual abilities in Parkinson’s disease. Neuropsychology. 1993;7:89–102. [Google Scholar]
- 34.Wheeler M, Stuss D, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- 35.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 36.Folstein MF, Folstein SE, McHugh PR. Mini mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Kwan LT, Reed BR, Eberling JL, et al. Effects of subcortical cerebral infarction on cortical glucose metabolism and cognitive function. Arch Neurol. 1999;56:809–814. doi: 10.1001/archneur.56.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 39.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 40.Braffman B, Zimmerman R, Trojanowski J, et al. Brain MR: pathologic correlation with gross and histopathology. 1. Lacunar infarction and Virchow-Robin spaces. AJR Am J Roentgenol. 1988;151:551–558. doi: 10.2214/ajr.151.3.551. [DOI] [PubMed] [Google Scholar]
- 41.Pullicino P, Miller L, Alexandrov A, Ostrow P. Infraputaminal ‘lacunes’. Clinical and pathological correlations Stroke. 1995;26:1598–1602. doi: 10.1161/01.str.26.9.1598. [DOI] [PubMed] [Google Scholar]
- 42.Corwin J. On measuring discrimination and response bias: unequal numbers of targets and distractors and two classes of dis-tractors. Neuropsychology. 1994;8:110–117. [Google Scholar]
- 43.Wienhard K, Dahlbom M, Eriksson L, et al. The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr. 1994;18:110–118. [PubMed] [Google Scholar]
- 44.Klein GJ, Teng X, Jagust WJ, et al. A methodology for specifying PET VOIs using multi-modality techniques. IEEE Trans Med Imaging. 1997;16:405–415. doi: 10.1109/42.611350. [DOI] [PubMed] [Google Scholar]
- 45.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Meltzer CC, Leal JP, Mayberry HS, et al. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr. 1990;14:561–570. doi: 10.1097/00004728-199007000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Lezak MD. Neuropsychological assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 48.Lines C, Dawson C, Preston G, et al. Memory and attention in patients with senile dementia of the Alzheimer type and in normal elderly subjects. J Clin Exp Neuropsychol. 1991;13:691–702. doi: 10.1080/01688639108401083. [DOI] [PubMed] [Google Scholar]
- 49.Teuber H, Milner B, Vaughan H. Persistent anterograde amnesia after stab wound of the basal brain. Neuropsychologia. 1968;6:267–282. [Google Scholar]
- 50.Rueckert L, Grafman J. Sustained attention deficits in patients with right frontal lesions. Neuropsychologia. 1996;34:953–963. doi: 10.1016/0028-3932(96)00016-4. [DOI] [PubMed] [Google Scholar]
- 51.Guillem F, N’kaoua B, Rougier A, Claverie B. Functional heterogeneity of the frontal lobe: evidence from intracranial memory ERPs. Int J Psychophysiol. 1996;21:107–119. doi: 10.1016/0167-8760(95)00051-8. [DOI] [PubMed] [Google Scholar]
- 52.Shimamura A. Memory and frontal lobe function. In: Gazzaniga M, editor. The cognitive neurosciences. Cambridge: MIT Press; 1995. pp. 803–813. [Google Scholar]
- 53.Glosser G, Goodglass H. Disorders in executive control functions among aphasic and other brain-damaged patients. J Clin Exp Neuropsychol. 1990;12:485–501. doi: 10.1080/01688639008400995. [DOI] [PubMed] [Google Scholar]
- 54.Mendez MF, Cherrier MM, Perryman KM. Differences between Alzheimer’s disease and vascular dementia on information processing measures. Brain Cogn. 1997;34:301–310. doi: 10.1006/brcg.1997.0923. [DOI] [PubMed] [Google Scholar]
- 55.McPherson SE. Neuropsychological aspects of vascular dementia. Brain Cogn. 1996;31:269–282. doi: 10.1006/brcg.1996.0045. [DOI] [PubMed] [Google Scholar]
- 56.Kertesz A, Clydesdale S. Neuropsychological deficits in vascular dementia vs Alzheimer’s disease. Arch Neurol. 1994;51:1226–1231. doi: 10.1001/archneur.1994.00540240070018. [DOI] [PubMed] [Google Scholar]
- 57.Ishii N, Nishihara Y, Imamura T. Why do frontal lobe symptoms predominate in vascular dementia with lacunes? Neurology. 1986;36:340–345. doi: 10.1212/wnl.36.3.340. [DOI] [PubMed] [Google Scholar]
- 58.Alexander GE, DeLong RM, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 59.Chui HC, Willis L. Vascular disease of the frontal lobes. In: Miller BL, Cummings JL, editors. The frontal lobes. New York: Guilford Press; 1997. pp. 370–401. [Google Scholar]
- 60.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 61.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 62.Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzhei-mer’s disease. Ann Neurol. 1986;20:472–481. doi: 10.1002/ana.410200406. [DOI] [PubMed] [Google Scholar]
- 63.Grady CL, McIntosh AR, Bookstein F, et al. Age-related changes in regional cerebral blood flow during working memory for faces. Neuroimage. 1998;8:409–425. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- 64.Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 65.Brewer J, Zhao Z, Desmond J, et al. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 66.Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 67.Wagner AD, Koutstaal W, Schacter DL. When encoding yields remembering: insights from event-related neuroimaging. Philos Trans R Soc London B Biol Sci. 1999;354:1307–1324. doi: 10.1098/rstb.1999.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]


