Abstract
Rationale: Alveolar macrophages are inflammatory cells that may contribute to the pathogenesis of idiopathic pulmonary fibrosis (IPF), which is characterized by excessive alveolar aggregation of cells and extracellular matrix proteins.
Objectives: To identify potential molecular mechanisms of IPF.
Methods: To examine large-scale gene expression, messenger RNA isolated from alveolar macrophages and peripheral blood mononuclear cells from subjects with IPF and normal volunteers was hybridized to cDNA filters.
Measurements and Main Results: We showed that in IPF there is global down-regulation of gene expression in alveolar macrophages but not in blood monocytes. Nuclear run-on and pulse-chase studies showed that alveolar macrophages had significantly reduced transcription (p < 0.01). No significant difference in RNA degradation was found between subjects with IPF and normal volunteers. Western blot analyses revealed that concentrations of transcription factor II-H, a general transcription factor, were significantly lower in alveolar macrophages from subjects with IPF than in those from normal volunteers (p = 0.012).
Conclusions: Impaired transcription in IPF is associated with decreased concentrations of transcription factor II-H in alveolar macrophages and may alter the intraalveolar milieu in IPF.
Keywords: monocytes/macrophages, transcription factors, TFII-H, lung
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The etiology of idiopathic pulmonary fibrosis (IPF) is unknown. There are no published reports on large-scale gene expression of alveolar macrophages in IPF.
What This Study Adds to the Field
In IPF there is global down-regulation of gene expression in alveolar macrophages but not in blood monocytes. Aberrant transcription is a novel molecular mechanism that may contribute to development of a profibrotic alveolar milieu in idiopathic pulmonary fibrosis.
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, often fatal interstitial lung disease of unknown etiology. It is hypothesized that alveolar injury, perhaps as a result of inflammation, and dysregulated repair mechanisms are involved in the pathogenesis of pulmonary fibrosis. Histopathologic features of IPF include abnormal alveolar interstitial accumulations of inflammatory cells, mesenchymal cells, and extracellular matrix proteins. Patients with IPF are usually of middle age or older and present with chronic shortness of breath with exertion and nonproductive cough. Conventional treatment for IPF with glucocorticoids and immunosuppressive drugs is generally ineffective, and the prognosis of patients with IPF is poor. Despite therapy, patients often die of progressive lung fibrosis. Median survival is approximately 2 years from the date of diagnosis. Elucidating the molecular mechanisms underlying fibrotic lung disease may identify novel therapeutic targets and lead to improvement in the clinical outcome.
Alveolar macrophages, the predominant inflammatory cells in alveolar epithelial lining fluid, may contribute to the pathogenesis of pulmonary fibrosis by secreting cytokines, growth factors, extracellular matrix proteins, and inhibitors of matrix metalloproteases (1–3). For example, it has been demonstrated that many proteins with proinflammatory or profibrotic effects, including monocyte chemoattractant protein-1, macrophage inflammatory protein (MIP)-1α, IL-8, tumor necrosis factor-α, granulocyte/macrophage colony-stimulating factor, insulin-like growth factor-I, platelet-derived growth factor, basic fibroblast growth factor, transforming growth factor-β, fibronectin, and tissue inhibitor of metalloproteinases, are secreted by alveolar macrophages. These proteins may contribute to alveolar injury and dysregulated lung repair by stimulating mesenchymal cell proliferation and enhancing collagen and extracellular matrix protein synthesis.
Despite the availability of methods that enable simultaneous analysis of many genes, only a limited number of publications have focused on large-scale gene expression in pulmonary fibrosis or in alveolar macrophages (4–7). In two reports from different groups of investigators, 7,129 genes or approximately 46,000 genes in lung tissue derived from patients with interstitial lung disease, including IPF, and normal lung tissue were simultaneously analyzed (4, 5). These data demonstrated that many genes, including those encoding proteins involved with extracellular matrix turnover, are significantly increased in IPF. These investigators studied whole lung tissue, and not specific cell types, derived from patients with IPF. In another report, microarray analysis of alveolar macrophages from smokers and nonsmokers identified a group of genes modulated by smoking (7). There are no published reports focusing on large-scale gene expression of alveolar macrophages in IPF.
We examined large-scale gene expression by alveolar macrophages from patients with IPF and normal volunteers using cDNA filters. We observed a global reduction of mRNA levels due to impaired transcription in IPF alveolar macrophages. Decreased alveolar macrophage transcription in IPF was associated with low concentrations of the transcription factor II-H (TFII-H) protein complex, a general transcription factor that contributes to the pathogenesis of human diseases, including xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy (8–10). These data suggest that a general impairment of transcription may contribute to the development of a profibrotic alveolar milieu in IPF. Some of the results of these studies have been previously reported in the form of abstracts (11, 12).
METHODS
Subject Selection
Written, informed consent was obtained from subjects with IPF and healthy research volunteers without lung disease (normal volunteer [NV] subjects) who were enrolled in a protocol (99-H-0068) approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute. IPF was diagnosed by histopathologic findings of open lung biopsies, which demonstrated usual interstitial pneumonia. Subjects with IPF were enrolled with or without supplemental oxygen or medical treatment (see online supplement for details). Research volunteers had no symptoms of lung disease, had normal chest radiographs, and had pulmonary function tests in the normal range. Research volunteers and subjects with IPF were matched for age (within 5 yr) and sex. All subjects were nonsmokers or former smokers who had not smoked for at least 2 years.
Large-scale cDNA Analyses
Total RNA and mRNA were isolated from unstimulated alveolar macrophages and peripheral blood mononuclear cells (PBMCs) using RNAgents and the Poly A Tract mRNA Isolation System II, respectively, in accordance with manufacturer's instructions (Promega, Madison, WI). Large-scale cDNA analyses were performed using cDNA filters containing 5,184 human genes in accordance with manufacturer's instructions (GeneFilter 211; Invitrogen, Carlsbad, CA). Images were analyzed using Pathways 4 (Invitrogen). See the online supplement for additional details on methods.
Functional category enrichment analysis was performed by using Database for Annotation, Visualization and Integrated Discovery 2.1 (http://david.abcc.neifcrf.gov). One-way analysis of variance was used to determine differential expression of alveolar macrophage genes between subjects with IPF and NV subjects. Genes with significantly different expression were defined as those with a p value of less than or equal to 0.05 and an absolute change of at least 1.5-fold. Seventy-six differentially expressed genes were grouped into hierarchical clusters according to pattern of gene expression. See the online supplement for details about Northern blot analyses for human MIP-1α, HLA-C, fibronectin, and β-actin and about isolation and quantification of total RNA and mRNA.
Nuclear Run-On and Pulse–Chase Experiments
Nuclei from alveolar macrophages or PBMCs were isolated as previously described (13). For pulse–chase experiments, alveolar macrophages or PBMCs (∼ 1–5 × 106 cells/reaction) were incubated in 10 μM ATP, 10 μM cytidine triphosphate, 10 μM guanosine triphosphate, 20 mM glucosamine, and 50 μCi [α-32P]–uridine triphosphate. Cells were chased with 10 μM ATP, 10 μM cytidine triphosphate, 10 μM guanosine triphosphate, and 2.5 μM uridine triphosphate and collected after 0, 1, 7.5, 15, 30, 60, and 120 minutes. RNA was isolated, and 32P was quantified by scintillation spectrometry. See the online supplement for details on Western blot analyses for general transcription factors and immunohistochemistry and on immunoprecipitation and two-dimensional gel electrophoresis.
RESULTS
Alveolar Macrophage mRNA Expression Is Reduced Globally in IPF
To compare mRNA levels in alveolar macrophages from subjects with IPF and matched NV subjects without lung disease, samples were analyzed using large-scale cDNA filters. Differential expression of each normalized mRNA was demonstrated as differences between subjects with IPF and NV subjects in distributions of ratios of radioactivity in each mRNA. These findings were confirmed by Northern blot analyses for fibronectin, MIP-1α, and HLA-C (data not shown).
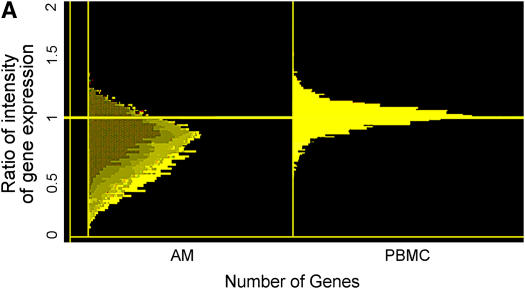
A striking finding of the large-scale cDNA analyses was that among mRNAs whose level differed 1.5-fold in alveolar macrophages from subjects with IPF and NV subjects, approximately 95% were lower in subjects with IPF, indicating that mRNA levels were globally reduced in alveolar macrophages from subjects with IPF. A histogram (Figure 1A) shows levels of individual mRNAs in alveolar macrophages from subjects with IPF expressed relative to that of the cognate NV = 1.0. The data for PBMC mRNA levels revealed no systematic difference between IPF and NV samples. Histograms demonstrated that alveolar macrophage and PBMC mRNA expression was in a normal, unimodal, bell-shaped distribution. Consistent with global reduction of mRNA levels in alveolar macrophages from subjects with IPF, the ratio of values at the center of the distribution was 0.85; more than 70% of the mRNAs had a ratio of less than 1.0. In contrast, the ratio of values at the center of the distribution for PBMC was approximately 1.0, demonstrating that mRNA levels are globally decreased in IPF alveolar macrophages but not in PBMCs.
Figure 1.
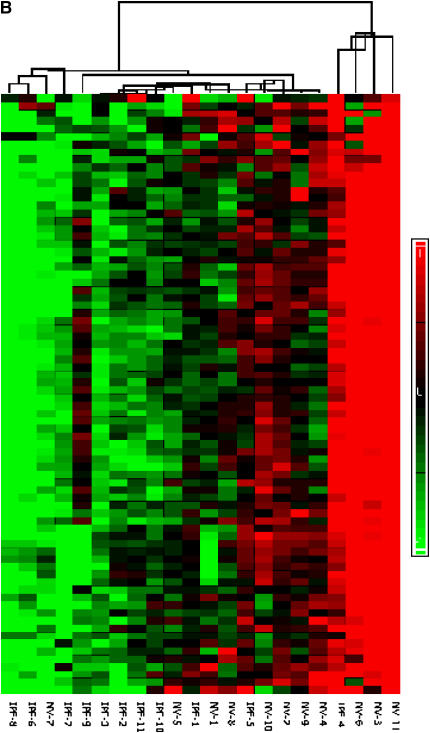
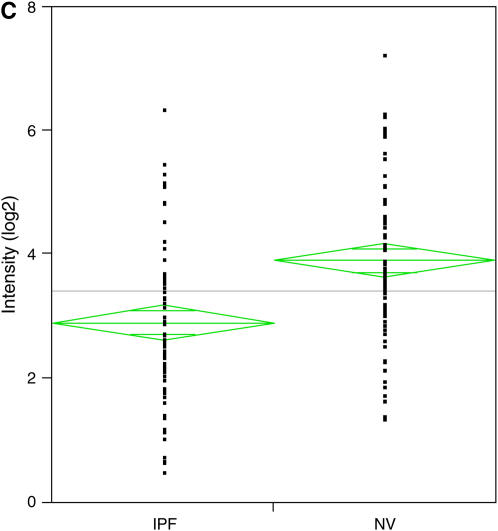
Global down-regulation of alveolar macrophage mRNA expression in idiopathic pulmonary fibrosis (IPF). (A) Histograms of mRNA levels in alveolar macrophages (AM) or peripheral blood mononuclear cells (PBMC) from subjects with IPF (n = 12) compared with matched normal healthy research (normal volunteer [NV]) subjects are shown. mRNA levels in cells from NV subjects are normalized to 1. (B) Using one-way analysis of variance to select mRNAs with a 1.5-fold change in mRNA expression and a p value of less than or equal to 0.05, 76 genes were identified and are clustered in a heatmap. Messenger RNA expression in 9 of 11 subjects with IPF demonstrated down-regulation (green). (C) Dot plot of these 76 mRNAs showed highly significant down-regulation of gene expression in subjects with IPF (p < 0.001).
Messenger RNAs with a 1.5-fold increase or 50% decrease in expression and a p value of at least 0.05 were identified using one-way analysis of variance. We found dysregulation of 76 genes, which are demonstrated in a heatmap (Figure 1B). mRNA expression was down-regulated in 9 of 11 subjects with IPF. A dot plot of these 76 mRNAs shows highly significant down-regulation of gene expression in subjects with IPF compared with NV subjects (Figure 1C) (p < 0.001).
An analysis to determine the biological function of the mRNAs dysregulated in subjects with IPF was performed. Eight functional groups with a p value of at least 0.05 were identified and included immune response, protein binding, cell adhesion, transcription, cell proliferation, receptor binding, cell metabolism, and signal transduction genes (Table 1).
TABLE 1.
FUNCTIONAL ANALYSIS OF DYSREGULATED GENES IN ALVEOLAR MACROPHAGES
| Biologic Function | Number of Genes | p Value |
|---|---|---|
| Immune response | 8 | 0.007 |
| Protein binding | 18 | 0.009 |
| Cell adhesion | 8 | 0.017 |
| Transcription | 5 | 0.019 |
| Cell proliferation | 5 | 0.028 |
| Receptor binding | 7 | 0.036 |
| Cell metabolism | 14 | 0.043 |
| Signal transduction | 22 | 0.046 |
Impaired Transcription Is Responsible for Global Down-regulation of Alveolar Macrophage mRNA Expression in IPF
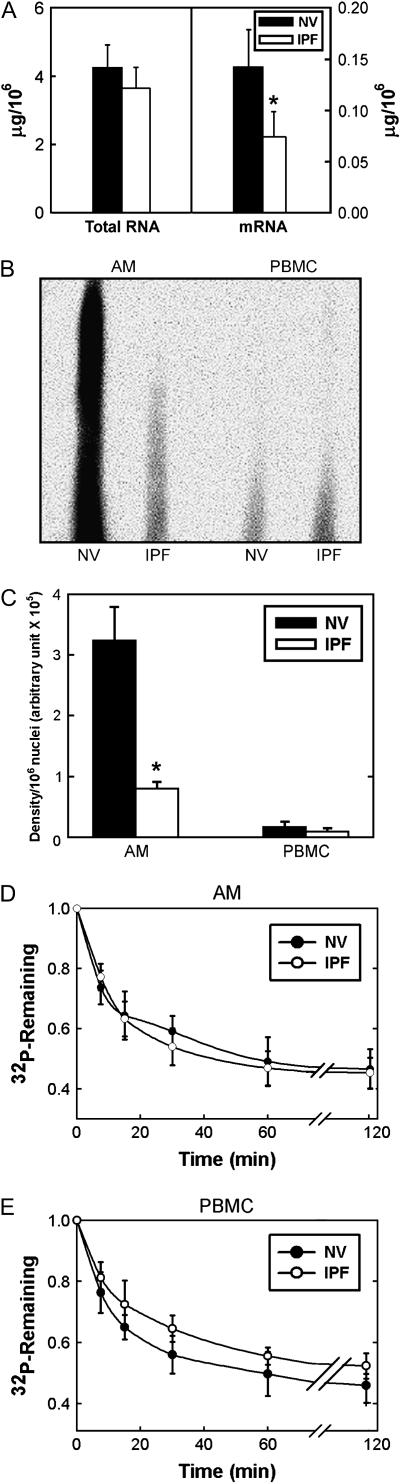
To ascertain whether alveolar macrophage mRNA expression was globally impaired in IPF, amounts of total RNA and mRNA in alveolar macrophages were quantified. Total RNA content tended to be lower, and mRNA content was significantly lower in IPF than in NV cells (p = 0.01) (Figure 2).
Figure 2.
Reduction of alveolar macrophage transcription in idiopathic pulmonary fibrosis (IPF). (A) Total RNA content of 1 × 106 alveolar macrophages (AM) from subjects with IPF (n = 10) and matched normal healthy research (normal volunteer [NV]) subjects did not differ significantly (p > 0.05). However, mRNA content of 1 × 106 alveolar macrophages from subjects with IPF was significantly lower than that of matched NV subjects (p = 0.01). (B) Representative nuclear run-on blot. (C) Quantification of nuclear run-on experiments demonstrated significantly lower amounts of newly synthesized mRNA in alveolar macrophages from subjects with IPF (n = 6) than from matched NV subjects (p = 0.001). Amounts of newly synthesized mRNA from peripheral blood mononuclear cells (PBMC) in subjects with IPF (n = 6) and matched NV subjects were not significantly different (p > 0.05). Pulse–chase experiments from alveolar macrophages (D) and PBMCs (E) demonstrated no significant difference in rates of RNA degradation in subjects with IPF (n = 8) and matched NV subjects (p > 0.05).
To assess the contribution of impaired transcription to the low levels of alveolar macrophage mRNA in IPF, nuclear run-on studies were performed. Results of a representative nuclear run-on experiment are shown in Figure 2B. Overall, these studies demonstrated that newly synthesized mRNA was significantly less in alveolar macrophages from subjects with IPF than in those from NV subjects (Figure 2C) (p = 0.001); there was no difference between PBMCs from subjects with IPF and NV subjects.
To determine the rates of degradation of newly synthesized RNA, pulse–chase experiments were performed. There were no significant differences between subjects with IPF and NV subjects in RNA stability in alveolar macrophages or PBMCs (Figures 2D and 2E). Thus, these data demonstrate that impaired transcription, not RNA degradation, is responsible for globally decreased alveolar macrophage mRNA levels in IPF.
Levels of TFII-H p89 Protein Are Low in IPF Alveolar Macrophages
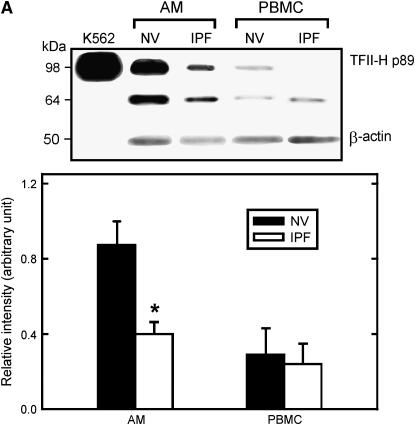
Because transcription was globally impaired in IPF alveolar macrophages, we hypothesized that there was differential expression of general transcription factors in these cells. Consistent with this hypothesis, mRNA levels of a polypeptide subunit of general TFII-H were significantly lower in alveolar macrophages from subjects with IPF than from NV subjects (data not shown). Thus, Western blot analyses were performed for several general transcription factors, including TFII-H. We found that levels of TFII-H p89, a subunit of TFII-H, were significantly lower in alveolar macrophages of subjects with IPF than in NV subjects (Figure 3A) (p = 0.012). Levels of TFII-H p89 were not significantly different in PBMCs of subjects with IPF and NV subjects.
Figure 3.
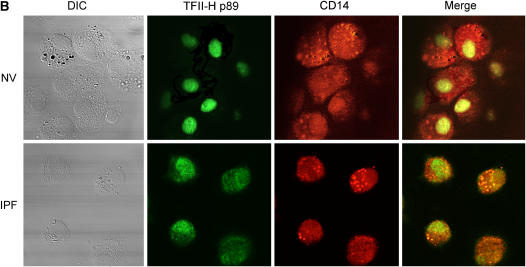
Lower levels of transcription factor II-H (TFII-H) p89 in idiopathic pulmonary fibrosis (IPF) alveolar macrophages (AM). (A) Representative Western blot analysis for TFII-H p89. Positions of standard proteins (kDa) are on the left. Nuclear extract from human chronic myelogenous leukemia cells (K562; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) is included as a positive control. Quantification of TFII-H p89 levels expressed relative to amount of β-actin protein in each sample demonstrated that levels of TFII-H p89 in alveolar macrophages, not peripheral blood mononuclear cells, were significantly lower in cells from subjects with IPF (n = 10) than in matched normal healthy research (normal volunteer [NV]) subjects (p = 0.012). (B) Representative confocal microscopy (original magnification: ×400) of cytospin preparations of bronchoalveolar lavage cells immunostained for TFII-H p89 (green) and CD14 (red). Nuclear staining of TFII-H p89 was less in alveolar macrophages from subjects with IPF (n = 6) than in macrophages from matched NV subjects. DIC = differential interference contrast.
Confocal microscopy of cytospin preparations of cells from bronchoalveolar lavage immunostained for TFII-H p89 revealed less staining intensity in nuclei of alveolar macrophages from subjects with IPF than in those from matched NV subjects (Figure 3B). Thus, low levels of TFII-H p89 protein were associated with impaired transcription in alveolar macrophages from subjects with IPF.
Low Levels of TFII-H p89 Affect Formation of TFII-H Complex
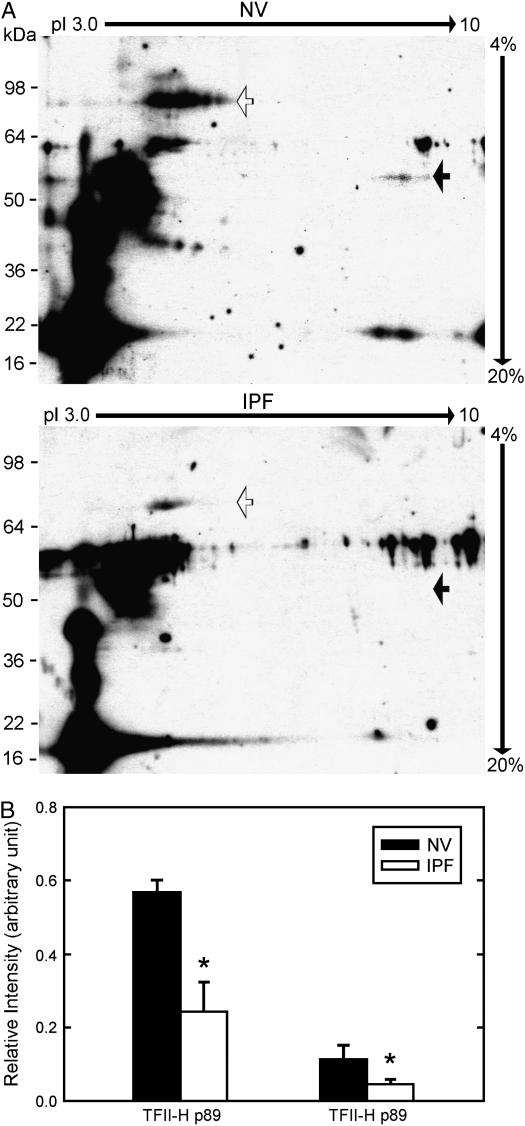
TFII-H complex, but not individual subunits such as TFII-H p89, exhibits basal transcriptional activity. To determine whether low levels of TFII-H p89 were associated with changes in TFII-H complex content, we separated coimmunoprecipitated proteins by two-dimensional gel electrophoresis. Anti–TFII-H p44 immunoprecipitated TFII-H p89 and TFII-H p62 (Figure 4A). Amounts of TFII-H p89 and p62 immunoprecipitated from IPF alveolar macrophages were significantly less than those immunoprecipitated from NV alveolar macrophages (Figure 4B) (p = 0.032 and 0.042, respectively), consistent with a reduced content of TFII-H complex in alveolar macrophages from subjects with IPF.
Figure 4.
Lower levels of transcription factor II-H (TFII-H) complex in alveolar macrophages in idiopathic pulmonary fibrosis (IPF). Alveolar macrophage TFII-H protein complexes from subjects with IPF (n = 10) and matched normal healthy research (normal volunteer [NV]) subjects were immunoprecipitated and separated by two-dimensional polyacrylamide gel electrophoresis. (A) Representative immunoblots using anti–TFII-H p89 (open arrow) and anti–TFII-H p62 (closed arrow) are shown. (B) Quantitative data demonstrate that the amounts of TFII-H p89 and TFII-H p62 were significantly lower in alveolar macrophages from subjects with IPF than matched NV subjects (p = 0.032 and p = 0.042, respectively).
DISCUSSION
The etiology of IPF, a chronic progressive interstitial lung disease, is unknown. Alveolar macrophages are inflammatory cells capable of secreting numerous proteins, and thus have the potential to contribute to the pathogenesis of pulmonary fibrosis. A novel finding of this study is the identification of a global reduction in mRNA levels due to impairment of transcription by alveolar macrophages, and not PBMCs, in individuals with IPF. We also demonstrated that TFII-H p89 and TFII-H complex, a general transcription factor, were reduced in IPF alveolar macrophages. These data suggest that broad reduction of general transcription in alveolar macrophages may contribute to the development of an alveolar milieu that encourages fibrosis.
Alveolar macrophages, localized to the interface between exogenous air and alveolar epithelium, are important in maintaining lung homeostasis (1). These phagocytic cells have an important role in host defense and possess antimicrobial activity against bacteria, viruses, and fungi. Alveolar macrophages, constituting approximately 90% of cells in bronchoalveolar lavage fluid, secrete numerous cytokines, chemokines, growth factors, and proteases, which contribute to the composition of the alveolar microenvironment.
Dysregulation of alveolar macrophages has been demonstrated in animal models of pulmonary fibrosis. For example, pale ear mice have a mutation in HPS1, which is associated with pulmonary fibrosis in patients with Hermansky-Pudlak syndrome, an autosomal recessive disease characterized by vesicular trafficking defects and abnormal accumulation of ceroid lipofuscin in alveolar macrophages (14). Inhalational exposure of pale ear mice to silica induced pulmonary fibrosis, persistent alveolar macrophage infiltration, and low proteolytic activity of cathepsins L and B, which are potent alveolar macrophage fibrinolytic lysosomal enzymes (15). In addition, in a murine model of pulmonary fibrosis induced by exposure to the hapten 2,4,6-trinitrobenzene sulphonic acid, selective depletion of alveolar macrophages attenuated pulmonary fibrosis (16). Furthermore, intratracheal instillation of apoptotic macrophages into the lungs of rats induced pulmonary infiltration of alveolar macrophages and pulmonary fibrosis (17).
Disruption of transcriptional activity is involved in the pathogenesis of several human diseases. For example, dysregulated transcription has been reported in Huntington's disease, which is an inherited neurodegenerative disease caused by the expansion of polyglutamine sequences in the huntingtin protein (18). Soluble mutant huntingtin has been shown to inhibit binding of the transcriptional activator Sp1 to DNA in brain tissue from patients with Huntington's disease, and oligonucleotide DNA microarray analyses of striatal mRNAs from transgenic murine models of Huntington's disease demonstrated broad reduced expression of genes involved in signaling pathways important to neuronal function (19, 20).
TFII-H is a multimeric complex with general transcription and DNA repair functions. TFII-H p89 (XPB), a helicase encoded by ERCC3 on chromosome 2q21–22, is a subunit that is required for TFII-H DNA repair and transcription functions. Investigations of the transcriptional role of TFII-H p89 have shown, through mutation analyses, that TFII-H p89 is important in transcription initiation and promoter escape (21).
Defects of TFII-H subunits, including TFII-H p89, have been reported in hereditary diseases, including xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy (8–10). Although the mutations associated with these diseases affect the same protein, their phenotypes are variable. Clinical manifestations include early development of changes in skin pigmentation, predisposition to UV radiation–induced skin cancer, progressive neurologic degeneration, developmental delay, short stature, and severe wasting. Although pulmonary disease has not been reported in patients with these rare inherited diseases, interstitial fibrosis of the renal parenchyma has been reported in patients with Cockayne syndrome (22). In patients with sporadic diseases, such as lung cancer, loss of heterozygosity for TFII-H p89 has been identified (23).
Impaired transcription associated with genetic mutations has been found in several hereditary diseases, and our data demonstrate that reduced transcription is a potential mechanism in the etiology of seemingly sporadic diseases such as IPF. In IPF, a disease with manifestations that seem to be restricted to the lung, transcription was reduced in alveolar macrophages and in a subpopulation of monocytes but not in PBMCs. Defective transcription in alveolar macrophages was associated with low concentrations of TFII-H, a general transcription factor, which may contribute to the development of a profibrotic alveolar microenvironment.
Supplementary Material
Acknowledgments
The authors thank Drs. Joel Moss, Martha Vaughan, and Vincent Manganiello for their insightful and critical review of the manuscript and Mr. Michael Spencer for his assistance with the figures. They also thank the patients, the Pulmonary–Critical Care Medicine Branch nurse practitioners, and the Clinical Center bronchoscopy nurses for their contributions to this research.
Supported by the Division of Intramural Research, National Institutes of Health, National Heart, Lung, and Blood Institute.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200607-958OC on March 1, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lohmann-Matthes ML, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J 1994;7:1678–1689. [PubMed] [Google Scholar]
- 2.Wynes MW, Riches DW. Induction of macrophage insulin-like growth factor-I expression by the Th2 cytokines IL-4 and IL-13. J Immunol 2003;171:3550–3559. [DOI] [PubMed] [Google Scholar]
- 3.Welgus HG, Campbell EJ, Bar-Shavit Z, Senior RM, Teitelbaum SL. Human alveolar macrophages produce a fibroblast-like collagenase and collagenase inhibitor. J Clin Invest 1985;76:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002;99:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, Aziz N, Kaminski N, Zlotnik A. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med 2006;173:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaminski N, Rosas IO. Gene expression profiling as a window into idiopathic pulmonary fibrosis pathogenesis: can we identify the right target genes? Proc Am Thorac Soc 2006;3:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heguy A, O'Connor TP, Luettich K, Worgall S, Cieciuch A, Harvey BG, Hackett NR, Crystal RG. Gene expression profiling of human alveolar macrophages of phenotypically normal smokers and nonsmokers reveals a previously unrecognized subset of genes modulated by cigarette smoking. J Mol Med 2006;84:318–328. [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen W, Scott RJ, Rodgers S, Muller HJ, Cole J, Arlett CF, Kleijer WJ, Bootsma D, Hoeijmakers JH, Weeda G. Clinical heterogeneity within xeroderma pigmentosum associated with mutations in the DNA repair and transcription gene ERCC3. Am J Hum Genet 1994;54:191–200. [PMC free article] [PubMed] [Google Scholar]
- 9.Henning KA, Li L, Iyer N, McDaniel LD, Reagan MS, Legerski R, Schultz RA, Stefanini M, Lehmann AR, Mayne LV. The Cockayne syndrome group A gene encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell 1995;82:555–564. [DOI] [PubMed] [Google Scholar]
- 10.Weeda G, Eveno E, Donker I, Vermeulen W, Chevallier-Lagente O, Taieb A, Stary A, Hoeijmakers JH, Mezzina M, Sarasin A. A mutation in the XPB/ERCC3 DNA repair transcription gene, associated with trichothiodystrophy. Am J Hum Genet 1997;60:320–329. [PMC free article] [PubMed] [Google Scholar]
- 11.Ren P, Rosas I, Wu HP, Travis WD, MacDonald SD, Gochuico BR. Global down-regulation of alveolar macrophage gene expression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;167:A982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren P, Rosas I, Wu HP, MacDonald SD, Gochuico BR. Reduction of TFII H complex is associated with global down-regulation of alveolar macrophage transcription and gene expression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2004;169:A740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groudine M, Peretz M, Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol 1981;1:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gahl WA, Brantly M, Kaiser-Kupfer MI, Iwata F, Hazelwood S, Shotelersuk V, Duffy LF, Kuehl EM, Troendle J, Bernardini I. Genetic defects and clinical characteristics of patients with a form of oculocutaneous albinism (Hermansky-Pudlak syndrome). N Engl J Med 1998;338:1258–1264. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka Y, Kumasaka T, Ishidoh K, Kominami E, Mitani K, Hosokawa Y, Fukuchi Y. Inflammatory response and cathepsins in silica-exposed Hermansky-Pudlak syndrome model pale ear mice. Pathol Int 2004;54:322–331. [DOI] [PubMed] [Google Scholar]
- 16.Zhang-Hoover J, Sutton A, van Rooijen N, Stein-Streilein J. A critical role for alveolar macrophages in elicitation of pulmonary immune fibrosis. Immunology 2000;101:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Antonini JM, Rojanasakul Y, Castranova V, Scabilloni JF, Mercer RR. Potential role of apoptotic macrophages in pulmonary inflammation and fibrosis. J Cell Physiol 2003;194:215–224. [DOI] [PubMed] [Google Scholar]
- 18.Trottier Y, Lutz Y, Stevanin G, Imbert G, Devys D, Cancel G, Saudou F, Weber C, David G, Tora L, et al. Polyglutamine expansion as a pathological epitope in Huntington's disease and four dominant cerebellar ataxias. Nature 1995;378:403–406. [DOI] [PubMed] [Google Scholar]
- 19.Dunah AW, Jeong H, Griffin A, Kim YM, Standaert DG, Hersch SM, Mouradian MM, Young AB, Tanese N, Krainc D. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease. Science 2002;296:2238–2243. [DOI] [PubMed] [Google Scholar]
- 20.Luthi-Carter R, Strand A, Peters NL, Solano SM, Hollingsworth ZR, Menon AS, Frey AS, Spektor BS, Penney EB, Schilling G, et al. Decreased expression of striatal signaling genes in a mouse model of Huntington's disease. Hum Mol Genet 2000;9:1259–1271. [DOI] [PubMed] [Google Scholar]
- 21.Bradsher J, Coin F, Egly JM. Distinct roles for the helicases of TFIIH in transcript initiation and promoter escape. J Biol Chem 2000;275:2532–2538. [DOI] [PubMed] [Google Scholar]
- 22.Hirooka M, Hirota M, Kamada M. Renal lesions in Cockayne syndrome. Pediatr Nephrol 1988;2:239–243. [DOI] [PubMed] [Google Scholar]
- 23.Takebayashi Y, Nakayama K, Kanzaki A, Miyashita H, Ogura O, Mori S, Mutoh M, Miyazaki K, Fukumoto M, Pommier Y. Loss of heterozygosity of nucleotide excision repair factors in sporadic ovarian, colon and lung carcinomas: implication for their roles of carcinogenesis in human solid tumors. Cancer Lett 2001;174:115–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.