Abstract
Rationale: Obstructive lung disease, the major cause of mortality in cystic fibrosis (CF), is poorly correlated with mutations in the disease-causing gene, indicating that other factors determine severity of lung disease.
Objectives: To quantify the contribution of modifier genes to variation in CF lung disease severity.
Methods: Pulmonary function data from patients with CF living with their affected twin or sibling were converted into reference values based on both healthy and CF populations. The best measure of FEV1 within the last year was used for cross-sectional analysis. FEV1 measures collected over at least 4 years were used for longitudinal analysis. Genetic contribution to disease variation (i.e., heritability) was estimated in two ways: by comparing similarity of lung function in monozygous (MZ) twins (∼ 100% gene sharing) with that of dizygous (DZ) twins/siblings (∼ 50% gene sharing), and by comparing similarity of lung function measures for related siblings to similarity for all study subjects.
Measurements and Main Results: Forty-seven MZ twin pairs, 10 DZ twin pairs, and 231 sibling pairs (of a total of 526 patients) with CF were studied. Correlations for all measures of lung function for MZ twins (0.82–0.91, p < 0.0001) were higher than for DZ twins and siblings (0.50–0.64, p < 0.001). Heritability estimates from both methods were consistent for each measure of lung function and ranged from 0.54 to 1.0. Heritability estimates generally increased after adjustment for differences in nutritional status (measured as body mass index z-score).
Conclusions: Our heritability estimates indicate substantial genetic control of variation in CF lung disease severity, independent of CFTR genotype.
Keywords: genetics, pulmonary function
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Severity of cystic fibrosis (CF) lung disease is poorly correlated with CFTR mutation. Although numerous candidates have been investigated, the role for modifier genes in CF lung disease severity has not been verified or quantified.
What This Study Adds to the Field
A significant portion of variability in CF lung disease is due to modifier genes.
Cystic fibrosis (CF) is a lethal autosomal recessive disorder characterized by chronic obstructive pulmonary disease and caused by mutations in the CF transmembrane conductance regulator (CFTR) gene (1). However, lung function is poorly correlated with CFTR mutation (2, 3), indicating that environmental, genetic, and/or stochastic factors are the major determinants of lung disease severity (2, 4). Numerous studies have linked environmental factors, including tobacco smoke exposure (5), bacterial infection (6, 7), and socioeconomic status (8, 9), with reduced pulmonary function. Conversely, aggressive nutritional intervention has been associated with improved outcomes (10). Although numerous candidate genes have been investigated as CF modifiers (11, 12), a role for genes other than CFTR in CF lung disease severity has not been verified or quantified. Family-based studies involving identical (monozygous [MZ]) twins, nonidentical (dizygous [DZ]) twins, and siblings provide an opportunity to assess contribution of genetic and nongenetic factors to disease variation. To this end, we have obtained detailed medical and environmental information from CF twins and CF siblings throughout the United States.
One of the major challenges in any study of the factors underlying disease variation is to accurately define the phenotype. Traditionally, pulmonary function testing (PFT) has been used to determine severity of and monitor progression in CF lung disease (13, 14). The FEV1 is the PFT measurement that is most predictive of survival in CF (15, 16). Expressing FEV1 as a percent-predicted value based on a normal reference population illustrates the reduction in pulmonary function of patients with CF relative to healthy individuals (17). However, patients with CF have variable growth abnormalities, including reduced height and delayed puberty (18, 19), that distort the values predicted from healthy populations and complicate comparisons of lung function from one patient with CF to another (20, 21). Disease-specific reference equations for FEV1 have been developed to compare patients of different age and sex with CF (22, 23). In the current study, the contribution of modifier genes to variation in CF lung disease severity (i.e., heritability) was estimated from cross-sectional and longitudinal measures of lung function derived from both percent-predicted FEV1 values and from disease-specific measures of FEV1 in affected twins and siblings. Some of the results of this study have been previously reported in the form of abstracts (24, 25).
METHODS
All subjects were recruited on the basis of having an affected sibling. Except for two sets of MZ twins, all patients attended U.S. CF care centers. All patients met CF diagnostic criteria (26). Zygosity for all twin pairs was determined by the AmpFlSTR Profiler kit (Applied Biosystems, Foster City, CA). CFTR genotype was obtained from medical records, by typing for 31 common CF alleles or by sequencing of the coding and flanking regions (27, 28). Ethnicity was determined by chart review. Written, informed consent or assent was obtained from all subjects.
Pairs of twins or siblings were included in the analysis if both members of the pair had a minimum of four quarterly PFT measurements. A quarter was defined as a 3-month block beginning with the subject's month of birth. PFT data obtained in patients younger than 6 years, after lung transplantation, or when living apart from their affected twin or sibling(s) were excluded. FEV1 values in liters were converted into percent-predicted values (FEV1%pred) (29) and into CF-specific percentiles for FEV1 (FEV1CF%) (23). For cross-sectional analysis, the best FEV1 measure within the last year of available data was termed MaxFEV1CF%. Siblings of different ages were compared using the best FEV1 for the older sibling from the year when the age of the older sibling matched the current age of the younger sibling. Longitudinal measures were derived from all years of available PFT data for each study subject, using the best FEV1 measurement per quarter. Rates of change for FEV1CF% were calculated by linear regression of FEV1CF% on test age in years using FEV1 data obtained after 1993. The best quarterly FEV1CF percentages for subjects with a minimum of 4 years of PFT data were used to calculate average FEV1CF% (AvgFEV1CF%). The estimated FEV1%pred at 20 years of age (EstFEV1%@20yrs) was calculated from a minimum of 5 years of FEV1 data using mixed modeling and Bayes estimation as described by Schluchter and colleagues (22). The AvgFEV1CF% and EstFEV1%@20yrs for each individual were used as a single numbers representing lung disease severity over time (longitudinal measures). To assess agreement between the two methods of defining longitudinal lung disease severity, AvgFEV1CF% and EstFEV1%@20yrs were converted into z-scores based on our patient population. The z-transformed values were compared using Bland-Altman analysis. The average of all body mass index (BMI) z-scores (AvgBMIZ) for each subject was derived from height and weight measurements between 2 and 20 years of age and was used as a longitudinal measure of nutritional status.
Intrapair similarity was determined for MZ twins, DZ twins, and siblings using Pearson pairwise correlation coefficients (r). Assignment of twins or siblings as “A” or “B” was permuted 106 times. The mean and standard deviation of correlation coefficients obtained from the permutations are reported. Statistical significance of the correlation coefficients was determined using the corresponding t value, calculated using the equation t = r/(sqrt[(1 − r2)(n − 2)]). Multiple linear regression was used to determine the contributions of sex, genotype, age at most recent PFT, pancreatic status, and nutritional status to variability in longitudinal measures of lung function. Genotype was defined as 0, 1, or 2 based on the number of ΔF508 CFTR mutations carried by each individual. Pancreatic status was insufficient if the subject had physician-diagnosed pancreatic insufficiency or was taking supplemental pancreatic enzymes. Heritability was estimated by subtracting the correlation coefficient for the combined DZ twin/sibling group from the correlation coefficient for MZ twins and multiplying the difference by two (30). Heritability was also estimated from the siblings alone by dividing additive trait variance among related siblings by total trait variance for all siblings using maximum likelihood estimates as implemented in Sequential Oligogenic Linkage Analysis Routines (SOLAR; http://www.sfbr.org/solar) (31). The significance of heritability estimates from SOLAR was determined by likelihood ratio tests, in which the obtained likelihood of the model with the stated additive genetic variance is compared with the likelihood of the model with the additive genetic variance constrained to zero. Statistical calculations and graphing were performed using Intercooled Stata 8 (Stata Corp., College Station, TX). p values less than 0.05 were deemed significant. Additional supporting data, including intrapair correlations for ΔF508 homozygotes and for age-dependent intrapair correlations are included in the online supplement.
RESULTS
Demographics
Fifty-seven CF twin pairs and 231 CF sibling pairs (of a total patient population of 526) from 61 fully accredited CF centers and 10 affiliate CF centers throughout the United States and a CF center in Australia were studied (Table 1). One hundred sixty-one subjects were excluded because either the subject or the affected twin or sibling had fewer than four quarterly PFT measurements. The average number of PFT observations per individual was 23 ± 14, with a range of 4 to 100. The average number of years of PFT data was 8.8 ± 5.3, with a range of 0.7 to 30.6 years. Four hundred twenty-six patients had at least 4 years of PFT data, and 391 had 5 years of data or more. The average age for the entire group was 17.4 ± 7.1 years, with a range of 6.8 to 46.7 years. The MZ twins were slightly older than the DZ twins and siblings, although age ranges for the different groups overlapped considerably (Table 1). Males represented 54.2% of the total study population. The majority (91.3%) of subjects had pancreatic insufficiency. Individuals homozygous for the ΔF508 CFTR mutation represented 51.4% of the entire study population. The distribution of ΔF508 homozygotes within each class is within expected variance given the sample size. Most subjects were white (89.9%), whereas a small minority were Hispanic/Latino (2.7%), African American (1.1%), Asian (0.6%), Middle Eastern (0.4%), or of mixed racial descent (3.0%). Ethnicity was unknown for 2.3% of study subjects. The demographic features of the study subjects mirror those of the population of patients with CF in the United States in 2004, except for a younger mean age (32).
TABLE 1.
CHARACTERISTICS OF STUDY SUBJECTS
| MZ Twins | DZ Twins | Siblings | Combined DZ Twins/Siblings* | |
|---|---|---|---|---|
| Individuals | 94 (47 pairs) | 20 (10 pairs) | 412 (231 pairs)† | 149 (79 pairs)‡ |
| Average age ± SD at most recent PFT (range) | 20.2 ± 8.2 yrs (7.8–46.7 yr) | 17.6 ± 7.1 yrs (10.8–34.5 yrs) | 16.8 ± 6.7 yrs (6.8–46.0 yrs) | 16.9 ± 6.3 (7.8–39.0 yrs) |
| Sex | ||||
| Female | 44 | 7 | 190 | 59 |
| Male | 50 | 13 | 222 | 90 |
| Pancreatic status§ | ||||
| Insufficient | 93 | 19 | 368 | 139 |
| Sufficient | 1 | 1 | 43 | 9 |
| Unknown | 0 | 0 | 1 | 1 |
| CFTR genotype | ||||
| ΔF508/ΔF508 | 56 (59.6%) | 10 (50%) | 203 (49.3%) | 75 (50.3%) |
| Non-ΔF508 homozygotes | 38 (40.4%) | 10 (50%) | 209 (50.7%) | 74 (49.7%) |
Definition of abbreviations: DZ = dizygous; MZ = monozygous; PFT = pulmonary function testing.
Same-sex DZ twins and same-sex siblings with fewer than 3 years' difference in age.
Fourteen families with three affected children (counted as three pairs), one family with four affected children (counted as six pairs).
Three families with three affected children (counted as three pairs).
Physician-diagnosed pancreatic insufficiency or individual taking supplemental pancreatic enzymes.
Measures of the Severity of CF Lung Disease
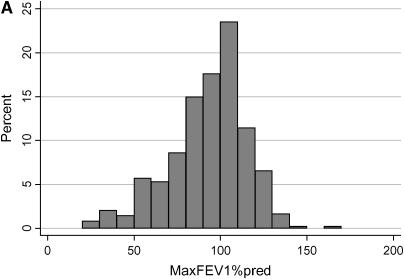
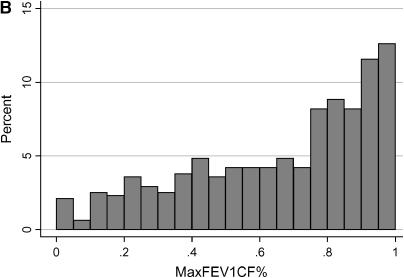
The distribution of the cross-sectional measures of lung function in the study population is shown in Figure 1. The mean MaxFEV1%pred for the study patients was 87.8% (± 25.7; range, 16–165). The mean MaxFEV1CF% for the study patients was 0.66 (± 0.29; range, 0–1). Because the CF-specific percentiles for FEV1 represent the entire CF population, an increment of 0.05 would be expected to represent 5% of patients if the disease severity of the study population exactly mirrored that of the CF population as a whole. The study subjects encompass the entire spectrum of severity, although a substantial fraction have moderate to mild lung disease, compared with the entire CF population.
Figure 1.
Distribution of the cross-sectional measures of lung function in the cystic fibrosis (CF) twins and siblings. (A) The x axis represents FEV1 expressed as percent-predicted based on the equations of Knudson (MaxFEV1%pred), in increments of 10%. The y axis shows the percentage of study patients with a MaxFEV1%pred that falls within each 10% increment. (B) FEV1 expressed as CF-specific percentile based on the equations of Kulich (FEV1CF%), in increments of 0.05. The y axis shows the percentage of study patients with an FEV1CF% that falls within each 0.05 increment. FEV1CF% is expressed as a fraction, with 0 corresponding to the most severe lung disease and 1 corresponding to the mildest lung disease (i.e., the equivalent of the 100th percentile).
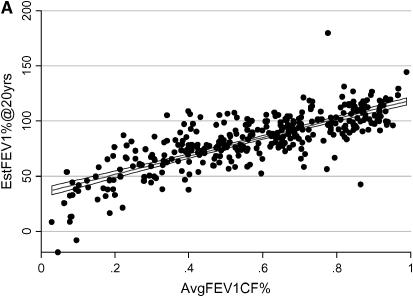
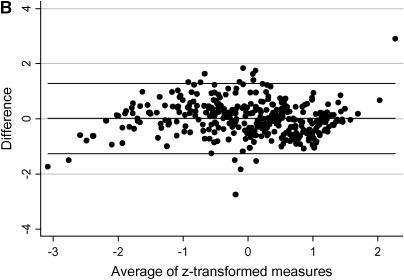
To determine if FEV1CF% changes over time, we plotted the best quarterly FEV1CF% for all study subjects versus age at time of PFT measurement. The mean linear rate of change for the entire group was 0.00 ± 0.03. The rate of change in FEV1CF% was between −0.03 and 0.03 for 68% of study subjects, and 98% had a rate of change of less than 0.10 per year. To evaluate the ability of AvgFEV1CF% to predict the actual FEV1CF% at age 20, we compared AvgFEV1CF% to the known MaxFEV1CF% at age 20 for the 120 subjects for whom these data were available. The average absolute difference in actual and AvgFEV1CF% was 0.096 ± 0.087 (range, 0.002–0.578). The majority (64.7%) of the subjects had a MaxFEV1CF% at age 20 that differed by less than 0.10 from the AvgFEV1CF%, and 89.9% differed by less than 0.20. Because FEV1CF% remains relatively stable for a number of years for many patients with CF and is predictive of lung function at age 20, we chose to use AvgFEV1CF% as a longitudinal measurement of the severity of lung disease. A Bayesian model that predicts FEV1 at 20 years of age (EstFEV1%@20yrs) was used as a second longitudinal measure of lung function (22). To evaluate the validity of this model for our population, we compared the known value of FEV1%pred for the 87 subjects who had a PFT measurement at age 20 with the value predicted by the model. The mean absolute difference between the EstFEV1%@20yrs and the MaxFEV1%pred at age 20 was 10.2 ± 15.2, with a range of 0.1 to 97.5. The majority (69%) of subjects had predicted values that differed from actual values by 10% or less, and 87.4% differed by less than 20%. These two longitudinal models of CF lung disease were highly correlated (r = 0.80, p < 0.0001) for the 341 individuals for whom both measures were available (Figure 2A). When considering z-transformed longitudinal measures, the mean difference between the two measurements is small. However, there is wide variation in difference between the two measures for any given mean, suggesting that the two longitudinal measures represent slightly different aspects of lung disease severity. There is no systematic bias between the two measures (Figure 2B).
Figure 2.
Agreement between longitudinal measures of lung function. (A) Each point on the graph represents an individual subject with a minimum of 5 years of pulmonary function testing data while living at home with an affected sibling. The x axis represents the average FEV1CF% (AvgFEV1CF%) and the y axis represents the estimated FEV1% at 20 years (EstFEV1%@20yrs) for each individual. The best-fit line with 95% confidence intervals are shown. (B) Each point on this Bland-Altman plot represents a single subject. The x axis represents the mean of the two longitudinal measures and the y axis represents the difference between the two longitudinal measures for each subject. The horizontal lines on the graph represent the mean difference between the two measures for all subjects (center line) and +2 and −2 standard deviations from that mean (upper and lower horizontal lines, respectively).
Covariate Analysis
Previous studies evaluating genetic contribution to variability in longitudinal measures of pulmonary function have found pulmonary function to be closely related to nutritional status (10, 33, 34). We used the AvgBMIZ as an estimate of nutritional status. Regression analysis was performed to evaluate the contributions of AvgBMIZ, pancreatic status, genotype, and age at most recent PFT measurement (maximum test age) to variability in longitudinal measures of severity of CF lung disease. When considered independently, AvgBMIZ and pancreatic status were significant covariates of EstFEV1%@20yrs (p < 0.001), whereas AvgBMIZ was the only significant covariate for AvgFEV1CF% (p < 0.001). When all covariates were included in a single model using multiple linear regression, AvgBMIZ remained a highly significant covariate for both measures, whereas pancreatic status was also a significant covariate for EstFEV1%@20yrs (Table 2). The best-fit model for EstFEV1%@20yrs was 88.4 + 13.8 (if pancreatic sufficient) + (15.4 × AvgBMIZ). For AvgFEV1CF%, the best-fit model was 0.626 + (0.124 × AvgBMIZ). AvgBMIZ accounts for 20% of the total variation in AvgFEV1CF% and 28% of the total variation in EstFEV1%@20yrs.
TABLE 2.
THE MAGNITUDE AND SIGNIFICANCE OF FACTORS INFLUENCING LONGITUDINAL MEASURES OF LUNG FUNCTION
| Coefficient* | 95% CI† | p Value‡ | ||||
|---|---|---|---|---|---|---|
| EstFEV1%@20yrs | ||||||
| AvgBMIZ | 15.6 ± 1.6 | 12.5 to 18.7 | < 0.001 | |||
| ΔF508 | −3.4 ± 2.2 | −7.7 to 1.0 | 0.132 | |||
| Pancreatic status | 11.2 ± 5.8 | −0.2 to 22.7 | 0.053 | |||
| Max. test age | 0.2 ± 0.3 | −0.4 to 0.7 | 0.550 | |||
| Constant | 90.7 ± 6.0 | 78.8 to 102.6 | < 0.001 | |||
| Best-fit model: EstFEV1% @20yrs = 88.4 + 13.8 (if pancreatic sufficient) + [15.46 × AvgBMIZ] | ||||||
| AvgFEV1CF% | ||||||
| AvgBMIZ | 0.126 ± 0.013 | 0.100 to 0.152 | < 0.001 | |||
| ΔF508 | 0.001 ± 0.019 | −0.036 to 0.038 | 0.972 | |||
| Pancreatic status | 0.034 ± 0.051 | −0.067 to 0.134 | 0.508 | |||
| Max. test age | 0.004 ± 0.002 | −0.000 to 0.008 | 0.074 | |||
| Constant | 0.555 ± 0.048 | 0.460 to 0.649 | < 0.001 | |||
| Best-fit model: AvgFEV1CF% = 0.626 + [0.124 × AvgBMIZ] | ||||||
Definition of abbreviations: AvgBMIZ = average of all body mass index z-scores; AvgFEV1CF% = average cystic fibrosis–specific percentile for FEV1; CI = confidence interval; EstFEV1%@20yrs = estimated FEV1%pred at 20 years.
Coefficient is the magnitude of the effect of each factor derived from multivariate analysis. Standard deviation for each coefficient is shown.
The 95% confidence interval for each coefficient.
p value refers to significance of the coefficient.
Estimation of Genetic Effect
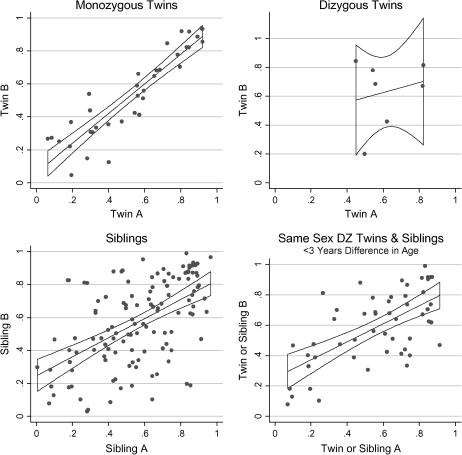
The intrapair correlations of the longitudinal measure of CF-specific lung function AvgFEV1CF% for MZ twins, DZ twins, siblings, and the combined group are shown in Figure 3. Similar trends were observed for the cross-sectional measure MaxFEV1CF% and the other longitudinal measure of EstFEV1%@20yrs (Table 3). The combined group of same-sex DZ twins and same-sex siblings within 3 years of age had similar or higher correlation than the entire group of siblings for each measurement (Table 3). To assess the effect of age on intrapair similarity, we calculated correlations for MZ twin and combined DZ twin/sibling pairs who were both younger than 15 years and for pairs who were both older than 15 years. Intrapair similarity for the younger and the older pairs did not differ significantly (see Table E1 in the online supplement). The high correlation among MZ twin pairs (∼ 100% gene sharing) compared with DZ twin pairs and sibling pairs (∼ 50% gene sharing) indicates strong genetic contribution to variation in each measure of lung function (Table 4). Estimates of heritability for longitudinal measures increased after adjusting for their significant covariates. Using the same techniques described above, correlations were calculated for twins and siblings homozygous for the common CFTR mutation ΔF508 (see Table E2). Twins and siblings homozygous for ΔF508 demonstrate strong genetic control of variation in lung disease (Table 4). Heritability estimates from siblings using variance components methods also demonstrate substantial genetic contribution to variation in lung function (Table 4). Estimates obtained from the sibling analysis were generally equal to or higher than those obtained by comparing MZ twins to DZ twins and siblings.
Figure 3.
Correlation of the longitudinal measure of CF-specific lung function in twins and siblings. For each plot, the x axis represents the AvgFEV1CF% for twin or sibling A and the y axis represents the AvgFEV1CF% for twin or sibling B. The points on each graph represent one twin pair or one sibling pair. The best-fit lines with 95% confidence intervals are shown. DZ = dizygous.
TABLE 3.
INTRAPAIR CORRELATIONS FOR CROSS-SECTIONAL AND LONGITUDINAL MEASURES OF CF LUNG DISEASE SEVERITY IN TWINS AND SIBLINGS LIVING TOGETHER
| MaxFEV1CF% (n) | EstFEV1%@20yrs* (n) | Adjusted† EstFEV1%@20yrs (n) | AvgFEV1CF%‡ (n) | Adjusted§ AvgFEV1CF% (n) | |
|---|---|---|---|---|---|
| MZ twins | 0.88 ± 0.08‖ (38) | 0.81 ± 0.01‖ (34) | 0.80 ± 0.01‖ (34) | 0.91 ± 0.01‖ (36) | 0.93 ± 0.00‖ (32) |
| DZ twins | 0.58 ± 0.08 (8) | 0.16 ± 0.11 (7) | 0.49 ± 0.09 (7) | 0.30 ± 0.12 (7) | 0.65 ± 0.07 (7) |
| Siblings | 0.36 ± 0.00‖ (184) | 0.41 ± 0.01¶ (90) | 0.43 ± 0.01‖ (87) | 0.55 ± 0.00‖ (124) | 0.49 ± 0.00‖ (117) |
| Same-sex Siblings, < 3 yr difference in age | 0.53 ± 0.01‖ (61) | 0.51 ± 0.03** (37) | 0.36 ± 0.03 (37) | 0.65 ± 0.01‖ (47) | 0.54 ± 0.02** (45) |
| Same-sex DZ twins and siblings, < 3 yr difference in age | 0.54 ± 0.03‖ (67) | 0.50 ± 0.02¶ (42) | 0.40 ± 0.03†† (42) | 0.64 ± 0.07‖ (52) | 0.58 ± 0.06‖ (50) |
Definition of abbreviations: AvgBMIZ = average of all body mass index z-scores; AvgFEV1CF% = average cystic fibrosis–specific percentile for FEV1; CI = confidence interval; DZ = dizygous; EstFEV1%@20yrs = estimated FEV1%pred at 20 years; MaxFEV1CF% = best cystic fibrosis–specific percentile for FEV1 within the last year of available data; MZ = monozygous.
Using minimum of 5 years of PFT data.
EstFEV1%@20yrs adjusted for AvgBMIZ and for pancreatic status.
Using minimum of 4 years of PFT data.
AvgFEV1CF% adjusted for AvgBMIZ.
p value < 0.0001.
p value < 0.001.
p value < 0.005.
p value < 0.01.
TABLE 4.
HERITABILITY ESTIMATES FOR CROSS-SECTIONAL AND LONGITUDINAL MEASURES OF LUNG FUNCTION
| Twins and Siblings*
|
All Siblings† (SE)
|
|||
|---|---|---|---|---|
| All Subjects | ΔF508 Homozygotes | All Subjects | ΔF508 Homozygotes | |
| MaxFEV1CF% | 0.68 | 0.86 | 0.68 (0.14)‡ | 0.54 (0.22)§ |
| AvgFEV1CF% | 0.54 | 0.80 | 1.00 (no SE)‡ | 0.96 (0.19)‡ |
| AdjAvgFEV1CF% | 0.70 | 0.78 | 0.96 (0.14)‡ | 0.89 (0.17)‡ |
| EstFEV1%@20yrs | 0.62 | 0.56 | 0.73 (0.19)§ | 0.65 (0.28) |
| AdjEstFEV1%@20yrs | 0.82 | 0.76 | 0.83 (0.20)§ | 1.00 (no SE)‡ |
Definition of abbreviations: AdjAvgFEV1CF% = adjusted average cystic fibrosis–specific percentile for FEV1; AdjEstFEV1%@20yrs = adjusted estimated FEV1%pred at 20 years; AvgFEV1CF% = average cystic fibrosis–specific percentile for FEV1; EstFEV1%@20yrs = estimated FEV1%pred at 20 years; MaxFEV1CF% = best cystic fibrosis–specific percentile for FEV1 within the last year of available data.
Heritability estimated by multiplying by 2 the correlation in MZ twins minus the correlation in combined same-sex DZ twin and same-sex siblings with < 3 years' difference in age (30)
Heritability estimated by dividing additive trait variance among related siblings by total trait variance for the entire group of siblings using maximum likelihood estimates as implemented in Sequential Oligogenic Linkage Analysis Routines (SOLAR) (31). Standard errors for each estimate are shown in parentheses.
p value < 0.0001.
p value < 0.001.
DISCUSSION
Identifying the underlying causes of variation in lung disease severity is a major goal of CF research. The discovery of the CFTR gene and characterization of its mutant alleles revealed that pancreatic status and, to some degree, sweat gland dysfunction are sensitive to variability in CFTR function (2, 35). However, CFTR genotype correlates poorly with pulmonary phenotype (36). Realization of the latter combined with the challenge posed by CFTR replacement therapy has intensified study of the mechanisms responsible for progression of obstructive airway disease, the primary cause of morbidity and mortality in patients with CF. Affected twins and siblings demonstrate that genetic control of both cross-sectional and longitudinal measures of lung function is substantial. The results of this study validate searches for CF modifier genes and, more importantly, provide a basis to quantify the contribution of identified modifiers to the heritable fraction of variation in pulmonary function. This discovery should lead to new insights into the pathophysiology of CF lung disease, and ultimately to development of new CF therapies.
The related patients with CF in this study are representative of the wide spectrum of disease severity observed in the entire CF population. However, the CF twins and siblings have better lung function than the measures reported to the CF Foundation patient registry by CF care centers in the United States (32). We used the best quarterly FEV1 measures to minimize variability in FEV1 measures due to intercurrent illnesses, insufficient patient effort, or inherent test variability. Although CF centers typically report the best FEV1, we cannot verify that the CF Foundation patient registry represents only optimum PFT measures. The bias toward milder lung disease could also be a consequence of recruiting only patients with CF who have at least one surviving sibling, thereby excluding siblings of deceased patients who potentially have more severe disease and the severely affected offspring whose parents elected to forego additional childbearing. The genetic contribution to early and severe lung disease is unknown. For many conditions, early-onset, severe disease usually has a higher likelihood of significant genetic effect (37). Absence of some sibling pairs with severe disease might have reduced estimates of genetic effect. On the other hand, the estimates of genetic effect presented here could be inflated. First, this study had an insufficient number of DZ twins from which to derive meaningful estimates of intrapair similarity. For this reason, we combined the DZ twins with siblings to achieve robust correlation coefficients. However, unlike DZ twins, siblings do not share an in utero environment nor does their home environment exactly match that of their sibling during critical periods of lung development. Using siblings as a proxy for DZ twins may have substantially lowered correlations from “actual” levels among those sharing 50% of their genes, thereby inflating heritability estimates. Second, it is plausible that MZ twins have higher levels of shared environment than DZ twins or siblings by virtue of their “identical” status (38). Although experimental evidence from behavioral studies counters this argument (39, 40), we did not test for differences in shared environment among twin pairs. Finally, error in estimating heritability from twins and siblings can arise from differences in the distribution of phenotypes among the groups of related patients (41). To minimize this source of inaccuracy, heritability was estimated only for lung function measures that did not differ significantly (p > 0.2) in means and variances between the MZ and combined DZ twin/sibling groups.
Correlation of all measures of lung function for MZ twins were high but were not 100%, suggesting a role for environmental and/or stochastic factors in CF lung disease variation. To minimize difference in environmental factors among twins and siblings, we analyzed lung function data collected while study subjects were living at home with their affected twin or sibling. Shared home environment is likely to control for significant environmental covariates, such as socioeconomic status (8, 9), ambient air pollution (42), and tobacco smoke (5). However, the increase in correlation coefficients in siblings selected for same sex and similarity in age suggests that there are additional sources of variation, even in a shared home environment. Future goals for this project will be to investigate the contribution of unique environmental factors, such as infection history, compliance with treatment regimens, and tobacco use to variation in CF lung disease.
The two predictive models for lung disease progression used in this study were derived from different CF populations, yet had similar predictive power and were highly correlated. Bland-Altman analysis of agreement between the two measures, however, indicates wide variation between the two methods of defining lung disease severity. This fact may be explained partially by the populations from which the two models were derived. EstFEV1%@20yrs was based on lung function data from 188 ΔF508 homozygotes born after 1965 and monitored at a single center (22). In contrast, FEV1CF% values were based on more than 25,000 patients with a variety of CFTR genotypes monitored from 1994 to 2001 at centers throughout the United States (23). Although EstFEV1%@20yrs was derived from ΔF508 patients only, CFTR genotype was not a significant covariate for this measure in our subjects. This finding is likely explained by the significance of pancreatic status to the EstFEV1%@20yrs model and the observation that CFTR genotype is highly correlated with pancreatic function (2). The similarity of these longitudinal prediction models may be explained by relative homogeneity in patterns of disease progression in CF. Indeed, different samples of the CF population have reported similar annual rates of decline in FEV1%pred, with mean values ranging from −1.5 to −3.6 (7, 14, 43–46). The results presented here indicate that AvgFEV1CF% or EstFEV1%@20yrs corrected for pancreatic status can be used to test genes that are candidate modifiers of CF lung disease.
Nutritional status has been shown to be associated with severity of lung disease in CF, but the exact nature of the relationship between nutritional status and lung function is unknown (10, 18, 33, 34). Evidence of genetic influence on both traits was reported by the European CF Twin and Sibling Study when the investigators noted that concordance for a composite cross-sectional measure of lung function and nutritional status was higher in 29 MZ twin pairs than in 12 DZ twin pairs (p < 0.04) (47). However, concordance rates did not differ when lung function and nutritional status were considered independently (47). Furthermore, genetic effect on longitudinal measures was not evaluated (47). Recently, Drumm and colleagues associated alleles of TGF-β1 with lung disease severity in patients with CF (12). The dichotomization strategy used to group patients by lung function measures also segregated patients by nutritional status (12). The Drumm and colleagues' study did not discern whether TGF-β1 alleles were associated with severity in lung disease, malnutrition, or both. Regression analysis was used here to quantify the interrelatedness of lung function and nutritional status. Longitudinal lung function measures adjusted for variation in nutritional status were less similar in pairs of DZ twins and siblings than unadjusted measures. On the other hand, pairs of MZ twins were very similar for unadjusted and adjusted measures. Thus, differences in nutritional status caused a fraction of the pairs of DZ twins and siblings to appear to have similar lung function, whereas nearly all of the MZ twins were similar in both respects. The disparity between the MZ and DZ/sibling groups indicates the presence of factors, possibly genetic, that modulate nutritional status independent of the genetic modifiers of lung function.
Studies of healthy individuals suggest that genes play a significant role in determining FEV1, even among individuals in different environments. Estimates of heritability obtained for cross-sectional FEV1 in various healthy adult populations (0.5–0.77) are comparable to our estimates from individuals with CF (0.68) (48–51). Whether the same or different genes contribute to cross-sectional measures of lung function in healthy individuals and those with a chronic and progressive obstructive disorder such as CF remains to be determined. Likewise, genes that influence lung function over time may differ from those that determine cross-sectional measures. Only one study evaluating genetic effect on longitudinal measures of lung function in healthy individuals has been published (52). The aforementioned study used FEV1 measured at two time points to derive linear rates of change and demonstrated only small genetic effect. As shown here, longitudinal measures derived from modeling disclosed strong genetic control of the progression of CF lung disease. If pulmonary response to chronic injury follows predictable genetically determined paths, then similar processes may underlie loss of lung function in the more common complex lung diseases.
Supplementary Material
Acknowledgments
The authors thank Beryl Rosenstein, M.D., for his thoughtful manuscript review; Michael Knowles, M.D., for helpful discussions; and Nulang Wang for CFTR genotyping. Special thanks to Mark D. Schluchter and to Michael Kulich for providing conversion programs for Bayes estimation of FEV1 at age 20 and for CF-specific percentiles for FEV1, respectively. The authors are indebted to the patients with CF and their families, research coordinators, and CF physicians who are participating in the CF Twin and Sibling Study. Without their commitment to CF research, this work would not have been possible.
The following individuals and Centers are participating in the CF Twin and Sibling Study:Frank Accurso, M.D., Shelley Mann, R.N., Advocate Hope Children's Hospital, Oak Lawn, IL; Steve Boas, M.D., Melinda Brechinella, R.N., Chicago CF Care Specialists, Glenview, IL; Titus Sheers, M.D., Deborah Ouellette, R.N., Children's Hospital and Medical Center of Akron, Akron, OH; James Acton, M.D., Patricia Joseph, M.D., Lorrie Duan, R.N., Children's Hospital and Medical Center of Cincinnati, Cincinnati, OH; Ron Gibson, M.D., Moira Aitken, M.D., Alan Genatossio, R.N., Children's Hospital and Medical Center, Seattle, WA; Joseph Cronin, M.D., Jeanne Smith, R.N., Children's Hospital of Buffalo, Buffalo, NY; Frank Accurso, M.D., Shelley Mann, R.N., Children's Hospital of Denver, Denver, CO; Arnold Platzker, M.D., Evelyn Hsu, R.N., Children's Hospital of Los Angeles, Los Angeles, CA; Karen Hardy, M.D., Deborah Kaley, N.P., Children's Hospital of Oakland, Oakland, CA; David Hicks, M.D., Bruce Nickerson, M.D., Candace Ramos, R.T., Children's Hospital of Orange County, Orange, CA; Ron Rubenstein, M.D., Maria Skotleski, B.S., Children's Hospital of Philadelphia, Philadelphia, PA; David Orenstein, M.D., Sandy Hurban, R.N., Elizabeth Hartigan, C.R.N.P., Children's Hospital of Pittsburgh, Pittsburgh, PA; William Gershan, M.D., Mary Ellen Freeman, C.R.N.P., Tami Miller, R.N., Children's Hospital of Wisconsin, Milwaukee, WI; Susanna McColley, M.D., Cathy Powers, R.D., Children's Memorial Medical Center, Chicago, IL; Peter Black, M.D., Lora Bear, C.R.N.P., Children's Mercy Hospital, Kansas City, MO; Karen McCoy, M.D., Terri Johnson, R.N., Columbus Children's Hospital, Columbus, OH; Craig Lapin, M.D., Connecticut Children's Medical Center, Hartford, CT; James Cunningham, M.D., Sara Scott, M.A., Cook Children's Medical Center, Fort Worth, TX; William Boyle, Jr., M.D., H. Worth Parker, M.D., Dennis Stokes, M.D., Dartmouth-Hitchcock Medical Center, Lebanon, NH; Thomas Lever, M.D., Rebecca Welch, R.N., Eastern Maine Medical Center, Bangor, ME; Arlene Stencenko, M.D., Carrie Cutchins, R.N., Emory University School of Medicine, Atlanta, GA; Tom Lahiri, M.D., Jackie Swartz, R.N., Fletcher Allen Health Care, Burlington, VT; Peter Scott, M.D., Barbara Crews, C.R.N.P., Georgia Pediatric Pulmonology Associates, Atlanta, GA; Jerrold Eichner, M.D., Laura Hodges, Great Falls Clinic, Great Falls, MT; Mary Passero, M.D., Mike Schechter, M.D., Hasbro Children's Hospital, Providence, RI; W. Stuart Warren, M.D., Melissa McClure, R.N., Hershey Medical Center, Hershey, PA; Morton Schwartzman, M.D., Joe DiMaggio Pulmonary Center, Hollywood, FL; Pamela Zeitlin, M.D., Peter Mogayzel, M.D., Michael Boyle, M.D., Johns Hopkins Hospital, Baltimore, MD; Greg Shay, M.D., Mary Seastrand, R.N., Kaiser Permanente Medical Center, Oakland, CA; Richard Cohen, M.D., Megan Danish, C.R.N.P., Kaiser-Permanente, Portland, OR; Allan Lieberthal, M.D., Joan Franco, R.N., Kaiser-Permanente, Panorama City, CA; Hank Dorkin, M.D., Monica Ulles, C.R.N.P., Massachusetts General Hospital, Boston, MA; Margaret Guill, M.D., Kathy Dyer, R.N., Medical College of Georgia, Augusta, GA; Patrick Flume, M.D., Susan Gray, R.N., Medical University of South Carolina, Charleston, SC; William Richards, M.D., Memphis Lung Physicians, South Haven, MS; Martha Morse, M.D., Julie Garcia, C.R.N.P., Methodist Children's Hospital, San Antonio, TX; Katharine Hale, M.D., Methodist Hospital, Houston, TX; Richard Honicky, M.D., Kathleen King, C.R.N.P., Michigan State University, Lansing, MI; Robert Zanni, M.D., Bridget Marra, R.N., Monmouth Medical Center, Long Branch, NJ; Stanley Fiel, M.D., Paula Lomas, R.N., Morristown Memorial Hospital, Morristown, NJ; Kathryn Moffett, M.D., Linda Baer, R.N., Mountain State CF Center, Morgantown, WV; Col. Joel Schmidt, M.D., Monica Leupold, R.N., National Naval Medical Center, Bethesda, MD; Capt. Henry Wojtczak, M.D., Naval Medical Center San Diego, San Diego, CA; Diana Lowenthal, M.D., Ingrid Gerson, B.S., Madelint Heydendael, R.N., New York Medical College, Valhalla, NY; James Royall, M.D., Oklahoma Health Science Center, Oklahoma City, OK; Chris Landon, M.D., Ann Thompson, R.N., Pediatric Diagnostic Center, Ventura, CA; Roxanne Marcille, M.D., Dan Brown, M.D., Carolyn Turner, R.N., Pediatric Pulmonary Associates, Columbia, SC; Cdr. Reese Lee, M.D., Fletcher Pierce, M.D., Portsmouth Naval Medical Center, Portsmouth, VA; Michael Konstan, M.D., Colette Bucur, R.N., Rainbow Babies and Children's Hospital, Cleveland, OH; Michelle Howenstine, M.D., Mary Blagburn, R.N., Delana Terrill, R.R.T., Riley Children's Hospital, Indianapolis, IN; Anne Devenny, M.B.Ch., Royal Hospital for Sick Children, Yorkhill, Scotland; Lucille Lester, M.D., Rush Presbyterian St. Luke's Medical Center, Chicago, IL; Maria Berdella, M.D., Patricia Walker, M.D., Elinor Langfelder Schwind, M.S., St. Vincent's Hospital and Medical Center, New York, NY; Joan DeCelie-Germana, M.D., Susan Galvin, R.N., Schneider Children's Hospital, New Hyde Park, NY; Louay Nassri, M.D., Janet Austin, R.N., Sparks Regional Medical Center, Fort Smith, AK; Suzanne Beck, M.D., Laurie Varlotta, M.D., Joanne Gambo, C.R.N.P., St. Christopher's Hospital for Children, Philadelphia, PA; Sue Goldfinger, M.D., Lisa Middleton, R.N., St. Mary's Medical Center, West Palm Beach, FL; Thomas Ferkol, M.D., Daniel Rosenbluth, M.D., Mary Boyle, R.N., St. Louis Children's Hospital and the Barnes Jewish Hospital, St. Louis, MO; Peter Holzwarth, M.D., Sumedha Ghate, M.S., St. Vincent Medical Center, Green Bay, WI; Richard Moss, M.D., Zoe Davies, C.R.N.P., Colleen Dunne, R.N., Stanford University Medical Center, Palo Alto, CA; Ran Anbar, M.D., Donna Lindner, R.N., SUNY Pediatric Pulmonary and CF Center, Syracuse, NY; Bradley Chipps, M.D., Kasey Pearson, C.R.N.P., Sutter Medical Center, Sacramento, CA; Joel Ledbetter, M.D., Karen Saranosky, N.P., TC Thompson Children's Hospital, Chattanooga, TN; David Waltz, M.D., Meredith Little, B.S., The Children's Hospital of Boston, Boston, MA; Pierre Vauthy, M.D., Mary Vauthy, N.P., Toledo Children's Hospital, Toledo, OH; Scott Bell, M.D., The Prince Charles Hospital, Brisbane, Queensland, Australia; Raymond Lyrene, M.D., Hector Guitierrez, M.D., Valerie Tarn, R.N., University of Alabama CF Center, Birmingham, AL; Wayne Morgan, M.D., Joanne Douthit, R.N., University of Arizona, Tucson, AZ; Dennis Neilson, M.D., Ph.D., University of California at San Francisco, San Francisco, CA; Terry Spencer, M.D., Lisa Magary, R.N., University of Florida, Gainsville, FL; Richard Ahrens, M.D., Mary Teresi, Pharm.D., University of Iowa Hospitals and Clinics, Iowa City, IA; Gaylin Perry, M.D., Adrienne Liebergen, M.N., A.R.N.P., University of Kansas Medical Center, Kansas City, KS; Jamshed Kanga, M.D., University of Kentucky, Lexington, KY; Brian O'Sullivan, M.D., Rhona Spaulding, C.R.N.P., University of Massachusetts Medical School, Worcester, MA; Samya Nasr, M.D., Dawn Kruse, University of Michigan, Ann Arbor, MI; Carlos Milla, M.D., Brooke Noren, R.N., University of Minnesota, Minneapolis, MN; Lynn Walker, M.D., Kim Adcock, Pharm.D., University of Mississippi Medical Center, Jackson, MS; John Colombo, M.D., Peter Murphy, M.D., Dee Acquazino, R.N., University of Nebraska, Omaha, NE; Elizabeth Perkett, M.D., Kyria Ramey, C.R.N.P., University of New Mexico Health Sciences Center, Albuquerque, NM; George Retsch-Bogart, M.D., Michael Knowles, M.D., Sally Wood, B.S., University of North Carolina at Chapel Hill, Chapel Hill, NC; David Lipson, M.D., Marianne Ferrin, C.R.N.P., University of Pennsylvania Adult CF Center, Philadelphia, PA; Richard Boswell, M.D., University of Tennessee, Memphis, TN; Rodolfo Amaro-Galvez, M.D., Bettina Hillman, M.D., University of Texas Health Center, Tyler, TX; Barbara Chatfield, M.D., Sue Griffiths, R.N., University of Utah, Salt Lake City, UT; Deborah Froh, M.D., Lauren Ahrens, R.N., University of Virginia Health System, Charlottesville, VA; Michael Rock, M.D., Linda Makholm, M.T., University of Wisconsin Hospital, Madison, WI; Tom Hazinski, M.D., Stephanie Rushing, R.N., Vanderbilt University Medical Center, Nashville, TN; Bruce Rubin, M.D., Wake Forest University, Baptist Medical Center, Winston-Salem, NC; Hugh Black, M.D., William Ashe, M.D., Western Carolina CF Center, Charlotte, NC; Maria Riva, M.D., David Doornbos, M.D., Janet Messamore, R.N., Wichita Clinic, PA, Wichita, KS; Andrew Lipton, M.D., Wilford Hall Medical Center, San Antonio, TX; Marie Egan, M.D., Tina Tolomeo, R.N., Yale University, New Haven, CT.
Supported by NHLBI grant HL68927 and CF Foundation funding (CUTTIN06PO).
The views expressed in this article are those of the authors and do not represent the official policy of the Department of Defense or other Departments of the U.S. Government. L.L.V. is a military service member. This work was prepared as part of her official duties.
This article has an online supplement, which is accessible from this issue's table of contents at www.thoracic.org
Originally Published in Press as DOI: 10.1164/rccm.200608-1164OC on March 1, 2007
Conflict of Interest Statement: L.L.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.M.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.H.-F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.R.C. serves as a consultant to Roche Molecular Systems and receives less than $1,000 per year from 1992 to present.
References
- 1.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073–1080. [DOI] [PubMed] [Google Scholar]
- 2.Kerem E, Corey M, Kerem B-S, Rommens J, Markiewicz D, Levison H, Tsui LC, Durie P. The relation between genotype and phenotype in cystic fibrosis–analysis of the most common mutation (deltaF508). N Engl J Med 1990;323:1517–1522. [DOI] [PubMed] [Google Scholar]
- 3.The Cystic Fibrosis Genotype-Phenotype Consortium. Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med 1993;329:1308–1313. [DOI] [PubMed] [Google Scholar]
- 4.Koch C, Cuppens H, Rainisio M, Madessani U, Harms H, Hodson M, Mastella G, Navarro J, Strandvik B, McKenzie S. European Epidemiologic Registry of Cystic Fibrosis (ERCF): comparison of major disease manifestations between patients with different classes of mutations. Pediatr Pulmonol 2001;31:1–12. [DOI] [PubMed] [Google Scholar]
- 5.Rubin BK. Exposure of children with cystic fibrosis to environmental tobacco smoke. N Engl J Med 1990;323:782–788. [DOI] [PubMed] [Google Scholar]
- 6.Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol 1996;143:1007–1017. [DOI] [PubMed] [Google Scholar]
- 7.Kerem E, Corey M, Gold R, Levison H. Pulmonary function and clinical course in patients with CF after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr 1990;116:714–719. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor GT, Quinton HB, Kneeland T, Kahn R, Lever T, Maddock J, Robichaud P, Detzer M, Swartz DR. Median household income and mortality rate in cystic fibrosis. Pediatrics 2003;111:e333–e339. [DOI] [PubMed] [Google Scholar]
- 9.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med 2001;163:1331–1337. [DOI] [PubMed] [Google Scholar]
- 10.Steinkamp G, von der Hardt H. Improvement of nutritional status and lung function after long-term nocturnal gastrostomy feedings in cystic fibrosis. J Pediatr 1994;124:244–249. [DOI] [PubMed] [Google Scholar]
- 11.Cutting GR. Cystic fibrosis. Annu Rev Genomics Hum Genet 2005;6:237–260. [DOI] [PubMed] [Google Scholar]
- 12.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, Zariwala M, Fargo D, Xu A, Dunn JM, et al. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med 2005;353:1443–1453. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey BW, Boat TF, Accurso FJ, Bennett W, Boucher R, Brody A, Crystal R, Cutting GR, Davis P, Dorkin HL, et al. Outcome measures for clinical trials in cystic fibrosis: summary of Cystic Fibrosis Foundation Consensus Conference. J Pediatr 1994;124:177–192. [DOI] [PubMed] [Google Scholar]
- 14.Davis PB, Byard PJ, Konstan MW. Identifying treatments that halt progression of pulmonary disease in cystic fibrosis. Pediatr Res 1997;41:161–165. [DOI] [PubMed] [Google Scholar]
- 15.Beaty TH, Cohen BH, Newill CA, Menkes HA, Diamond EL, Chen CJ. Impaired pulmonary function as a risk factor for mortality. Am J Epidemiol 1982;116:102–113. [DOI] [PubMed] [Google Scholar]
- 16.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med 1992;326:1187–1191. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld M, Pepe MS, Longton G, Emerson J, Fitzsimmons S, Morgan W. Effect of choice of reference equation on analysis of pulmonary function in cystic fibrosis patients. Pediatr Pulmonol 2001;31:227–237. [DOI] [PubMed] [Google Scholar]
- 18.Zemel BS, Jawad AF, Fitzsimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the cystic fibrosis foundation national CF patient registry. J Pediatr 2000;137:374–380. [DOI] [PubMed] [Google Scholar]
- 19.Aswani N, Taylor CJ, McGaw J, Pickering M, Rigby AS. Pubertal growth and development in cystic fibrosis: a retrospective review. Acta Paediatr 2003;92:1029–1032. [PubMed] [Google Scholar]
- 20.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–968. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 1991;144:1202–1218. [DOI] [PubMed] [Google Scholar]
- 22.Schluchter MD, Konstan MW, Davis PB. Jointly modelling the relationship between survival and pulmonary function in cystic fibrosis patients. Stat Med 2002;21:1271–1287. [DOI] [PubMed] [Google Scholar]
- 23.Kulich M, Rosenfeld M, Campbell J, Kronmal R, Gibson RL, Goss CH, Ramsey B. Disease-specific reference equations for lung function in patients with cystic fibrosis. Am J Respir Crit Care Med 2005;172:885–891. [DOI] [PubMed] [Google Scholar]
- 24.Vanscoy LL, Lai T, Bowers A, Cutting GR. Modifier genes may contribute substantially to variation in cystic fibrosis (CF) lung disease estimated by CF-specific % for FEV1 [abstract]. Proc Am Thorac Soc 2006;3:A408. [Google Scholar]
- 25.Vanscoy LL, Blackman S, Collaco JM, Bowers A, Naughton K, Cutting GR. Modifier genes substantially influence variability in severity of CF lung disease, independent of CFTR genotype and variation in body mass index. Pediatr Pulmonol Suppl 2006;29:270. [Google Scholar]
- 26.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. J Pediatr 1998;132:589–595. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Myers A, Saiki RK, Cutting GR. Development and evaluation of a PCR-based, line probe assay for the detection of 58 alleles in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Clin Chem 2002;48:1121–1123. [PubMed] [Google Scholar]
- 28.Groman JD, Meyer ME, Wilmott RW, Zeitlin PL, Cutting GR. Variant cystic fibrosis phenotypes in the absence of CFTR mutations. N Engl J Med 2002;347:401–407. [DOI] [PubMed] [Google Scholar]
- 29.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis 1983;127:725–734. [DOI] [PubMed] [Google Scholar]
- 30.Falconer DS, Mackay TFC. Introduction to quantitative genetics, 4th ed. Essex, UK: Pearson Education Ltd; 1996. pp. 160–183.
- 31.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998;62:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation patient registry annual data report 2004. Bethesda MD: Cystic Fibrosis Foundation; 2004.
- 33.Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, Johnson CA, Morgan WJ. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr 2003;142:624–630. [DOI] [PubMed] [Google Scholar]
- 34.Milla CE. Association of nutritional status and pulmonary function in children with cystic fibrosis. Curr Opin Pulm Med 2004;10:505–509. [DOI] [PubMed] [Google Scholar]
- 35.Wilschanski M, Zielenski J, Markiewicz D, Taui LC, Corey M, Levison H, Durie PR. Correlation of sweat chloride concentration with classes of the cystic fibrosis transmembrane conductance regulator gene mutations. J Pediatr 1995;127:705–710. [DOI] [PubMed] [Google Scholar]
- 36.The Cystic Fibrosis Genotype–Phenotype Consortium. Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med 1993;329:1308. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez-Sanchez G, Childs B, Valle D. Human disease genes. Nature 2001;409:853–855. [DOI] [PubMed] [Google Scholar]
- 38.Kyvik KO. Generalisability and assumptions of twin studies. In: Spector TD, Snieder H, MacGregor AJ, editors. Advances in twin and sib-pair analysis. London, UK: Greenwich Medical Media Ltd; 2000. pp. 67–77.
- 39.Scarr S. Environmental bias in twin studies. Eugen Q 1968;15:34–40. [DOI] [PubMed] [Google Scholar]
- 40.Matheny AP Jr, Wilson RS, Dolan AB. Relations between twins' similarity of appearance and behavioral similarity: testing an assumption. Behav Genet 1976;6:343–351. [DOI] [PubMed] [Google Scholar]
- 41.Christian JC, Williams CJ. Comparison of analysis of variance and likelihood models of twin data analysis. In: Spector TD, Snieder H, MacGregor AJ, editors. Advances in twin and sib-pair analysis. London, UK: Greenwich Medical Media Ltd; 2000. pp. 103–118.
- 42.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med 2004;169:816–821. [DOI] [PubMed] [Google Scholar]
- 43.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med 1995;332:848–854. [DOI] [PubMed] [Google Scholar]
- 44.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr 1997;131:809–814. [DOI] [PubMed] [Google Scholar]
- 45.Eigen H, Rosenstein BJ, Fitzsimmons S, Schidlow DV, Beckerman R, Canny G, Caplan D, Fink R, Glasser L, Harley F, et al. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. J Pediatr 1995;126:515–523. [DOI] [PubMed] [Google Scholar]
- 46.Merkus PJ, Tiddens HA, de Jongste JC. Annual lung function changes in young patients with chronic lung disease. Eur Respir J 2002;19:886–891. [DOI] [PubMed] [Google Scholar]
- 47.Mekus F, Ballmann M, Bronsveld I, Bijman J, Veeze H, Tummler B. Categories of deltaF508 homozygous cystic fibrosis twin and sibling pairs with distinct phenotypic characteristics. Twin Res 2000;3:277–293. [DOI] [PubMed] [Google Scholar]
- 48.Hubert HB, Fabsitz RR, Feinleib M, Gwinn C. Genetic and environmental influences on pulmonary function in adult twins. Am Rev Respir Dis 1982;125:409–415. [DOI] [PubMed] [Google Scholar]
- 49.McClearn GE, Svartengren M, Pedersen NL, Heller DA, Plomin R. Genetic and environmental influences on pulmonary function in aging Swedish twins. J Gerontol 1994;49:264–268. [DOI] [PubMed] [Google Scholar]
- 50.Whitfield KE, Grant J, Ravich-Scherbo I, Marytuina T, Iboutolina A. Genetic and environmental influences on forced expiratory volume in midlife: a cross-cultural replication. Exp Aging Res 1999;25:255–265. [DOI] [PubMed] [Google Scholar]
- 51.Wilk JB, Djousse L, Arnett DK, Rich SS, Province MA, Hunt SC, Crapo RO, Higgins M, Myers RH. Evidence for major genes influencing pulmonary function in the NHLBI family heart study. Genet Epidemiol 2000;19:81–94. [DOI] [PubMed] [Google Scholar]
- 52.Gottlieb DJ, Wilk JB, Harmon M, Evans JC, Joost O, Levy D, O'Connor GT, Myers RH. Heritability of longitudinal change in lung function: the Framingham study. Am J Respir Crit Care Med 2001;164:1655–1659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.