Abstract
Rationale: Long-acting β-agonists (LABAs) and inhaled corticosteroids administered together appear to be complementary in terms of effects on asthma control. The elements of asthma control achieved by LABAs (improved lung function) and leukotriene receptor antagonists (LTRAs; protection against exacerbations) may be complementary as well.
Objective: We sought to determine whether the combination of the LTRA montelukast and the LABA salmeterol could provide an effective therapeutic strategy for asthma.
Methods and Measurements: In a randomized, placebo-controlled, crossover study of 192 subjects with moderate asthma, we compared the clinical efficacy of regular treatment over 14 weeks with the combination of montelukast and salmeterol to that with the combination of beclomethasone and salmeterol in moderate asthma. The primary efficacy outcome was time to treatment failure.
Main Results: Three months after the randomization of the last subject, the Data and Safety Monitoring Board determined that the primary research question had been answered and terminated the trial. The combination of montelukast and salmeterol was inferior to the combination of beclomethasone and salmeterol as judged by protection against asthma treatment failures (p = 0.0008), lung function (26 L/min difference in a.m. peak expiratory flow rate, p = 0.011), asthma control score (0.22 difference in Asthma Control Questionnaire score, p = 0.038), and markers of inflammation and airway reactivity.
Conclusions: Patients with moderate asthma similar to those we studied should not substitute the combination of an LTRA and an LABA for the combination of inhaled corticosteroid and an LABA.
Keywords: combination therapy, leukotriene, beta-agonists, inhaled corticosteroids
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Previous studies in asthma suggest that the most robust clinical effects of long-acting β-agonists (improved lung function) are distinct from those of the leukotriene receptor antagonists (protection against exacerbations). The efficacy of combination therapy with these agents, as compared with the usual combination therapy with a long-acting β-agonist and an inhaled corticosteroid, is not known.
What This Study Adds to the Field
In patients with moderate asthma, the combination of a leukotriene receptor antagonist and a long-acting β-agonist was inferior to the combination of an inhaled corticosteroid and a long-acting β-agonist as judged by protection against asthma treatment failures, lung function, and markers of inflammation and airway reactivity. Patients similar to those we studied should not substitute the combination of a leukotriene receptor antagonist and a long-acting β-agonist for the combination of an inhaled corticosteroid and a long-acting β-agonist.
Inhaled corticosteroids (ICS) are effective in the treatment of asthma and are considered to be generally safe. Although current guidelines recommend the use of these agents as first-line therapy for individuals with persistent symptoms, these documents acknowledge that, because systemic adverse effects may occur with prolonged use at higher doses, ICS should be prescribed at the lowest effective dose (1, 2). Strategies to limit ICS exposure have included the use of other controller agents, such as long-acting β-agonists (LABAs) or leukotriene receptor antagonists (LTRAs) (3). This approach is supported by studies demonstrating that, compared with increasing the dose of ICS in patients with asthma whose disease is not well controlled while using lower dose ICS, addition of an LABA is associated with superior clinical outcomes with less ICS exposure (4–6).
In a previous study, we investigated whether an LABA could replace ICS therapy in patients with well-controlled asthma using low-dose ICS and found that, although LABAs and ICS produced similar effects on lung function, ICS achieved greater suppression of biological markers associated with airway inflammation as well as superior protection against asthma treatment failure in this population (7). In contrast to this clinical profile, LTRAs have antiinflammatory properties as judged by suppression of markers of airway inflammation in asthma, even in subjects previously treated with combination therapy (8, 9). Furthermore, in some reports, these agents have been shown to reduce asthma exacerbations to a degree comparable to an ICS, although ICS produce superior effects on lung function (10, 11). In this regard, because the elements of asthma control achieved by an LTRA (suppression of inflammation and maintenance of control) and an LABA (improvement in lung function) appear to be complementary, we hypothesized that the combination of an LTRA and an LABA might demonstrate synergistic beneficial clinical properties and thus provide an effective therapeutic strategy for asthma. Although our understanding of the appropriate role of LABA in asthma therapy is evolving (12–14), currently published treatment guidelines recommend that individuals with asthma requiring more than low–moderate doses of ICS receive concomitant LABA therapy (1). Thus, we compared clinical outcomes of treatment with the novel combination of an LTRA and an LABA to those of treatment with the standard combination of an ICS and an LABA in subjects with moderate asthma.
METHODS
Patients
We recruited male and female subjects aged 12 to 65 years with a history of physician-diagnosed asthma at the Asthma Clinical Research Network centers and screened them using methods previously reported (7). At enrollment, subjects were required to have an FEV1 of at least 40% of the predicted value and demonstrate hyperresponsiveness to methacholine (PC20 ⩽ 8 mg/ml) or a 12% or greater improvement in FEV1 after the administration of a β-agonist bronchodilator (if FEV1 was < 55% of predicted at enrollment). Subjects not using an ICS or LTRA at the time of enrollment were required to have an FEV1 of 80% or less of the predicted value. Exclusion criteria included cigarette smoking (⩾ 10 pack-years or any cigarette use within the last 12 mo), respiratory tract infection, or asthma exacerbation (i.e., a need for oral corticosteroid or urgent care visit) within the previous 6 weeks. All subjects gave their written, informed consent as required by the institutional review board of each center, which also reviewed the protocol.
Study Design
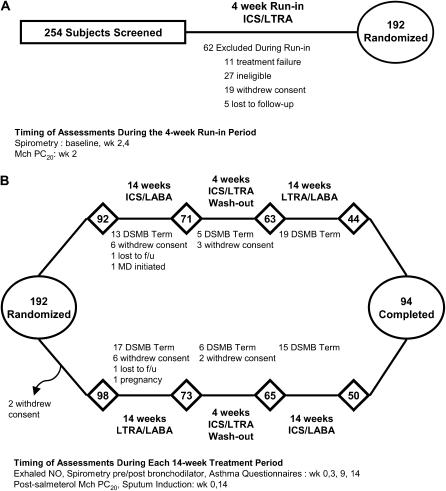
Subjects meeting the above criteria entered a 36-week randomized, double-blind, placebo-controlled cross-over trial (Figure 1). During an initial 4-week run-in period, all subjects received single-blind treatment with beclomethasone hydrofluoroalkane (HFA) (80 μg twice daily) and montelukast (10 mg by mouth at bedtime) as well as “as needed” albuterol. Physiologic and symptom measures recorded during this run-in provided baseline data for use in defining subsequent asthma treatment failures. To avoid preselecting subjects preferentially responsive to a specific controller agent (i.e., ICS only or an LTRA only), the treatment regimen during the run-in period included both an ICS and an LTRA. In this regard, subjects with the capacity to achieve reasonable asthma control while using either an ICS or an LTRA were enrolled. Subjects without any treatment failure criteria and an FEV1 of 55% or greater of predicted at the conclusion of the 4-week run-in period were randomized to receive double-blind treatment with either the combination of beclomethasone HFA (QVAR, 80 μg twice daily; IVAX Labs, Miami, FL) and salmeterol (Serevent, 50 μg inhaled twice daily via Diskus; GlaxoSmithKline, Research Triangle Park, NC) and placebo LTRA or the combination of montelukast (Singulair, 10 mg by mouth at bedtime; Merck, Whitehouse Station, NJ) and salmeterol (50 μg inhaled twice daily via Diskus) and placebo beclomethasone HFA for 14 weeks of active treatment. Subsequently, subjects entered a second 4-week run-in period during which time they again received single-blind therapy with beclomethasone HFA 80 μg twice daily in addition to montelukast 10 mg by mouth at bedtime. Subjects then crossed over to the alternate combination therapy as compared with that which they had received in the first active treatment and completed the second active treatment period over the subsequent 14 weeks.
Figure 1.
Timing of the assessments and disposition of all enrolled subjects during the (A) 4-week run-in and (B) the two 14-week randomized treatment periods. DSMB = Data and Safety Monitoring Board; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LTRA = leukotriene receptor antagonist; Mch = methacholine.
The primary outcome for this trial was time to treatment failure. Treatment failure was defined by criteria, listed in Table 1, that we have used in previous trials (7, 15). Consistent with these previous trials, subjects meeting treatment failure criteria received additional therapy and continued study participation. Prespecified secondary outcomes included spirometric values, scheduled recordings of morning and evening peak expiratory flow rate (PEFR), use of rescue medications, biologic markers associated with airway inflammation in asthma (methacholine PC20, exhaled nitric oxide, sputum eosinophils), a six-question asthma control score (16), asthma quality of life questionnaire (AQLQ), and asthma symptom utility index (ASUI). The techniques for these measures and the protocols for establishing and monitoring quality control have been described previously (7, 17). In this trial, spirometry was performed using Masterscope spirometers and JLAB Bronchial Test software (Erich Jaeger, Milbury, OH).
TABLE 1.
TREATMENT FAILURE CRITERIA
| In-clinic measures |
|
| At-home measures |
|
| Additional criteria |
PEF rate measurements were scheduled on awakening (before bronchodilator use) and between 8 and 11 p.m.
Baseline values defined by the average daily measurement obtained during Weeks 3 and 4 of the run-in period.
Subjects meeting these criteria were also designated as experiencing an asthma exacerbation.
Protocol Approval and Study Monitoring
Prior to beginning the trial, a National Institutes of Health–appointed review committee approved the protocol. A Data and Safety Monitoring Board (DSMB) reviewed adverse outcomes and data quality throughout the study. Three months after the randomization of the last study subject, the DSMB determined that the study had generated sufficient data to answer the primary research question and terminated the trial.
Statistical Analysis
The primary response variable was the time until treatment failure. Because of the crossover design, each subject provided a failure time or a censoring time for each of the two treatment regimens. For each subject, one of the treatment regimens was considered to be superior if the time to failure was longer when the subject used that regimen as compared with the time to failure when that subject used the alternate regimen. Subjects eligible for inclusion in this primary analysis are those who (1) completed the trial, (2) failed on at least one treatment and had enough data during treatment with the second regimen to determine a treatment superiority, or (3) did not fail during treatment with the one regimen, had not failed during treatment with the second regimen through the time of the last completed study visit, and had at least as much follow-up with the second regimen as with the first. If a subject did not meet failure criteria during either treatment, then neither treatment was considered superior for that subject. Superiority of one treatment over the other was assessed by McNemar's test for paired binary data applied in an intent-to-treat manner. The target sample size of 180 randomized subjects provided 90% statistical power to detect such a difference in failure rates, with a two-sided 0.05 significance level test, while accounting for a 10% withdrawal rate.
Secondary outcome variables measured serially over the trial were analyzed in a longitudinal intent-to-treat manner using mixed-effects linear models (18, 19). Proportions of subjects with longer time to failure with ICS/LABA as compared with that with LTRA/LABA were examined between study subgroups using a two-sided Fisher's exact test. Proportions of subjects with an increase in Asthma Control Questionnaire (ACQ) score of at least 0.5 during each treatment regimen were compared using an exact two-sided McNemar's test. Additional information regarding the statistical analysis can be found in the online supplement.
Role of the Funding Source
The National Heart, Lung, and Blood Institute of the National Institutes of Health funded the study, including the purchase of salmeterol. IVAX Laboratories, Inc., provided beclomethasone HFA and matching placebo, and Merck, Inc., provided montelukast and matching placebo. Neither entity had input into the trial, including design, data collection, analysis, or interpretation.
RESULTS
Patient Enrollment and Demographics
Two hundred fifty-four subjects were recruited between September 2002 and January 2004. Of these, 62 subjects did not complete the 4-week run-in for reasons indicated in Figure 1A; therefore, 192 subjects were randomized. In March 2004, the DSMB stopped the trial after determining that no further information was needed to establish superiority, as reflected by the primary outcome, for one of the treatment arms as compared with the other. The baseline characteristics of the randomized subjects are shown in Table 2.
TABLE 2.
CHARACTERISTICS OF THE STUDY SUBJECTS*
| Age at enrollment, yr | 34.3 ± 10.1 |
| Female sex, n (%) | 117 (61) |
| Race/ethnicity, n (%) | |
| White | 105 (54.7) |
| Black | 55 (28.6) |
| Asian of Pacific Islander | 10 (5.2) |
| Hispanic | 21 (10.9) |
| Other | 1 (0.5) |
| Atopic, n (%) | 180 (94) |
| FEV1, L | 2.87 ± 0.78 |
| FEV1, % predicted | 83.3 ± 14.7 |
| a.m. PEFR, L/min† | 424 ± 112 |
| FEV1 improvement 60 min after salmeterol, % | 8.1 ± 8.2 |
| Methacholine PC20,‡ mg/ml, n = 189§ | 0.94 (0.47, 3.30) |
| Rescue albuterol use, no. daily puffs†§ | 0.24 (0.00, 1.04) |
| ACQ§ | 0.83 (0.33, 1.33) |
| IgE, IU/ml, n = 179§ | 233 (91, 534) |
| Exhaled nitric oxide, ppb, at 350 ml/s (n = 188)§ | 13.1 (8.9, 20.9) |
| Induced sputum parameters§ | |
| Eosinophils, % (n = 153) | 0.20 (0.00, 0.90) |
| ECP, μg/L (n = 147)§ | 62 (32, 149) |
| Tryptase, μg/L (n = 148)§ | 1.0 (1.0, 2.6) |
| Baseline asthma medication use | |
| Short-acting β-agonists only, n (%) | 37 (19) |
| Long-acting β-agonists, n (%) | 81 (42) |
| Leukotriene receptor antagonists, n (%) | 25 (13) |
| ICS, n (%) | 127 (66) |
| ICS μg/d§‖ | 200 (114, 320) |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; ECP = eosinophil cationic protein; ICS = inhaled corticosteroids; PEFR = peak expiratory flow rate.
Values represent mean ± SD, measured at randomization, unless otherwise noted; n = 192 unless otherwise noted.
Average during last 2 weeks of run-in.
Measured at Week 2 of the run-in period.
Median and first and third quartiles are reported.
Fluticasone equivalent.
The disposition of the study subjects is shown in Figure 1B. Of the 192 subjects that were randomized, 144 completed at least one treatment arm and 110 subjects completed a portion of the trial sufficient to be included in the primary prespecified time-to-treatment failure analysis. Of the 98 subjects who did not complete the trial, 75 subjects terminated because the DSMB stopped the trial. There were no differences in the numbers or reasons for withdrawal from the trial between the two treatment arms.
Treatment Failures
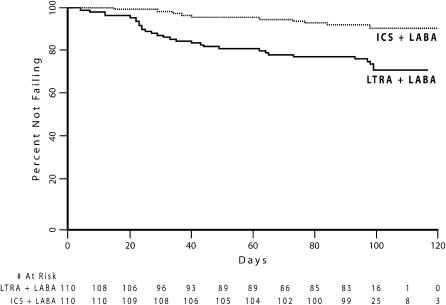
Of the 110 subjects eligible for the prespecified primary analysis, 73 (66%) did not fail while receiving either therapy. Ten subjects (9%) failed while receiving an ICS and an LABA, and 29 individuals (26%) met treatment failure criteria while receiving an LTRA and an LABA. In the two subjects who failed while receiving both treatment regimens, the time to failure was shorter during treatment with the combination of an LTRA and an LABA as compared with that during treatment with the combination of an ICS and an LABA. Significantly more subjects experienced a shorter time to treatment failure while using an LTRA and an LABA in combination as compared with using an ICS and an LABA in combination (29 vs. 8 subjects, p = 0.0008; Figure 2). The reasons for treatment failure in these subjects were similar to those observed in previous studies and are detailed in Table 3.
Figure 2.
Kaplan-Meier survival curves for treatment failure during treatment with LTRA + LABA or ICS + LABA in the subjects eligible for the prespecified primary analysis (n = 110). p = 0.0008 (McNemar's test) for differential prolongation of time to failure between the treatments.
TABLE 3.
REASONS FOR TREATMENT FAILURE
| Treatment
|
||
|---|---|---|
| LTRA/LABA (29 events) | ICS/LABA (10 events) | |
| Post-BD FEV1 ⩽ 80% of baseline | 11 (38%) | 2 (20%) |
| Corticosteroid treatment | 9 (31%) | 5 (50%) |
| Pre-BD PEF ⩽ 65% of baseline (2 of 3 consecutive assessments) | 7 (24%) | 3 (30%) |
| Physician clinical judgment for safety reasons | 6 (21%) | 4 (40%) |
| Albuterol use of ⩾ 8 puffs per 24 h over baseline | 7 (24%) | 1 (10%) |
| Persistent asthma symptoms despite rescue use | 5 (17%) | 2 (20%) |
| Physician judgment or use of corticosteroids only | 2 (7%) | 4 (40%) |
| Pre-BD FEV1 ⩽ 80% of baseline (2 consecutive assessments) | 3 (10%) | 1 (10%) |
| Emergency department treatment | 1 (3%) | 3 (30%) |
| Post-BD PEF ⩽ 80% of baseline | 1 (3%) | 2 (20%) |
| No PEF improvement despite rescue use | 1 (3%) | 0 |
| ⩾ 16 Puffs albuterol/24 h for 48 h | 0 | 0 |
Definition of abbreviations: BD = bronchodilator; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LTRA = leukotriene receptor antagonist.
Multiple reasons are possible for each event. Reasons for events contributing to the primary efficacy outcome are given.
Secondary Outcomes
The differences in asthma exacerbations were consistent with those observed in treatment failures. Six percent of those eligible for the primary analysis experienced an asthma exacerbation while receiving an ICS and an LABA, whereas 14% met exacerbation criteria while receiving an LTRA and an LABA. More subjects experienced a shorter time to exacerbation while using an LTRA and an LABA in combination as compared with using an ICS and an LABA in combination (p = 0.041).
The changes in other secondary outcomes mirrored the pattern observed for treatment failures and exacerbations (Table 4). The combination of an ICS and an LABA produced significant improvements in a.m. PEFR (26 L/min), methacholine responsiveness (∼ 1 doubling dilution shift), and exhaled NO (5.9 ppb) as compared with the combination of montelukast and salmeterol. We also observed smaller, but statistically significant, treatment differences favoring beclomethasone and salmeterol in pre- and post-bronchodilator FEV1, the need for supplemental short-acting β-agonist use, daytime asthma symptoms, ACQ score, and sputum parameters, including eosinophil counts, eosinophil cationic protein (ECP), and tryptase.
TABLE 4.
CHANGES FROM BASELINE IN THE SECONDARY OUTCOME VARIABLES WITH EACH TREATMENT OVER 14 WEEKS AND DIFFERENCES BETWEEN TREATMENTS
| LTRA/LABA
|
ICS/LABA
|
LTRA/LABA vs. ICS/LABA
|
||
|---|---|---|---|---|
| Variable | Change (95% CL) | Change (95% CL) | Difference (95% CL) | p Value |
| FEV1, L | −0.04 (−0.09, 0.01) | 0.05 (0.01, 0.09) | −0.09 (−0.15, −0.02) | 0.007 |
| FEV1, % predicted | −1.27 (−2.78, 0.25) | 1.54 (0.35, 2.74) | −2.81 (−4.69, −0.93) | 0.003 |
| Postsalmeterol FEV1, L | −0.12 (−0.17, −0.07) | −0.03 (−0.07, 0.01) | −0.09 (−0.15, −0.02) | 0.007 |
| a.m. peak flow, L/min | −5.69 (−25.46, 14.08) | 20.18 (14.80, 25.55) | −25.87 (−46.26, −5.48) | 0.011 |
| p.m. peak flow, L/min | −11.06 (−19.10, −3.02) | 9.96 (4.83, 15.09) | −21.02 (−30.32, −11.72) | < 0.001 |
| Peak flow variability, % | −1.11 (−2.34, 0.13) | −2.22 (−3.13, −1.31) | 1.11 (−0.44, 2.66) | 0.152 |
| Rescue use, no. daily puffs | 0.12 (−0.09, 0.33) | −0.25 (−0.41, −0.08) | 0.36 (0.10, 0.62) | 0.006 |
| Daily a.m. symptoms (0–3) | 0.02 (−0.03, 0.06) | −0.05 (−0.08, −0.01) | 0.06 (0.01, 0.12) | 0.025 |
| ACQ* | 0.01 (−0.16, 0.17) | −0.21 (−0.35, −0.08) | 0.22 (0.01, 0.43) | 0.038 |
| AQLQ† | 0.05 (−0.11, 0.21) | 0.14 (0.01, 0.26) | −0.09 (−0.29, 0.11) | 0.370 |
| ASUI‡ | −0.01 (−0.04, 0.02) | 0.03 (0.01, 0.055) | −0.04 (−0.08, 0.00) | 0.057 |
| Exhaled nitric oxide difference from baseline, ppb§ | 3.23 (0.05, 6.40) | −2.69 (−5.44, 0.06) | 5.91 (1.59, 10.24) | 0.006 |
| Salmeterol-protected methacholine PC20 (n = 88)‖¶ | −1.46 (−1.85, −1.08) | −0.47 (−0.80, −0.14) | −0.99 (−1.45, −0.54) | < 0.0001 |
| Sputum eosinophils, % (n = 60)¶ | 0.80 (0.30, 1.60) | 0.15 (0.0, 0.25) | 0.70 (0.20, 1.40) | 0.0019 |
| Sputum ECP, μg/L (n = 53)¶ | 133 (79, 191) | 10 (−4, 42) | 90 (17, 166) | 0.0147 |
| Sputum tryptase, μg/L (n = 53)¶ | 5.5 (3.3, 9.4) | 0.2 (0.0, 0.5) | 5.0 (3.0, 8.1) | < 0.0001 |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; AQLQ = asthma quality of life questionnaire; ASUI = asthma symptoms utility index; CL = confidence limits; ECP = eosinophil cationic protein; ICS = inhaled corticosteroids; LABA = long-acting β-agonist; LTRA = leukotriene receptor antagonist.
Unless otherwise noted, values represent the results of a modeled, intent-to-treat analysis; n = 190.
Scale: 0–6; higher scores indicate poorer control.
Scale: 1–7; higher scores indicate improved asthma-related quality of life.
Scale 0–1: higher scores indicate fewer symptoms.
Measured at 350-ml/s expiratory flow rate.
Values represent doubling dilution changes; negative values indicate increased responsiveness.
Aligned analysis of the individuals with complete data.
Subgroup Analysis
Differences in initial asthma control, as defined in previous studies by lung function and diurnal PEFR variability, did not affect the relative efficacy of the treatment regimens (7, 15). Of the 55 subjects with well-controlled asthma (as determined by normal lung function and low diurnal PEFR variability) when using an LTRA and an ICS at randomization, 4 (7%) met treatment failure criteria while receiving beclomethasone and salmeterol, and 12 individuals (22%) met treatment failure criteria while receiving an LTRA and an LABA. More well controlled subjects experienced a longer time to treatment failure when using beclomethasone and salmeterol in combination than when using montelukast and salmeterol in combination (12 vs. 3, p = 0.035).
Of the 55 subjects who had less well controlled asthma (abnormal lung function or elevated diurnal PEFR variability), 5 individuals (9%) met treatment failure criteria while receiving beclomethasone and salmeterol, and 17 (31%) met treatment failure criteria while receiving an LTRA and an LABA. In this less well controlled subgroup, more subjects experienced a longer time to treatment failure when using an ICS and an LABA in combination than when using an LTRA and LABA in combination (17 vs. 5, p = 0.017). There was no difference in the proportion of subjects in this less well controlled subgroup with preferential protection against treatment failure while using an ICS/LABA (relative to an LTRA/LABA) as compared with that in the subjects with well-controlled asthma (p = 1.0).
Because the results of a large salmeterol surveillance study suggest that the clinical effects of salmeterol may differ in distinct ethnic populations (12), we also assessed whether the treatment differences we observed were similar in white subjects as compared with African Americans. In the 60 white individuals, more subjects experienced a longer time to treatment failure when using beclomethasone and salmeterol in combination than when using montelukast and salmeterol in combination (10 vs. 2, p = 0.039). Thirty-two subjects identified themselves as African American. In these subjects, more individuals experienced a longer time to treatment failure when using beclomethasone and salmeterol in combination than when using montelukast and salmeterol in combination (15 vs. 3, p = 0.0075). There was no difference in proportion of white subjects with preferential protection against treatment failure while using an ICS/LABA (relative to an LTRA/LABA) as compared with that in the African-American subjects (p = 1.0).
DISCUSSION
The hypothesis that the combination of an LTRA and an LABA could provide effective asthma treatment was based on the observation that each agent has been demonstrated to produce beneficial effects similar to those of ICS on complementary parameters of asthma control (7, 10, 11). We speculated that combining an LTRA and an LABA could produce synergistic beneficial effects, resulting in acceptable control of both lung function and asthma stability.
Our crossover study, which directly compared the combination of an LTRA (montelukast) and an LABA (salmeterol) with the combination of an ICS (beclomethasone) and an LABA in the same individuals, demonstrates that in subjects with moderate asthma, the combination of an LTRA and an LABA is inferior to the combination an ICS and an LABA as judged by protection against asthma treatment failures, lung function, and markers associated with inflammation. The difference in protection against treatment failure between the two regimens is substantial. As reflected by relative prolongation of time to failure, the proportion of subjects with superior asthma control while using an ICS/LABA (29/110) was over 3.5-fold higher than the proportion of subjects with preferential control during use of an LTRA/LABA (8/110). Because the criteria for determining treatment failure status were designed to capture elements of asthma instability that would induce most clinicians to augment therapy, the inferior protection against these events we document with the combination of an LTRA/LABA suggests that this combination cannot be generally substituted for an ICS/LABA combination in patients with asthma similar to those we studied.
The effects of the two combination treatments on the secondary outcomes studied mirrored those observed for treatment failures. As compared with the LTRA/LABA combination, an ICS/LABA combination was associated with improved lung function (FEV1 before and after a bronchodilator and a.m. PEFR), symptoms, need for supplemental rescue medication, and suppressed markers associated with airway inflammation. The relative improvement in a.m. PEFR (25 L/min) associated with the ICS/LABA as compared with the LTRA/LABA is clinically meaningful and is consistent with the magnitude of the treatment failure differences. Although statistically significant, the magnitude of the relative improvements in other measures, including prebronchodilator FEV1 and quality-of-life scores, was small and of uncertain clinical relevance. This variability in treatment effect across asthma outcome measures is consistent with that recently documented by Jenkins and colleagues (20).
Previous data suggest that the response to controller therapies is heterogeneous and specific individuals may be preferentially responsive to a specific class of controller therapy (21, 22). In this regard, we designed the run-in period of this trial to include combined treatment with an ICS and an LTRA so as not to bias enrollment against subjects whose asthma could not be controlled with either an LTRA or an ICS alone. Furthermore, we retrospectively stratified our data according to baseline asthma control and ethnicity, two factors that may influence the likelihood of beneficial response to either a leukotriene modifier or an ICS (12, 23). We determined that the magnitude of the difference between the regimens as judged by treatment failures was similar in those with well-controlled asthma as compared with those with poorer control, as well as in whites as compared with African Americans. Additional analyses may determine if responses to the regimens studied are associated with other phenotypic and genotypic markers.
We recognize that because the present study did not include an LABA monotherapy arm, we cannot directly compare the combination of an LTRA/LABA to an LABA alone. Prior studies, including our own, have demonstrated that, in comparison to ICS, LABA monotherapy is associated with increased risk of asthma treatment failures, exacerbations, and, in some individuals, increased risk of death (7, 12, 24). However, the percentage of subjects experiencing treatment failures over 14 weeks of therapy we observed with the LTRA/LABA combination (26%) is considerably smaller than that we previously reported in a comparable population of patients with asthma treated for 16 weeks with salmeterol during ICS withdrawal (46%). Taking these data together, it appears likely that the addition of an LTRA to an LABA provides improved suppression of treatment failures as compared with an LABA alone, although not to the degree achieved by the combination of an ICS and an LABA. Whether either therapy reduces the putative idiosyncratic risk of severe exacerbations, or death, attributed to the use of LABAs cannot be determined from our data.
Although the primary endpoint of the trial—treatment failure—is a composite designed to capture multiple aspects of asthma control, which we have used in multiple previous studies, we note that the relative effects of the two treatment regimens on the ACQ, another composite measure of asthma control, were modest. Although the average group difference in ACQ (0.22) between the treatments reached statistical significance, this value is below the reported minimal clinically important difference (0.5) in this measure for an individual (16). This discrepancy may reflect the different aspects of asthma control captured by treatment failures—as we define them—as compared with the ACQ. The treatment failure endpoint is designed to capture intermittent deteriorations in asthma stability that would induce many clinicians to intensify asthma care. In this respect, whereas the ACQ (which was assessed only during clinic visits) captures the subjects' retrospective assessment of their overall asthma control in the preceding week, treatment failures assess whether an individual's asthma control has ever deteriorated below a clinically relevant threshold. Importantly, the proportion of subjects with a worsening of asthma control as reflected by an increase in ACQ of at least 0.5 during LTRA/LABA treatment was larger than that during treatment with the ICS/LABA combination (19 vs. 9%, p = 0.04). However, the magnitude of the treatment effect as judged by preferential protection against treatment failures (∼ 3.5:1) was still larger than that as judged by preferential improvements in ACQ (p = 0.005 by a generalized estimating equation). In this respect, our primary endpoint may be more sensitive to significant, but transient, alterations in asthma control that are not retrospectively reported by the subjects using the ACQ.
In summary, despite previous data suggesting a strong rationale for combining an LABA with an LTRA (an alternate antiinflammatory agent), the results of our study indicate that the clinical synergy produced with this combination is inferior to the synergistic properties associated with an ICS and an LABA as judged by protection from treatment failures, lung function, and suppression of markers associated with airway inflammation. Although future studies may identify subgroups of individuals for whom the LTRA/LABA combination is effective, patients with moderate asthma should not substitute this combination of agents for ICS/LABA treatment.
Supplementary Material
Supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (U10-HL51810, U10-HL51834, U10-HL51831, U10-HI51823, U10-HL51845, U10-HL51843, U10-HL56443, U10-HL, M01-RR03186) and by IVAX Laboratories, Inc., and Merck, Inc. See details under Methods.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200601-112OC on September 14, 2006
Conflict of Interest Statement: A.D. received $4,500 in 2005 for consulting services to Aerocrine US. He is also the principal investigator of research projects sponsored by Merck for which the Brigham and Women's Hospital has been paid $54,000 in 2004, and will be paid $94,000 in 2006. He received $6,000 in speaking fees from Merck in 2006. M.E.W. has received less than $10,000/year in 2003–2005 from Merck, Novartis, and GlaxoSmithKline (GSK) for consultancies, advisory boards, and lecture fees; he received $25,000/year in 2004–2005 from Merck to study Churg-Strauss syndrome genetics. H.A.B. received $19,200 in payments during 2003 and 2004 and 2005 from GSK for services on a GOAL Steering Committee for a multicenter study and for chairing and speaking at conferences, and directs a research project funded by the company at University of California, San Francisco, and received $3,000 in 2005 for the Food and Drug Administration Advisory Committee meeting. He has received payments for honoraria and for consulting from Sanofi-Aventis, Boehringer-Ingelheim (BI), Novartis, Genentech, Altana, and Sumitomo. V.M.C. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.J.K. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.J.C. is a speaker for Merck (ongoing, $10,000), Genentech/Novartis (ongoing, $8,000), Pfizer (ongoing, $3,000), GSK (ongoing, $5,000), Schering (ongoing, $7,000), and Sanofi-Aventis (ongoing, $7,500). He has received or is receiving research or education grant money from Schering, Merck, ZLB, and GSK (2003, $50,000). He is on an advisory board for Sanofi-Aventis (2005, $5,000). He participates in industry-sponsored research as an investigator for AstraZeneca (2005, $125,000), BI (2006, $325,000), Novartis (2005, $75,000), Genentech (2005, $50,000), Dyax (2002–2006, $85,000), Lev (2005–2006, $50,000), ZLB (2006, $25,000), Centacore (2002–2006, $25,000), Sanofi-Aventis (2005, $200,000), Altana (2004–2005, $150,000), and Pharming (2006, $7,500). E.D. received a research grant from Genentech/Novartis in the amount of $110,000 for 2004–2006. Between 2003 and 2006, J.V.F. provided consulting services on asthma and drug development for asthma to biotechnology (Xoma, Tilarik, Arriva, Abgenix) and pharmaceutical companies (AstraZeneca, Sanofi-Aventis). M.K. received $3,000 in 2005 from GSK for consulting, $3,000 in 2004 and 2005 from AstraZeneca, $2,000 from BI in 2005, $2,000 from Genentech in 2005, and $1,500 from Merck for participation in advisory board activities; she received the following for participation as a speaker at scientific meetings: $5,000 in 2004 and 2005 and $4,000 in 2006 from Merck; $2,000 in 2004 and $2,000 in 2005 from GSK; $3,000 from Sepracor in 2005, $6,000 in 2004, $3,000 in 2005; and $4,000 in 2006 from Genentech/Novartis. She has received research funding from GSK, Altana, Genentech, Novartis, BI, Medicinova, and Merck for participation in clinical trials. F.L. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.C.L. received $9,000 in 2003 and $3,000 in 2004 from Merck and $2,000 in 2004 from Critical Therapeutics for serving on advisory boards. He received $2,500 in 2004 from GSK and $5,500 in 2005 from Merck for participating as a speaker in scientific programs. He received $36,000 in 2003 from Abaris Pharma as a research grant for participating in a clinical trial. R.F.L. received speaker honoraria from GSK, Merck & Co., Aventis, and AstraZeneca in the last 3 years; in 2002, this totaled $22,000 and in 2003, $12,000. All the other yearly amounts for each company were under $10,000. He also received consultant fees from AstraZeneca, Aventis, GSK, and Novartis/Genentech; in 2004, he received $11,000 from AstraZeneca whereas all the other amounts for all years totaled under $10,000. R.J.M. received less than $10,000 per year per company (GSK, IVAX, Sanofi-Aventis, Schering, Merck) for lecture fees and/or advisory board combined. He also received a research grant from GSK for $50,000 in 2003–2005. G.R.P. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.P.P. has the following consulting involvements: Adelphi (Respiratory Digest, Associate Editor), AstraZeneca Pharmaceuticals, Discovery, Genentech, Novartis, Omnicare, RAD Foundation, PRI-Med, UpToDate, Sanofi-Aventis: none of these involved more that $10,000 per annum in 2003–2005. He has also participated in educational programs sponsored by AstraZeneca Pharmaceuticals, Merck Pharmaceuticals, Genentech, Novartis, and Practicome: none of these relationships with pharmaceutical companies involved more than $10,000 per annum in 2003–2006 except for Merck (sponsor of Visiting Professorships and educational programs), which involved approximately $12,000 in 2004, $28,000 in 2005, and 20,000 in 2006; and AstraZeneca, $17,000 in 2006. He also participated as a member of a Wake Forest University Clinical Trials Group, sponsored by Abaris, AstraZeneca, Altana, BI, Centocor, Genentech, GSK, Novartis, Pfizer, and Wyeth. C.A.S. received $5,000 annually for speaking at conferences sponsored by GSK and AstraZeneca (2002–2005), $5,000 annually (2003–2005) for a GSK advisory board, and $50,000 from GSK in grant support (2002–2005). S.J.S. has served as a consultant and member of an advisory board for GSK, AstraZeneca, and Aventis for the last 3 years and received approximately $6,000 per year from each company, and from Merck for 2 years at $5,000 per year. He also received research funds for clinical trials performance from AstraZeneca for $90,000 for 2002–2004 and from Ross Pharmaceuticals for $1,200,000 for 2003–2005. E.I. served as a consultant for Asthmatx in 2005 and received between $10,000 and $20,000. A multicenter clinical research project at his institution is currently pending. He received advisory board fees from Merck, and speaker's fees from Merck between 2003 and 2005, totaling $10,000–$20,000 per year. He received speaker's fees from Genentech, and serves on a Genentech advisory board for a total compensation of $10,000–$20,000 per year, and his institution is conducting a multicenter clinical trial with Genentech. In the past 3 years, he has participated in multicenter clinical trials with AstraZeneca, BI, Centocor, GSK, and Merck & Co. He received a medical school grant from Merck & Co. for support and research.
References
- 1.National Heart, Lung, and Blood Institute; World Health Organization. NHLBI/WHO Workshop Report. Global strategy for asthma management and prevention: global initiative for asthma. Bethesda, MD: National Institutes of Health; 2002. NIH Publication No. 02-3659.
- 2.National Heart, Lung, and Blood Institute. Guidelines for the diagnosis and management of asthma. National Asthma Education Program. Expert Panel Report 2. Bethesda, MD: National Institutes of Health; 1997. NIH Publication No. 97-4051.
- 3.National Asthma Education and Prevention Program (NAEPP) Expert Panel Report. Guidelines for the diagnosis and management of asthma: update on selected topics 2002. Bethesda, MD: National Institutes of Health; 2003. NIH Publication No. 02-5074. [PubMed]
- 4.Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996;153:1481–1488. [DOI] [PubMed] [Google Scholar]
- 5.Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet 1994;344:219–224. [DOI] [PubMed] [Google Scholar]
- 6.O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, Tattersfield A. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001;164:1392–1397. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, Kraft M, Fish JE, Peters SP, Craig T, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285:2583–2593. [DOI] [PubMed] [Google Scholar]
- 8.Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. Evaluation of salmeterol or montelukast as second-line therapy for asthma not controlled with inhaled corticosteroids. Chest 2001;119:1021–1026. [DOI] [PubMed] [Google Scholar]
- 9.Currie GP, Lee DK, Haggart K, Bates CE, Lipworth BJ. Effects of montelukast on surrogate inflammatory markers in corticosteroid-treated patients with asthma. Am J Respir Crit Care Med 2003;167:1232–1238. [DOI] [PubMed] [Google Scholar]
- 10.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma: a randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999;130:487–495. [DOI] [PubMed] [Google Scholar]
- 11.Israel E, Chervinsky PS, Friedman B, Van Bavel J, Skalky CS, Ghannam AF, Bird SR, Edelman JM. Effects of montelukast and beclomethasone on airway function and asthma control. J Allergy Clin Immunol 2002;110:847–854. [DOI] [PubMed] [Google Scholar]
- 12.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129:15–26. [DOI] [PubMed] [Google Scholar]
- 13.Martinez FD. Safety of long-acting beta-agonists: an urgent need to clear the air. N Engl J Med 2005;353:2637–2639. [DOI] [PubMed] [Google Scholar]
- 14.Szefler SJ, Whelan GJ, Leung DY. “Black box” warning: wake-up call or overreaction? J Allergy Clin Immunol 2006;117:26–29. [DOI] [PubMed] [Google Scholar]
- 15.Lemanske RF Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, Drazen JM, Chinchilli VM, Craig T, Fish JE, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA 2001;285:2594–2603. [DOI] [PubMed] [Google Scholar]
- 16.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005;99:553–558. [DOI] [PubMed] [Google Scholar]
- 17.Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, Chinchilli VM, Craig TJ, Dimango E, Deykin A, et al. Regular vs. intermittent controller therapy for mild persistent asthma. N Engl J Med 2005;352:1519–1528. [DOI] [PubMed] [Google Scholar]
- 18.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- 19.Vonesh E, Chinchilli VM. 1997. Linear and Non-Linear Models for the Analysis of Repeated Measurements Marcel Decker, Inc, New York, NY.
- 20.Jenkins CR, Thien FC, Wheatley JR, Reddel HK. Traditional and patient-centred outcomes with three classes of asthma medication. Eur Respir J 2005;26:36–44. [DOI] [PubMed] [Google Scholar]
- 21.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, Craig TJ, Dolovich M, Drazen JM, Fagan JK, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002;109:410–418. [DOI] [PubMed] [Google Scholar]
- 22.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, Zeiger RS, Larsen G, Spahn JD, Bacharier LB, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 2005;115:233–242. [DOI] [PubMed] [Google Scholar]
- 23.Israel E, Cohn J, Dube L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zileuton Clinical Trial Group. JAMA 1996;275:931–936. [PubMed] [Google Scholar]
- 24.Castle W, Fuller R, Hall J, Palmer J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ 1993;306:1034–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.