Abstract
Rationale: Nitrogen oxide (NO) species are markers for oxidative stress that may be pathogenic in acute lung injury (ALI).
Objectives: We tested two hypotheses in patients with ALI: (1) higher levels of urine NO would be associated with worse clinical outcomes, and (2) ventilation with lower Vt would reduce urine NO as a result of less stretch injury.
Methods: Urine NO levels were measured by chemiluminescence in 566 patients enrolled in the National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome Network trial of 6 ml/kg versus 12 ml/kg Vt ventilation. The data were expressed corrected and uncorrected for urine creatinine (Cr).
Results: Higher baseline levels of urine NO to Cr were associated with lower mortality (odds ratio, 0.43 per log(10) increase in the ratio), more ventilator-free days (mean increase, 1.9 d), and more organ-failure–free days (mean increase, 2.3 d) on multivariate analysis (p < 0.05 for all analyses). Similar results were obtained using urine NO alone. NO to Cr levels were higher on Day 3 in the 6 ml/kg than in the 12 ml/kg Vt group (p = 0.04).
Conclusions: Contrary to our hypothesis, higher urine NO was associated with improved outcomes in ALI at baseline and after treatment with the 6 ml/kg Vt strategy. Higher endogenous NO may reflect less severe lung injury and better preservation of the pulmonary and systemic endothelium or may serve a protective function in patients with ALI.
Keywords: acute respiratory distress syndrome, nitrogen oxide species, pulmonary endothelium, tidal volume, pulmonary edema
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Higher levels of nitrogen oxide species in lavage and pulmonary edema fluid correlated with worse outcomes in human and animal studies of acute lung injury.
What This Study Adds to the Field
In a large-scale, multicenter trial of patients with the acute respiratory distress syndrome, higher urine nitric oxide levels correlated with better outcomes. This may reflect better preservation of the endothelium or a protective effect of nitric oxide.
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) define a clinical disorder that causes acute respiratory failure in critically ill patients. There is evidence for lung endothelial damage and alveolar epithelial damage in ALI/ARDS that may be the result of oxidative injury (1–6). Important markers of oxidative injury in the lung include nitrogen oxide species (NOx), particularly nitric oxide (NO), nitrite 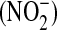 , and nitrate
, and nitrate 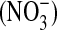 (7). NO readily reacts with superoxide ion to form a highly reactive intermediate known as peroxynitrite (4, 7, 8). Peroxynitrite rapidly oxidizes and nitrates proteins such as α1-antitrypsin and surfactant protein A, respectively, thereby inhibiting their function (6). Inhibition of these proteins may contribute to the proinflammatory environment believed to be pathogenic in ALI (3, 9). Peroxynitrite cannot be measured directly because of its short half-life, but its presence can be inferred by measuring metabolites such as
(7). NO readily reacts with superoxide ion to form a highly reactive intermediate known as peroxynitrite (4, 7, 8). Peroxynitrite rapidly oxidizes and nitrates proteins such as α1-antitrypsin and surfactant protein A, respectively, thereby inhibiting their function (6). Inhibition of these proteins may contribute to the proinflammatory environment believed to be pathogenic in ALI (3, 9). Peroxynitrite cannot be measured directly because of its short half-life, but its presence can be inferred by measuring metabolites such as 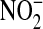 and
and 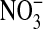 (6).
(6).
Prior research in ALI has demonstrated the importance of NOx. Rats that underwent a ventilator-induced lung injury protocol demonstrated elevations in whole-lung lavage NOx levels (10). Similarly, sheep that underwent combined burn and smoke inhalation injury to model ARDS had higher plasma NOx levels than control sheep (11). In a single-center clinical study, higher NOx levels in bronchoalveolar lavage (BAL) samples from patients with ALI were associated with increased mortality on Days 3 and 7 of the study, leading to the conclusion that NOx elevation occurs early and is associated with worse outcomes (12). In another observational clinical study, patients with ALI had higher NOx levels in pulmonary edema samples than control patients with hydrostatic pulmonary edema (13). In summary, some observational studies have shown that higher levels of NOx are associated with more severe illness, but a definitive evaluation in a multicenter study with large numbers of patients has not been done.
Urine samples were chosen for the current study as a novel medium in which to assess NOx levels in patients with ALI. Two prior studies have demonstrated the utility of studying urine biomarkers in ALI. Mathru and colleagues (14) demonstrated that higher urine H2O2 levels in patients with ALI were associated with worse clinical outcomes, perhaps reflecting greater oxidative injury in these patients. Parikh and colleagues (15) recently showed that higher urine IL-18 levels predicted which patients with ALI would develop acute kidney injury.
The current study investigated urine NO levels in a subgroup of 566 of the 861 patients included in the NHLBI ARDS Network study of lower Vt ventilation (16). We tested two hypotheses. First, we hypothesized that baseline urine NO levels would be elevated in patients with more severe clinical indices and worse clinical outcomes. Second, we hypothesized that urine NO levels would be lower in patients who received lower Vt ventilation because this intervention reduced mortality in the lower Vt ventilation study. Some of the results from this study have been previously reported in the form of abstracts (17, 18).
METHODS
Subjects
The ARDS network trial of lower Vt ventilation (16) included 861 patients, but only 590 had urine samples available for analysis. Most of the excluded patients (235 of 271) were in a lisofylline versus placebo arm of the trial, and these patients did not have urine collected per protocol (19). Fourteen of the 590 patient samples were grossly contaminated on visual inspection or did not have enough urine available for analysis. Therefore, 576 patients underwent assay of urine NO and urine creatinine (Cr) levels. For this study, Day 0 and Day 3 urine samples were analyzed. Clinical data were obtained from the ARDS Network Clinical Coordinating Center. Risk factors predisposing to ALI (sepsis, pneumonia, aspiration, trauma, and other) were determined by investigators at study enrollment. Study protocols with informed consent to participate in the ARDS Network trial of lower Vt ventilation were approved by the institutional review board at each hospital before enrolling patients into the clinical trial. Permission for collection and analysis of biological samples was granted as part of the original Institutional Review Board approval of this study for all participating medical centers.
NO and Cr Measurements
Urine samples were obtained from the National Heart Lung and Blood Institute repository, thawed, and aliquotted into 0.5-ml vials. The aliquots were relabeled using sequential integer values to maintain blinding of the individuals performing the laboratory analysis. One 0.5-ml aliquot of each urine sample was used to measure NO. The urine aliquots were heated at 90°C in an acidic environment (1 M HCl) in the presence of vanadium (III) chloride to catalyze the reduction of 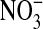 to
to 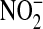 and then to NO (20–26). NO levels were measured with a chemiluminescent analyzer (Sievers Nitric Oxide Analyzer, model 280i; Sievers, Boulder, CO). These measurements were repeated in duplicate and compared with a standard curve. None of the NO levels were above or below the limit of detection for the NO analyzer. Urine Cr was measured in a second set of urine aliquots from the same patients as previously described (27). Ten patients had urine Cr levels below the lower limit of detection for this competitive assay. These patient samples were excluded from the analysis to avoid the hazards of imputing an arbitrary value to a sample that was not reliable for evaluation. The Cr results obtained were also used to evaluate urine desmosine in these patients; those results have been reported elsewhere (27). The ratio of urine NO to urine Cr (NO/Cr) was used in the statistical analyses to control for urine dilution, a standard method when evaluating urine biomarkers (28–31). Analyses using urine NO without Cr correction were performed for comparison.
and then to NO (20–26). NO levels were measured with a chemiluminescent analyzer (Sievers Nitric Oxide Analyzer, model 280i; Sievers, Boulder, CO). These measurements were repeated in duplicate and compared with a standard curve. None of the NO levels were above or below the limit of detection for the NO analyzer. Urine Cr was measured in a second set of urine aliquots from the same patients as previously described (27). Ten patients had urine Cr levels below the lower limit of detection for this competitive assay. These patient samples were excluded from the analysis to avoid the hazards of imputing an arbitrary value to a sample that was not reliable for evaluation. The Cr results obtained were also used to evaluate urine desmosine in these patients; those results have been reported elsewhere (27). The ratio of urine NO to urine Cr (NO/Cr) was used in the statistical analyses to control for urine dilution, a standard method when evaluating urine biomarkers (28–31). Analyses using urine NO without Cr correction were performed for comparison.
Statistics
Statistical analyses were done using SAS software. The data were logarithmically (log10) transformed before statistical analysis because the urine NO (corrected and uncorrected for urine Cr) was not normally distributed. Ventilator-free days (VFD) and organ failure–free days (OFFD) to Day 28 were calculated as previously described (16). Spearman correlation coefficients were used to compare markers of disease severity with urine NO levels. Direct comparisons between groups were made using unpaired t tests. Analysis of covariance was used to analyze the impact of ventilator group on change in urine NO from baseline to Day 3 of the study. Statistical significance was defined as p < 0.05.
RESULTS
Demographics
Table 1 shows the baseline demographics and clinical variables for the included and excluded subjects. Baseline demographics were not significantly different between the groups, although there was a trend for more women in the included group. APACHE (Acute Physiology and Chronic Health Evaluation) III and serum Cr levels were significantly higher, and platelet count showed a trend toward being lower in the patients who were excluded from the current study.
TABLE 1.
CHARACTERISTICS OF INCLUDED VERSUS EXCLUDED STUDY SUBJECTS WITH ACUTE LUNG INJURY
| Clinical Data | Included Subjects (n = 566) | Excluded Subjects (n = 295) | p Value* |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD | 52 ± 17 | 51 ± 18 | 0.94 |
| Sex, % male | 57 | 64 | 0.08 |
| Race, % white | 74 | 72 | 0.57 |
| Sepsis as ALI risk, % | 25 | 30 | 0.17 |
| Ventilation group | |||
| 6 ml/kg, % | 51 | 48 | 0.39 |
| Clinical variables, mean ± SD | |||
| APACHE III | 74 ± 26 | 80 ± 29 | 0.002 |
| Creatinine | 1.6 ± 1.4 | 1.8 ± 1.9 | 0.02 |
| Platelets | 164 ± 115 | 150 ± 99 | 0.08 |
| PaO2/FiO2 | 135 ± 62 | 129 ± 62 | 0.15 |
| No. of organ failures | 0.9 ± 0.9 | 1.0 ± 1.0 | 0.28 |
Definition of abbreviations: ALI = acute lung injury; APACHE III = Acute Physiology and Chronic Health Evaluation III.
Statistical comparison between included and excluded subjects was by two-sided t test for continuous demographic or clinical variables and by χ2 analysis for dichotomous variables.
Raw Urine NO Data
The raw urine NO data by study day and survival group are shown in Table 2 (corrected for urine Cr level) and Table 3 (uncorrected for urine Cr). Because the data for urine NO/Cr and the urine NO alone were abnormally distributed, these data are presented using median, 25th and 75th percentiles, and highest and lowest values. All statistical analyses used log-transformed data.
TABLE 2.
RAW URINE NITRIC OXIDE TO CREATININE DATA
| Survivors
|
Nonsuvivors
|
|||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 0 | Day 3 | |
| Lowest value | 1.4 | 0.6 | 0.5 | 0.9 |
| 25% value | 32 | 44 | 15 | 22 |
| Median (50%) value | 53 | 69 | 40 | 56 |
| 75% value | 88 | 113 | 73 | 103 |
| Highest value | 2,873 | 666 | 591 | 680 |
This table includes the raw data for the urine nitric oxide to creatinine ratio by survival group and study day. Because of the abnormal distribution of these data, the median values, 25th and 75th percentiles, and the highest and lowest values for the group are shown to summarize the data. Values are given as μmol NO/mg creatinine.
TABLE 3.
RAW URINE NITRIC OXIDE DATA
| Survivors
|
Nonsurvivors
|
|||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 0 | Day 3 | |
| Lowest value | 20 | 5 | 4 | 11 |
| 25% value | 192 | 170 | 85 | 96 |
| Median (50%) value | 338 | 346 | 182 | 220 |
| 75% value | 598 | 602 | 424 | 395 |
| Highest value | 6,333 | 5,230 | 5,346 | 1,952 |
This table includes the raw data for the urine nitric oxide values separated by survival group and study day rounded to the nearest whole number. Because of the abnormal distribution of these data, the median values, 25th and 75th percentiles, and the highest and lowest values for the group are shown to summarize the data. Values are given as μmol NO/L.
Baseline Urine NO and Clinical Outcomes
The bivariate relationship between urine NO (corrected and uncorrected for urine Cr levels) on each study day and clinical outcomes was analyzed after log transformation of the data (Table 4). Logistic regression analysis showed that for each log10 increase in urine NO/Cr, the risk of death on Day 0 decreased by more than half (odds ratio [OR], 0.39; 95% confidence interval [CI], 0.27–0.57; p < 0.0001). This effect persisted on Day 3 of the study, when the OR for death was 0.47 for each log10 increase in urine NO/Cr (95% CI, 0.32–0.71; p = 0.0003). Analysis of urine NO alone showed that the risk of death dropped to less than one-third for each log10 increase in urine NO, a result that was highly statistically significant on Day 0 and Day 3 (p < 0.0001 for both).
TABLE 4.
URINE NITRIC OXIDE (CORRECTED AND UNCORRECTED FOR URINE CREATININE) AND RISK OF DEATH IN ACUTE LUNG INJURY
| Type of Sample | Study Day | Number of Patients | Risk of Death OR (95% CI) | p Value |
|---|---|---|---|---|
| Urine NO/Cr | Day 0 | 566 | 0.39 (0.27–0.57) | < 0.0001 |
| Urine NO/Cr | Day 3 | 504 | 0.47 (0.32–0.71) | 0.0003 |
| Urine NO | Day 0 | 566 | 0.27 (0.18–0.42) | < 0.0001 |
| Urine NO | Day 3 | 504 | 0.33 (0.23–0.55) | < 0.0001 |
Definition of abbreviations: CI = confidence interval; NO/Cr = urine nitric oxide to creatinine ratio; OR = odds ratio.
Mortality analysis performed using logistic regression analysis of the baseline NO/Cr to determine an OR for mortality adjusted for ventilator group assignment. For each log10 increment in urine NO/Cr or urine NO level, the risk of death decreased on each study day.
Linear regression analyses tested the association between urine NO (corrected and uncorrected for urine Cr) and other clinical outcomes, namely VFD and OFFD. Higher levels of urine NO/Cr were associated with better outcomes, including more OFFD and more VFD. On average, for each log10 increase in the ratio of urine NO/Cr at baseline, there were 3.6 fewer days of organ failure (95% CI, 1.7–5.4) and 2.5 fewer days on the ventilator (95% CI, 0.7–4.3). The increases in VFD and OFFD were more striking when urine NO alone was evaluated (Table 5). Similar results were obtained on Day 3 analyses for urine NO/Cr and urine NO alone (Table 5).
TABLE 5.
URINE NITRIC OXIDE (CORRECTED AND UNCORRECTED FOR URINE CREATININE) AND IMPROVEMENT IN OTHER CLINICAL OUTCOMES
| Type of Sample | Study Day | Organ-Failure–Free Days (95% CI) | p Value | Ventilator-Free Days (95% CI) | p Value |
|---|---|---|---|---|---|
| Urine NO/Cr | Day 0 | 3.6 (1.7–5.4) | 0.0001 | 2.5 (0.7–4.3) | 0.0059 |
| Urine NO/Cr | Day 3 | 4.4 (2.3–6.4) | < 0.0001 | 2.8 (0.8–4.7) | 0.0056 |
| Urine NO | Day 0 | 6.3 (4.3–8.3) | < 0.0001 | 3.9 (2.0–5.9) | < 0.0001 |
| Urine NO | Day 3 | 6.7 (4.6–8.8) | < 0.0001 | 3.6 (1.6–5.7) | 0.0006 |
Definition of abbreviations: CI = confidence interval; NO/Cr = urine nitric oxide to creatinine ratio.
Linear regression analysis controlling for ventilator group assignment was used to determine mean change in organ-failure–free days and ventilator-free days for each log10 increase in urine NO/Cr levels or urine NO levels.
Correlation with Markers of Disease Severity
Several clinical parameters are known to have prognostic value or to be associated with disease severity in ALI (2, 16, 32). Spearman correlation coefficients were determined between several of these variables and urine NO/Cr levels. The strongest correlation was between Day 0 and Day 3 urine NO/Cr levels (r = 0.5; p < 0.0001). There were weak, inverse relationships between urine NO/Cr levels and serum Cr (r = −0.27; p < 0.0001) and age (r = −0.10; p = 0.02). The inverse correlation indicated that higher urine NO/Cr was associated with lower serum Cr and younger age. Urine NO/Cr levels showed no significant correlation with APACHE III, PaO2/FiO2 ratio, platelets, or number of baseline organ failures (p ⩾ 0.1 for all analyses).
In separate analyses, urine NO/Cr levels were compared in patients with and without vasopressor use as a surrogate for the presence or absence of shock, respectively. There was no difference in urine NO/Cr levels when comparing patients who required vasopressors with those who did not (p = 0.48; unpaired t test analysis). Although there was no significant difference between groups, this analysis may have missed a more subtle interaction between urine NO/Cr levels and shock. Therefore, we included vasopressor use in our multivariate models as a surrogate for shock.
Urine NO and Conditions Predisposing to ALI
The conditions predisposing to ALI recorded in the original trial of lower Vt ventilation were aspiration, pneumonia, sepsis, trauma, and other (16). The urine NO/Cr level was compared between different conditions predisposing to ALI using analysis of variance. Only patients with sepsis showed a significant difference in urine NO/Cr when compared with each of the alternate predisposing conditions (p = 0.006). Patients with sepsis as a primary risk factor for ALI had significantly higher levels of urine NO/Cr (p = 0.0008), a finding that would be expected based on prior studies (33, 34). Because sepsis was the only predisposing condition with significantly different urine NO/Cr levels, only sepsis was included in the multivariate models.
Multivariate Analysis of NO and Clinical Outcomes
The association between higher levels of urine NO (corrected and uncorrected for urine Cr) and better clinical outcomes was assessed in a multivariate model that included other clinical variables known to have predictive value in ALI or suspected to contribute to urine NO levels. The multivariate analyses included age, sex, ventilator group assignment, estimated glomerular filtration rate (GFR) (35, 36), APACHE III score, vasopressor use, and sepsis as a primary risk factor for ALI. GFR was estimated using the modified MDRD equation (Modified Diet in Renal Disease) because this equation provides a more accurate estimate of GFR for patients with normal renal function and patients with mild, moderate, or end-stage renal disease than older methods, such as the Cockcroft-Gault equation for creatinine clearance (35, 36–38). Even when controlling for multiple potential contributing factors, higher urine NO (corrected and uncorrected for urine creatinine) was independently predictive of lower mortality. Specifically, for each log10 increase in urine NO (corrected and uncorrected for urine Cr), the risk of death decreased by more than half. The OR for death when NO was corrected for Cr was 0.43 per log10 increase in urine NO/Cr; the OR for death without correcting for urine Cr was 0.33 per log10 increase in urine NO (Table 6).
TABLE 6.
MULTIVARIATE ANALYSIS OF BASELINE URINE NITRIC OXIDE (CORRECTED AND UNCORRECTED FOR URINE CREATININE) AND OUTCOMES IN ACUTE LUNG INJURY
| Outcomes | Urine NO/Cr | p Value | Urine NO Alone | p Value |
|---|---|---|---|---|
| Risk of death, OR (95% CI) | 0.43 (0.28–0.66) | 0.0001 | 0.33 (0.20–0.54) | < 0.0001 |
| Mean difference in organ-failure–free days, n (95% CI) | 2.7 (0.9–4.5) | 0.003 | 4.2 (2.2–6.2) | < 0.0001 |
| Mean difference in ventilator-free days, n (95% CI) | 1.9 (0.1–3.7) | 0.03 | 2.1 (0.1–4.1) | 0.04 |
For definition of abbreviations, see Table 4.
Multivariate analysis included sex, ventilator group assignment, estimated glomerular filtration rate, age, APACHE III score, vasopressor use, and sepsis as primary risk factors for acute lung injury. Risk of death was calculated using logistic regression analysis. Mean differences in organ-failure–free days and ventilator-free days were calculated using linear regression analysis. ORs are per log10 increment in urine NO/Cr or urine NO alone.
We used multivariate linear regression analyses to assess the predictive value of baseline urine NO (corrected and uncorrected for urine Cr levels) for VFD and OFFD. When controlling for multiple clinical and demographic variables, higher urine NO/Cr and higher urine NO alone remained independently predictive of better outcomes. There were more OFFD (average, 2.7 corrected for urine Cr and 4.2 uncorrected for urine Cr) and more VFD (average, 1.9 corrected for urine Cr and 2.1 uncorrected for urine Cr) for each log10 increase in urine NO/Cr (Table 6).
Effect of Lower Vt Ventilation on Each Study Day
Lower Vt ventilation reduced mortality in the ARDS network trial of lower Vt ventilation (16). Therefore, urine NO/Cr levels were assessed by ventilator group assignment. Median values of untransformed urine NO/Cr levels by ventilator group and study day are shown in Table 7. Statistical analysis using unpaired t test of the log-transformed data showed no significant difference in values on Day 0 between the 6 ml/kg Vt group and the 12 ml/kg Vt group (p = 0.58). However, the NO/Cr levels were higher in the 6 ml/kg than in the 12 ml/kg groups by Day 3 (p = 0.05).
TABLE 7.
LOW Vt VERSUS HIGH Vt STRATEGY AND URINE NITRIC OXIDE IN PATIENTS WITH ACUTE LUNG INJURY
| 6 ml/kg Vt Group
|
12 ml/kg Vt Group
|
|||
|---|---|---|---|---|
| Day of Sample | No. of Patients | Median (IQR) | No. of Patients | Median (IQR) |
| Day 0 | 290 | 53 (28–88) | 276 | 46 (26–83) |
| Day 3 | 264 | 71 (41–113) | 240 | 59 (31–103) |
Definition of abbreviation: IQR = interquartile range.
t Test comparison of the mean values (log transformed) on Day 0 showed no significant difference between groups (p = 0.58). In contrast, t test comparison on Day 3 of the log-transformed mean values showed borderline significant differences between groups (p = 0.05).
Change in Urine NO over Time by Ventilator Group Assignment
The median increase in the non–log-transformed data from Day 0 to Day 3 was greater in the lower Vt group (Figure 1). The average percentage increase in urine NO/Cr levels in the 6 ml/kg group versus the 12 ml/kg group was 19% (95% CI, 0.9–40%) over the first 3 study days. Analysis of covariance of the log-transformed data showed a significantly greater rise in urine NO/Cr by study Day 3 in the 6 ml/kg Vt group (p = 0.04).
Figure 1.
Median of the change in urine NO/Cr from Day 0 to Day 3 by ventilator group. The median change from Day 0 to Day 3 was higher in the 6 ml/kg Vt group than the 12 ml/kg Vt group. This was analyzed statistically using analysis of covariance of the log-transformed urine NO/Cr data, which showed that the mean increase from Day 0 to Day 3 was significantly higher in the 6 ml/kg group than the 12 ml/kg group. Values are given in μmol/mg creatinine. **p = 0.04.
DISCUSSION
Contrary to our original hypothesis, higher urine NO, corrected or uncorrected for urine Cr, was strongly associated with better clinical outcomes, including improved survival, more VFD, and more OFFD, in patients with ALI. This association persisted even when controlling for other factors known to affect the severity and outcome of ALI. Therefore, elevation in urine NO was independently associated with better clinical outcomes in patients with ALI. As further validation of the significance of the higher NO values seen in survivors, the lower Vt ventilation strategy, which reduced mortality in the original study, was associated with higher urine NO/Cr levels after 3 days of treatment. To our knowledge, this study is the first large-scale, multicenter investigation of endogenous NO in ALI and the first to provide evidence for a strong association between higher levels of endogenous NO and better outcomes in ALI.
Why might NO be associated with better outcomes in patients with ALI? It is possible that NO or NOx are protective during ALI. NO scavenges oxygen free radicals that are generated during oxidative stress (4, 33). NO vasodilates the microcirculation, which allows for increased perfusion of tissue beds (33). Prior research has demonstrated an elevation in pulmonary dead space in patients with ALI, in part because some alveoli are being ventilated but not perfused (39). By increasing flow to the microcirculation, particularly to the alveoli that are well ventilated, higher levels of NO would allow for better ventilation to perfusion matching. Regarding the alveolar epithelium, NO has been shown to protect type II alveolar cells from stretch injury (40).
An alternate hypothesis to explain our findings is that endogenous NO has a beneficial effect in organs other than the lung during ALI. Higher NO levels could help prevent further tissue damage by improving oxygen and nutrient delivery to the tissues while helping decrease the amount of toxic oxygen species. NO may also protect endothelial tissue by decreasing platelet and leukocyte adhesion to the endothelium (33). NO would thereby decrease multiorgan failure, which contributes to mortality in ALI. Finally, NO and NOx have antibacterial effects that may be important in infectious conditions that predispose patients to ALI (33). Based on this evidence, NO species may be protective by several different mechanisms in patients with ALI.
A third interpretation of the findings is that higher NO levels are biologic markers of less severe injury. NO is produced by alveolar epithelial cells, alveolar macrophages, and endothelial cells of the lung (41–44). The presence of higher levels of NO could reflect a greater percentage of intact lung endothelium and epithelium as a result of a less severe initial insult. This explanation does not exclude the possibility that endogenous NO production is also protective during ALI. Both explanations may be valid in patients with ALI.
The finding of an association between higher urine NO (corrected and uncorrected for urine Cr levels) and better clinical outcomes was initially unexpected, given the prior experimental and clinical work that had correlated higher levels of NOx with adverse outcomes in ALI (10, 12, 13). Prior work on exhaled breath condensate showed that higher levels of 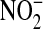 correlated with higher Vt (40), in contrast to our results. There are several possible explanations for the differences between the current study and prior studies. Animal models of ALI may differ significantly from human physiology. Also, most of the earlier human studies included small numbers of patients and were done at single research centers, perhaps limiting the statistical power to detect a protective effect. Some of these studies also used intermediate outcomes as a surrogate for important clinical outcomes, which is often not an effective strategy. For instance, better oxygenation was not a surrogate for better clinical outcomes in the ARDS Network trial of lower Vt ventilation (16). Finally, a phase III study of a NO synthase inhibitor in patients with sepsis showed that patients who received the study drug had a lower incidence of shock and vasopressor use but had a higher mortality rate, leading to premature termination of the study (45). Therefore, it is important to consider the potential pitfalls in studying intermediate outcomes as a surrogate for important outcomes such as mortality.
correlated with higher Vt (40), in contrast to our results. There are several possible explanations for the differences between the current study and prior studies. Animal models of ALI may differ significantly from human physiology. Also, most of the earlier human studies included small numbers of patients and were done at single research centers, perhaps limiting the statistical power to detect a protective effect. Some of these studies also used intermediate outcomes as a surrogate for important clinical outcomes, which is often not an effective strategy. For instance, better oxygenation was not a surrogate for better clinical outcomes in the ARDS Network trial of lower Vt ventilation (16). Finally, a phase III study of a NO synthase inhibitor in patients with sepsis showed that patients who received the study drug had a lower incidence of shock and vasopressor use but had a higher mortality rate, leading to premature termination of the study (45). Therefore, it is important to consider the potential pitfalls in studying intermediate outcomes as a surrogate for important outcomes such as mortality.
There are some limitations to this study. We were not able to include all of the patients from the ARDS Network trial of lower Vt ventilation in the current study primarily because urine samples were not collected in the last 235 patients of the 861 patients in the original trial. A few patients from the first 626 enrolled in the original study did not have urine samples collected. It is possible that the some of these patients were not able to provide urine samples because of more severe disease, reflected by higher APACHE III scores, or because of renal failure, reflected by higher serum Cr levels. To account for these differences at baseline, a multivariate analysis was done that included the APACHE III score and GFR to estimate renal function. Urine NO, corrected and uncorrected for urine Cr, remained independently predictive for better outcomes even when taking these factors into account.
The use of urine samples limits our ability to determine the source of the NO measured in this study. Whole-body production of NO cannot be distinguished from that produced in the lung. Also, NOx produced by the body cannot be separated from dietary intake of 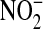 and
and 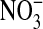 (7), although under conditions of low dietary
(7), although under conditions of low dietary 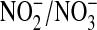 intake and control of other activities such as exercise, 24-hour urine levels of NOx have been established as a qualitative marker of NO production (7). The advantage of examining urine samples is that they provide a reasonable reflection of whole-body NO levels compared with a single-organ measurement, as would be obtained by BAL. Also, even BAL may not sample all of the NOx produced in the lung. Only the NOx present in the bronchioles and alveoli is measured in a lavage sample, meaning that potentially significant levels in the lung interstitium and vasculature may be missed. Finally, there is precedent for evaluating biomarkers of oxidative lung injury in urine samples of patients with ARDS. Mathru and colleagues (14) found that urine H2O2 levels were significantly higher in patients with sepsis and ARDS who died than those who survived or had other predisposing conditions for ARDS. They also identified a high correlation between urine H2O2 levels and the lung injury score, thus providing evidence that urine markers may reflect the severity of lung injury in patients with ARDS.
intake and control of other activities such as exercise, 24-hour urine levels of NOx have been established as a qualitative marker of NO production (7). The advantage of examining urine samples is that they provide a reasonable reflection of whole-body NO levels compared with a single-organ measurement, as would be obtained by BAL. Also, even BAL may not sample all of the NOx produced in the lung. Only the NOx present in the bronchioles and alveoli is measured in a lavage sample, meaning that potentially significant levels in the lung interstitium and vasculature may be missed. Finally, there is precedent for evaluating biomarkers of oxidative lung injury in urine samples of patients with ARDS. Mathru and colleagues (14) found that urine H2O2 levels were significantly higher in patients with sepsis and ARDS who died than those who survived or had other predisposing conditions for ARDS. They also identified a high correlation between urine H2O2 levels and the lung injury score, thus providing evidence that urine markers may reflect the severity of lung injury in patients with ARDS.
We used urine Cr to control for urinary dilution. Although it is possible that this variable influenced the statistical analysis independent of NO, this is an unlikely scenario. First, the use of a ratio between a biomarker and urine Cr is a standard method to adjust for urinary dilution (28–31). Second, in multivariate analyses that included estimated GFR, baseline urine NO/Cr remained highly predictive of better outcomes. Third, urine desmosine, a marker of elastin breakdown, was studied in this group of patients with completely contrary results. Using the same urine Cr measurements as in the current study, higher urine desmosine to Cr was predictive of worse outcomes. Moreover, desmosine levels rose in patients who died compared with those who survived by Day 3 (27). If the evaluation of desmosine and NO simply reflected the inverse of Cr, then the results in both studies should be comparable rather than divergent. Finally, we repeated the important analyses using urine NO not corrected for Cr (urine NO alone) and found even stronger associations with better outcomes than the results using the urine NO/Cr data (Table 3). Alterations in renal function are thus unlikely to explain the current results.
Another potential limitation is the role of urinary tract infection. A large percentage of patients in the ARDS Network trial had sepsis, and urinary tract infection is a leading cause of sepsis. There were no culture data for patients in the current study, so we performed an additional analysis to address this issue. The urine NO levels we measured in this study were converted using the molecular weight of NO and compared with standard urine dipstick measurement of 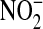 because NO can be oxidized to an equivalent concentration of
because NO can be oxidized to an equivalent concentration of 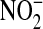 . Urine dipstick measurement is used clinically to screen for the presence of bacteria in the urine. The highest level of urine NO that was measured in this study (0.02 mg/dl) was below the limits of detection for
. Urine dipstick measurement is used clinically to screen for the presence of bacteria in the urine. The highest level of urine NO that was measured in this study (0.02 mg/dl) was below the limits of detection for  using the urine dipstick (0.06 mg/dl) (Multistix; Bayer, Pittsburgh, PA). Therefore, we do not believe that bacteria in the urine significantly contributed to the NO levels measured.
using the urine dipstick (0.06 mg/dl) (Multistix; Bayer, Pittsburgh, PA). Therefore, we do not believe that bacteria in the urine significantly contributed to the NO levels measured.
This study has a number of strengths. Patient data and samples in this study were prospectively collected in a large multicenter trial with the pre hoc design for subsequent biomarker measurements. The biomarkers were measured in duplicate in a blinded fashion and carefully decoded for evaluation. The results were in sharp contrast to our original hypotheses, but they were internally consistent with one another. Higher urine NO levels were strongly associated with better outcomes, including lower mortality, more VFD, and more OFFD, in several different evaluations. Lower Vt ventilation was associated with higher levels of urine NO, which is an important internal validation because lower Vt was associated with better survival in the ARDS Network trial. The results in patients with sepsis provided further validation for this study. NO has a well described role in sepsis (33, 34, 46). We anticipated and found higher urine NO/Cr levels in patients with sepsis.
In summary, higher baseline urine NO levels are strongly associated with better clinical outcomes, including increased survival, more VFD, and more OFFD. These associations remained significant even after controlling for potentially confounding factors. Furthermore, use of lower Vt for ventilation resulted in higher urine NO levels in addition to a decrease in mortality. Our findings suggest that (1) endogenous NO may be protective during ALI or (2) endogenous NO may serve as a marker of less severe organ injury or both.
Acknowledgments
The authors thank Barry Starcher for his contribution to this study regarding the urine creatinine results.
This study was supported in part by NHLBI HL51856 and HL74005 (M.A.M.), and by HL70521 and HL81332 (L.B.W.).
Originally Published in Press as DOI: 10.1164/rccm.200607-947OC on November 2, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network Participants: Cleveland Clinic Foundation—Herbert P. Wiedemann, M.D.*; Alejandro C. Arroliga, M.D.; Charles J. Fisher, Jr., M.D.; John J Komara, Jr., B.A., R.R.T.; Patricia Periz-Trepichio, B.S., R.R.T. Denver Health Medical Center—Polly E. Parsons, M.D. Denver VA Medical Center—Carolyn Welsh, M.D. Duke University Medical Center—William J. Fulkerson, Jr., M.D.*; Neil MacIntyre, M.D.; Lee Mallatratt, R.N.; Mark Sebastian, M.D.; John Davies, R.R.T.; Elizabeth Van Dyne, R.N.; Joseph Govert, M.D. Johns Hopkins Bayview Medical Center—Jonathan Sevransky, M.D.; Stacey Murray, R.R.T. Johns Hopkins Hospital—Roy G. Brower, M.D.; David Thompson, M.S., R.N.; Henry E. Fessler, M.D. LDS Hospital—Alan H. Morris, M.D.*; Terry Clemmer, M.D.; Robin Davis, R.R.T.; James Orme, Jr., M.D.; Lindell Weaver, M.D.; Colin Grissom, M.D.; Frank Thomas, M.D.; Martin Gleich, M.D. (posthumous). McKay-Dee Hospital—Charles Lawton, M.D.; Janice D'Hulst, R.R.T. MetroHealth Medical Center of Cleveland—Joel R. Peerless, M.D.; Carolyn Smith, R.N. San Francisco General Hospital Medical Center—Richard Kallet, M.S., R.R.T.; John M. Luce, M.D. Thomas Jefferson University Hospital—Jonathan Gottlieb, M.D.; Pauline Park, M.D.; Aimee Girod, R.N., B.S.N.; Lisa Yannarell, R.N., B.S.N. University of California, San Francisco—Michael A. Matthay, M.D.*; Mark D. Eisner, M.D., M.P.H.; Brian Daniel, R.C.P., R.R.T. University of Colorado Health Sciences Center—Edward Abraham, M.D.*; Fran Piedalue, R.R.T.; Rebecca Jagusch, R.N.; Paul Miller, M.D.; Robert McIntyre, M.D.; Kelley E. Greene, M.D. University of Maryland—Henry J. Silverman, M.D.*; Carl Shanholtz, M.D.; Wanda Corral, B.S.N., R.N. University of Michigan—Galen B. Toews, M.D.*; Deborah Arnoldi, M.H.S.A.; Robert H. Bartlett, M.D.; Ron Dechert, R.R.T.; Charles Watts, M.D. University of Pennsylvania—Paul N. Lanken, M.D.*; Harry Anderson, III, M.D.; Barbara Finkel, M.S.N., R.N.; C. William Hanson, III, M.D. University of Utah Hospital—Richard Barton, M.D.; Mary Mone, R.N. University of Washington/Harborview Medical Center—Leonard D. Hudson, M.D.*; Greg Carter, R.R.T.; Claudette Lee Cooper, R.N.; Annemieke Hiemstra, R.N.; Ronald V. Maier, M.D.; Kenneth P. Steinberg, M.D. Utah Valley Regional Medical Center—Tracy Hill, M.D.; Phil Thaut, R.R.T. Vanderbilt University—Arthur P. Wheeler, M.D.*; Gordon Bernard, M.D.*; Brian Christman, M.D.; Susan Bozeman, R.N.; Linda Collins; Teresa Swope, R.N.; Lorraine B. Ware, M.D.
Clinical Coordinating Center: Massachusetts General Hospital, Harvard Medical School—David A. Schoenfeld, Ph.D.*; B. Taylor Thompson, M.D.; Marek Ancukiewicz, Ph.D.; Douglas Hayden, M.A.; Francine Molay, M.S.W.; Nancy Ringwood, B.S.N., R.N.; Gail Wenzlow, M.S.W., M.P.H.; Ali S. Kazeroonin, B.S.
NHLBI Staff: Dorothy B. Gail, Ph.D.; Andrea Harabin, Ph.D.*; Pamela Lew; Myron Waclawiw, Ph.D.
*Steering Committee: Gordon R. Bernard, M.D., Chair; Principal Investigators from each center are indicated by an asterisk.
Data and Safety Monitoring Board: Roger G. Spragg, M.D., Chair; James Boyett, Ph.D.; Jason Kelley, M.D.; Kenneth Leeper, M.D.; Marion Gray Secundy, Ph.D.; Arthur Slutsky, M.D.
Protocol Review Committee: Joe G. N. Garcia, M.D., Chair; Scott S. Emerson, M.D., Ph.D.; Susan K. Pingleton, M.D.; Michael D. Shasby, M.D.; William J. Sibbald, M.D.
References
- 1.Matthay MA. Alveolar fluid clearance in patients with ARDS: does it make a difference? Chest 2002;122:340S–343S. [DOI] [PubMed] [Google Scholar]
- 2.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327–336. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo N. The role of intrapulmonary nitric oxide generation in the development of adult respiratory distress syndrome. Surg Today 1999;29:1068–1074. [DOI] [PubMed] [Google Scholar]
- 4.Muzaffar S, Jeremy JY, Angelini GD, Stuart-Smith K, Shukla N. Role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax 2003;58:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink MP. Role of reactive oxygen and nitrogen species in acute respiratory distress syndrome. Curr Opin Crit Care 2002;8:6–11. [DOI] [PubMed] [Google Scholar]
- 6.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 2002;122:314S–320S. [DOI] [PubMed] [Google Scholar]
- 7.Baylis C, Vallance P. Measurement of nitrite and nitrate levels in plasma and urine: what does this measure tell us about the activity of the endogenous nitric oxide system? Curr Opin Nephrol Hypertens 1998;7:59–62. [DOI] [PubMed] [Google Scholar]
- 8.Ehrhart IC, Zou L, Theodorakis MJ, Parkerson JB, Gu X, Caldwell RB, Catravas JD. Effect of nitrite on endothelial function in isolated lung. Gen Pharmacol 2000;34:401–408. [DOI] [PubMed] [Google Scholar]
- 9.Haddad IY, Pataki G, Hu P, Galliani C, Beckman JS, Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest 1994;94:2407–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA. High tidal volume ventilation induces nos2 and impairs camp-dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol 2003;284:L791–L798. [DOI] [PubMed] [Google Scholar]
- 11.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips GB, Parkinson JF, Cox R, Hawkins H, Herndon D, et al. The inducible nitric oxide synthase inhibitor bbs-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med 2003;167:1021–1026. [DOI] [PubMed] [Google Scholar]
- 12.Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:503–510. [DOI] [PubMed] [Google Scholar]
- 13.Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S. Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med 2001;163:166–172. [DOI] [PubMed] [Google Scholar]
- 14.Mathru M, Rooney MW, Dries DJ, Hirsch LJ, Barnes L, Tobin MJ. Urine hydrogen peroxide during adult respiratory distress syndrome in patients with and without sepsis. Chest 1994;105:232–236. [DOI] [PubMed] [Google Scholar]
- 15.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 2005;16:3046–3052. [DOI] [PubMed] [Google Scholar]
- 16.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome Network. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 17.McClintock DE, Ware LB, Eisner MD, Wickersham N, Thompson BT, Matthay MA, and the ARDS Network. Higher urine nitric oxide is associated with better outcomes in acute lung injury [abstract]. Proc Am Thorac Soc 2006;3:A570. [Google Scholar]
- 18.McClintock DE, Ware LB, Eisner MD, Wickersham N, Thompson BT, Matthay MA, and the ARDS Network. Higher urine nitric oxide is associated with better outcomes in acute lung injury. First Annual Pulmonary & Critical Care Research Fellows' Conference. Big Sky, MT: STI IME Innovations; 2006. p. 12.
- 19.Cocci F, Miniati M, Monti S, Cavarra E, Gambelli F, Battolla L, Lucattelli M, Lungarella G. Urinary desmosine excretion is inversely correlated with the extent of emphysema in patients with chronic obstructive pulmonary disease. Int J Biochem Cell Biol 2002;34:594–604. [DOI] [PubMed] [Google Scholar]
- 20.Ergenekon E, Gucuyener K, Erbas D, Aral S, Koc E, Atalay Y. Cerebrospinal fluid and serum vascular endothelial growth factor and nitric oxide levels in newborns with hypoxic ischemic encephalopathy. Brain Dev 2004;26:283–286. [DOI] [PubMed] [Google Scholar]
- 21.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L60–L68. [DOI] [PubMed] [Google Scholar]
- 22.Braman RS, Hendrix SA. Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (iii) reduction with chemiluminescence detection. Anal Chem 1989;61:2715–2718. [DOI] [PubMed] [Google Scholar]
- 23.Atochina EN, Beers MF, Hawgood S, Poulain F, Davis C, Fusaro T, Gow AJ. Surfactant protein-D, a mediator of innate lung immunity, alters the products of nitric oxide metabolism. Am J Respir Cell Mol Biol 2004;30:271–279. [DOI] [PubMed] [Google Scholar]
- 24.Cui X, Moayeri M, Li Y, Li X, Haley M, Fitz Y, Correa-Araujo R, Banks SM, Leppla SH, Eichacker PQ. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol 2004;286:R699–R709. [DOI] [PubMed] [Google Scholar]
- 25.Gonon AT, Erbas D, Broijersen A, Valen G, Pernow J. Nitric oxide mediates protective effect of endothelin receptor antagonism during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 2004;286:H1767–H1774. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro L. de Assuncao e Silva F, Kurihara RS, Schor N, Mieko E, Higa S. Evaluation of the nitric oxide production in rat renal artery smooth muscle cells culture exposed to radiocontrast agents. Kidney Int 2004;65:589–596. [DOI] [PubMed] [Google Scholar]
- 27.McClintock DE, Starcher B, Eisner MD, Thompson BT, Hayden DL, Church GD, Matthay MA. Higher urine desmosine levels are associated with mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L566–L571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabbani GH, Islam S, Chowdhury AK, Mitra AK, Miller MJ, Fuchs G. Increased nitrite and nitrate concentrations in sera and urine of patients with cholera or shigellosis. Am J Gastroenterol 2001;96:467–472. [DOI] [PubMed] [Google Scholar]
- 29.Girgis RE, Champion HC, Diette GB, Johns RA, Permutt S, Sylvester JT. Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am J Respir Crit Care Med 2005;172:352–357. [DOI] [PubMed] [Google Scholar]
- 30.Demoncheaux EA, Higenbottam TW, Kiely DG, Wong JM, Wharton S, Varcoe R, Siddons T, Spivey AC, Hall K, Gize AP. Decreased whole body endogenous nitric oxide production in patients with primary pulmonary hypertension. J Vasc Res 2005;42:133–136. [DOI] [PubMed] [Google Scholar]
- 31.Gosling P, Czyz J, Nightingale P, Manji M. Microalbuminuria in the intensive care unit: clinical correlates and association with outcomes in 431 patients. Crit Care Med 2006;34:2158–2166. [DOI] [PubMed] [Google Scholar]
- 32.Eisner MD, Thompson T, Hudson LD, Luce JM, Hayden D, Schoenfeld D, Matthay MA. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164:231–236. [DOI] [PubMed] [Google Scholar]
- 33.Cobb JP, Danner RL. Nitric oxide and septic shock. JAMA 1996;275:1192–1196. [PubMed] [Google Scholar]
- 34.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med 1993;328:1471–1477. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: modification of diet in renal disease study group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 36.Levey AS, Greene T, Kusek JW, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 2000;11:155A. [Google Scholar]
- 37.Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol 2003;14:2573–2580. [DOI] [PubMed] [Google Scholar]
- 38.Kuan Y, Hossain M, Surman J, El Nahas AM, Haylor J. GFR prediction using the MDRD and Cockcroft and Gault equations in patients with end-stage renal disease. Nephrol Dial Transplant 2005;20:2394–2401. [DOI] [PubMed] [Google Scholar]
- 39.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002;346:1281–1286. [DOI] [PubMed] [Google Scholar]
- 40.Gessner C, Hammerschmidt S, Kuhn H, Lange T, Engelmann L, Schauer J, Wirtz H, Schiller J, Meybaum M, Sandvoss T, et al. Exhaled breath condensate nitrite and its relation to tidal volume in acute lung injury influence of tidal volume on pulmonary no release, tissue lipid peroxidation and surfactant phospholipids. Chest 2003;124:1046–1052. [DOI] [PubMed] [Google Scholar]
- 41.Ricciardolo FL, Di Stefano A, Sabatini F, Folkerts G. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol 2006;533:240–252. [DOI] [PubMed] [Google Scholar]
- 42.Albertine KH, Wang ZM, Michael JR. Expression of endothelial nitric oxide synthase, inducible nitric oxide synthase, and endothelin-1 in lungs of subjects who died with ARDS. Chest 1999;116:101S–102S. [PubMed] [Google Scholar]
- 43.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA 1995;92:7809–7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dweik RA, Laskowski D, Abu-Soud HM, Kaneko F, Hutte R, Stuehr DJ, Erzurum SC. Nitric oxide synthesis in the lung: regulation by oxygen through a kinetic mechanism. J Clin Invest 1998;101:660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546c88: effect on survival in patients with septic shock. Crit Care Med 2004;32:21–30. [DOI] [PubMed] [Google Scholar]
- 46.Buwalda M, Ince C. Opening the microcirculation: can vasodilators be useful in sepsis? Intensive Care Med 2002;28:1208–1217. [DOI] [PubMed] [Google Scholar]



