Abstract
Background: Although failure of tuberculosis (TB) control in sub-Saharan Africa is attributed to the HIV epidemic, it is unclear why the directly observed therapy short-course (DOTS) strategy is insufficient in this setting. We conducted a cross-sectional survey of pulmonary TB (PTB) and HIV infection in a community of 13,000 with high HIV prevalence and high TB notification rate and a well-functioning DOTS TB control program.
Methods: Active case finding for PTB was performed in 762 adults using sputum microscopy and Mycobacterium tuberculosis culture, testing for HIV, and a symptom and risk factor questionnaire. Survey findings were correlated with notification data extracted from the TB treatment register.
Results: Of those surveyed, 174 (23%) tested HIV positive, 11 (7 HIV positive) were receiving TB therapy, 6 (5 HIV positive) had previously undiagnosed smear-positive PTB, and 6 (4 HIV positive) had smear-negative/culture-positive PTB. Symptoms were not a useful screen for PTB. Among HIV-positive and -negative individuals, prevalence of notified smear-positive PTB was 1,563/100,000 and 352/100,000, undiagnosed smear-positive PTB prevalence was 2,837/100,000 and 175/100,000, and case-finding proportions were 37 and 67%, respectively. Estimated duration of infectiousness was similar for HIV-positive and HIV-negative individuals. However, 87% of total person-years of undiagnosed smear-positive TB in the community were among HIV-infected individuals.
Conclusions: PTB was identified in 9% of HIV-infected individuals, with 5% being previously undiagnosed. Lack of symptoms suggestive of PTB may contribute to low case-finding rates. DOTS strategy based on passive case finding should be supplemented by active case finding targeting HIV-infected individuals.
Keywords: African community, case finding, HIV infection, incidence and prevalence, pulmonary tuberculosis
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The complex interaction between the dual HIV and TB epidemics at a community level is underreported.
What This Study Adds to the Field
Pulmonary tuberculosis is common in HIV-infected individuals. Specific active case finding strategies need to be targeted at HIV-infected individuals.
Present tuberculosis (TB) control strategies based on directly observed therapy short-course (DOTS) strategy is insufficient to contain TB in sub-Saharan Africa, largely because of the parallel epidemic of HIV in the region (1). An estimated 2.4 million new TB cases and 540,000 TB-related deaths now occur in sub-Saharan Africa annually. Consequently, in August 2005, the World Health Organization declared the epidemic in Africa to be a regional emergency (2).
The DOTS strategy has been shown to be effective in many settings, particularly where levels of HIV are relatively low (3–5). However, the reasons for increasing TB rates where HIV is prevalent have not been clearly defined. Failure of TB control may in part be due to poor implementation of the DOTS strategy because of lack of infrastructure or adequate resources (6). However, TB control may be less effective in communities with high HIV prevalence because of alterations in patterns of transmission or disease presentation. By examining where DOTS has been less successful (7), we may understand the reasons for the breakdown in TB control efforts and suggest modifications and improvement in current approaches and design new control strategies.
The interactions between HIV and TB are complex; however, understanding the interplay between these two epidemics is particularly important in countries of southern Africa, where HIV has fueled an unprecedented increase in TB notification rates (8). Because infectivity decreases rapidly after initiation of anti-TB therapy (9), TB control is based on rapid identification and treatment of infectious cases, thereby reducing the mean duration of infectiousness and the resultant number of secondary infections (10). However, HIV may affect TB case finding due to atypical presentations of TB (11–16) and the presence of coinfections, which may mimic the symptoms of TB. HIV-mediated immune deficiency also increases risk for development of disease (17), and the vulnerable population increases during the course of a maturing HIV epidemic as the proportion of those with advanced immune deficiency increases (18, 19).
We have previously reported an increasing TB notification rate in a community with high HIV and TB prevalence despite implementation of a DOTS TB control program with good outcome measures (20). In this population of approximately 13,000 individuals, data collected over 10 yr have shown a 2.5-fold increase in TB notifications, to unprecedented levels exceeding 1,400 of 100,000, coincident with HIV prevalence in the community increasing from 6 to 22% (20). In the present study, we conducted a cross-sectional, population-based survey of both HIV and TB prevalence to estimate the burden of undiagnosed TB among HIV-infected people in this community. To avoid selection bias, all individuals in the study sample were screened for TB without any preselection on the basis of symptoms. In conjunction with notification data, these data allow the estimation of case-finding rates under the local DOTS program and the population burden of undiagnosed TB disease. Some of the results of this study have been previously reported as an abstract (21).
METHODS
Study Community
The study community comprised 13,000 black African individuals living mainly in shacks in a high-density residential area sited within well-demarcated boundaries, where unemployment exceeds 50% (20). The estimated mid-2005 adult population (> 14 yr) was derived from a household survey performed in December 2004, adjusted for population growth. There is a single public-sector primary care clinic, which incorporates voluntary HIV counseling and testing, and a TB clinic for the diagnosis and management of TB in accordance with the South African national TB control program protocols.
TB and HIV Prevalence Survey
Between February and June 2005, a total of 1,150 adults included in the 2004 census were selected using simple random sampling. This sample size was chosen to calculate an anticipated prevalence of TB of 2.5%, with precision of 1.0%, and with corrections for anticipated nonresponse (22). An initial home visit confirmed 971 of the selected adults were living permanently on the identified plots, and these individuals were invited to participate in the study. Of these, 762 adults (78%) consented to participate in the study, 61 were unable to be contacted after five home visits, and 148 declined to participate.
Participants were requested to bring an early-morning sputum sample to the study site. At the study visit, a second saline nebulized sputum sample was collected and an oral transudate was taken for anonymous linked HIV testing; HIV test results were available for 758 individuals (two patients declined testing and two specimens were unsuitable for analysis). Voluntary counseling, testing, and disclosure were offered to all participants wishing to know their HIV status. HIV counseling, care, treatment, and prevention services are run from the primary health care clinic on site. A structured questionnaire investigating participant demographic characteristics, TB symptoms (cough, loss of appetite, weight loss, and night sweats), risk factors for TB (including housing, alcohol, smoking, recreational drug use, incarceration, previous contact with health services, time away from community, and employment history), and risk factors for HIV infection (including number of sexual partners and condom use) was completed. Questionnaires were administered in participants' home language (predominantly isiXhosa) by trained interviewers who used standardized isiXhosa phrasing for all questions. All interviews took place in a private room within the clinic. Draft questionnaires, including translated versions, were piloted before the study began to ensure appropriate translation and participant comprehension. All participants gave written, informed consent. The study received approval from the Research Ethics Committee of the University of Cape Town.
Case Definitions for TB Disease
TB definitions used for notification data were as defined by the South African TB Control Program (23). Definitive prevalent smear-positive TB was defined as a positive sputum smear confirmed with a second positive smear or culture of Mycobacterium tuberculosis; and smear-negative pulmonary TB (PTB) was defined by two positive cultures of M. tuberculosis with confirmed identical spoligotype patterns.
TB Incidence and Treatment Outcomes
TB is a notifiable condition in South Africa, and each TB clinic is required to maintain and report TB statistics, including patient demographics, TB diagnostic criteria, treatment regimen prescribed, results of laboratory monitoring, voluntary HIV counseling and testing results, and treatment completion and interruptions.
The adult TB case notification rate was determined as the number of cases notified and initiating TB therapy during the 2005 calendar year. Program performance criteria were extracted from the TB register for all patients commencing therapy from 2002 to 2004.
Laboratory Procedures
Sputum smears were examined for acid-fast bacilli (AFB) using the auramine O fluorescent stain. Mycobacterial growth indicator tubes (Becton-Dickinson, Sparks, MD) were inoculated according to the manufacturer's instructions and incubated for 8 wk before being recorded as negative. Positive cultures were screened for the presence of AFB by Ziehl-Neelsen staining, and identified as M. tuberculosis complex by inhibition of growth on p-nitrobenzoic acid. A polymerase chain reaction assay specific for M. tuberculosis (24) was performed on those cultures that had AFB visible on microscopy, but were contaminated and could thus not be identified using the p-nitrobenzoic acid test. DNA isolated from all M. tuberculosis cultures underwent spoligotyping.
An oral mucosal transudate for HIV testing was collected using the OraSure (OraSure Technologies, Bethlehem, PA) oral fluid collection device. The Vironostika Uni-Form II (bioMérieux, Marcy-l'Etoile, France) HIV-1 and HIV-2 plus O ELISA test was used to test for HIV-1 and HIV-2 antibodies.
Statistical Methods
Data were analyzed using Stata version 9.0 (StataCorp, College Station, TX). Notification rates are based on the mid-2005 population estimates. Median time receiving treatment in the TB program was calculated from date of commencing treatment to date of completion, death, and loss to follow-up, or transfer out of the program. Kaplan-Meier mortality proportions at 6 mo were estimated from the start of treatment to death, treatment completion, loss to follow-up, or transfer. Because the notification data come from routine service delivery where HIV testing is optional, test results were available for only 81% of notified PTB cases; for subsequent calculations, we made a conservative assumption that the prevalence of HIV in notified PTB cases of unknown HIV status was similar to the observed prevalence in notified cases with HIV data available. The mean time that PTB cases spent in the community before diagnosis and treatment (for both smear-positive disease and all PTB) was estimated as the observed PTB prevalence divided by the incidence of TB treatment (notification data). The time before treatment was corrected for the excess mortality associated with HIV infection in individuals with TB (as observed in the community DOTS program between 2002 and 2004). In turn, the total person-time (i.e., the time that different types of PTB cases spent in the community before diagnosis) is estimated as the product of the number of affected individuals in the community and the mean time before diagnosis. Case-finding proportions were calculated as the quotient of the prevalence of treated PTB divided by the sum of the prevalence of treated and the prevalence of untreated PTB. Exact 95% confidence intervals (CI) are based on the hypergeometric distribution (25). We evaluated risk factors for TB using Fisher's exact tests, and Mann-Whitney U tests were used to compare proportions and medians, respectively; all statistical tests were two-sided at α = 0.05.
RESULTS
TB Notifications 2005
The mid-2005 adult population was projected to be 10,408 with an HIV seroprevalence of 23% using existing data (20). Thus, approximately 8,000 HIV-negative and 2,400 HIV-infected individuals constituted the study population. A total of 259 TB cases were notified in adults over the age of 14 yr and 201 (78%) of these were categorized as PTB. The direct sputum smear–positive and smear–negative adult PTB treatment notifications, stratified by HIV status, are shown in Table 1. HIV testing was performed in 81% of notified TB cases, with 66% testing positive. The HIV-related odds ratio (OR) for smear-positive TB was 4.7 (95% CI, 3.3–6.7) and for smear-negative TB was 8.1 (95% CI, 4.7–14.7).
TABLE 1.
2005 ADULT PULMONARY TUBERCULOSIS TREATMENT NOTIFICATION RATES PER 100,000 PER ANNUM, CALCULATED FOR TOTAL ADULT POPULATION (n = 10,408) AND HIV-POSITIVE (n = 2,432) AND HIV-NEGATIVE (n = 7,976) POPULATIONS
| Adult Population (95% CI) | HIV-positive Adults (95% CI) | HIV-negative Adults (95% CI) | |
|---|---|---|---|
| Pulmonary tuberculosis case notifications | 1,931 (1,676–2,214) | 5,140 (4,296–6,093) | 953 (751–1,191) |
| Sputum smear–positive tuberculosis | 1,307 (1,097–1,544) | 3,248 (2,580–4,032) | 715 (542–925) |
| Sputum smear–negative tuberculosis | 625 (482–795) | 1,891 (1,388–2,515) | 238 (143–372) |
Definition of abbreviation: CI = confidence interval.
HIV test results were available for only 81% of notified PTB cases; a conservative assumption has been made that the prevalence of HIV in notified PTB cases of unknown HIV status is similar to the observed prevalence in notified cases with HIV data available.
In the 2002–2004 performance criteria for the TB control program among HIV-negative and HIV-positive individuals, treatment completion was obtained in 84 and 73%, mortality in 3 and 13%, transfer out in 9 and 12%, and treatment interruption occurred in 4 and 1%, respectively. The median length of TB treatment was 178 d and the Kaplan-Meier estimations of mortality among HIV-negative individuals were 4 and 5% at 6 mo for smear-positive and smear-negative TB, respectively. The corresponding mortality estimates for HIV-positive TB were 10.5 and 11% at 6 mo, respectively.
Prevalence Survey
Of the 762 participants in the community survey, 174 (23%) tested HIV positive, confirming the modeled HIV prevalence estimate. The survey sample also closely matched the predicted 2005 age, race, and sex distribution (data not shown). Among study participants, 11 individuals with TB were currently notified and receiving TB treatment. A further 12 previously undiagnosed definitive TB cases were identified: 6 smear-positive cases had either a second positive smear (n = 1) or culture of M. tuberculosis (n = 5); and 6 smear-negative cases had positive M. tuberculosis cultures obtained from two sputum specimens. Spoligotyping of DNA from these cultures demonstrated six distinct M. tuberculosis subtype patterns, with paired specimens from each individual showing identical patterns.
The HIV status of previously and newly identified cases of smear-negative and smear-positive TB is shown in Table 2. Of the 12 previously undiagnosed TB cases, 9 (75%) tested positive for HIV. Demographic characteristics of all participants with and without TB are shown in Table 3, together with symptoms and risk factors for TB. Prior imprisonment and HIV infection were significantly associated with notified TB, and HIV infection with previously undiagnosed TB. Cough, night sweats, loss of appetite, and weight loss were absent among 67% of those with previously undiagnosed TB. Two or more symptoms were present in 25% of previously undiagnosed TB cases compared with 22% among the no-TB group (p = 0.826).
TABLE 2.
PREVALENCE SURVEY OF 762 RANDOMLY SELECTED ADULTS
| Survey Sample (n = 762)* | HIV-positive Subjects (n = 174) | HIV-negative Subjects (n = 588) | |
|---|---|---|---|
| Total pulmonary tuberculosis cases | 23 (3.3) | 16 (9.2) | 7 (1.2) |
| Pulmonary tuberculosis cases on treatment | 11 (1.4) | 7 (4.0) | 4 (0.7) |
| Newly identified | |||
| Pulmonary tuberculosis | 12 (1.6) | 9 (5.2) | 3 (0.5) |
| New direct sputum smear–positive cases | 6 (0.8) | 5 (2.9) | 1 (0.2) |
| New culture positive | |||
| New culture-positive smear-negative cases | 6 (0.8) | 4 (2.3) | 2 (0.3) |
Numbers (%) of identified treated cases of tuberculosis, previously undiagnosed pulmonary tuberculosis, previously undiagnosed direct sputum smear–positive and Mycobacterium tuberculosis culture-positive/smear-negative cases stratified by HIV infection status.
Two patients refused HIV testing and two oral transudates were inadequate for testing. These four patients were all in the non-TB category.
TABLE 3.
CHARACTERISTICS OF STUDY SAMPLE, OVERALL AND BY PREVALENT TUBERCULOSIS INFECTION, AMONG 762 PARTICIPANTS
| All Surveyed (n = 762) | No TB (n = 739) | Nonnotified TB (n = 12) | p Value | Notified TB (n = 11) | p Value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Median age, yr | 27 | 27 | 26.5 | 0.81 | 30 | 0.12 |
| Median school grade completed | 11 | 11 | 11.5 | 0.31 | 8 | 0.06 |
| Presently employed | 398 (52) | 390 (53) | 7 (58) | 0.78 | 1 (9) | 0.004 |
| Median household income, South African rands | 1,300 | 1,300 | 1,400 | 0.98 | 578 | 0.009 |
| Median residence, yr | 5 | 5 | 5 | 0.83 | 6 | 0.33 |
| Time away from home in last 6 mo | 209 (27) | 204 (28) | 2 (17) | 0.53 | 3 (27) | 0.99 |
| Median residents in household | 3 | 3 | 3 | 0.07 | 3 | 0.85 |
| Median persons sleeping in same room | 2 | 2 | 2 | 0.81 | 2 | 0.65 |
| Symptoms | ||||||
| Cough | 142 (19) | 134 (18) | 2 (17) | 0.99 | † | — |
| Night sweats | 130 (17) | 121 (16) | 1 (8) | 0.70 | † | — |
| Loss of appetite | 144 (19) | 137 (19) | 1 (8) | 0.71 | † | — |
| Loss of weight | 207 (27) | 193 (26) | 4 (33) | 0.52 | † | — |
| Risk factors | ||||||
| Ever had TB in past | 61 (8) | 58 (8) | 0 | 0.61 | 3 (27) | 0.053 |
| Alcohol intake in past 6 mo | 324 (43) | 313 (42) | 5 (42) | 0.99 | 6 (55) | 0.54 |
| Visited shebeen (bar) in past 6 mo | 180 (24) | 175 (24) | 3 (25) | 0.99 | 2 (18) | 0.99 |
| Smoked in past 6 mo | 205 (27) | 196 (27) | 4 (33) | 0.53 | 5 (45) | 0.18 |
| Recreational drugs in past 6 mo | 29 (4) | 28 (4) | 0 | 0.99 | 1 (9) | 0.35 |
| Employment history | ||||||
| Past mining | 43 (6) | 41 (6) | 1 (8) | 0.50 | 1 (9) | 0.47 |
| Health care worker | 21 (3) | 20 (3) | 0 | 0.99 | 1 (9) | 0.27 |
| Prison | 11 (1) | 8 (1) | 1 (8) | 0.14 | 2 (18) | 0.008 |
| HIV positive | 174 (23)* | 158 (22) | 9 (75) | < 0.001 | 7 (64) | 0.003 |
Definition of abbreviation: TB = tuberculosis.
All values are n (%) unless otherwise specified. p values are for comparing nonnotified TB (n = 12) and notified TB (n = 11) against no-TB (n = 739).
Two patients refused HIV testing and two oral transudates were inadequate for testing. These four patients were all in the non-TB category.
Symptoms questionnaire results do not represent prediagnosis symptoms and have been excluded.
Case-finding Proportions
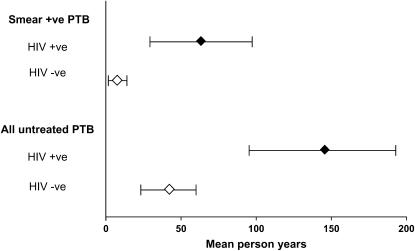
In 2005, the point prevalence of adult PTB in the community (2,517/100,000; 95% CI, 2,225–2,837) was calculated by addition of notified case prevalence (notified incidence rate/median time on treatment = 942/100,000; 95% CI, 765–1146) and survey-determined untreated TB prevalence (1,575/100,000; 95% CI, 816–2,735). Based on this point prevalence, the proportion of cases identified and receiving TB treatment was only 37%. The prevalence of newly diagnosed smear-positive PTB among HIV-positive and -negative individuals was 2,837 of 100,000 and 175 of 100,000, respectively. Estimates of smear-positive PTB case-finding proportions for HIV-positive and -negative individuals, together with estimated time in the community before initiation of TB therapy, are given in Tables 4 and 5. The community burden of disease, estimated as the product of the number of affected individuals and the mean time before diagnosis and treatment, is presented in Figure 1. HIV-infected individuals were found to contribute 87% of the total person-years of undiagnosed disease.
TABLE 4.
PREVALENCE PER 100,000 OF NOTIFIED, TREATED ADULT CASES WITH PULMONARY TUBERCULOSIS AND UNTREATED CASES WITH PULMONARY TUBERCULOSIS IN THE COMMUNITY, WITH ESTIMATED CASE-FINDING PROPORTION AND TIME IN YEARS BEFORE INITIATION OF TREATMENT
| Total Population HIV Positive (95% CI) | Total Population HIV Negative (95% CI) | |
|---|---|---|
| Prevalence of treated PTB | 2,508 (1,924–3,210) | 464 (327–639) |
| Prevalence of total (treated and untreated) PTB | 7,648 (6,623–8,777) | 978 (774–1,219) |
| Case-finding proportion* | 0.34 (0.26–0.46) | 0.48 (0.32–0.70) |
| Estimated mean time (yr) of TB patients before treatment† | 1.19 | 1.02 |
Definition of abbreviations: CI = confidence interval: PTB = pulmonary tuberculosis.
Prevalence of treated PTB/prevalence of treated and untreated PTB.
Mean time untreated = PTB prevalence/(PTB incidence + HIV mortality rate), where the HIV mortality rate is the excess mortality associated with HIV infection in individuals notified with PTB 2002–2004.
TABLE 5.
PREVALENCE PER 100,000 OF NOTIFIED, TREATED ADULT SPUTUM SMEAR–POSITIVE CASES WITH PULMONARY TUBERCULOSIS AND UNTREATED SPUTUM SMEAR–POSITIVE CASES WITH PULMONARY TUBERCULOSIS IN THE COMMUNITY, WITH ESTIMATED CASE-FINDING PROPORTION AND TIME IN YEARS BEFORE INITIATION OF TREATMENT
| HIV Positive, Smear Positive (95% CI) | HIV Negative, Smear Positive (95% CI) | |
|---|---|---|
| Prevalence of treated PTB | 1,563 (1,108–2,138) | 352 (233–507) |
| Prevalence of total (treated and untreated) PTB | 4,400 (3,619–5,299) | 527 (380–711) |
| Case-finding proportion* | 0.37 (025–0.53) | 0.67 (0.41–1.0) |
| Estimated mean time (yr) of TB patients before treatment† | 0.98 | 0.73 |
For definition of abbreviations, see Table 4.
Prevalence of treated PTB/prevalence of treated and untreated PTB.
Mean time untreated = PTB prevalence/(PTB incidence + HIV mortality rate), where the HIV mortality rate is the excess mortality associated with HIV infection in individuals notified with PTB 2002–2004.
Figure 1.
Community burden of undiagnosed pulmonary tuberculosis (PTB) by HIV and smear status. The closed diamonds (HIV +ve) and open diamonds (HIV −ve) indicate the estimated number of person-years, and the horizontal lines the 95% confidence intervals around these estimates. Estimated time before diagnosis is adjusted for increased mortality (13/100 patient-years) for HIV-positive individuals.
DISCUSSION
This is the first population-based active case-finding survey of HIV and TB in sub-Saharan Africa. The major finding of this study was that, despite a well-run DOTS-based TB control program, 63% of community adult cases with PTB remained unrecognized to the TB treatment services. Among HIV-negative individuals, passive case finding identified 67% of prevalent smear-positive cases, which is close to the target of 70% suggested for adequate DOTS implementation (26). In contrast, among individuals with HIV infection, passive case finding only identified 33% of those with smear-positive TB. Thus, our study identified a huge unrecognized burden of TB in the community, predominantly among HIV-infected people. In this group, untreated smear-positive and smear-negative/culture-positive prevalence rates of TB were 2.9 and 2.3%, respectively. Among those with sputum smear–positive TB, the mean time spent in the community before accessing TB treatment was estimated to be as long for HIV-positive as for HIV-negative individuals, even after accounting for increased HIV mortality. HIV-infected individuals accounted for 87% of total person-years of untreated smear-positive TB before diagnosis and treatment. This finding is in contrast to an active case-finding study in South African gold miners, which estimated mean duration of smear-positive TB before diagnosis to be only 2 mo for HIV-positive miners (15). In that study, HIV-positive miners attended an HIV clinic and resulting ascertainment bias and use of isoniazid prophylaxis may have lowered the rate of previously undiagnosed smear-positive TB among the HIV-positive subjects (0.4%), resulting in a low calculated mean TB duration before diagnosis. The mean duration of infectiousness is a key determinant of the dynamics of the TB epidemic. In our study, the combination of high prevalence of untreated PTB among HIV-infected people, together with a prolonged exposure time in the community, may account for the lack of success of the DOTS program. However, TB infectivity may be lower for HIV-infected compared with HIV-uninfected individuals (27).
The study was performed in a community characterized by high HIV prevalence and increasing TB notification rates (20), and was sited within well-demarcated boundaries. There was a single community health clinic, making it likely that diagnosis and reporting of TB was uniform, thereby minimizing reporting biases. The TB DOTS control program performance over the 3 yr immediately before the survey demonstrated reasonable treatment completion rates but with a significant TB case fatality rate, which was higher in HIV-infected patients and those with smear-negative disease. The results of this survey reflect the epidemiology of TB and HIV in this community before access to highly active antiretroviral therapy (HAART) and without use of isoniazid prophylaxis.
The population-based survey identified individuals from whom M. tuberculosis was isolated but who did not perceive themselves to have symptoms of PTB. These individuals would therefore be less likely to present early to a TB control program. Our finding of a large burden of undiagnosed TB in this population is in contrast to a prior study in South Africa, which found a modest burden of undiagnosed TB in a rural population. However, in that study, laboratory investigation of TB was limited to only those with chronic cough and may therefore have underestimated the true TB burden (28).
Isolation of M. tuberculosis from asymptomatic individuals may initially appear to be in conflict with the international standards of TB care, which emphasize the investigation for TB among those with chronic cough (29). However, in a prospective population-based survey in the United States, symptoms of prolonged cough and fever were found to be insensitive predictors of TB (30). HIV infection has also been recognized to be a major modifier of the clinical presentation of TB, such that TB symptoms may be relatively minor (15, 16), atypical (11–13), or subclinical in HIV coinfected individuals (14). Furthermore, post mortem studies from Africa have reported TB as the cause of death in 38 to 47% of HIV-infected individuals in whom the diagnosis was suspected in only half while they were alive (31–33). In this poor community in our study, cough, night sweats, loss of appetite, and weight loss were reported frequently even in the absence of TB.
This study also highlights the problem of applying diagnostic tests developed primarily for clinic-based diagnosis to a population-based survey. Demonstration of acid-fast organisms on direct sputum smear lacks sensitivity (34) but has high specificity when sufficient organisms are present (35, 36). In contrast, M. tuberculosis culture from sputum is sensitive but may be subject to false-positive results due to laboratory cross-contamination (37, 38). The performance characteristics of these diagnostic tests are of particular importance in population surveys. Limiting testing only to those with symptoms may increase the positive predictive value of TB tests; however, this strategy may miss a significant proportion of TB cases (30). In our study, restricting the case definition to those individuals with two positive laboratory results increased the specificity of diagnosis. Furthermore, false-positive cultures due to laboratory cross-contamination were very unlikely in our study because of the demonstration of multiple M. tuberculosis spoligotypes and 100% concordance among duplicate spoligotypes from each individual.
These data should be interpreted in light of several limitations. Although TB events are frequent in this population, the sample size was modest and therefore calculated prevalences have relatively wide confidence intervals.
There is evidence that many black African communities in this region have high degrees of mobility (39); however, time spent away from the community was not correlated with TB infection (either notified or nonnotified), suggesting that any such bias would have a minimal impact on the results. The response rate to the prevalence survey was not complete, although this response rate (78%) was relatively high and there were no differences in the distribution of age, sex, or race between our sample and the community based on population census data. Furthermore, the HIV prevalence observed in our sample (23%) was identical to modeling of the HIV epidemic in this setting (20). In addition, because our TB notification data come from routine service in which HIV testing is optional, HIV test results were available for only 81% of the notified TB cases; because we have assumed that the HIV prevalence among TB cases whose HIV status is unknown is similar to that of known individuals, these missing data are unlikely to affect our results substantially. Last, the generalizabilty of our findings will be dependent on the confirmation of our findings in other populations with very high HIV and TB burdens.
The results of this study have implications for individual management of HIV-infected individuals and the TB control program. Active screening of known HIV-infected individuals for TB, with induced sputum for sputum microscopy and culture, is indicated in this setting, even in the absence of symptoms suggestive of TB. Active screening for TB should precede initiation of isoniazid prophylaxis to avoid monotherapy of active TB, and screening based on symptoms may be inadequate. It will need to be prospectively evaluated whether screening for TB at entry to ART programs can identify the approximately 5% of individuals who develop TB soon after initiation of ART (40). TB control in this and similar communities will be dependent on the ability of the health system to identify HIV infections. Voluntary counseling and testing will need to be combined with subsequent active screening for TB of those who test HIV positive.
We have reported a large burden of HIV-associated undiagnosed TB in a community despite a well-functioning DOTS TB control program. Further population-based prevalence studies are required to confirm these findings in similar communities where HIV/TB coinfection is common, but our data indicate that such studies should not be limited to those with symptomatic disease. The passive case-finding aspect of the DOTS program performed inadequately for those with HIV infection and resulted in lower case-finding proportions in HIV-infected compared with HIV-uninfected individuals. The lack of symptoms suggestive of PTB may in part have contributed to this failure. Our data confirm that more intensive TB case finding in HIV-infected individuals is required, as has been previously suggested (21, 41–43).
Acknowledgments
The authors thank the research and clinic staff and participants for their contributions.
Supported by NIH CIPRA grant 1 U19AI53217-01 (principal investigator, J.M.). R.W. is supported in part by NIH grant ROI A1058736-01A1 (principal investigator, Ken Freedberg). S.D.L. is funded by the Wellcome Trust, London, UK, through grant 074641/Z/04/Z. G.K. is supported in part by NIAID RO1 54361. The study was partially funded by the National Institutes of Health (NIH). The NIH did not participate in the data analysis or the decision to publish.
Originally Published in Press as DOI: 10.1164/rccm.200606-759OC on September 14, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the manuscript.
References
- 1.World Health Organization. Global tuberculosis control: surveillance, planning, financing. Geneva, Switzerland: World Health Organization; 2005. WHO Report No. WHO/HTM/TB/2005.349.
- 2.World Health Organization. WHO declares TB an emergency in Africa: call for “urgent and extraordinary actions” to halt a worsening epidemic. September 2, 2005. Available from: http://www.who.int/mediacentre/news/africa_emergency/en/ (accessed September 6, 2005).
- 3.China Tuberculosis Control Program. Results of directly observed short-course chemotherapy in 112,842 Chinese patients with smear-positive tuberculosis. Lancet 1996;350:169–172. [PubMed] [Google Scholar]
- 4.Netto EM, Dye C, Raviglione M. Progress in tuberculosis control 1995–1996, with emphasis on 22 high-income countries. Int J Tuberc Lung Dis 1999;3:310–320. [PubMed] [Google Scholar]
- 5.Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB, Gomez E, Foresman BH. The effect of directly observed therapy on the rates of drug resistance and relapses in tuberculosis. N Engl J Med 1994;330:1179–1184. [DOI] [PubMed] [Google Scholar]
- 6.Maher D, Borgdorff M, Boerma T. HIV-related tuberculosis: how well are we doing with current control efforts? Int J Tuberc Lung Dis 2005; 9:17–24. [PubMed] [Google Scholar]
- 7.Whalen CC. Failure of directly observed treatment for tuberculosis in Africa: a call for new approaches. Clin Infect Dis 2006;42:1048–1050. [DOI] [PubMed] [Google Scholar]
- 8.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 2006;367:926–937. [DOI] [PubMed] [Google Scholar]
- 9.Brindle RJ, Nunn PP, Githi W, Allen BW, Gathua S, Waiyaki P. Quantitative bacillary response to treatment in HIV-associated pulmonary tuberculosis. Am Rev Respir Dis 1993;147:948–961. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press; 1991. pp. 13–26.
- 11.Archibald LK, den Dulk MO, Pallangyo KJ, Reller LB. Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis 1998;26:290–296. [DOI] [PubMed] [Google Scholar]
- 12.Banda HT, Harries AD, Welby S, Boeree MJ, Wirima JJ, Subramanyam VR, Maher D, Nunn PA. Prevalence of tuberculosis in TB suspects with short duration of cough. Trans R Soc Trop Med Hyg 1998;92:161–163. [DOI] [PubMed] [Google Scholar]
- 13.Mwachari C, Batchelor BI, Paul J, Waiyaki PG, Gilks CF. Chronic diarrhoea among HIV-infected adult patients in Nairobi, Kenya. J Infect 1998;37:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mtei L, Matee M, Herfort O, Bakari M, Horsburgh CR, Waddell R, Cole BF, Vuola JM, Tvaroha S, Kreisworth B, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis 2005;40:1500–1507. [DOI] [PubMed] [Google Scholar]
- 15.Corbett EL, Charalambous S, Moloi VM, Fielding K, Grant A, Dye C, De Cock KM, Hayes RJ, Williams BG, Churchyard GJ. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med 2004;170:673–679. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey-Faussett P, Sonnenberg P, Shearer S, Bruce MC, Mee C, Morris L, Murray J. Tuberculosis control and molecular epidemiology in a South African gold-mining community. Lancet 2000;356:1066–1071. [DOI] [PubMed] [Google Scholar]
- 17.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study Lancet 2002;359:2059–2064. [DOI] [PubMed] [Google Scholar]
- 18.Glynn JR, Crampin AC, Ngwira BM, Mwaungulu FD, Mwafulirwa DT, Floyd S, Ponnighaus JM, Warndorff DK, Fine PE. Trends in tuberculosis and the influence of HIV-infection in northern Malawi, 1988–2001. AIDS 2004;18:1459–1463. [DOI] [PubMed] [Google Scholar]
- 19.Wood R, Maartens G, Lombard CL. Risk factors for developing tuberculosis in HIV-1 infected adults from communities with a low or very high incidence of tuberculosis. AIDS 2000;23:75–80. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Bekker L-G, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. CID 2006;42:1040–1046. [DOI] [PubMed] [Google Scholar]
- 21.Bekker L-G, Middelkoop K, Myer L, Whitelaw A, Grant A, Kaplan G, Lawn S, Wood R. Prevalence of HIV and undiagnosed tuberculosis in a peri-urban community in South Africa. Presented at the 13th Conference on Retroviruses and Opportunistic Infections, Denver, Colorado, February 5–8, 2006. Abstract No. 69.
- 22.Levy PS, Lemshow S. Sampling of populations: methods and applications. New York: Wiley Interscience; 1999.
- 23.The South African Tuberculosis Control Program; Department of Health, South Africa. Practical guidelines 2000. Available from: http://www.doh.gov.za/tb/docs/ntcpguidelines.pdf (accessed April 5, 2006).
- 24.De Wit D, Steyn L, Shoemaker S, Sogin M. Direct detection of Mycobacterium tuberculosis in clinical specimens by DNA amplification. J Clin Microbiol 1990;28:2437–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleiss JL, Levin B, Cho Paik M. Statistical methods for rates and proportions, 3rd edition. New York: Wiley Science; 2003.
- 26.World Health Organization. Framework for effective tuberculosis control. Geneva, Switzerland; World Health Organization; 1994. WHO Document No. WHO/TB/94.179.
- 27.Elliott AM, Hayes RJ, Halwindii B, Luo N, Tembo G, Pobee JO, Nunn PP, McAdam KP. The impact of HIV on infectiousness of pulmonary tuberculosis: a community study in Zambia. AIDS 1993;7:981–987. [DOI] [PubMed] [Google Scholar]
- 28.Pronyk PM, Joshi B, Hargreaves JR, Madonsela T, Collinson MA, Mokoena O, Tollman SM, Hausler HR. Active case finding: understanding the burden of tuberculosis in rural South Africa. Int J Tuberc Lung Dis 2001;5:611–618. [PubMed] [Google Scholar]
- 29.Tuberculosis Coalition for Technical Assistance. International standards for tuberculosis care (ISTC). The Hague, Netherlands: Tuberculosis Coalition for Technical Assistance; 2006.
- 30.Miller LG, Asch S, Yu EI, Knowles L, Gelberg L, Davidson P. A population-based survey of tuberculosis symptoms: how atypical are atypical presentations? Clin Infect Dis 2000;30:293–299. [DOI] [PubMed] [Google Scholar]
- 31.Ansari NA, Kombe AH, Kenyon TA, Hone NM, Tappero JW, Nyirenda ST, Binkin NJ, Lucas SB. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis 2002;6:55–63. [PubMed] [Google Scholar]
- 32.Lucas SB, Hounnou A, Peacock C, Beaumel A, Djomand G, N'Gbichi JM, Yeboue K, Honde M, Diomande M, Giordano C, et al. The mortality and pathology of HIV-infection in a West African city. AIDS 1993;7:1569–579. [DOI] [PubMed] [Google Scholar]
- 33.Rana FS, Hawken MP, Mwachari C, Bhatt SM, Abdullah F, Ng'ang'a LW, Power C, Githui WA, Porter JD, Lucas SB. Autopsy study of HIV-1 positive and HIV-negative adult medical patients in Nairobi, Kenya. J Acquir Immune Defic Syndr 2000;24:23–29. [DOI] [PubMed] [Google Scholar]
- 34.Harries AD, Kamenya A, Subramanyam VR, Maher D, Squire SB, Wirima JJ, Nyangulu DS, Nunn P. Screening pulmonary tuberculosis suspects in Malawi: testing different strategies. Trans R Soc Trop Med Hyg 1997;91:416–419. [DOI] [PubMed] [Google Scholar]
- 35.Hargreaves NJ, Kadzakumanja O, Phiri S, Nyangulu DS, Salaniponi FM, Harries AD, Squire SB. What causes smear-negative pulmonary tuberculosis in Malawi, an area of high HIV seroprevalence? Int J Tuberc Lung Dis 2001;5:113–122. [PubMed] [Google Scholar]
- 36.Apers L, Wijarajah C, Mutsvanga J, Chigara N, Mason P, van der Styft P. Accuracy of routine diagnosis of pulmonary tuberculosis in an area of high HIV prevalence. Int J Tuberc Lung Dis 2004;8:945–951. [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC). Multiple misdiagnoses of tuberculosis resulting from laboratory error–Wisconsin, 1996. MMWR Morb Mortal Wkly Rep 1997;46:797–801. [PubMed] [Google Scholar]
- 38.Battacharya M, Dietrich S, Mosher L, Siddiqui F, Reisberg BE, Paul WS, Warren JR. Cross contamination of specimens with Mycobacterium tuberculosis: clinical significance, causes and prevention. Am J Clin Pathol 1998;109:324–330. [DOI] [PubMed] [Google Scholar]
- 39.Cape Metropolitan Council. Townships and informal settlements in the Cape metropolitan region: visual illustration, development, background and present issues. Cape Town, South Africa: Regional Planning, Engineering Service, Cape Metropolitan Council; 1995.
- 40.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis (TB) in an antiretroviral treatment (ART) service in sub-Saharan Africa: impact on ART outcomes and implications for TB control. AIDS 2006;20:1605–1612. [DOI] [PubMed] [Google Scholar]
- 41.De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis 1999;3:457–465. [PubMed] [Google Scholar]
- 42.World Health Organization. Interim policy on collaborative TB/HIV activities. Geneva, Switzerland: World Health Organization; 2004. Available from: http://whqlibdoc.who.int/hq/2004/who_htm_tb_2004.330.pdf (accessed July 4, 2006).
- 43.World Health Organization. An expanded DOTS framework for effective tuberculosis control. Geneva, Switzerland: World Health Organization; 2002. Publication No. WHO/CDS/TB/2002.297.