Abstract
Rationale: Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrotic lung disorder of unknown cause. Several studies suggest an association between Epstein-Barr virus pulmonary infection and the development of IPF.
Objectives: To determine whether reduction of γ-herpesvirus reactivation from latency would alter progressive lung fibrogenesis in an animal model of virus-induced pulmonary fibrosis.
Methods: IFN-γ receptor–deficient (IFN-γR−/−) mice infected intranasally with murine γ-herpesvirus 68 (MHV68) develop lung fibrosis that progresses for up to at least 180 days after initial infection. Viral replication during the chronic phase of infection was controlled by two methods: the administration of cidofovir, an antiviral drug effective at clearing lytic but not latent virus, and by using a mutant γ-herpesvirus defective in virus reactivation from latency.
Measurements and Main Results: Ten percent of the asymptomatic MHV68-infected animals that received antiviral treatment beginning on Day 45 postinfection had severe pulmonary fibrosis compared with 40% of the control saline-treated animals. Absence of severe fibrosis was also observed in IFN-γR−/− mice infected with the defective reactivation mutant MHV68 v-cyclin stop. Decreased fibrosis was associated with lower levels of transforming growth factor-β, vascular endothelial growth factor, and markers of macrophage alternative activation. When antiviral treatment was administered on Day 60 in symptomatic animals, survival improved from 20 to 80% compared with untreated symptomatic animals, but lung fibrosis persisted in 60% of the mice.
Conclusions: MHV68-induced fibrosis is a result of viral lytic replication during chronic lung herpesvirus infection in mice. We speculate that antiviral therapy might help to control lung fibrosis in humans with IPF and associated herpesvirus infection.
Keywords: lung, fibrosis, herpesvirus, antiviral
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive disease without a proven treatment. Several studies suggest an association between herpesvirus infection in the lung and IPF.
What This Study Adds to the Field
Using a murine herpesvirus–induced lung fibrosis model, we show that antiviral therapy controls virus replication during chronic infection and prevents lung fibrosis. Patients with IPF with associated herpesvirus infection might benefit from antiviral therapy.
Idiopathic pulmonary fibrosis (IPF) is a progressive, lethal, interstitial lung disease with no proven effective therapy other than lung transplantation (1). Although the cellular and molecular pathways that drive the pathogenesis of IPF are complex and not fully delineated, increasing evidence suggests that a key event in its pathogenesis is ongoing alveolar epithelial injury in association with an abnormal host repair response. Alveolar epithelial injury induces the proliferation of fibroblasts and their differentiation into myofibroblasts and increased deposition of extracellular matrix that results in distortion of alveolar capillary units, fibrosis, and loss of lung function (2, 3).
Several studies have implicated viral infection as a cause of ongoing epithelial injury in IPF and therefore an important factor in pathogenesis. Specifically, Epstein-Barr virus (EBV) protein and DNA have been detected in 40–70% of lung tissue from patients with IPF compared with 10–17% of lung tissue from control subjects (4–7). Using polymerase chain reaction to detect viral DNA in lung specimens, we detected cytomegalovirus, EBV, and human herpesvirus-8 (HHV-8) at a significantly higher frequency in patients with IPF compared with patients with non-IPF lung diseases (5). Using immunohistochemistry analysis for detection of viral protein, we could detect HHV-8 and EBV viral antigen in epithelial cells of patients with IPF, confirming infection of lung tissue rather than amplification by polymerase chain reaction of infected lymphocytes traversing the lung. Finally, treatment of a patient shedding EBV in the respiratory tract, using an agent that controls lytic EBV infection, resulted in decreased viral load in the lung and concomitant stabilization of lung function (5).
We have developed a model of chronic herpesvirus-induced pulmonary fibrosis infection using the herpesvirus MHV68, a natural pathogen of rodents that is closely related to the human γ-herpesviruses, HHV-8 and EBV (8–10). Because IFN-γ plays an important role in controlling chronic MHV68 infection in rodents and has antifibrotic functions, we studied MHV68 pulmonary infection in IFN-γ receptor (IFN-γR)-deficient mice (IFN-γR−/−) (11–13). Acute replication of the virus in lung epithelial cells resulted in a lymphocytic pneumonia followed by establishment of virus latency in B cells, dendritic cells, macrophages, and lung epithelial cells. We found that chronic infection in IFN-γR−/− mice causes moderately severe, progressive pulmonary fibrosis with histologic features reminiscent of IPF (14). In addition, there is increased expression of the profibrotic cytokines transforming growth factor (TGF)-β and IL-13 in these mice. Finally, derangement of alveolar type II cells and surfactant proteins is found in infected animals, similar to what has been reported in some families with familial IPF (15).
MHV68 reactivation from latency to lytic replication could cause repetitive lung injury and generation of dysregulated reparative responses. In this article, we use this unique model to determine whether control of virus replication, using an antiviral agent during the chronic phase of the infection, would prevent progression of lung fibrosis. The antiviral agent chosen, cidofovir, is a nucleoside analog that has been shown to be more efficacious in blocking MHV68 replication than other antiviral nucleosides (16). We found that prevention of viral reactivation from latency arrested the fibrogenic process induced by the virus.
METHODS
Animals and Animal Treatment
IFN-γR−/− mice were inoculated intranasally on Days 0, 15, and 30 with 1 × 105 plaque-forming units of MHV68 mixed with Dulbecco's modified Eagle's medium–10% fetal calf serum to a total volume of 40 μl. Inoculation of supernatant of homogenated uninfected NIH3T12 cells was used as a mock-infection control. Virus-infected and mock-infected mice were each divided into two groups: phosphate-buffered saline (PBS) or cidofovir (Gilead Sciences, Foster City, CA). Cidofovir was given at 7.5 or 15 mg/kg of body weight, and was mixed in saline solution and administered subcutaneously in the scruff of the neck. Mice received cidofovir daily for 2 days, or an equivalent volume of saline, and then every third day until the time of sacrifice. Similarly, mock-infected mice received cidofovir or saline according to the same schedule (16). Additional details on the methods are provided in the online supplement.
Histology and Immunofluorescence
Five or six mice were used per group for histopathology analysis. After inflation and fixation with 4% paraformaldehyde for 24 hours, lung tissue was paraffin embedded, sectioned, and stained with hematoxylin and eosin for routine histologic examination and Masson trichrome staining to delineate collagen. Indirect immunofluorescence was performed in sections from frozen blocks. Slides were fixed with 4% paraformaldehyde for 20 minutes at room temperature. Anti-MHV68 was used as described previously and anti-vascular endothelial growth factor (VEGF; Calbiochem/EMD Chemicals, San Diego, CA) antibody was used according to the recommendations of the manufacturer (17).
Morphometric Analysis: Pathology Score
Lung pathology was evaluated by an independent blinded veterinary pathologist, who scored Masson trichrome–stained lung sections on the basis of a scale from 0 to 4. Additional details on the method are included in the online supplement.
Hydroxyproline Assay
Hydroxyproline content in whole mouse lung was used to quantify lung collagen content and was measured colorimetrically by a method described previously, with modifications (18). Additional detail on the method for making these measurements is provided in the online supplement.
Determination of Cytokine Levels
Mouse tumor necrosis factor-α, IL-5, IL-6, IL-13, IFN-γ, macrophage inflammatory protein-1α, and monocyte chemotactic protein (MCP)-1 levels were measured in bronchoalveolar lavage (BAL) fluid and serum, using a multiplex bead immunoassay (Linco/Millipore, St. Charles, MO) according to the manufacturer's recommendations. Additional detail on the method for making these measurements is provided in the online supplement.
Western Blot
Whole cell extracts from lung tissue samples were prepared (0.15% Nonidet P-40, 50 mM N-2-hydroxyethylpiperazine-N′-ethanesulfonic acid [pH 7.0], 250 mM NaCl, 5 mM ethylenediaminetetraacetic acid [pH 8.0]). Aliquots of lung lysates (40 μg) and BAL fluid (12 μl) were resolved in 4–20% sodium dodecyl sulfate–polyacrylamide gels, and transferred onto nitrocellulose membranes. Western blotting for TGF-β (BD Biosciences Immunocytometry Systems, San Jose, CA), VEGF (Calbiochem/EMD Chemicals), and fibronectin (Sigma, St. Louis, MO) was performed according to the manufacturer's recommendations. Antibody against Ym1/2 (kindly provided by T. Iwanaga, Hokkaido University, Japan) was used at a 1:2,000 dilution. Filters were stripped and reprobed with an antiserum against β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) and against surfactant A (Chemicon International/Millipore, Temecula, CA) as a loading control for lung homogenates and BAL lavage samples, respectively. Films were scanned with a FluorChem MultiImage apparatus and analyzed with AlphaEase-FC software (Alpha Innotech, San Leandro, CA).
Fibronectin Gene Transcription
We determined fibronectin transcription as previously described (19, 20). Additional detail on the method is provided in the online supplement.
Gelatin Zymography
Gelatin zymography was performed with a 9% sodium dodecyl sulfate–polyacrylamide gel saturated with gelatin (300 bloom; Sigma) at 1 mg/ml as described previously (21). Additional detail on the method for making these measurements is provided in the online supplement.
Arginase Activity
Arginase activity was measured in lung lysates as previously described (19). Additional detail on the method is provided in the online supplement.
MHV68 Glycoprotein B, Fizz1, and Ym1/2 Expression in the Lung
Total RNA was extracted from lung tissue with an RNeasy mini kit according to the manufacturer's recommendations (Qiagen, Valencia, CA). cDNA was generated from 0.5 to 5 μg of total RNA, using random hexamers and ThermoScript reverse transcriptase (Invitrogen, Carlsbad, CA). Additional detail on the method is provided in the online supplement.
Bleomycin Instillation and Cidofovir Treatment
Mice were given a single intratracheal instillation of bleomycin or PBS and then killed 21 days post-treatment as we previously described (22). Beginning 1 day after intratracheal instillation of bleomycin or PBS, mice received cidofovir (15 mg/kg) or the equivalent volume of saline solution subcutaneously. Treatment was given every third day until the time of sacrifice. An average of five mice was allocated per group in two independent experiments. Additional details on the method are provided in the online supplement.
Statistical Analyses
Data were plotted and statistically analyzed with InStat 3 and GraphPad Prism 4 (GraphPad Software, San Diego, CA). Nonparametric analysis of variance and Dunn's multiple comparison tests were performed for cytokine concentrations. Tidal volume, arginase activity, number of cells, and fibronectin transcription results were analyzed by unpaired t test.
RESULTS
Treatment with Cidofovir Is Associated with Clearance of Viral Antigen and Decreased Fibrosis
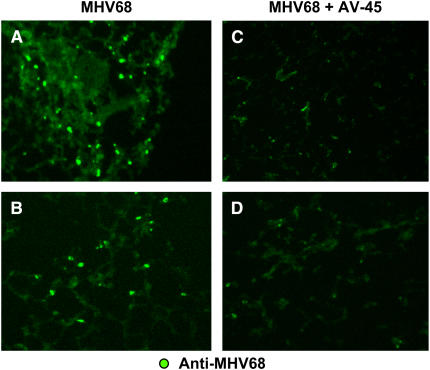
We have shown previously that MHV68 infection in IFN-γR−/− mice causes severe pneumonia during the acute phase of the infection (< 15 d) and persistent lymphocytic perivascular, peribronchial, and subpleural infiltrates during the early chronic phase of the infection. Interstitial inflammation is followed by progressive pulmonary fibrosis that is evident by Day 120–180 of infection (17). To study the effect of antiviral treatment on virus-induced fibrosis, we initiated antiviral treatment in mock- and virus-infected animals on Day 45 after infection, a time point before fibrosis is seen. Mock- and virus-infected mice were treated subcutaneously with cidofovir (or saline) biweekly for 4 months and compared with mock- and virus-infected animals receiving saline solution. Mice were killed 120 days after initial infection. Viral antigen clearance by the antiviral treatment was confirmed by immunofluorescence analysis with an anti-MHV68 polyclonal antibody. Frozen lung sections from infected mice without antiviral treatment showed abundant positive signals corresponding to viral proteins. In contrast, lung preparations from animals receiving antiviral treatment resulted in a marked decrease in detection of viral antigens (Figures 1A–1D).
Figure 1.
Viral antigen clearance after cidofovir treatment in murine γ-herpesvirus 68 (MHV68)-infected IFN-γ receptor–deficient (IFN-γR−/−) mice. (A–D) Immunostained frozen lung sections from MHV68-infected IFN-γR−/− mice, taken on Day 120 postinfection: (A and B) Mice that received saline solution beginning on Day 45; (C and D) mice that received antiviral treatment beginning on Day 45 (AV-45). Each panel represents a different animal. Original magnification: (A–D) ×10.
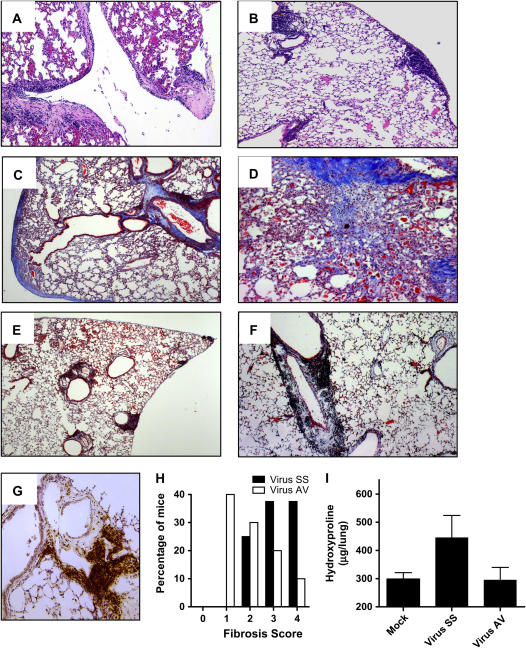
Histopathologic analysis of lungs from chronic virus-infected mice shows that a high percentage of the mice have subpleural and perivascular lymphocytic infiltrates associated with interstitial and subpleural fibrosis (Figures 2A, 2C, and D); and a lower percentage showed predominantly inflammatory infiltrates with minimal collagen deposition. In sharp contrast, 90% of the mice that received antiviral treatment lacked alveolar remodeling and fibrosis despite the presence of lymphocytic infiltrates in subpleural and perivascular areas (Figures 2B, 2E, and 2F). The majority of cells in the lymphocytic infiltrates were B cells, as demonstrated by immunohistochemical analysis with an antibody that detects the B-cell marker B220 (Figure 2G). As can be seen in Figure 2H, morphometric analysis of lung sections of infected mice indicates that fibrosis was greater in mice infected without antiviral treatment. The significant reduction of pulmonary fibrosis in antiviral agent-treated animals was supported by determination of hydroxyproline levels in lung samples. By this measurement, mice that received cidofovir have less accumulation of collagen than do infected mice receiving saline solution (Figure 2I). After 8 weeks of treatment lung function was measured with a whole body plethysmograph. As we have reported previously and consistent with a restrictive pulmonary defect, lung function showed significant reduction in tidal volume in infected IFN-γR−/− animals. Antiviral treatment improved the pulmonary function of virus-infected animals in parallel with the diminution of lung fibrosis (data not shown).
Figure 2.
Prevention of lung fibrosis and persistent lymphocytic infiltrates in virus-infected mice treated with cidofovir. (A) Hematoxylin and eosin (H&E) staining of MHV68-infected lung on Day 120. Notice the thickening of the pleura and alveolar walls. (B) H&E-stained section from MHV68-infected mouse receiving antiviral treatment from Day 45 of infection. Lymphocytic infiltrates are observed in the subpleural, perivascular, and peribronchial areas, but there is no thickening of the pleura or alveolar walls. (C and D) Masson trichrome staining of lung section from MHV68-infected mice on Day 120; mice were treated with saline solution. Collagen deposition is demonstrated by blue staining. (E and F) Masson trichrome staining of lung section from MHV68-infected lung receiving antiviral treatment. Notice the absence of fibrosis. Each panel represents a different animal. Original magnification: (A–C and E), ×10; (D and F) ×20. (G) Immunohistochemical analysis of lung from antiviral-treated mice, using anti-B220–specific antibody. (H) Semiquantitative morphometric analysis of lung histopathology in virus-infected mice with (Virus AV) or without (Virus SS) antiviral therapy; analyzed on Day 120 of infection. Infected mice showed higher pathology scores corresponding to thickening of the interalveolar septa and thickening of the pleura. In contrast, mice receiving antiviral treatment had lymphocytic infiltrates. (n = 18 for Virus SS and n = 12 for Virus AV). AV = antiviral agent; SS = saline solution. (I) Hydroxyproline determination in lung lysates from mock and infected mice shows that the antiviral treatment decreases collagen content compared with virus-infected lungs of saline-treated animals.
Decreased Inflammatory Responses in MHV68-infected IFN-γR−/− Mice Treated with Cidofovir
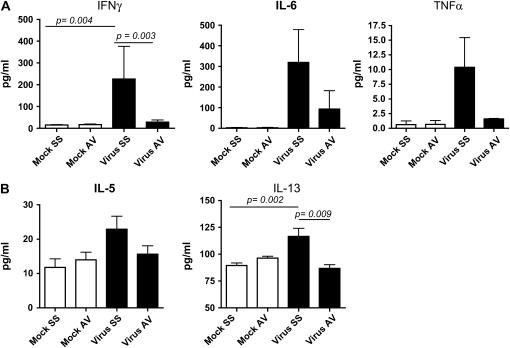
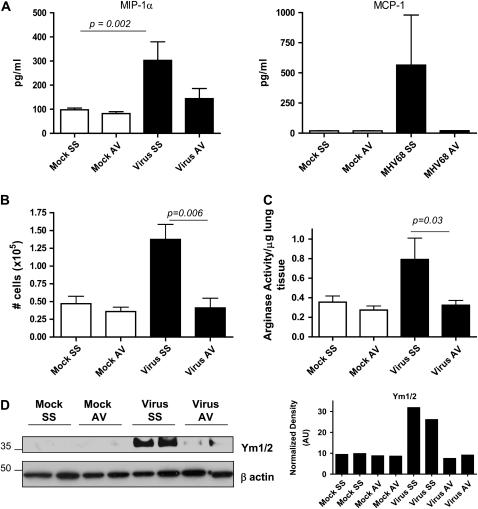
We also determined whether control of viral replication diminished immune responses such as macrophage recruitment and helper T-cell type 2 (Th2) differentiation, two processes that have been correlated directly with the virus-induced fibrogenic process. Analysis of cytokine levels in BAL fluid on Day 120 postinfection demonstrated that antiviral drug-treated animals had lower levels of IFN-γ (p = 0.001), IL-6, and tumor necrosis factor-α, as well as the Th2 cytokines IL-5 (p = 0.031) and IL-13 (0.005), than did MHV68-infected mice without antiviral treatment and were similar to levels in mock-infected animals treated with either saline or cidofovir (Figure 3). BAL fluid levels of the monocyte chemokines macrophage inflammatory protein-1α (p = 0.0042) and MCP-1 were also decreased by antiviral treatment (Figure 4A). The total number of cells in BAL fluid was lower in antiviral-treated animals compared with infected mice receiving saline solution (Figure 4B).
Figure 3.
Decreased levels of cytokines after treatment in MHV68-infected IFN-γR−/− mice. (A) IFN-γ, IL-6, and tumor necrosis factor (TNF)-α levels were measured in bronchoalveolar lavage (BAL) fluid from mock and MHV68-infected IFN-γR−/− mice after treatment with saline solution (SS) or the antiviral agent (AV), which was begun on Day 45 postinfection. Levels of cytokines were determined in a multiplex bead immunoassay on Day 120. (B) IL-5 and IL-13 levels were measured in BAL fluid 120 days postinfection in mock and MHV68-infected IFN-γR−/− mice treated with saline solution or antiviral agent. Shown are means and SEM (n = 4–10 mice in each group).
Figure 4.
Antiviral treatment decreases recruitment and activation via the alternative pathway of macrophages in MHV68-infected IFN-γR−/− mice. (A) Macrophage inflammatory protein (MIP)-1α and monocyte chemotactic protein (MCP)-1 levels were measured in bronchoalveolar lavage (BAL) fluid from mock and MHV68-infected IFN-γR−/− mice on Day 120 after chronic treatment with saline solution (SS) or antiviral agent (AV) begun on Day 45. Levels of cytokines were determined in a multiplex bead immunoassay. Shown are means and SEM (n = 4–10 per group). (B) BAL was performed in mock and virus-infected animals after 4 months of saline solution or antiviral therapy (n = 4–8 mice in each group). Cell counts show a significant diminution in the numbers of cells after antiviral therapy. (C) Measurement of arginase I activity in lung samples of mock and infected animals on Day 120 after treatment with saline solution or antiviral begun on Day 45. Arginase activity significantly diminished after antiviral treatment. Shown are means and SEM (n = 3–7 per group). (D) Lung homogenate from mock and virus-infected mice treated with saline solution or antiviral were subjected to Western blot analysis for Ym1/2 proteins. Antiviral treatment in infected mice was associated with lower levels of Ym proteins in the lungs. Blots were stripped and reprobed with an anti–β-actin antibody to normalize expression of Ym1/2.
Lower Arginase Activity in MHV68 IFN-γR−/− Mice Treated with Antiviral
Macrophages involved in healing and repair are activated by an alternative pathway associated with up-regulation of arginase, an enzyme that promotes cell proliferation and collagen synthesis through the production of l-proline (23, 24). We have established that macrophage recruitment and high levels of arginase I activity in the lung are associated with chronic MHV68 infection and the development of lung fibrosis (19). We extended these studies to antiviral-treated animals and we found that arginase activity was significantly decreased after cidofovir treatment (Figure 4C). Antiviral treatment also diminished the expression of other virus-induced markers of alternative activation in macrophages such as the chitinase-like secretory lectins Ym1 and Ym2 (25, 26) (Figure 4D).
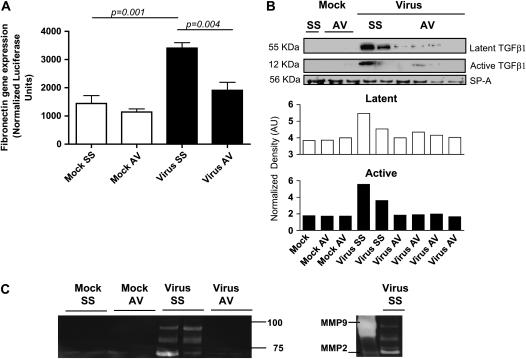
Alternatively, activated macrophages contribute to wound repair releasing extracellular components such as fibronectin, metalloproteinases, and profibrotic growth factors (27, 28). We analyzed induction by BAL of fibronectin transcription in NIH3T3 cells transfected with a luciferase reporter under the control of the fibronectin promoter. We found higher induction of fibronectin gene transcription with BAL fluid samples from MHV68-infected mice compared with samples from mock-treated mice and a significant reduction in samples from animals treated with cidofovir begun on Day 45 (Figure 5A). We have also shown that virus-induced lung fibrosis is linked with high levels of TGF-β1, one of the most potent regulators of connective tissue synthesis. Analysis of TGF-β1 levels in BAL fluid from infected mice receiving saline solution or antiviral treatment demonstrated that cidofovir treatment begun on Day 45 reduced the expression of latent and active TGF-β1 in the lungs of infected animals (Figure 5B). Activation of TGF-β1 is induced by high levels of IL-13 using a plasmin/serine protease and matrix metalloproteinase (MMP)-9–dependent mechanism. IL-13 also is a potent stimulator of other MMPs such as MMP-2, -12, -13, and -14 (29, 30). We determined MMP-2 and MMP-9 gelatinolytic activity in BAL samples from MHV68-infected IFN-γR−/− mice during the chronic phase of infection. BAL fluid samples from infected animals showed high gelatinolytic activity, compared with mock-treated animals, that corresponded with MMP-9 and MMP-2 activity (Figure 5C). The use of an antiviral agent in infected animals markedly diminished this gelatinolytic activity in BAL samples (Figure 5C).
Figure 5.
Control of virus replication diminished fibrogenesis in MHV68 IFN-γR−/− infected mice. (A) NIH3T3 cells stably transfected with a fibronectin reporter were cultured for 24 hours in the presence of bronchoalveolar lavage (BAL) fluid from mock and virus-infected animals treated with saline solution (SS) or antiviral (AV). Afterward, the cells were harvested and fibronectin gene transcription was measured by luminescence (n = 4 per group). MHV68 infection stimulates gene transcription of a reporter under control of the fibronectin promoter. AV treatment significantly diminished fibronectin transcription. (B) Western blot analysis for the latent and active forms of transforming growth factor (TGF)-β in BAL fluid samples collected on Day 120. The blot was stripped and reprobed with an anti-surfactant A (SP-A) antibody to normalize expression of latent (open columns) and active (solid columns) TGF-β. Decreased levels of active TGF-β were found in infected mice treated with the antiviral agent. (C) Gelatin zymography of BAL fluid samples from mock and MHV68-infected animals at the indicated times points after infection. High gelatinolytic activity was observed in samples from infected mice compared with mock animals and infected animals treated with antiviral agent. Purified matrix metalloproteinase (MMP)–2 and MMP-9 were used to identify zymography bands in the samples from virus-infected animals treated with SS.
Antiviral Treatment in Symptomatic Animals Improved Clinical Disease and Severity of Lung Fibrosis
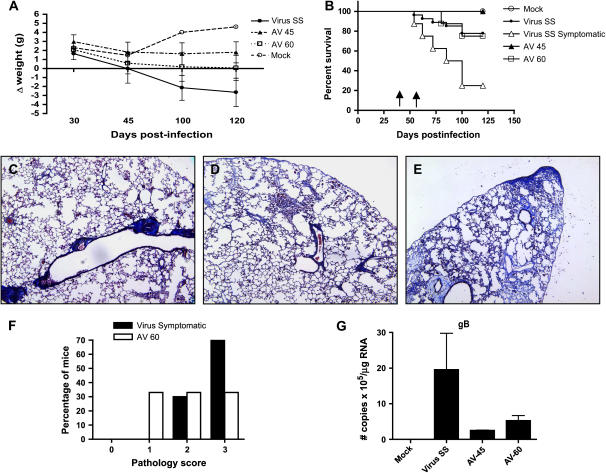
MHV68 infection of IFN-γR−/− mice resulted in substantial decline in body weight after Day 45 postinfection. This weight loss was associated with progressive clinical deterioration assessed by observation of ruffled fur and lethargy. Antiviral treatment begun on Day 45 to asymptomatic mice prevented progression of fibrosis, loss of weight, and mortality (Figures 6A and 6B). To determine whether antiviral treatment was effective in improving clinical disease and fibrosis in symptomatic mice, we administrated cidofovir on Day 60 postinfection in a group of infected mice, who were showing clinical signs of disease. This therapeutic regimen prevented the weight loss and diminished the mortality compared with symptomatic mice receiving saline solution (Figures 6A and 6B).
Figure 6.
Antiviral treatment in symptomatic mice improved clinical disease and survival. (A) Body weight was tracked for mock (open circles) and MHV68-infected mice treated with saline solution (Virus SS; solid circles) or antiviral from Day 45 (AV-45; solid triangles) or from Day 60 (AV-60; open squares). Data are presented as the difference in body weight from Day 0 of infection. More severe illness was observed in SS-treated mice. A beneficial effect was observed with the antiviral treatment. Number of mice: mock (n = 10), SS (n = 9), AV-45 (n = 5), AV-60 (n = 6). Data are representative of three different experiments. (B) Survival is plotted versus time postinfection for mock (open circles), MHV68-infected mice treated with saline solution (Virus SS; solid circles), MHV68-infected mice treated with antiviral begun on Day 45 (AV-45; solid triangles), and symptomatic MHV68-infected mice treated with antiviral (AV-60; open squares) or saline solution begun on Day 60 (virus symptomatic; open triangles). Number of mice: mock (n = 20), SS (n = 26), AV-45 (n = 19), AV-60 (n = 8), virus symptomatic (n = 8). Data represent three pooled different experiments. The Kaplan-Meier survival curves were significantly different as follows: mock versus SS (p = 0.0087), SS versus AV-45 (p = 0.03), virus symptomatic versus AV-60 (p = 0.03). Arrows indicate the time points when AV treatment was begun on Day 45 and Day 60. (C–E) Masson trichrome staining of lung sections from MHV68-infected mice receiving antiviral treatment from Day 60 postinfection. Each panel represents a different animal. Notice the perivascular fibrosis in (C), mild interstitial fibrosis in (D), and the severe interstitial and subpleural deposition of collagen in (E). Original magnification: ×10. (F) Semiquantitative morphometric analysis of lung histopathology in symptomatic virus-infected mice with or without antiviral therapy analyzed on Day 120 of infection. Virus-infected symptomatic mice showed a high proportion of multiple fibrotic foci with thickness of the pleura (score, 4). A reduction in the severity of the fibrosis was found in symptomatic mice receiving antiviral treatment. Number of mice: virus symptomatic (n = 8); AV-60 (n = 8). (G) Real-time reverse transcription-polymerase chain reaction (RT-PCR) quantifying viral transcripts for the late lytic gene gB in the lungs of mock and virus-infected mice treated with SS or AV (n = 3). Negative amplification was obtained in mock-infected mice. A lower number of copies was observed in MHV68-infected mice treated with cidofovir.
Histopathology analysis of the lungs of mice with antiviral treatment begun on Day 60 postinfection showed lung fibrosis limited to perivascular and peribronchial areas in 30% of the mice (Figure 6C), and some degree of interstitial fibrosis with or without pleural thickening in 70% of them (Figures 6D and 6E). Conversely, 70% of the symptomatic mice without antiviral treatment had lung fibrosis with pleura thickening (Figure 6F). The severity of the fibrosis correlated with detectable virus in the spleen and higher number of copies of transcripts of the viral lytic gene glycoprotein B (gB) in the lungs, suggesting virus reactivation from latency. Antiviral treatment begun on Day 45 and 60 diminished the number of gB transcripts by eight- and fourfold, respectively, compared with saline solution–treated animals (Figure 6G).
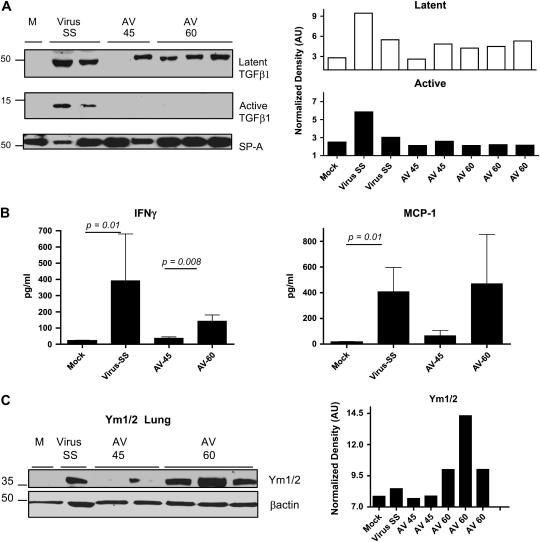
The reduction in the severity of the fibrosis and virus replication in symptomatic mice receiving antiviral was associated with reduced levels of active TGF-β and lower levels of IFN-γ in BAL fluid (Figures 7A and 7B). The antiviral treatment begun on Day 60 was ineffective in diminishing levels of monocytic chemokines such as MCP-1 (Figure 7B). Lungs of infected mice receiving antiviral beginning on Day 60 had high levels of the chitinase-like protein Ym1/2, indicating the presence of macrophages activated by the alternative pathway (Figure 7C).
Figure 7.
Antiviral treatment in symptomatic mice fails to control alternative activation of macrophages. (A) Western blot analysis for the latent and active forms of TGF-β in bronchoalveolar lavage (BAL) fluid samples collected on Day 120. Blot was stripped and reprobed with an anti-surfactant A (SP-A) antibody to normalize expression of latent (open columns) and active (solid columns) TGF-β. Decreased levels of active TGF-β were found in symptomatic infected mice treated with the antiviral agent. (B) IFN-γ and monocyte chemotactic protein (MCP)–1 levels were measured in BAL fluid from mock and MHV68-infected IFN-γR−/− mice after treatment with saline solution (SS) or antiviral, which was begun on Day 45 or 60 postinfection (AV-45 and AV-60, respectively). Levels of cytokines were determined in a multiplex bead immunoassay on Day 120. Shown are means and SEM. Number of mice: mock (n = 5); AV-45 (n = 5); virus SS (n = 6); AV-60 (n = 6). (C) Lung homogenate from mock and virus-infected mice treated with saline solution or antiviral were subjected to Western blot analysis for Ym1/2 proteins. Antiviral begun on Day 60 postinfection failed to lower levels of Ym proteins in the lungs. Blot was stripped and reprobed with an anti–β-actin antibody to normalize expression of Ym1/2.
IFN-γR−/− Mice Infected with Reactivation-deficient Virus (Mutant v-Cyclin Stop MHV68) Failed to Develop Lung Fibrosis
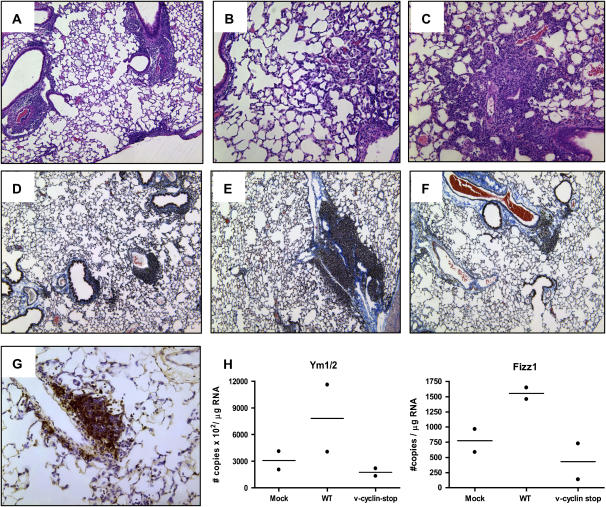
γ-Herpesviruses encode a homolog of mammalian D-type cyclins. The v-cyclin encoded by MHV68 induces cell cycle progression and is an oncogene (31). MHV68 containing a translation stop codon within the v-cyclin gene has been generated and this mutant virus (mutant v-cyclin stop MHV68) has been shown to be significantly compromised in its capacity to reactivate from latency (32). v-Cyclin stop virus has been reported to replicate normally in fibroblasts in vitro and during acute infection in the spleen, liver, and lungs in vivo (32). Thus, v-cyclin stop has the normal progression of acute infection followed by latent infection, like wild-type virus, but does not reactivate from latency and undergo replication. To confirm that lung fibrosis is associated with virus reactivation and lytic replication, we infected IFN-γR−/− mice with the v-cyclin stop MHV68. In concordance, histopathology analysis of lungs of mice infected with v-cyclin stop virus showed, during the acute phase of infection, lymphocytic pneumonia characterized by the presence of peribronchial and perivascular lymphocytic infiltrates, macrophages, and fibrotic areas (Figures 8A–8C). On Day 150 lungs from IFN-γR−/− mice infected with v-cyclin stop virus had predominantly lymphocytic infiltrates and vasculitis with mild fibrosis limited to perivascular, peribronchial, and paraseptal areas (Figures 8D–8F). Similar to antiviral-treated wild-type virus–infected mice, immunohistochemical analysis in infected lungs with the v-cyclin mutant virus showed lymphocytic infiltrates containing predominantly B220-positive cells (Figure 8G). Accumulation of alveolar macrophages in the lung was not evident and low expression of alternative activation markers was found (Figure 8H). Thus, infection with a virus that does not reactivate from latency resulted in limited fibrosis and failure to activate alveolar macrophages by the alternative pathway.
Figure 8.
Infection with the reactivation-deficient v-cyclin stop MHV68 failed to produce lung fibrosis and alternative activation of macrophages. (A–C) Hematoxylin-and-eosin staining of v-cyclin stop MHV68–infected lung on Day 20. v-Cyclin stop MHV68 has an acute replication similar to that of wild-type virus. Notice the lymphocytic infiltrates around blood vessels and airways, and the accumulation of alveolar macrophages and fibroblasts. (D–F) Masson trichrome staining of lung sections from v-cyclin stop MHV68–infected mice on Day 150. Collagen deposition is demonstrated by blue staining. Notice the absence of interstitial fibrosis. Each panel represents a different animal. Original magnification: (A and D–F) ×10; (B and C) ×20. (G) Immunohistochemical analysis of v-cyclin stop virus–infected lung, using an anti-B220 antibody. (H and I) Quantitative reverse transcription–polymerase chain reaction was used to determine the levels of Ym and Fizz1, respectively, in lungs of mock, wild-type (WT)–infected, and v-cyclin stop MHV68–infected IFN-γR−/− mice on Day 120 postinfection. Data are normalized against β-actin.
Cidofovir Fails to Control VEGF Expression and Lung Fibrosis in Bleomycin Model
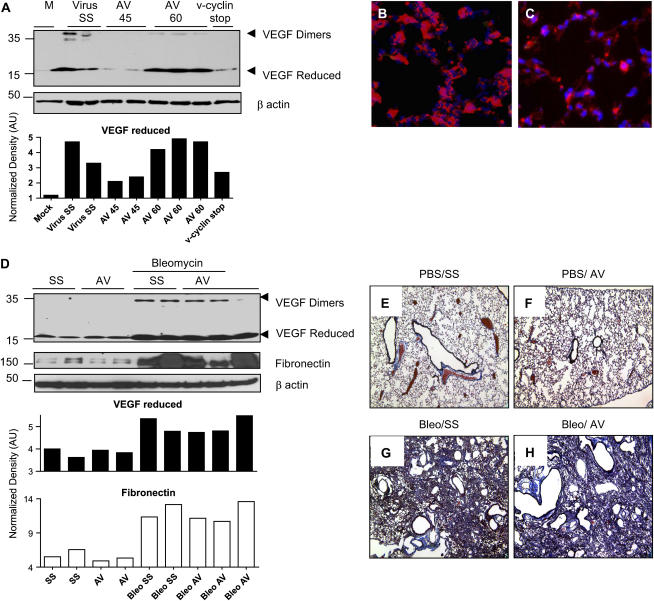
Stimulation of VEGF has been shown to be associated with Th2 inflammatory responses and lung remodeling in animal models and human patients (33–35). Western blot analysis of lung lysates from chronic MHV68-infected IFN-γR−/− mice showed high levels of expression of VEGF. Antiviral therapy begun on Day 45 in asymptomatic animals prevented up-regulation of VEGF but was ineffective when the antiviral was administrated to symptomatic animals (AV-60) (Figure 9A). Levels of VEGF were also diminished in IFN-γR−/− mice infected with the mutant v-cyclin stop MHV68. To determine the source of VEGF in infected mice, we performed an immunofluorescence assay. We found high expression of VEGF in hyperplastic alveolar epithelial cells and macrophages of infected animals. In contrast, low signal was obtained in lung samples from mice treated with antiviral from Day 45 postinfection (Figures 9B and 9C). Cidofovir treatment has been associated with inhibition of VEGF in human papillomavirus-18 (HPV-18) cervical carcinoma cell lines. To determine whether cidofovir inhibited VEGF expression and fibrosis in another lung fibrosis model, we administered cidofovir to IFN-γR−/− mice after bleomycin instillation. Cidofovir was initiated at 15 mg/kg of body weight on Day 1 after bleomycin instillation and continued every third day until sacrifice. VEGF expression was determined in lung lysates on Day 21 after bleomycin instillation. High levels of VEGF were obtained in bleomycin-treated animals with or without antiviral treatment (Figure 9D). Furthermore, cidofovir treatment failed to decrease fibrogenesis in bleomycin-treated animals as analyzed by the expression of the extracellular matrix component fibronectin and by histopathology analysis of the lungs, using Masson trichrome staining (Figures 9D–9H).
Figure 9.
Cidofovir treatment in a bleomycin fibrosis model is ineffective in controlling vascular endothelial growth factor (VEGF) expression and fibrosis. (A) Western blot analysis, using an anti-VEGF antibody in lung homogenates collected on Day 120–150. High levels of VEGF were found in wild-type MHV68–infected IFN-γR−/− mice receiving saline solution (Virus SS) and symptomatic infected mice treated with the antiviral agent from Day 60 of infection (AV-60). Low VEGF levels were obtained in infected mice treated with antiviral from Day 45 of infection (AV-45) and also in mice infected with the v-cyclin stop mutant MHV68. The blot was stripped and reprobed with an anti–β-actin antibody to normalize expression of reduced VEGF. (B) VEGF expression was detected in hyperplastic alveolar epithelial cells and alveolar macrophages by immunofluorescence analysis of lung of MHV68-infected mice on Day 120 (red). Slides were counterstained with 4′,6-diamidino-2-phenyindole, which stains nuclei blue. (C) Frozen section from a mouse treated with antiviral from Day 45 and stained with anti-VEGF antibody (red) shows decreased VEGF expression. (D) VEGF and fibronectin expression were determined in lung lysates from mock and bleomycin-treated mice receiving saline solution (SS) or antiviral (AV). Comparable VEGF and fibronectin up-regulation was observed after bleomycin treatment in mice with or without antiviral treatment. The blot was stripped and reprobed with an anti–β-actin antibody to normalize expression of reduced VEGF and fibronectin. (E–H) Masson trichrome staining of lungs of IFN-γR−/− mice on Day 21 after intratracheal inoculation of phosphate-buffered saline or bleomycin and after receiving subcutaneously cidofovir (antiviral, AV) or saline solution (SS) every 3 days. Collagen deposition is denoted in blue.
DISCUSSION
The pathogenesis of IPF is not fully delineated, but a critical event may be ongoing injury of the lung epithelium. Chronic herpesvirus infection is a potential cause of epithelial cell dysfunction, either by causing direct epithelial injury via virus lytic replication or by altering cell phenotype via a latent infection that induces immune responses that promote abnormal repair and fibrosis. We found that chronic herpesvirus lung infection in a mouse biased toward a Th2-type response resulted in progressive pulmonary fibrosis. Using this animal model, we demonstrate that active MHV68 replication during the chronic phase of infection is required for the development of virus-induced lung fibrosis. This finding has significant implications when developing an antiviral strategy in patients with IPF and infected with herpesvirus, as current antiherpesvirus treatments control only viruses undergoing lytic but not latent infection.
Prevention of viral replication with the antiviral cidofovir in chronically infected mice, beginning on Day 45 postinfection, mediated virus clearance, decreased lung levels of proinflammatory and profibrotic cytokines, and had a dramatic effect of lung fibrosis. These findings were associated with prevention of mortality and improvement of the clinical disease. Histopathologic analyses of the lungs of MHV68-infected mice treated with cidofovir showed persistence of lymphocytic infiltrates that in the past we have shown to be B cells (17). Although antiviral treatment is effective only against lytic forms of the virus, ongoing productive replication is essential for maintaining high levels of latently infected cells. Thus, we found that mice treated with cidofovir had a reduction in the number of copies of transcripts of the viral latent genes M2 and M11, as expected (data not shown). Studies showed that mice infected intranasally with MHV68 and treated with cidofovir from Day 2 postinfection established long-term infection in lung B cells but were unable to establish latency in the spleen. Similar results were obtained when mice were infected intranasally with a gene 50 stop. MHV68 gene 50 encodes Rta, the major trans-activator of the lytic program (36, 37). The role of the persistently latent infected B cells in lung fibrosis is unclear. B cells have been found to confer a protective role against silica-induced lung fibrosis by the production of prostaglandin E2 (38). On the other hand, B-cell–deficient mice have markedly reduced collagen deposition in a model of liver fibrosis produced by chronic treatment with CCl4 (39).
It is known that some viral proteins expressed during latency can modify the virus-mediated pathology. For instance, mice infected with MHV76, a virus that is deficient in expression of the unique set of latent viral proteins M1 to M4, or mice infected with an MHV68 virus that does not express the M1 latent protein, do not develop splenomegalia or chronic pathology (40, 41). Preliminary studies with the M1 mutant MHV68 show that IFN-γR−/− mice infected with this virus have acute pneumonitis but no lung and spleen fibrosis on Day 180 postinfection. Analyses to discern the mechanism of M1-mediated virus pathology are in progress. Expression of M2 viral latent protein down-regulates Stat1 and Stat2, resulting in inhibition of interferon-mediated transcriptional activation that might enhance the Th2 profibrotic responses (42). M3 is a chemokine-binding protein that can regulate the chemotaxis of neutrophils, lymphocytes, and monocytes (43–45). T-cell responses and macrophages have been implicated in the development of virus-mediated pathology. Finally, the absence of chronic arteritis is also observed in IFN-γR−/− mice infected with an MHV68 deficient in the M11 viral gene. M11 is a bcl-2 homolog with antiapoptotic activity required for efficient reactivation from latency (46). M11 prevents apoptosis induced either by expression of viral genes critical for ex vivo reactivation or by proapoptotic host genes that come into play during ex vivo reactivation.
Persistent lymphocytic infiltrates without fibrosis were also found in lungs of mice infected with the mutant MHV68, v-cyclin stop. This virus has the capacity to establish latency, but it is defective in reactivation from latency. Taken together, these results suggest that active lytic replication in the chronic phase of infection is a driving mechanism for the fibrogenic process. A common finding in animals treated with antiviral agent beginning on Day 45 and in v-cyclin stop MHV68–infected animals is the lack of macrophage recruitment and lack of expression of alternative activation markers. Studies show expression of markers of alternative macrophage activation in the lungs of patients with IPF (47). Our experimental model shows a similar pattern of activation for alveolar macrophages in chronically infected animals (19). Macrophages activated by the alternative pathway have been implicated in wound repair (24, 27). These macrophages have up-regulated arginase activity and high expression of chitinase-like lectins Ym1/2 as well as of TGF-β and extracellular matrix proteins including fibronectin. We demonstrate here that by controlling lung injury by antiviral treatment or diminution of virus reactivation from latency, Th2-mediated activation of macrophages is prevented, and pulmonary fibrosis as well. These data suggest that alternatively activated macrophages have an active role in the exaggerated reparative response to lung injury associated with fibrosis.
Another mediator of collagen deposition that is associated with Th2 responses is VEGF. A longitudinal study evaluating VEGF levels in the plasma of patients with IPF showed a direct correlation between VEGF levels and clinical and radiologic deterioration (33). In our model of pulmonary fibrosis, we demonstrated that chronic γ-herpesvirus infection is associated with high levels of VEGF in the lung that diminished with control of lytic infection by antiviral treatment or by infection with the v-cyclin stop mutant virus. These results support the concept that lytic infection mediates up-regulation of VEGF expression. Similarly, enhancement of VEGF expression has been reported during EBV reactivation (48). On the other hand, the maintenance of high levels of VEGF in mice receiving antiviral from Day 60 postinfection and the studies with the bleomycin lung fibrosis model eliminate a direct effect of cidofovir on VEGF expression regulation.
Finally, we analyzed the effectiveness of antiviral treatment in symptomatic mice undergoing viral replication as demonstrated by high copy numbers of gB transcripts, a product of lytic replication. This group of mice had high mortality that improved with the antiviral treatment. The control of viral replication was incomplete in these symptomatic animals, as well as in the asymptomatic animals, probably because a low dose of antiviral agent was used to avoid the nephrotoxicity of this compound. Pilot experiments conducted with 25 mg/kg of body weight resulted in 50% mortality after the first week of treatment. Antiviral treatment failed to reverse lung fibrosis and alternative activation of macrophages, although there was a significant reduction in the severity of the fibrosis. It is possible that increasing the dose of antiviral or adding IFN-γ in the therapeutic regimen could result in better control of virus replication.
No current therapies for IPF have been proven to alter lung fibrosis or survival. Corticosteroids, and immunosuppressive or cytotoxic agents, have not proven to be of benefit and have potentially serious toxicities (49). Our data in the animal model demonstrate that antiviral therapy aimed at replicating virus can prevent disrepair and fibrosis in a susceptible host. We also show that antiviral treatment in herpesvirus-infected mice improves clinical disease and survival. It is possible that treatment of γ-herpesvirus infection in patients with IPF with associated viral infection might help to control the progression of the fibrotic process. Future studies in this mouse model will be required to determine the impact of combination therapies in ameliorating pulmonary fibrosis.
In summary, using agents that stop replication of the virus and a replication-defective virus, we show that lytic infection is an important mechanism for virus-induced fibrosis. Furthermore, our data support the notion that activation of alveolar macrophages by the alternative pathway is a critical partner in the development of virus-induced fibrosis. Finally, the potential therapeutic ramification of our study is that antiviral therapy in herpesvirus-infected patients with IPF may be effective.
Supplementary Material
Acknowledgments
The authors thank Debra Haas and Robert Joodi for technical assistance and Dr. Anapatricia Garcia (Yerkes National Research Center, Emory University) for pathologic analyses.
Supported by NHLBI NIH KO1 HL073154-01, an ALA Dalsemer Research Grant, NHLBI R21 HL 080284-01, and the McKelvey Lung Transplantation Center at Emory University.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200610-1426OC on March 15, 2007
Conflict of Interest Statement: A.L.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.T.-G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.X. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R. (Jeffrey Ritzenthaler) does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.H.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R. (Jesse Roman) received $100,000 in 2005 and 2006 from Intermune in research grants to participate in multicenter clinical trials. K.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society; European Respiratory Society.Idiopathic pulmonary fibrosis: diagnosis and treatment [International Consensus Statement]. Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 2.Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S93–S97. [PubMed] [Google Scholar]
- 3.Perez A, Rogers RM, Dauber JH. The prognosis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S19–S26. [PubMed] [Google Scholar]
- 4.Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1999;159:1336–1341. [DOI] [PubMed] [Google Scholar]
- 5.Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 2003;41:2633–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan JJ, Stewart JP, Hasleton PS, Arrand JR, Carroll KB, Woodcock AA. Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax 1995;50:1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:510–513. [DOI] [PubMed] [Google Scholar]
- 8.Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, Gompels UA. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol 1990;71:1365–1372. [DOI] [PubMed] [Google Scholar]
- 9.Virgin HW IV, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol 1997;71:5894–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash AA, Dutia BM, Stewart JP, Davison AJ. Natural history of murine γ-herpesvirus infection. Philos Trans R Soc Lond B Biol Sci 2001;356:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weck KE, Dal Canto AJ, Gould JD, O'Guin AK, Roth KA, Saffitz JE, Speck SH, Virgin HW. Murine γ-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-γ responsiveness: a new model for virus-induced vascular disease. Nat Med 1997;3:1346–1353. [DOI] [PubMed] [Google Scholar]
- 12.Tibbetts SA, van Dyk LF, Speck SH, Virgin HW IV. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J Virol 2002;76:7125–7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steed AL, Barton ES, Tibbetts SA, Popkin DL, Lutzke ML, Rochford R, Virgin HW IV. Gamma interferon blocks gammaherpesvirus reactivation from latency. J Virol 2006;80:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora AL, Woods CR, Garcia A, Xu J, Rojas M, Speck SH, Roman J, Brigham KL, Stecenko AA. Lung infection with γ-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L711–L721. [DOI] [PubMed] [Google Scholar]
- 15.Thomas AQ, Lane K, Phillips J III, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med 2002;165:1322–1328. [DOI] [PubMed] [Google Scholar]
- 16.De Clercq E. Potential of acyclic nucleoside phosphonates in the treatment of DNA virus and retrovirus infections. Expert Rev Anti Infect Ther 2003;1:21–43. [DOI] [PubMed] [Google Scholar]
- 17.Mora AL, Woods CR, Garcia A, Xu J, Rojas M, Speck SH, Roman J, Brigham KL, Stecenko AA. Lung infection with gamma herpesvirus induces progressive pulmonary fibrosis in Th2 biased mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L711–L721. [DOI] [PubMed] [Google Scholar]
- 18.Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961;93:440–447. [DOI] [PubMed] [Google Scholar]
- 19.Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol 2006;35:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman J, Rivera HN, Roser-Page S, Sitaraman SV, Ritzenthaler JD. Adenosine induces fibronectin expression in lung epithelial cells: implications for airway remodeling. Am J Physiol Lung Cell Mol Physiol 2005;290:L317–L325. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 1994;218:325–329. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Mora AL, LaVoy J, Brigham KL, Rojas M. Increased bleomycin-induced lung injury in mice deficient in the transcription factor T-bet. Am J Physiol Lung Cell Mol Physiol 2006;291:L658–L667. [DOI] [PubMed] [Google Scholar]
- 23.Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett 2003;85:173–180. [DOI] [PubMed] [Google Scholar]
- 24.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35. [DOI] [PubMed] [Google Scholar]
- 25.Raes G, Noel W, Beschin A, Brys L, de Baetselier P, Hassanzadeh GH. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev Immunol 2002;9:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb DC, McKenzie AN, Foster PS. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J Biol Chem 2001;276:41969–41976. [DOI] [PubMed] [Google Scholar]
- 27.Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, Goerdt S. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein βIG-H3. Scand J Immunol 2001;53:386–392. [DOI] [PubMed] [Google Scholar]
- 28.Noel W, Raes G, Hassanzadeh Ghassabeh G, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol 2004;20:126–133. [DOI] [PubMed] [Google Scholar]
- 29.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med 2001;194:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanone S, Zheng T, Zhu Z, Liu W, Lee CG, Ma B, Chen Q, Homer RJ, Wang J, Rabach LA, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13–induced inflammation and remodeling. J Clin Invest 2002;110:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dyk LF, Hess JL, Katz JD, Jacoby M, Speck SH, Virgin HI. The murine gammaherpesvirus 68 v-cyclin gene is an oncogene that promotes cell cycle progression in primary lymphocytes. J Virol 1999;73:5110–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dyk LF, Virgin HW IV, Speck SH. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J Virol 2000;74:7451–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simler NR, Brenchley PE, Horrocks AW, Greaves SM, Hasleton PS, Egan JJ. Angiogenic cytokines in patients with idiopathic interstitial pneumonia. Thorax 2004;59:581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keane MP, Belperio JA, Arenberg DA, Burdick MD, Xu ZJ, Xue YY, Strieter RM. IFN-γ–inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol 1999;163:5686–5692. [PubMed] [Google Scholar]
- 35.Hamada N, Kuwano K, Yamada M, Hagimoto N, Hiasa K, Egashira K, Nakashima N, Maeyama T, Yoshimi M, Nakanishi Y. Anti-vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J Immunol 2005;175:1224–1231. [DOI] [PubMed] [Google Scholar]
- 36.Gangappa S, Kapadia SB, Speck SH, Virgin HW IV. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell–deficient mice. J Virol 2002;76:11460–11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moser JM, Farrell ML, Krug LT, Upton JW, Speck SH. A gammaherpesvirus 68 gene 50 null mutant establishes long-term latency in the lung but fails to vaccinate against a wild-type virus challenge. J Virol 2006;80:1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arras M, Louahed J, Simoen V, Barbarin V, Misson P, van den Brule S, Delos M, Knoops L, Renauld JC, Lison D, et al. B-lymphocytes are critical for lung fibrosis control and PGE2 regulation in IL-9 transgenic mice. Am J Respir Cell Mol Biol 2006;34:573–580. [DOI] [PubMed] [Google Scholar]
- 39.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, Shlomchik MJ, Koteliansky V, Hochman PS, Ibraghimov A. Attenuated liver fibrosis in the absence of B cells. J Clin Invest 2005;115:3072–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macrae AI, Dutia BM, Milligan S, Brownstein DG, Allen DJ, Mistrikova J, Davison AJ, Nash AA, Stewart JP. Analysis of a novel strain of murine gammaherpesvirus reveals a genomic locus important for acute pathogenesis. J Virol 2001;75:5315–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clambey ET, Virgin HW IV, Speck SH. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J Virol 2000;74:1973–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang X, Shin YC, Means RE, Jung JU. Inhibition of interferon-mediated antiviral activity by murine gammaherpesvirus 68 latency-associated M2 protein. J Virol 2004;78:12416–12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bridgeman A, Stevenson PG, Simas JP, Efstathiou S. A secreted chemokine binding protein encoded by murine gammaherpesvirus-68 is necessary for the establishment of a normal latent load. J Exp Med 2001;194:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin AP, Canasto-Chibuque C, Shang L, Rollins BJ, Lira SA. The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J Immunol 2006;177:7296–7302. [DOI] [PubMed] [Google Scholar]
- 45.van Berkel V, Preiter K, Virgin HW IV, Speck SH. Identification and initial characterization of the murine gammaherpesvirus 68 gene M3, encoding an abundantly secreted protein. J Virol 1999;73:4524–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gangappa S, van Dyk LF, Jewett TJ, Speck SH, Virgin HW IV. Identification of the in vivo role of a viral bcl-2. J Exp Med 2002;195:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, Vollmer E, Muller-Quernheim J, Zissel G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med 2006;173:781–792. [DOI] [PubMed] [Google Scholar]
- 48.Hong GK, Kumar P, Wang L, Damania B, Gulley ML, Delecluse HJ, Polverini PJ, Kenney SC. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J Virol 2005;79:13984–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP III. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs 2004;64:405–430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.