Abstract
Rationale: Bronchiolitis obliterans syndrome (BOS), defined by loss of lung function, develops in the majority of lung transplant recipients. However, there is a paucity of information on the subsequent course of lung function in these patients.
Objectives: To characterize the course of FEV1 over time after development of BOS and to determine the predictors that influence the rate of functional decline of FEV1.
Methods: FEV1% predicted (FEV1%pred) trajectories were studied in 111 lung transplant recipients with BOS by multivariate, linear, mixed-effects statistical models.
Measurements and Main Results: FEV1%pred varied over time after BOS onset, with the steepest decline typically seen in the first 6 months (12% decline; p < 0.0001). Bilateral lung transplant recipients had significantly higher FEV1%pred at BOS diagnosis (71 vs. 47%; p < 0.0001) and at 24 months after BOS onset (58 vs. 41%; p = 0.0001). Female gender and pretransplant diagnosis of idiopathic pulmonary fibrosis were associated with a steeper decline in FEV1%pred in the first 6 months after BOS diagnosis (p = 0.02 and 0.04, respectively). A fall in FEV1 greater than 20% in the 6 months preceding BOS (termed “rapid onset”) was associated with shorter time to BOS onset (p = 0.01), lower FEV1%pred at BOS onset (p < 0.0001), steeper decline in the first 6 months (p = 0.03), and lower FEV1%pred at 2 years after onset (p = 0.0002).
Conclusions: Rapid onset of BOS, female gender, pretransplant diagnosis of idiopathic pulmonary fibrosis, and single-lung transplantation are associated with worse pulmonary function after BOS onset.
Keywords: bronchiolitis obliterans syndrome, FEV1, pulmonary function, prognosis
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The effect of various clinical variables on the course of FEV1 after bronchiolitis obliterans syndrome onset in lung transplant recipients remains to be completely determined.
What This Study Adds to the Field
Rapid onset of BOS, female gender, pretransplant diagnosis of idiopathic pulmonary fibrosis, and single-lung transplantation are associated with worse pulmonary function after BOS onset.
Lung transplantation is the only viable option for many patients with chronic, end-stage lung diseases. However, 60% of patients within 5 years of transplant develop bronchiolitis obliterans syndrome (BOS) (1–4), a disease syndrome defined as a fall in FEV1 of greater than 20% from baseline determined by the average of two measurements made at least 3 weeks apart (5). Median survival after onset of BOS is 3 to 4 years, with a range of 0 to 9.4 years (6–8). These survival numbers are comparable to other chronic lung diseases, such as idiopathic pulmonary fibrosis (IPF). Similarly, although development of BOS is associated with progressive worsening of lung function, the rate of decline in FEV1 after onset of BOS is believed to be variable among patients and over time (9). Although significant effort has been made to understand factors associated with the risk of development of BOS, little information has been published regarding the course of FEV1 after onset of BOS and the effect of various variables on it. This information is important for patient counseling and management, but is also crucial for designing diagnostic and therapeutic clinical trials in the field of BOS.
The objectives of this study were to characterize the course of FEV1 over time after development of BOS, to describe the natural history of spirometric dysfunction, and to determine the predictors that influence the rate of functional decline of FEV1 in a large cohort of well-characterized lung transplant recipients.
METHODS
A total of 317 lung transplants were performed in the University of Michigan Health System between November 1990 and December 2004. All lung transplant recipients alive 3 months after transplantation with available post-transplant pulmonary measures were screened for presence of BOS according to the published guidelines (5) and as described previously (4). A total of 111 lung transplant recipients who developed BOS (BOS stages 1 or higher) comprised the study group.
The study was approved by the University of Michigan Institutional Review Board. All patients were followed by a standardized protocol, as previously described (4, 10). Pulmonary function testing was performed following standards established by the American Thoracic Society at each clinic visit (11). To study the course of FEV1 after onset of BOS, all follow-up pulmonary function tests were analyzed. Follow-up was complete until time of death or July 1, 2005. The dataset included 10,317 spirometric observations, with a mean number of 93 (± 102) observations per patient. Median follow-up time was 5.5 years (95% confidence interval, 4.3–7.1 yr), with a range of 0.1 to 11.1 years after onset of BOS.
The predictors analyzed for a potential influence on FEV1 trajectory after BOS onset included recipient age and gender, transplant date, type of transplantation (single- [SLT] vs. bilateral lung transplant [BLT]), indication for transplantation (chronic obstructive pulmonary disease [COPD] vs. IPF), rate of decline of FEV1 before BOS onset (rapid vs. gradual), time of BOS onset (early vs. late), and presence of histologic findings of acute rejection and lymphocytic bronchitis in the post-transplantation period before BOS onset. These factors were chosen on the basis of previous literature, hypothesized associations, and their potential importance in guiding pretransplant decisions, as elaborated in Discussion. In addition, each variable that was analyzed contained only information that was available at the time of BOS onset. Hence, the results will be practically useful to the clinician at the time a patient is diagnosed with BOS.
Time of BOS onset was defined as “early” if the onset of BOS occurred within 2 years of transplantation; otherwise it is termed “late” (8). The BOS onset date was defined as the date of the first of the two FEV1 measurements used to establish the BOS diagnosis. FEV1 values at the BOS onset date and those collected approximately 6 months earlier were used to calculate percent change in the 6 months before BOS onset. Patients with 20% or greater change in the 6 months before BOS onset were defined as having a rapid course of BOS onset; the remaining patients were defined as experiencing a gradual onset. Histologic findings of acute rejection and lymphocytic bronchitis were classified according to the International Society of Heart and Lung Transplant grading scheme (12). Two categories of pretransplant diagnosis were evaluated: patients with underlying restrictive physiology and diagnosis of IPF, and patients with underlying chronic obstructive pulmonary disease (COPD). The small number of patients in other categories limited our ability to meaningfully evaluate the impact of those diagnoses.
Statistical Analysis
Multivariate, linear, mixed-effects statistical models (PROC MIXED, SAS version 9.1.3; SAS Institute, Cary, NC) were used to estimate FEV1%pred trajectories for each group of patients, adjusted for confounding factors (13). This method was used to estimate the mean FEV1%pred during the time from 0 to 24 months post-BOS, while allowing for correlation between measurements on a particular BOS patient. In addition, the method adjusts for the relative absence of spirometric values for those patients who die early or otherwise leave the study. This particular analysis incorporated linear spline terms to allow trajectories to change every 6 months from the time of development of BOS. Cited percent increases or decreases in FEV %pred are reported as relative changes rather than absolute changes, unless otherwise noted. In addition, average times to BOS were compared across groups using the two-sample t test.
RESULTS
Characteristics of the Study Cohort
The 111 lung transplant recipients with BOS included 49 males and 62 females, with a mean age at the time of transplantation of 52 years (range, 19–64 yr). The cohort included 91 SLT and 20 BLT patients. Primary indications for transplantation in the SLT cohort included emphysema (n = 76), IPF (n = 12), and primary pulmonary hypertension (n = 3). Indications for BLT included emphysema (n = 14), IPF (n = 2), primary pulmonary hypertension (n = 1), and cystic fibrosis (n = 3).
Average Course of FEV1 over Time after BOS Onset
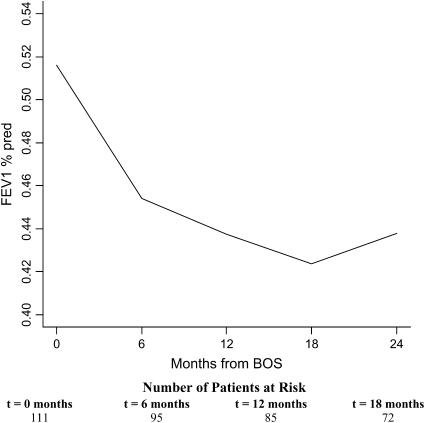
The course of FEV1%pred was evaluated for changes in trajectories at 6, 12, and 18 months after BOS onset. Figure 1 demonstrates the FEV1%pred for the average patient profile adjusted for age, gender, pretransplant diagnosis, time-to-BOS, type of transplant, transplant date, and 6-month pre-BOS relative change in FEV1 (rapid vs. gradual onset of BOS). The rate of decline of FEV1%pred (trajectory) changed significantly during the first 2 years after BOS onset (p < 0.0001). The steepest decline in FEV1%pred was seen in the first 6 months and was highly statistically significant (12% decline; p < 0.0001). This was followed by continuing significant decline between 6 and 12 months (4% decline; p = 0.01), a decrease in the rate of decline of FEV1%pred between 12 and 18 months (3% decline; p = 0.17), and stabilization of FEV1%pred from 18 to 24 months (3% increase; p = 0.23) (Figure 1).
Figure 1.
Course of FEV1% predicted (FEV1%pred) after bronchiolitis obliterans syndrome (BOS) onset in lung transplant recipients. FEV1%pred for the average patient profile adjusted for age, pretransplant diagnosis, time-to-BOS, type of transplant, transplant date, and 6-month pre-BOS relative change in FEV1 (rapid vs. gradual onset of BOS) is shown. Numbers of patients at the onset of each time interval are displayed at the bottom. The rate of decline of FEV1%pred changed significantly during the first 2 years after BOS onset (p < 0.0001). The steepest decline was seen in the first 6 months (12% decline; p < 0.0001). The decline between 6 and 12 months was also significant (4% decline; p = 0.01).
Effect of Gender on the Course of FEV1 after BOS Onset
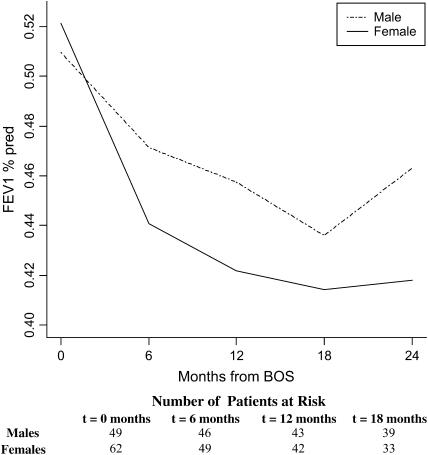
Time from transplant to BOS onset was similar in males (889 d) versus females (891 d; p = 0.99), and no significant difference was observed in FEV1%pred at BOS onset (p = 0.62). However, subsequent trajectories for FEV1%pred differed by gender after adjusting for age, pretransplant diagnosis, time-to-BOS, type of transplant, transplant date, and 6-month pre-BOS relative change in FEV1 (rapid vs. gradual onset of BOS) (Figure 2). In particular, the 0–6 months declining trajectory for FEV1%pred was significantly less steep for male versus female patients (8 vs. 15% decrease for males vs. females, p = 0.02). Gender-specific trajectories between 6 and 24 months were similar (p = 0.76), and differences in FEV1%pred at 24 months did not reach statistical significance (p = 0.13), although, at this time, FEV1%pred was 11% higher in males than in females.
Figure 2.
Gender-specific course of FEV1%pred after BOS onset in lung transplant recipients. Trajectories for FEV1%pred after BOS onset for male and female patients are shown, adjusted for age, pretransplant diagnosis, time-to-BOS, type of transplant, transplant date, and 6-month pre-BOS relative change in FEV1 (rapid vs. gradual onset of BOS). The declining trajectory between 0 and 6 months was significantly less steep for male versus female patients (p = 0.02). This difference remained significant after adjusting for the FEV1 value at BOS onset (p = 0.046). Numbers of male and female patients at the onset of each time interval are displayed at the bottom.
Effect of Pretransplant Diagnosis on the Course of FEV1 after BOS Onset
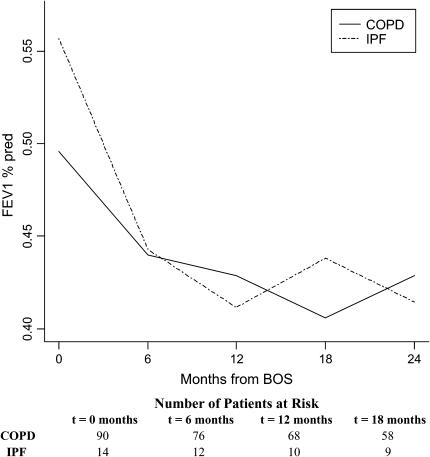
Average time from transplantation to development of BOS was 910 days in the patients with COPD (n = 90) and 737 days in the IPF cohort (n = 14) (p = 0.35). No statistically significant difference was noted in the FEV1%pred at BOS onset in the two groups (p = 0.09) (Figure 3). However, a steeper decline of FEV1%pred was seen during the first 6 months after BOS onset for recipients with IPF compared with recipients with COPD, which was statistically significant when adjusted for age, gender, time-to-BOS, type of transplant, and 6-month pre-BOS relative change in FEV1 (p = 0.04). Observed differences in the shape of the FEV1%pred trajectories after the first 6 months were not statistically significant (p = 0.41).
Figure 3.
Course of FEV1%pred after BOS onset in lung transplant recipients by pretransplant diagnosis. Trajectories for FEV1%pred after BOS onset for patients with pretransplant diagnosis of idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD) are shown, adjusted for age, gender, time-to-BOS, type of transplant, transplant date, and 6-month pre-BOS relative change in FEV1 (rapid vs. gradual onset of BOS). The steeper decline during the 6 months after BOS onset for recipients with IPF compared with those with COPD was statistically significant when adjusted for age, gender, time-to-BOS, type of transplant, transplant date, and 6-month pre-BOS relative change in FEV1 (p = 0.04). Numbers of patients with COPD and IPF at the onset of each time interval are displayed at the bottom.
Effect of Type of Transplant on the Course of FEV1 after Onset of BOS
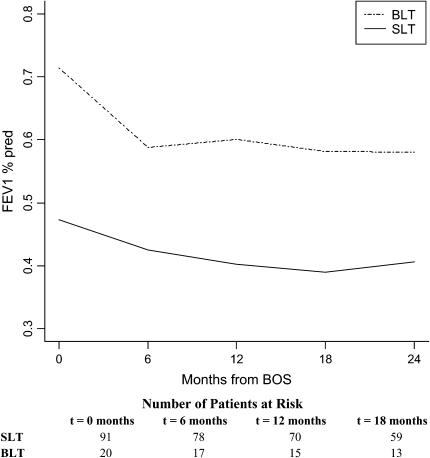
There was a nonsignificant trend toward longer time to development of BOS in BLT recipients (n = 20) compared with SLT recipients (n = 91) (1,178 vs. 827 d; p = 0.16). Significantly higher FEV1%pred was seen at BOS onset in BLT compared with SLT recipients (71 vs. 47%; p < 0.0001). Figure 4 shows the course of FEV1%pred after BOS onset for SLT versus BLT patients, adjusted for gender, age, diagnosis, time-to-BOS, transplant date, and 6-month pre-BOS relative change in FEV1. A significantly steeper slope seen in BLT recipients during the first 6 months (p = 0.004) vanished when adjusted for differences in FEV1%pred at BOS onset (p = 0.17), suggesting that this steeper decline in BLT patients is due more to their typically higher initial FEV1%pred value at BOS onset than to the type of transplant procedure. This is known as a “floor effect” in statistical parlance, because those with lower FEV1%pred values at onset have less opportunity to decline further. No significant difference was seen in the rate of decline of FEV1%pred after 6 months (p = 0.23). At 24 months after BOS onset, BLT recipients maintained a 42% higher FEV1%pred compared with SLT recipients (p = 0.0001). A study of the interaction between transplant type and pretransplant diagnosis echoed the aforementioned observations in the COPD population (data not shown). Small sample size limited the ability to verify the direction and magnitude of changes due to transplant type in the remaining diagnoses.
Figure 4.
Course of FEV1%pred after BOS onset in lung transplant recipients by type of transplant. Course for FEV1%pred after BOS onset for single- (SLT) and bilateral lung transplant (BLT) recipients are shown, adjusted for age, gender, time-to-BOS, pretransplant diagnosis, transplant date, and 6-month pre-BOS relative change in FEV1 (rapid vs. gradual onset of BOS). The steeper slope seen in BLT recipients during the first 6 months was not significant when adjusted for differences in FEV1%pred at BOS onset (p = 0.17). Twenty-four months after BOS onset, BLT recipients maintained a 42% higher FEV1%pred compared with SLT recipients (p = 0.0001). Numbers of SLT and BLT recipients at the onset of each time interval are displayed at the bottom.
Effect of Pre-BOS FEV1 Change and Time to BOS Onset on the Subsequent Course of FEV1
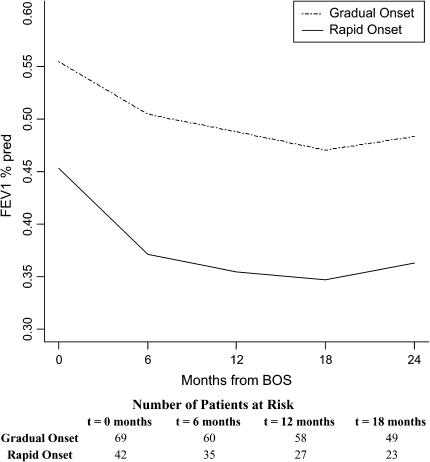
A total of 42 of 111 patients (38%) had FEV1 values decreasing 20% or more during the 6 months before BOS onset (rapid onset); the remaining 69 patients were considered gradual onset patients. Patients with rapid onset had a significantly shorter time from transplant to BOS (643 d) compared with those with gradual onset (1,010 d; p = 0.01), and had an 18% lower FEV1%pred at onset compared with gradual-onset patients (p < 0.0001) (Figure 5). Furthermore, these patients continued to exhibit a steeper decline in FEV1%pred during the first 6 months of BOS, adjusting for gender, age, type of transplant, transplant date, pretransplant diagnosis, time-to-BOS, and level of FEV1%pred at BOS onset (p = 0.03). FEV1%pred at 24 months after BOS onset was 33% higher in the patients with gradual versus rapid onset (p = 0.0002).
Figure 5.
Course of FEV1%pred after BOS onset in lung transplant recipients by pre-BOS FEV1 change (rapidity of BOS onset). FEV1 values at the BOS onset date and FEV1 values collected approximately 6 months earlier were used to calculate percent change in the 6 months before BOS onset. Patients with 20% or greater change in the 6 months before BOS onset were defined as having a rapid course of BOS onset; the remaining patients were defined as experiencing a gradual onset. Both FEV1%pred trajectories are adjusted for age, gender, pretransplant diagnosis, time-to-BOS, transplant date, and type of transplant. Patients with rapid onset had an 18% lower FEV1%pred at onset compared with gradual-onset patients (p < 0.0001). These patients continued to exhibit a steeper decline in FEV1%pred during the first 6 months of BOS after adjusting for gender, age, type of transplant, pretransplant diagnosis, time-to-BOS, transplant date, and baseline FEV1%pred at BOS onset (p = 0.03). FEV1%pred at 24 months after BOS onset was higher in the patients with gradual versus rapid onset (p = 0.0002). Numbers of patients with gradual-onset and rapid-onset BOS at the onset of each time interval are displayed at the bottom.
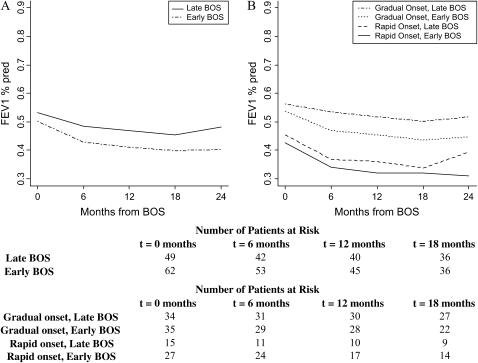
Patients were also compared on the basis of time to BOS onset. A total of 62 patients (56%) developed BOS within 2 years of transplant (early onset). Patients with early onset were observed to have lower FEV1%pred values over time (Figure 6A), although this trend did not reach statistical significance (difference in FEV1%pred at BOS onset, p = 0.22; difference in decline during Months 0–6 of BOS, p = 0.19). However, 24 months after BOS onset, patients with early onset had 16% lower FEV1%pred compared with patients with late onset (p = 0.01).
Figure 6.
(A) Course of FEV1%pred after BOS onset in lung transplant recipients by time-to-BOS. Time of BOS onset was defined as “early” if the onset of BOS occurred within 2 years of transplantation; otherwise it is termed “late.” Both FEV1%pred trajectories are adjusted for age, gender, pretransplant diagnosis, type of transplant, transplant date, and 6-month pre-BOS relative change in FEV1 (rapid vs. gradual onset of BOS). At 24 months after BOS onset, patients with early onset had a lower FEV1%pred compared with patients with late onset (p = 0.01). (B) The estimated interaction of time-to-BOS onset and the rapidity of onset (rapid vs. gradual). All trajectories are adjusted for other significant variables, including age, gender, pretransplant diagnosis, transplant date, and type of transplant. Lowest to highest post-BOS FEV1%pred patterns over time belonged to patients with early and rapid onset of BOS, followed by patients with rapid onset and late BOS, gradual onset and early BOS, and, finally, gradual onset and late BOS. Numbers of patients with late and early BOS, and those with gradual-onset (late and early) and rapid-onset (late and early) BOS at the onset of each time interval are displayed at the bottom.
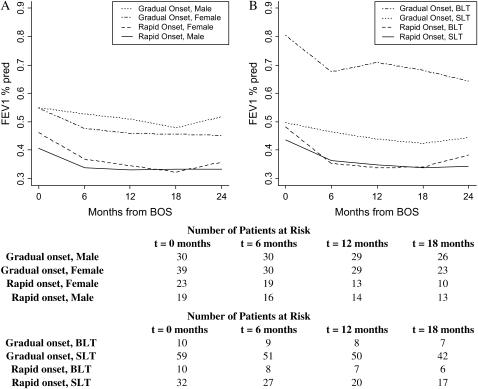
The estimated interaction of time to BOS onset and the rapidity of onset displays the relative importance of rapid onset compared with early onset in the future course of FEV1%pred. Lowest to highest post-BOS FEV1%pred patterns over time belonged to patients with early and rapid onset of BOS (Figure 6B), followed by patients with rapid onset and late BOS, gradual onset and early BOS, and, finally, gradual onset and late BOS. Patients with rapid and early onset of BOS had significantly lower FEV1%pred at BOS onset (p < 0.0001), steeper decline in the first 6 months (p = 0.03), and lower FEV1%pred 24 months after onset of BOS (p < 0.0001) as compared with the cohort with gradual and late onset of BOS. Estimated interactions of rapid BOS onset with gender and type of transplant showed a particularly poor prognosis, as demonstrated by significantly lower FEV1%pred at 24 months after BOS onset for male patients (p = 0.0002) and BLT recipients (p = 0.0005) with rapid BOS onset compared with their gradual-onset counterparts (Figures 7A and 7B).
Figure 7.
Estimated interaction of rapidity of BOS onset (rapid vs. gradual) with gender (males vs. females) and type of transplantation (bilateral lung transplant [BLT] vs. single-lung transplant [SLT]). Trajectories for these interactions are adjusted for the remaining significant variables including age, pretransplant diagnosis, time-to-BOS onset, transplant date, and type of transplant (A) or gender (B). Particularly poor prognosis for male patients and BLT recipients with rapid BOS onset compared with their gradual-onset counterparts is noted. Numbers of male and female patients with gradual and rapid BOS, and those with gradual-onset (BLT and SLT) and rapid-onset (BLT and SLT) BOS at the onset of each time interval are displayed at the bottom.
Effect of Histologic Diagnosis and Era of Transplantation on the Course of FEV1 after BOS Onset
A total of 743 transbronchial biopsies performed between transplantation and BOS onset were examined for acute rejection and lymphocytic bronchitis in the study cohort. The histologic diagnosis of lymphocytic bronchitis before BOS onset had no significant effect on FEV1%pred at or after onset of BOS (data not shown). The presence, number of episodes, or grade of acute rejection also gave nonsignificant results in terms of predicting FEV1%pred after BOS onset (data not shown).
To determine if calendar year had an influence on the course of FEV1 after onset of BOS, transplant date was studied as a variable. Later transplant years were associated with lower FEV1%pred at BOS onset (p = 0.03), with each additional transplant year corresponding to an approximate decrease of 1% in FEV1%pred at time of BOS. The course of FEV1%pred after BOS onset (0–6 mo and 6–24 mo trajectories) was not significantly different by transplant date (p = 0.227 and p = 0.240, respectively). Similarly, the era of BOS onset (studied either as a continuous or a categorical variable) was found to have no significant effect on FEV1%pred at or after onset of BOS.
DISCUSSION
BOS is an important and frequent clinical entity in lung transplant recipients that poses a challenge for physicians, researchers, and patients. Patients with BOS are identified at various stages with different patient histories, and hence exhibit varying values of FEV1%pred at onset and beyond (9). Although predictors of time-to-BOS and estimates of survival in BOS patients have been studied extensively, very few studies have addressed the impact of various clinical variables on the course of FEV1%pred after BOS onset.
In this study, we examined the course of FEV1 after BOS onset in a large cohort of lung transplant recipients and the effect of various clinical variables on its natural history. We demonstrated that (1) FEV1%pred varies over time after BOS onset with the steepest decline typically seen in the first 6 months; (2) BLT recipients have significantly higher FEV1%pred at BOS diagnosis and continue to maintain that benefit for at least 2 years after onset of BOS; (3) female gender and pretransplant IPF diagnosis are associated with steeper decline in FEV1%pred in the first 6 months after BOS diagnosis; and (4) rapid BOS onset (⩾ 20% decline in FEV1 6 mo before onset of BOS) is a strong predictor of worse course of disease and is associated with lower FEV1%pred at onset, steeper decline in FEV1%pred during the first 6 months of BOS, and significantly lower FEV1%pred at 24 months after BOS onset.
This study is the first to investigate the multivariate relationship between predictors known at BOS diagnosis and the course of subsequent FEV1%pred in a large cohort of patients. Clinical risk factors, such as type of transplantation, gender, and pretransplant diagnosis, have been demonstrated to influence various post–lung transplantation outcomes, such as survival, time-to-onset of BOS, and functional status (14–17). However, the impact of these important variables on course of FEV1 after BOS onset had not been previously described. Heng and colleagues studied progression of BOS in a cohort of patients who were in stage 1 at diagnosis (8). Progression was defined as advancement to stages 2 or 3, or death. Era of transplantation and number of episodes of acute rejection were found to be significant predictors of progression. In the present study, the impact of year of transplantation and histologic diagnosis of acute rejection, as well as lymphocytic bronchitis, was studied on the linear course of FEV1 after BOS onset. Furthermore, factors such as rapidity (18) and time (19) of onset of BOS, which have been previously associated with increased mortality after BOS onset, were studied for their impact on course of FEV1 after onset of BOS in a multivariate model adjusting for other confounding variables. The use of splines with the ability to change every 6 months gave us a unique ability to assess, for the first time, the impact of these variables on the course of FEV1 over time.
A clinically relevant finding of this study is that the course of FEV1%pred for BOS patients is significantly improved in BLT versus SLT recipients. A majority of the reports studying the impact of transplant type have focused on survival as an outcome. Hadjiliadis and colleagues reported increased risk of BOS among SLT recipients (14). Earlier BOS onset and decreased survival after diagnosis of BOS was also seen in SLT recipients with COPD (20). However, the association between type of transplant and course of FEV1%pred after BOS onset has not been studied. We demonstrate that, at 24 months after BOS onset, BLT recipients continued to have 42% higher FEV1%pred compared with SLT patients. This higher FEV1%pred could potentially translate to a quality of life benefit, and is also consistent with the longer survival of BLT recipients after BOS onset reported previously (20). Our work also confirms previous work done by Hadjiliadis and colleagues (20) noting a trend toward longer BOS onset time in patients with BLT versus SLT. Importantly, the difference in course of FEV1 after BOS onset was especially strong in patients with COPD. The small number of IPF cases with BLT makes it difficult to reach firm conclusions about this group. Because of small numbers of BLT recipients in our cohort, further studies validating this finding and establishing the impact of BLT versus SLT on quality of life and survival after BOS onset are warranted.
An additional important finding of our study is the effect of gender on the natural history of BOS. The issue of gender and its implications on graft function have not been completely evaluated in lung transplant recipients. In the analysis of post–lung transplant survival, female gender was found to be associated with increased mortality (21). Gender-related differences have been reported in studies of quality of life in lung transplant recipients (15). Female gender has been associated with significantly lower 6-min walk distance values after lung transplantation (16). However, the impact of gender on the natural history of FEV1 after BOS onset is not known. As such, we document that female recipients experienced a significantly steeper decline in FEV1%pred in the first 6 months after BOS onset compared with their male counterparts and had lower FEV1%pred 2 years after BOS diagnosis. Although the exact mechanism for these differences is not known, several factors may contribute. Studies in other solid organ transplantations point to a difference in allograft rejection after transplantation in humans, with females demonstrating a higher incidence of acute rejection (22, 23). Female rat recipients of rat cardiac or skin allografts have shorter graft survival compared with males (24–26), believed to be secondary to alteration of immunologic responses by the female sex hormone, estrogen (24, 26). These data extend available data supporting clinically relevant gender differences to lung transplantation.
Another important, practical observation reflects the finding that patients with IPF demonstrated significantly more rapid decline in FEV1 compared with recipients with COPD after BOS diagnosis. This expands on the findings in a previously studied, small cohort of recipients with COPD (n = 10) and IPF (n = 6) with BOS (17). These investigators suggested a survival benefit in SLT recipients with a pretransplant diagnosis of COPD. Native lung physiology impacts pulmonary functions measured after SLT. We have previously shown that early change in FEV1 [BOS (potential-BOS stage CBOS O-P)] in patients with underlying restrictive physiology has a higher predictive ability for further progression to BOS than in patients with underlying obstructive physiology (4). Similarly, differences in native lung physiology (restrictive vs. obstructive) can potentially alter the course of FEV1 after BOS onset in SLT recipients. Furthermore, progressive airflow obstruction in patients with SLTs with obstructive lung disease might not always indicate the presence of BOS, as factors such as native lung hyperinflation will also present with worsening obstructive defect (5). It can be postulated that new onset of obstruction with decline in FEV1 in a patient with initial underlying restriction better identifies the true cohort of patients with bronchiolitis obliterans, and hence the significant decline seen in this group. Further confirmation of this finding in an even larger multicentric cohort of IPF cases is suggested.
Our study identifies rapid BOS onset as a very important predictor of poorer subsequent FEV1%pred. In particular, a fall in FEV1 greater than 20% in the 6 months preceding BOS onset is associated with (1) lower FEV1%pred at the time of BOS diagnosis, (2) steeper decline in the first 6 months after onset, and (3) lower FEV1%pred 2 years after onset when compared with those with a more gradual onset. The impact of rapidity of onset of BOS on survival has been demonstrated, with poorer prognosis seen in patients with acute onset of BOS; however, the course of FEV1 after onset of BOS in these groups was not reported (18). The authors classified patients as having rapid (acute) versus gradual (chronic) BOS based on knowing FEV1 values from BOS onset to the end of follow-up, which is of limited use to the clinician attempting to classify patients when they initially present with BOS. In our study, we define rapid and gradual onset based on FEV1 information available in the 6-month period preceding BOS diagnosis. For example, if a patient with a BOS FEV1 value of 2.5 L had an FEV1 value of 3.2 L 6 months earlier, it was determined that this patient decreased 20% or more during this period (i.e., decrease was [(3.2 − 2.5 L)/3.2 L] × 100 = 22%). This simple variable can be easily determined at bedside, and provides valuable information regarding the further course of pulmonary function.
The effect of BOS onset time on prognosis after onset has also been studied with varying conclusions. In one study, this variable was not found to be a significant predictor of disease progression, defined as advancement of BOS stage 1 to stages 2 or 3, or death (8). However, another group demonstrated differences in FEV1 course after BOS onset in 29 SLT recipients (19). In our cohort, a trend toward a greater decline in FEV1%pred was observed in patients with early BOS onset. Our findings were consistent with those of Brugiere and colleagues in that lung transplant recipients with early onset of BOS had significantly lower FEV1%pred (17%) at 24 months after onset of BOS as compared with those with late onset of disease (19). Further study of acuteness of onset (rapid vs. gradual) and time-to-onset (early vs. late) demonstrates lowest trajectories of FEV1%pred in the cohort with early, rapid BOS onset.
This study provides important insight into the longitudinal behavior of FEV1%pred in a large cohort of lung transplant recipients; these results have potential implications for patient care, pretransplant lung allocation, and the design of therapeutic trials aimed at ameliorating BOS-associated loss of lung function. These findings include documentation of a greater decline within the first 6 months after BOS diagnosis, gender-specific differences in the rate of progression, procedure- and pretransplant diagnosis–related differences (SLT vs. BLT and IPF vs. COPD), and additional predictors of an accelerated post-BOS decline (steeper pre-BOS decline or rapid onset, and early onset of BOS). Investigators on BOS clinical studies should strongly consider stratification of patients by factors that are most likely to influence post-BOS FEV1 behavior so as to minimize confounders in interpretation of therapeutic response. Similarly, the factors that predict future FEV1%pred behavior should be considered in assessing each newly diagnosed BOS patient's prognosis and therapeutic options.
Supported in part by National Institutes of Health grants K23 HL077719 (V.N.L.) and K24 HL04212 (F.J.M.), and by a grant from the American Society of Transplantation/Chest Foundation (V.N.L.).
Originally Published in Press as DOI: 10.1164/rccm.200609-1344OC on March 8, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Boehler A, Kesten S, Weder W, Speich R. Bronchiolitis obliterans after lung transplantation: a review. Chest 1998;114:1411–1426. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med 2002;166:440–444. [DOI] [PubMed] [Google Scholar]
- 3.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Mohacsi PJ, Keck BM, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twentieth official adult lung and heart-lung transplant report—2003. J Heart Lung Transplant 2003;22:625–635. [DOI] [PubMed] [Google Scholar]
- 4.Lama VN, Murray S, Mumford JA, Flaherty KR, Chang A, Toews GB, Peters-Golden M, Martinez FJ. Prognostic value of bronchiolitis obliterans syndrome stage 0-p in single-lung transplant recipients. Am J Respir Crit Care Med 2005;172:379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297–310. [DOI] [PubMed] [Google Scholar]
- 6.Reichenspurner H, Girgis RE, Robbins RC, Conte JV, Nair RV, Valentine V, Berry GJ, Morris RE, Theodore J, Reitz BA. Obliterative bronchiolitis after lung and heart–lung transplantation. Ann Thorac Surg 1995;60:1845–1853. [DOI] [PubMed] [Google Scholar]
- 7.Valentine VG, Robbins RC, Berry GJ, Patel HR, Reichenspurner H, Reitz BA, Theodore J. Actuarial survival of heart–lung and bilateral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant 1996;15:371–383. [PubMed] [Google Scholar]
- 8.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant 1998;17:1255–1263. [PubMed] [Google Scholar]
- 9.Nathan SD, Ross DJ, Belman MJ, Shain S, Elashoff JD, Kass RM, Koerner SK. Bronchiolitis obliterans in single-lung transplant recipients. Chest 1995;107:967–972. [DOI] [PubMed] [Google Scholar]
- 10.DiGiovine B, Lynch JP III, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol 1996;157:4194–4202. [PubMed] [Google Scholar]
- 11.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 12.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant 1996;15:1–15. [PubMed] [Google Scholar]
- 13.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- 14.Hadjiliadis D, Davis RD, Palmer SM. Is transplant operation important in determining posttransplant risk of bronchiolitis obliterans syndrome in lung transplant recipients? Chest 2002;122:1168–1175. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigue JR, Baz MA. Are there sex differences in health-related quality of life after lung transplantation for chronic obstructive pulmonary disease? J Heart Lung Transplant 2006;25:120–125. [DOI] [PubMed] [Google Scholar]
- 16.Sager JS, Kotloff RM, Ahya VN, Hadjiliadis D, Simcox R, Blumenthal NP, Mendez J, Bilker WB, Pochettino A, Christie JD. Association of clinical risk factors with functional status following lung transplantation. Am J Transplant 2006;6:2191–2201. [DOI] [PubMed] [Google Scholar]
- 17.Haider Y, Yonan N, Mogulkoc N, Carroll KB, Egan JJ. Bronchiolitis obliterans syndrome in single lung transplant recipients—patients with emphysema versus patients with idiopathic pulmonary fibrosis. J Heart Lung Transplant 2002;21:327–333. [DOI] [PubMed] [Google Scholar]
- 18.Jackson CH, Sharples LD, McNeil K, Stewart S, Wallwork J. Acute and chronic onset of bronchiolitis obliterans syndrome (BOS): are they different entities? J Heart Lung Transplant 2002;21:658–666. [DOI] [PubMed] [Google Scholar]
- 19.Brugiere O, Pessione F, Thabut G, Mal H, Jebrak G, Leseche G, Fournier M. Bronchiolitis obliterans syndrome after single-lung transplantation: impact of time to onset on functional pattern and survival. Chest 2002;121:1883–1889. [DOI] [PubMed] [Google Scholar]
- 20.Hadjiliadis D, Chaparro C, Gutierrez C, Steele MP, Singer LG, Davis RD, Waddell TK, Hutcheon MA, Palmer SM, Keshavjee S. Impact of lung transplant operation on bronchiolitis obliterans syndrome in patients with chronic obstructive pulmonary disease. Am J Transplant 2006;6:183–189. [DOI] [PubMed] [Google Scholar]
- 21.Hertz MI, Taylor DO, Trulock EP, Boucek MM, Mohacsi PJ, Edwards LB, Keck BM. The registry of the International Society for Heart and Lung Transplantation: nineteenth official report—2002. J Heart Lung Transplant 2002;21:950–970. [DOI] [PubMed] [Google Scholar]
- 22.Fabbri A, Bryan AJ, Sharples LD, Dunning J, Caine N, Schofield P, Wallwork J, Large SR. Influence of recipient and donor gender on outcome after heart transplantation. J Heart Lung Transplant 1992;11:701–707. [PubMed] [Google Scholar]
- 23.Meier-Kriesche HU, Ojo AO, Leavey SF, Hanson JA, Leichtman AB, Magee JC, Cibrik DM, Kaplan B. Gender differences in the risk for chronic renal allograft failure. Transplantation 2001;71:429–432. [DOI] [PubMed] [Google Scholar]
- 24.Hirasawa K, Enosawa S. Effects of sex steroid hormones on sex-associated differences in the survival time of allogeneic skin grafts in rats: evidence that testosterone enhances and estradiol reverses the immunosuppressive activity of cyclosporine. Transplantation 1990;50:637–641. [DOI] [PubMed] [Google Scholar]
- 25.Takami H, Backer CL, Crawford SE, Zales VR, Mavroudis C. Influence of gender on allograft rejection in a rat heart transplant model. J Heart Lung Transplant 1995;14:529–536. [PubMed] [Google Scholar]
- 26.Zou Y, Steurer W, Klima G, Obrist P, Margreiter R, Brandacher G. Estradiol enhances murine cardiac allograft rejection under cyclosporin and can be antagonized by the antiestrogen tamoxifen. Transplantation 2002;74:354–357. [DOI] [PubMed] [Google Scholar]