Abstract
The recently cloned human gene named “placental immunoregulatory ferritin” (PLIF) is a pregnancy-related immunomodulator. Recombinant PLIF and its bioactive domain C48 are immune-suppressive and induce pronounced IL-10 production by immune cells. PLIF is expressed in the placenta and breast cancer cells. Blocking PLIF in pregnant mice by anti-C48 antibodies inhibited placental and fetal growth and modulated the cytokine network. It has been revealed that anti-C48 treatment inhibited MCF-7 tumor growth in nude mice. However, this significant effect was observed only in those transfused with human peripheral blood mononuclear cells. Blocking PLIF in tumor-engrafted human immune cell transfused mice resulted in massive infiltration of human CD45+ cells (mainly CD8+ T cells), both intratumorally and in the tumor periphery, and a significant number of caspase-3+ cells. In vitro, anti-C48 treatment of MCF-7 tumor cells cocultured with human lymphocytes induced a significant increase in interferon-γ secretion. We conclude that blocking PLIF inhibits breast cancer growth, possibly by an effect on the cytokine network in immune cells and on breakdown of immunosuppression.
Keywords: Breast cancer, immunotherapy, antibody, immunomodulator, gene
Introduction
It is now accepted that the immune system can mediate the suppression of tumor growth in cancer-bearing patients. Target antigens have been identified, and cancer vaccines have been developed using mostly immunogenes that can prime T cells and immune responses that lead to destruction of tumor cells in animal models [1]. However, many of the responses are partial and transient. Tumor cells may employ immunosuppressive mechanisms resembling those in the placenta, which are believed to prevent rejection of the embryo [2,3].
In recent years, our group has reported the cloning and characterization of a novel human pregnancy-related immune modulator named “placental immunoregulatory ferritin” (PLIF). PLIF cDNA was isolated from human placenta cDNA library [4]. It has a unique molecular structure composed of an incomplete ferritin heavy-chain sequence (amino acids 1–117) linked to a novel nonferritin 48-amino acid domain named C48 (amino acids 118–165). PLIF is expressed in placental syncytiotrophoblast cells and decidual mononuclear cells [4]. PLIF codes for the unique p43 subunit of placental isoferritin (PLF), which has been previously identified using its specific monoclonal antibody (MoAb) CM-H9 [5,6]. The molecular mass of p43 is 43 kDa, and it represents a molecular dimer of PLIF (molecular mass, 22 kDa).
Accumulated data revealed that PLIF acts as an immunosuppressive cytokine and that C48 represents its bioactive domain [4]. The in vitro and in vivo regulatory functions of PLIF are not dependent on any ferritin sequence [4]. The functions of PLIF, as well as of its subcloned C48 fragment, were partially attributed to the induction of pronounced and rapid IL-10 production, as well as of granulocyte—macrophage colony-stimulating factor and IL-6, and to the modulation of cytokine—chemokine networks in immune cells [7,8]. This was demonstrated in vitro in peripheral blood mononuclear cells (PBMCs) [4,5] and in human bone marrow cells [8], as well as in vivo in animal models of rheumatoid arthritis [9].
The expression of PLIF in the placenta is associated with prevention of embryo rejection [10,11]. Indeed, blocking PLIF in pregnant mice by in vivo treatment with anti-C48 antibodies affected placental development and resulted in fetal growth retardation and resorption accompanied by modulation of the cytokine network from T helper (Th) 2 response to Th1 response [12].
In pathological conditions, PLIF has been shown to be expressed in malignant diseases such as lymphoproliferative diseases [13], in human breast cancer tissues, and in PBMCs [14] and breast cancer cell lines (T47D and MCF-7) [4], but not in benign breast disease [15]. Thus, expression of PLIF, similar to its function in the embryo, could manipulate the cytokine network and immune response in the tumor microenvironment and could enable tumor immune escape and growth.
Accordingly, the aim of the current study was to investigate whether in vivo blocking of PLIF in human breast cancer by treatment with anti-PLIF/C48 antibodies in a nude mouse model would affect tumor development and whether it is immune cell-dependent.
Materials and Methods
Prokaryotic Protein Expression and Purification of C48
The cDNA fragment coding for the 48-amino acid C-terminal (C48) of PLIF was subcloned into a pGEX 5X-1 prokaryotic expression vector (Amersham Biosciences, Piscataway, NJ) resulting in a glutathione-S-transferase (GST) — C48 fusion vector, as previously described [4]. This vector was transformed into Escherichia coli BL-21 strain. Bacterial cultures of transformants were harvested after induction with isopropylthiogalactoside and lysed in Triton X-100-based lysis buffer. Then fusion protein was absorbed from lysates using Glutathione Sepharose 4B beads and subsequently eluted (GE Healthcare, Bucks, UK) with an excess of free glutathione. After dialysis, factor Xa cleaved the fusion protein, and purified C48 was obtained by removal of the cleaved GST part using Glutathione Sepharose beads.
Control GST protein was prepared by using the empty pGEX 5X-1 expression vector transformed into E. coli BL-21 strain, as described above.
Preparation of Rabbit Anti-C48 Immunoglobulin (Ig)
Rabbits were immunized with purified recombinant C48 or with GST and control anti-GST Ig [11]. Each rabbit was immunized with 50 µg of purified protein mixed (vol/vol) with complete Freund's adjuvant on days 1, 7, and 21. On day 28, rabbits were bled, and Igs were isolated from anti-C48 and anti-GST sera by salt precipitation. Control Igs from preimmunized rabbits were also purified. Endotoxin levels in purified anti-C48 Ig and anti-GST Ig preparations used for treatment were < 0.1 EU/Ég protein. This was determined by the Limulus amebocyte lysate assay (Biological Industries, Beit Haemek, Israel).
The specificity of anti-C48 Ig was tested on breast cell lines. It was revealed that C48 Ig does not react with cells derived from a normal lactating breast (HBL-100), but reacts with breast cancer cell lines T47D and MCF-7, which express PLIF [4]. Anti-C48 Ig reacts by Western blot analysis with C48 and PLIF [4], but does not react with ferritin H chain (unpublished results). Anti-C48 reacts by enzyme-linked immunosorbent assay (ELISA) with sera from pregnant women (unpublished results) and sera from pregnant mice [12], but does not react with normal human sera (unpublished) and normal mouse sera [12].
Cell Cultures
The MCF-7 human breast carcinoma cell line was maintained in monolayer cultures in RPMI 1640 medium supplemented with 10% fetal calf serum. For passages, confluent monolayer cultures were trypsinized with trypsin/EDTA solution (0.25%/0.05%, respectively), washed once, and seeded in culture medium.
Preparation of Human PBMCs
Buffy coats from blood bank donors were layered onto Lymphoprep solution (Nycomed, Oslo, Norway) and spun at 2000 rpm for 20 minutes. The interface layer was collected, washed twice, counted, and resuspended in phosphate-buffered saline (PBS; pH 7.4) to the desired cell concentration.
MCF-7 and PBMC Coculture
Trypsinized MCF-7 cells were seeded into six-well plates at 4 x 105 cells/well and incubated for 1 hour in a 5% CO2 incubator. Furthermore, supernatants containing nonadherent cells were removed and replaced with fresh medium containing PBMCs at 4 x 106 cells/well at a final volume of 2 ml. Antibodies (100 µg/ml) were added daily to the coculture with MCF-7 cells. The supernatants were collected at 24, 48, and 72 hours of culture, centrifuged at 500g for 10 minutes to remove nonadherent cells, and frozen at -20°C.
MCF-7 monolayer was washed twice with PBS and fixed for 1 hour with 4% paraformaldehyde solution for microscopic visualization.
Measurement of Cytokine Production By ELISA
ELISA kits for the human cytokines IL-10 and interferon-γ (IFN-γ) were purchased from DPC (Sigma, St. Louis, MO) and Peprotec Systems USA (Rocky Hill, NJ). These kits were used according to the manufacturer's instructions to quantify indicated cytokines produced in the supernatants.
In Vivo Studies
The study was approved by the animal review board of the Sackler School of Medicine, Tel Aviv University (Petah Tiqwa, Israel).
Athymic nude mice were purchased from Harlan Laboratories, Ltd. (Jerusalem, Israel). Mice were inoculated subcutaneously with MCF-7 human breast cancer cells (6 x 106) in the presence of an estrogen source from 0.72 mg of β-estradiol pellets (Innovative Research of America, Sarasota, FL). Slow-release pellets (60-day release) were implanted subcutaneously into the cervical scapular space. Human PBMCs (40 x 106/0.5 ml) were injected intravenously after the transplantation of MCF-7 tumor cells, as described above.
Treatment of Tumor-Bearing Mice with Anti-C48 Ig
In each experiment, mice were divided into groups of six mice. Anti-recombinant C48 Ig (anti-C48 Ig) was injected intraperitoneally to each mouse daily at an optimal dose of 2 mg/0.3 ml, as previously determined [11]. Control mice received daily injections of rabbit anti-GST Ig (control Ig) for nonspecific cytotoxic and cytopatic effects of rabbit Ig. Mice were sacrificed as indicated in Results.
Gross and Microscopic Pathology
Tumors were excised 20 days after tumor cell inoculation. They were measured by caliper measurements (using the formula: length x length), weighed, and fixed in phosphate-buffered formalin (pH 7.0) from which paraffin-embedded blocks were prepared.
Immunohistochemistry
Four-micron tissue sections were cut from paraffin-embedded blocks and mounted on coated Superfrost Plus slides (Electron Microscopy Sciences, Hatfield, PA). They were deparaffinized in xylene, rehydrated with graded alcohols, rinsed in H2O, incubated in 3% H2O2 for 10 minutes, and washed in H2O.
For antigen unmasking, the sections were heated in 10 mM sodium citrate buffer (pH 6.0) for 10 minutes in a microwave or pressure chamber, depending on the manufacturer's instructions, and then they were cooled at room temperature for 20 minutes. Slides were washed in H2O and PBS buffer for 5 minutes.
Slides were incubated in normal serum for 10 minutes with a kit (Zymed Laboratories, San Francisco, CA).
For immunohistochemical staining performed manually, slides were incubated with primary antibodies at previously determined optimal concentrations for 60 minutes at room temperature (Table 1).
Table 1.
Antibody Specificity By Immunohistochemical Staining.
| Positive Control | Origin | Cellular Distribution | Antibody |
| Tonsil | Biogenex, San Ramon, CA | All leukocytes | LCA |
| Tonsil | Zymed Laboratories | T cells (helper) | CD4 |
| Tonsil | Signet, Dedham, MA | T cells (suppressor) | CD8 |
| Tonsil | Zymed Laboratories | B cells | CD20 |
| Tonsil | Cell Signaling Technology, Beverly, MA | Apoptotic cells | Caspase-3 |
| Tonsil | R&D Systems, Minneapolis, MN | IL-10 | |
| Tonsil | R&D Systems | IFN-γ |
Slides were washed in H2O and PBS buffer for 5 minutes and further incubated for 10 minutes with biotinylated second antibody (Zymed Laboratories) followed by PBS.
Slides were further incubated for 10 minutes with conjugated streptavidin horseradish peroxidase (Zymed Laboratories), washed with PBS, incubated with 3,3′-diaminobenzidine (Zymed Laboratories) for 5 minutes, and further washed with PBS. Stained slides were counterstained with Mayer's hematoxylin and mounted with aqueous mounting solution.
Counting Procedure for Immunohistochemical Analysis
Immunohistochemistry results were obtained by counting the number of positively stained cells for each immunohistochemical stain.
Positive cells were counted in two areas: between tumoral cells and in the periphery of the tumor surrounded by a thin fibrous capsule and fat tissues.
We selected three areas for each stain: the most positively stained area and two other randomly stained areas. Stained cells appear to have brown cytoplasm. Counting areas were covered with a cover slip on which a 4-mm2 square was marked [16]. We counted positive cells within this square at x 400 magnification, and the results of three such squares were calculated as mean positive cells/4 mm2 x 3 and expressed as the mean percentage of positive cells in the marked area for all antibodies. For CD4 and CD8 cells, the ratio was indicated as the percentage of positive lymphocytes detected by LCA.
Statistical Analysis
Results were presented as mean ± standard deviation. Comparisons were made by Student's t test. P ≤ .05 was considered statistically significant.
Results
Effect of Therapeutic Anti-C48 Ig on MCF-7 Tumor Development in Nude Mice
The functional role of PLIF in tumor development was studied in a human breast cancer animal model. Nude mice were implanted subcutaneously with MCF-7 tumor cells, followed by daily intraperitoneal injections with anti-C48 Ig (2 mg) to block PLIF activity. Control mice received anti-GST Ig (2 mg) for comparison (control Ig).
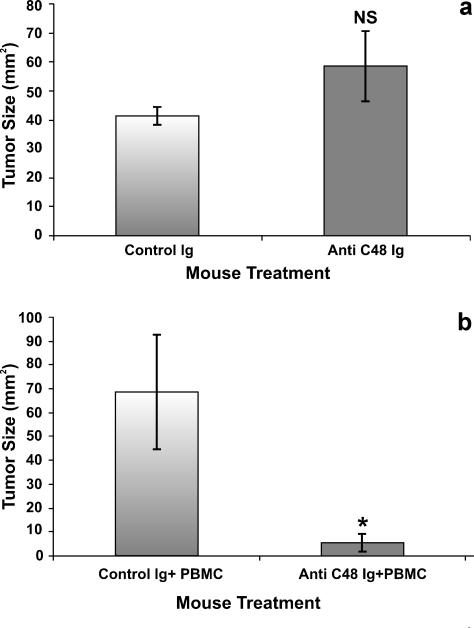
After 20 days, the mice were sacrificed, and the tumors were removed and measured. As seen in Figure 1a, there was no significant difference between the mean size of tumors removed from the anti-C48 Ig-treated group (58.6 ± 12 mm2) and the mean size of tumors removed from the control group (41.3 ± 3 mm2) (P = .27) (Figure 1a). These results indicated that there was no direct effect of anti-C48 antibodies on the growth and development of MCF-7 human breast cancer cells.
Figure 1.
The effect of anti-C48 Ig treatment without and with inoculated PBMC on the growth of MCF-7 breast tumors in nude mice. (a) Nude mice without PBMC inoculation. (b) Nude mice inoculated intravenously with human PBMCs (40 x 106/0.5 ml) (HICT) after the engraftment of MCF-7 breast cancer cells. All mice received daily intraperitoneal injections of anti-C48 Ig or control Ig (2 mg/0.3 ml). Bars represent mean ± S.E. (n = 6).
Because PLIF is an immunosuppressive regulator that affects the cytokine network in immune cells, we investigated whether, in the nude mouse model, passive transfer of lymphocytes and blocking of PLIF in MCF-7 tumor cells affect tumor growth.
In the following experiments, human PBMCs were injected intravenously to MCF-7-engrafted nude mice, and the mice were treated as described above. As seen in Figures 1b and 2A, in human immune cell transfused (HICT) mice, treatment with anti-C48 Ig significantly inhibited the growth of tumors (5.6 ± 3.6 mm2) compared with mice treated with control Ig (68.5 ± 23.9 mm2) (P = .005).
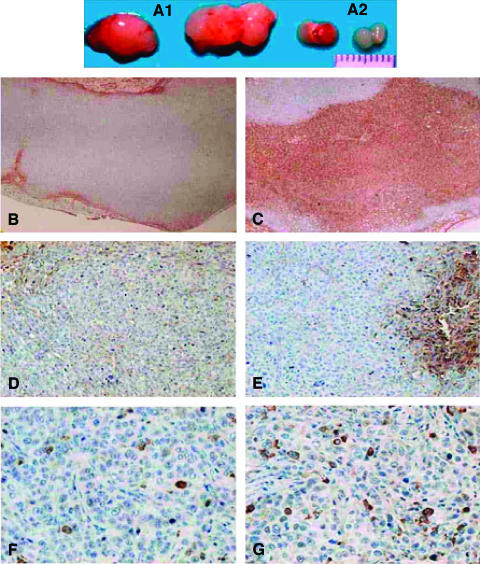
Figure 2.
Gross and microscopic immunohistochemical analyses of tumors removed from HICT mice treated with anti-C48 Ig (A2, C, E, and G) or control Ig (A1, B, D, and F). (A) Tumor size. (B and C) CD45+ inside tumors. (D and E) CD45+ in tumors. (F and G) Caspase-3+.
Furthermore, in MCF-7-engrafted HICT mice, treatment with anti-C48 Ig on days 1 to 30 after tumor engraftment prolonged their survival compared with control HICT mice. After 45 days, 100% of the mice treated with anti-C48 Ig were alive compared with only 20% in the control Ig-treated group (Figure 3).
Figure 3.
The effect of anti-C48 Ig treatment on the survival of MCF-7 tumor-bearing HICT nude mice. Mice engrafted as in Figure 1 were treated daily (1–30 days) with anti-C48 Ig or control Ig (2 mg/0.3 ml, i.p.) and followed up for 45 days.
Immunohistochemical Characterization
By staining with hematoxylin and eosin, there were large areas of tumoral necrosis in tumors removed from anti-C48 Ig-treated mice. In comparison, tumors removed from control Ig-treated mice showed nearly no necrosis.
Immunohistochemical analyses of tumors derived from HICT mice treated with anti-C48 Ig and control Ig are presented in Figure 2.
Phenotypic analysis of tumor-infiltrating leukocytes was carried out with the following MoAbs: anti-CD45, anti-CD4, anti-CD8, and anti-CD20. Figure 2 shows images obtained from representative tumors.
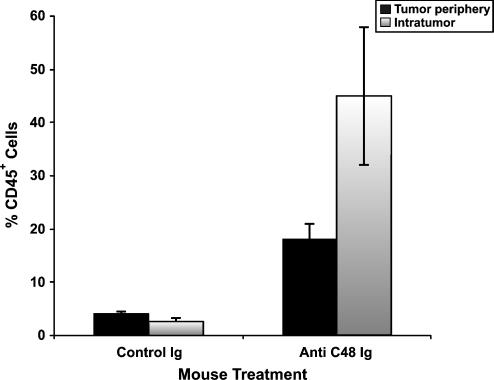
As seen in Figures 2C and 4, specimen obtained from the anti-C48 Ig-treated group exhibited massive infiltration of human CD45+ cells between tumor cells (mean/4 mm2 = 40 ± 1%) and in the tumor periphery (25 ± 3%) (Figure 4). In comparison, specimens obtained from the control Ig-treated group (Figure 2B) exhibited very low leukocyte infiltration either intratumorally (2.6 ± 0.7%) or in the tumor periphery (4.1 ± 0.5%) (Figure 4).
Figure 4.
Quantitative immunohistochemical analysis of human leukocytes (CD45+) infiltrating MCF-7 human breast tumors removed from HICT nude mice treated with anti-C48 Ig and control Ig. Data are expressed as the mean percentage of positive cells in area section (4 mm2) ± S.E. (n = 3) (as in Materials and Methods).
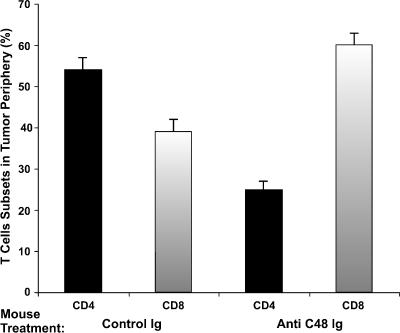
Furthermore, immunophenotyping with anti-CD4, anti-CD8, and anti-CD20 revealed that the majority of CD45+ cells were T lymphocytes (Figure 5), whereas only few stained B cells (CD20+) could be seen (not shown). Furthermore, most of those infiltrating the tumors derived from anti-C48 Ig-treated mice were CD8+ (Figure 5). A comparison of infiltrating T-cell subsets in the periphery of tumors removed from anti-C48 Ig and those from control Ig-treated mice revealed a reversed ratio of CD4+/CD8+ cells (Figure 5). Whereas the CD4/CD8 ratio in control Ig-treated tumors was 1.39, the ratio in anti-C48 Ig-treated tumors was 0.4 (Figure 5).
Figure 5.
Quantitative immunohistochemical analysis of human CD4 and CD8 T cells infiltrating human breast tumors removed from HITC nude mice treated with anti-C48 Ig and control Ig. Data are expressed as the mean proportion of CD45+ cells in area section (4 mm2) ± S.E. (n = 3).
Functional Immune Characterization
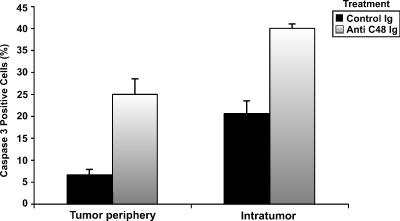
Immunohistochemical analyses of cytokines with antibodies to INF-γ and IL-10 did not yield a positive staining of tumor sections. Yet, immunostaining of apoptotic cells with anti-caspase-3 antibodies exhibited positive staining. A difference in the proportion of positively stained apoptotic cells was observed between tumors from anti-C48 Ig-treated mice and tumors from control Ig-treated mice (Figure 2, F and G). The mean numbers of caspase-3+ cells were significantly increased intratumorally (40 ± 1) and in the tumor periphery (25 ± 3) of anti-C48 Ig-treated tumors compared with control Ig-treated tumors (20 ± 3 and 6.6 ± 2, respectively) (Figure 6).
Figure 6.
Quantitative immunohistochemical analysis of caspase-3+ cells in human breast tumors removed from HITC nude mice treated with anti-C48 Ig and control Ig. Data are expressed as the mean percentage of positive cells in area (4 mm2) ± S.E. (n = 3).
IFN-γ and IL-10 Secretion in Cocultures of MCF-7 and PBMCs Treated In Vitro with Anti-C48 Ig
Because we could not demonstrate the immunostaining of cytokines (IL-10 or INF-γ) in sections of tumors removed from HICT mice treated in vivo with either anti-C48 Ig or control Ig, an attempt was made to investigate the effect of this treatment on cytokine secretion in vitro.
These experiments were carried out in cocultures of MCF-7 breast cancer cells and PBMCs from three healthy blood bank donors treated in vitro daily with anti-C48 Ig or control Ig. Amounts of INF-γ and IL-10 that accumulated during 24, 48, and 72 hours were measured in the supernatants of these cocultures.
The results of a representative experiment are presented in Figures 7 and 8.
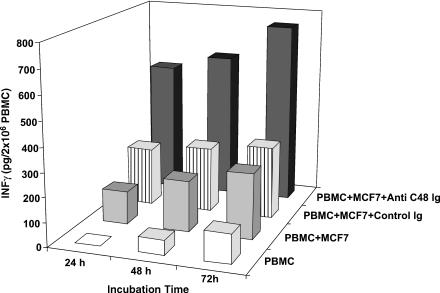
Figure 7.
Time course secretion of INF-γ in mixed cultures of MCF-7 cells and PBMCs (2 x 106 ml-1) treated daily with anti-C48 Ig or control Ig (100 µl). Culture supernatants were removed at 24, 48, and 72 hours of incubation and centrifuged at 500g for 10 minutes to remove cells and debris, and the supernatant (100 µg/ml) was subjected to an ELISA plate for quantitative measurement of INF-γ (a representative experiment).
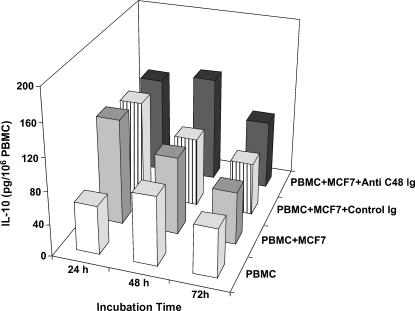
Figure 8.
Time course secretion of IL-10 in mixed cultures of MCF-7 cells and PBMCs (106 ml-1) treated daily with anti-C48 Ig or control Ig (100 µg/ml). Supernatants were collected as in Figure 7 and subjected (100 µl) to an ELISA plate for quantitative measurement of IL-10 (a representative experiment).
The level of INF-γ was measured in the following cell cultures: PBMC; PBMC + MCF-7 cells; PBMC + MCF-7 cells treated daily with control Ig (100 µg/ml); PBMC + MCF-7 cells treated daily with anti-C48 Ig (100 µg/ml). As seen in a representative experiment (Figure 7), a marked increase in INF-γ (540 µg/ml) was measured after 24 hours of incubation in cocultures of PBMCs and MCF-7 cells treated with anti-C48 Ig. This is compared with the level in the following control cultures: PBMC (0 pg/ml); PBMC + MCF-7 cells (135 pg/ml); PBMC + MCF-7 cells treated with control Ig (240 pg/ml). INF-γ level accumulated further at 72 hours up to 780 pg/ml in anti-C48 Ig-treated cultures, and proportionally lower levels were measured in control cultures (Figure 7). Measurements of IL-10 level in the respective cultures did not reveal a significant difference between them, and no accumulation of IL-10 was measured following further incubation time for 72 hours (Figure 8). It is noteworthy that treatment of PBMC cultures with anti-C48 Ig or control Ig did not affect the levels of INF-γ and IL-10 compared to non-treated PBMC cultures (not shown).
Discussion
In the present study, we analyzed different immunologic parameters associated with human breast tumors following treatment with antibodies to C48 (PLIF), a tumor-associated immunosuppressive protein.
The results demonstrated that treatment with anti-C48 (PLIF) antibodies significantly inhibited tumor growth and increased host survival. This was not a direct antitumor effect of anti-C48 (PLIF) antibodies, but was rather mediated by transfused human PBMCs into nude mice.
Furthermore, it was found that the anti-C48 (PLIF) treatment of tumor-engrafted HICT mice resulted in large necrotic areas and massive infiltration of human leukocytes (CD45+) intratumorally and in the tumor periphery, as opposed to lack of infiltration in control Ig-treated tumors. Most of the tumor-infiltrating leukocytes were T cells, and the majority were CD8+ (reversed CD4/CD8 ratio).
Despite the interest in immune prevention strategies, information on molecular events associated with prevention of tumor development is scarce. Our results may be analyzed in line with current views.
The presence of tumor-infiltrating leukocytes in residual tumors removed from C48-treated mice may indicate a tumor-related immune response [17]. Furthermore, the large necrotic areas induced by this treatment may possibly result in successful self-antitumor immunization if located in the correct cytokine environment, as has been previously suggested [18].
It was suggested that the cytokine profile of the tumor microenvironment may modulate tumor-host interaction. Hence, cytokine deregulation is likely to participate in the development or evolution of the malignant process [19].
It is noteworthy that IL-10 suppresses the function of antigen-presenting cells and T cells by inhibiting proinflammatory cytokine production. A role for IL-10 in the pathogenesis of cancer has been demonstrated by potent therapeutic antitumor effects of anti-IL-10 receptor MoAbs combined with anti-Toll-like receptor-9 [20].
Indeed, C48 (PLIF) is an inducer of IL-10 production and regulator of cytokine-chemokine networks in immunocytes [4,7,8]. It is expressed in MCF-7 cells and in the cytosol of human breast cancer tissue, where its level correlates with features of tumor cell differentiation [21]. Furthermore, PLIF is expressed on the surface of breast cancer cells [22], on the surface of PBMCs from breast cancer patients [15], and in the blood of patients with breast cancer [23]. Breast cancer-associated PLF downregulates lymphocyte mitogenesis [24], and activated lymphocytes from breast cancer patients express characteristics of Th2 cells [25].
Our working hypothesis suggests that PLIF expressed by syncytiotrophoblast cells in the feto-maternal interface or, alternatively, by breast cancer cells interacting with immune cells activates a Th2 response resulting in immunosuppression. Blocking this effect may tip the balance toward TH1 response and subsequent abortion or tumor rejection, respectively.
In a previous study, we have demonstrated that blocking PLIF in pregnant mice by treatment with anti-C48 antibodies affected placental and fetal growth in inbred and outbred allogeneic pregnancies, resulting in a high ratio of embryo resorption and diminished placental growth [12]. This effect was associated with an increased secretion of Th1 cytokines (IL-2, TNF, and IL-12) and a decrease in Th2 cytokines (IL-4, IL-5, IL-10, and IL-6) compared with normal outcome pregnancies.
In the current study, we could not demonstrate by immunohistochemical staining the expression of either human IL-10 (Th2) or INF-γ (Th1) in cytokine sections of tumors from HICT mice. Yet the in vitro experiments carried out in cocultures of MCF-7 cells and PBMCs revealed that blocking PLIF by anti-C48 Ig treatment induced a significant increase in INF-γ secretion (Th1 cytokine) compared with control cultures, but without affecting IL-10 production.
Previously, it has been shown that INF-γ induced in MCF-7 breast adenocarcinoma cells induces the expression of functional FasL, which is a major effector molecule used by CD8+ cytotoxic lymphocytes to kill target cells [26]. Furthermore, INF-γ has been shown to upregulate caspase-3 in MCF-7 breast cancer cells and to sensitize these cells to death receptor-mediated apoptosis [27,28].
The high ratio of human CD8 cells infiltrating tumors in anti-C48-treated mice and the significantly higher number of caspase-3+ cells in these tumors may indicate a mechanism of cytotoxicity and apoptosis mediated by infused human PBMCs.
Moreover, the current results, together with previous findings [7–9,12], suggest that modulation of cytokine profile following anti-C48 treatment affected tumor-host interaction, resulting in tumor rejection. This is in agreement with the findings of Gong et al. [29] in their study of T cells from patients with prostate cancer.
Hopefully, these results might guide the application of PLIF blockade by anti-C48 as a therapeutic approach in subgroups of human tumors that express the immunosuppressive PLIF protein.
Acknowledgement
The authors thank the Dvorson Fund for financial support.
Abbreviations
- GST
glutathione-S-transferase
- HICT
human immune cell transfused
- IFN-γ
interferon-γ
- MoAb
monoclonal antibody
- PBMCs
peripheral blood mononuclear cells
- PLIF
placental immunoregulatory ferritin
References
- 1.Emens LA, Reilly RT, Jaffee EM. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr Relat Cancer. 2005;12:1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- 2.Rouas-Freiss N, Paul P, Dausset J, Carosella ED. HLA-G promotes immune tolerance. J Biol Regul Homeost Agents. 2000;14:93–98. [PubMed] [Google Scholar]
- 3.Cavin LG, Venkatraman M, Factor VM, Kaur S, Schroeder I, Mercurio F, Beg AA, Thorgeirsson SS, Arsura M. Regulation of alpha-fetoprotein by nuclear factor-kappaB protects hepatocytes from tumor necrosis factor-alpha cytotoxicity during fetal liver development and hepatic oncogenesis. Cancer Res. 2004;64:7030–7038. doi: 10.1158/0008-5472.CAN-04-1647. [DOI] [PubMed] [Google Scholar]
- 4.Moroz C, Traub L, Maymon R, Zahalka MA. PLIF, a novel human ferritin subunit from placenta with immunosuppressive activity. J Biol Chem. 2002;277:12901–12905. doi: 10.1074/jbc.M200956200. [DOI] [PubMed] [Google Scholar]
- 5.Moroz C, Kupfer B, Twig S, Parhami-Seren B. Preparation and characterization of monoclonal antibodies specific to placenta ferritin. Clin Chim Acta. 1985;148:111–118. doi: 10.1016/0009-8981(85)90220-7. [DOI] [PubMed] [Google Scholar]
- 6.Parhami-Seren B, Moroz C. A unique subunit structure of human placenta ferritin identified by the use of monoclonal antibodies. Ital J Clin Pathol. 1986;1:17–23. [Google Scholar]
- 7.Zahalka MA, Barak V, Traub L, Moroz C. PLIF induces IL-10 production in monocytes; a calmodulin-p38 mitogen-activated protein kinase dependent pathway. FASEB J. 2003;17:955–957. doi: 10.1096/fj.02-0960fje. [DOI] [PubMed] [Google Scholar]
- 8.Moroz Ch, Grunspan A, Zahalka MA, Traub L, Kodman Y, Yaniv I. Treatment of human bone marrow with recombinant placenta immunoregulator ferritin results in myelopoiesis and T-cell suppression through modulation of the cytokine-chemokine networks. Exp Hematol. 2006;34:159–166. doi: 10.1016/j.exphem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Weinberger A, Halpern M, Zahalka MA, Quintana F, Traub L, Moroz C. Placental immunomodulator ferritin, a novel immunoregulator, suppresses experimental arthritis. Arthritis Rheum. 2003;48:846–853. doi: 10.1002/art.10850. [DOI] [PubMed] [Google Scholar]
- 10.Maymon R, Jauniaux E, Greenwold N, Moroz Ch. Localization of p43 placental isoferritin in human feto-maternal tissue interface. Am J Obstet Gynecol. 2000;182:670–674. doi: 10.1067/mob.2000.104145. [DOI] [PubMed] [Google Scholar]
- 11.Jauniaux E, Maymon R, Greenwold N, Hustin J, Moroz Ch. Diminished expression of placental isoferritin p43 component in first trimester abnormal pregnancies. Placenta. 2000;21:408–411. doi: 10.1053/plac.1999.0491. [DOI] [PubMed] [Google Scholar]
- 12.Nahum R, Brenner O, Zahalka MA, Traub L, Quintana F, Moroz C. Blocking of the placental immune-modulatory ferritin activates Th1 type cytokines and affects placenta development, fetal growth and the pregnancy outcome. Hum Reprod. 2004;19:715–722. doi: 10.1093/humrep/deh099. [DOI] [PubMed] [Google Scholar]
- 13.Moroz Ch, Bessler H, Lurie Y, Shaklai M. A new monoclonal antibody enzymoassay for the specific measurement of placental ferritin isotype in hematologic malignancies. Exp Hematol. 1987;15:258–262. [PubMed] [Google Scholar]
- 14.Rosen HR, Moroz Ch, Reiner A, Stierer M, Svec J, Reinerova M, Schemper M, Jakesz R. Expression of p43 in breast cancer tissue, correlation with prognostic parameters. Cancer Lett. 1992;67:35–45. doi: 10.1016/0304-3835(92)90006-h. [DOI] [PubMed] [Google Scholar]
- 15.Moroz Ch, Kan M, Chaimoff C, Marcus H, Kupfer B, Cuckle S. Ferritin-bearing lymphocytes in the diagnosis of breast cancer. Cancer. 1984;54:84–89. doi: 10.1002/1097-0142(19840701)54:1<84::aid-cncr2820540118>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Gal R, Rath-Wolfson L, Rosenblatt Y, Halpern M, Schwartz A, Koren R. An improved technique for mitosis counting. Int J Surg Pathol. 2005;13:161–165. doi: 10.1177/106689690501300206. [DOI] [PubMed] [Google Scholar]
- 17.Odunsi K, Old LJ. Tumor infiltrating lymphocytes: indicators of tumor-related immune responses. Cancer Immun. 2007;7:3. [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 19.Kurzrock R. Cytokine deregulation in cancer. Biomed Pharmacother. 2001;55:543–547. doi: 10.1016/s0753-3322(01)00140-8. [DOI] [PubMed] [Google Scholar]
- 20.Vicari AP, Trinchieri G. Interleukin-10 in viral diseases and cancer: exiting the labyrinth? Immunol Rev. 2004;202:223–236. doi: 10.1111/j.0105-2896.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosen HR, Moroz Ch, Reiner A, Reinerova M, Stierer M, Svec J, Schemper M, Jakesz R. Placental isoferritin associated p43 antigen correlates with features of high differential in breast cancer. Breast Cancer Res Treat. 1992;24:17–26. doi: 10.1007/BF01832354. [DOI] [PubMed] [Google Scholar]
- 22.Shterman N, Kupfer B, Moroz Ch. Comparison of transferring receptors, iron content and isoferritin profile in normal and malignant human breast cell lines. Pathobiology. 1991;59:19–25. doi: 10.1159/000163611. [DOI] [PubMed] [Google Scholar]
- 23.Rosen HR, Flex D, Stierer C, Moroz Ch. Monoclonal antibody CM-H-9 detects circulating placental isoferritin measured in the serum of patients with metastases of breast cancer. Cancer Lett. 1991;59:145–151. doi: 10.1016/0304-3835(91)90179-l. [DOI] [PubMed] [Google Scholar]
- 24.Rosen HR, Ausch C, Reiner G, Reinerova M, Svec J, Tuchler H, Schiessel R, Moroz C. Downregulation of lymphocyte mitogenesis by breast cancer-associated p43. Cancer Lett. 1994;82:105–111. doi: 10.1016/0304-3835(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 25.Rosen HR, Ausch Ch, Reinerova M, Zaspin E, Renner K, Rosen AC, Schiessel R, Moroz C. Activated lymphocytes from breast cancer patients express the characteristics of type 2 helper cells—a possible role for breast cancer associated p43. Cancer Lett. 1998;127:129–134. doi: 10.1016/s0304-3835(98)00017-2. [DOI] [PubMed] [Google Scholar]
- 26.Naujokat C, Sezer O, Possinger K. Tumor necrosis factor-alpha and interferon-gamma induce expression of functional Fas ligand on HT29 and MCF-7 adenocarcinoma cells. Biochem Biophys Res Commun. 1999;264:813–819. doi: 10.1006/bbrc.1999.1500. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Ruiz C, Ruiz de Almodovar C, Rodriguez A, Ortiz-Ferron G, Redondo JM, Lopez-Rivas A. The up-regulation of human caspase-8 by interferon-gamma in breast tumor cells requires the induction and action of the transcription factor interferon regulatory factor-1. J Biol Chem. 2004;279:19712–19720. doi: 10.1074/jbc.M313023200. [DOI] [PubMed] [Google Scholar]
- 28.Bouker KB, Skaar TC, Riggins RB, Harburger DS, Fernandez DR, Zwart A, Wang A, Clarke R. Interferon regulatory factor-1 (IRF-1) exhibits tumor suppressor activities in breast cancer associated with caspase activation and induction of apoptosis. Carcinogenesis. 2005;26:1527–1535. doi: 10.1093/carcin/bgi113. [DOI] [PubMed] [Google Scholar]
- 29.Gong MC, Latouche J-B, Krause A, Heston WDW, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]