Abstract
Rationale: Previous studies have demonstrated that dysregulated coagulation and fibrinolysis contribute to the pathogenesis of asthma.
Objective: The role of procoagulant factor X in a murine model of ovalbumin (OVA)-induced asthma was investigated.
Methods: Biochemical, cellular, and physiologic in vivo and in vitro approaches were used to determine effects of factor X on the asthmatic response in mice.
Measurements and Main Results: Factor X transcript levels and factor Xa activity were increased in lungs of asthmatic mice challenged with OVA, compared with controls treated with phosphate-buffered saline. Factor X was highly expressed in bronchoalveolar lavage fluid macrophages from asthmatic mice. Treatment of mice with the factor Xa inhibitor fondaparinux during the last 4 wk of OVA challenge resulted in the attenuation of airway hyperresponsiveness but did not alter infiltration of inflammatory cells into the lung. There was a significant decrease in the thickness of the mucosal layer and in lung collagen deposition in fondaparinux-treated mice. In vitro investigations using human mucus-producing NCI-H292 cells indicated that exogenous factor Xa enhanced mucin production in a dose-dependent manner. Levels of amphiregulin, a protein that induces mucin production, were also increased in cells stimulated by factor Xa.
Conclusions: The results of this study introduce a novel participant in the asthmatic response and indicate that factor Xa functions in airway remodeling in asthma by stimulating mucin production, through regulation of amphiregulin expression and collagen deposition.
Keywords: coagulation protein, airway hyperresponsiveness, mucus production, amphiregulin
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Previous results implied that an imbalance in coagulation and fibrinolysis contributes to the pathologic events associated with asthma, but their mechanistic roles in these processes have not been elucidated.
What This Study Adds to the Field
This study suggests that coagulation protein factor Xa may be involved in the regulation of asthma.
Previous investigations have indicated that coagulation- and fibrinolysis-related proteins contribute to the pathogenesis of asthma. Plasminogen activator inhibitor–1 levels are increased during asthma and contribute to fibrosis (1–3). Furthermore, activated protein C is associated with the chronic asthmatic response (4, 5). Recently, it was reported that fibrin deposition and abnormalities in the coagulation and fibrinolytic pathways in the distal airways of the lung significantly contribute to airway hyperresponsiveness and airway closure in asthma (6). In that study, fibrin deposits were found on the luminal surface of the airway epithelium in a patient who died in status asthmaticus. In related work, extravascular fibrinogen and thrombin have been identified in the sputum of patients with asthma (7, 8). Thrombin activity in bronchoalveolar lavage fluid (BALF) was increased by segmental antigen provocation in patients with asthma, and BALF from these patients was able to induce proliferation of fibroblasts (9). These results suggest an association between coagulation events and asthmatic responses. It is not known whether coagulation factors are directly associated with the asthmatic response. Fibrin formation is the result of thrombin generated by factor Xa (FXa), a key component of the intrinsic and extrinsic coagulation pathways. As a result, we hypothesize that FXa plays a role in asthmatic responses and tested this hypothesis using an ovalbumin (OVA) allergen mouse model (10, 11). Some of the results of these studies have been previously reported in the form of an abstract (12).
METHODS
Animals
All experimental procedures were approved by the Laboratory Animal Care and Use Committee of the University of Notre Dame. Male A/J mice (7–10 wk old) were used in this study because of the sensitive airway responses in this mouse strain.
Induction of Eosinophilic Inflammation
Mice were anesthetized by isoflurane inhalation, after which OVA (1 mg/ml) was administered intranasally (50 μl) 3 d/wk for 16 wk. Phosphate-buffered saline (PBS) was administered to mice as a vehicle control. At 24 h after the last OVA dose, the mice were anesthetized, and the lungs were lavaged with sterile PBS. Cell counts were performed on bronchoalveolar lavage cells. BALF was collected for analyses of FXa activity.
Isolation of Macrophages, Lymphocytes, and Eosinophils
Macrophages were purified from BALF by adhesion to a plastic surface, and T and B lymphocytes were purified from the peribronchial lymph nodes using Dynabeads specific for T and B cells (Dynal Biotech, Oslo, Norway). Eosinophils were purified from BALF by lectin affinity negative selection as previously reported (13). Additional details on these methods are provided in the online supplement.
Quantitative Real-time Reverse Transcriptase–Polymerase Chain Reaction
Quantitative reverse transcriptional–polymerase chain reaction (RT-PCR) was performed as previously described (14). Total RNA was isolated from lungs, BALF, and NCI-H292 cells by the RNeasy total RNA purification kit (Qiagen, Valencia, CA). For determining if FXa-mediated induction of mucin production is regulated through amphiregulin, a mouse monoclonal antibody to human amphiregulin (10 μg/ml) (R&D Systems, Minneapolis, MN) was added to NCI-H292 cells before the addition of FXa. Quantitative RT-PCR was performed with the primers and probes listed in Table 1 for FX, protein-activated receptor (PAR)1, PAR2, amphiregulin, MUC5AC, and the housekeeping gene RPL19.
TABLE 1.
SEQUENCES OF PRIMERS AND PROBES USED FOR QUALITATIVE REVERSE TRANSCRIPTIONAL–POLYMERASE CHAIN REACTION
| Gene and Type | Sequence | Position | Size (bp) |
|---|---|---|---|
| RPL19 (NM_009078) | |||
| Forward | ATGTATCACAGCCTGTACCTG | 355–375 | |
| Probe* | TTTCGTGCTTCCTTGGTCTTAGACCT (AS) | 487–512 | 233 |
| Reverse | TTCTTGGTCTCCTCCTCCTTG | 567–587 | |
| FX (NM_007972) | |||
| Forward | CTGCTGCCCACTGTCTCCAT | 809–828 | |
| Probe† | TCAAGGTGAGGGTAGGTGATCGGAACA | 842–868 | 101 |
| Reverse | GTCCACCTCGTGCACCATCT | 890–909 | |
| PAR-1 (NM_010169) | |||
| Forward | CAGCCAGAATCAGAGAGGACAGA | 141–163 | |
| Probe* | CGGTGAACCCCCGCTCATTCTTTCTAA (AS) | 169–195 | 136 |
| Reverse | CCAGCAGGACGCTTTCATTT | 257–276 | |
| PAR-2 (NM_007974) | |||
| Forward | AGCCGGACCGAGAACCTT | 165–182 | |
| Probe* | CTTCCTTTACTGTTGTTGCGTCCCGGT (AS) | 185–211 | 101 |
| Reverse | GGAACCCCTTTCCCAGTGATT | 245–265 | |
| MUC5AC (XM_001130382) | |||
| Forward | TACTCCACAGACTGCACCAACTG | 1272–1294 | |
| Probe* | TGTGCTTGGAGGTGCCCACTTCTCAA | 1352–1377 | 129 |
| Reverse | CGTGTATTGCTTCCCGTCAA | 1381–1400 | |
| Amphiregulin (NM_001657) | |||
| Forward | AGGCCATTATGCTGCTGGAT | 270–289 | |
| Probe* | ACCTCAATGACACCTACTCTGGGAAGCGT | 293–321 | 74 |
| Reverse | TGTGGTCCCCAGAAAATGGT | 324–343 |
Definition of abbreviation: AS = antisense.
Probes were labeled at the 5′ end with 6-FAM and at the 3′ end with BHQ1 or TAMRA.
Assay of FXa Activity
FXa activity was determined using a chromogenic assay with S-2222 (Chromogenix, Lexington, MA) as the substrate. Human FXa (Enzyme Research Laboratories, South Bend, IN) was used as the standard over the concentration range of 0.001–1 U/ml.
Fondaparinux Levels in Blood as a Function of Time
Fondaparinux (FPX) (GlaxoSmithKline, Triangle Park, NC) was administered subcutaneously to mice at doses of 10 or 30 μg. A standard curve of FXa inhibition was generated using FPX at 0.01–1 μg/ml and FXa at 0.2 U/ml in an FXa chromogenic assay to determine FPX levels in test serum samples at various times after its administration.
Assessment of Airway Responsiveness
Airway hyperresponsiveness was measured by lung resistance in anesthetized mice at 24 h after the last intranasal antigen challenge as previously reported (10). Increasing doses of methacholine (1–16 mg/ml) (Sigma-Aldrich, St. Louis, MO) were administered by inhalation. Additional details on this method are provided in the online supplement.
FPX Treatment
FPX was administered subcutaneously to a population of OVA-treated mice, from Week 13 to 16 during challenge, at a dose of 10 or 30 μg for 6 d/wk. Fluticasone (TOCRIS Bioscience, Ellisville, MO) was used as a positive control and administered intranasally at 10 μg/mouse for 6 d/wk.
Collagen Deposition, Mucus Production, and Smooth Muscle Cell Hyperplasia in Lung Tissue
Lungs were obtained at 24 h after the final OVA administration, fixed with periodate-lysine-paraformaldehyde, embedded in paraffin, and sectioned at a thickness of 4 μm. Slides were stained with periodic acid–Schiff or Masson's trichrome. Smooth muscle α-actin immunostains were performed as previously described (15).
Measurement of Airway Wall Thickness
Lung tissue was prepared as described previously, and slides were stained with Elastica van Gieson. Cross-sections of airways were used for measuring the airway wall thickness. Additional detail on the methods for making these measurements is provided in the online supplement.
Hydroxyproline Assay
Total lung collagen was determined by analysis of hydroxyproline (10).
Mucin Production from NCI-H292 Cells
NCI-H292 cells were seeded in 96-well plates and cultured for 2 d in RPMI 1640 media containing 10% fetal bovine serum. After reaching confluence, the cells were washed twice with PBS and incubated with fetal bovine serum–free RPMI 1640 for 2 h. NCI-H292 cells were then stimulated for 48 h with various concentrations of FXa, thrombin (ERL), epidermal growth factor (EGF) (PeproTech, Rocky Hill, NJ), or activation peptides for PAR1 and PAR2 (TOCRIS). After fixation, assays for MUC5AC production were performed. For FPX studies in FXa-stimulated cells, 12.8 μg/ml of antithrombin-III was added. Mouse–anti-human MUC5AC (45M1; Lab Vision, Fremont, CA) was used as the primary antibody. Maximal production of MUC5AC (100%) was identified as the absorbance obtained from cells stimulated with 1 μg/ml EGF.
Amphiregulin Protein Levels in the Supernatant of NCI-H292 Cells
Human amphiregulin levels in supernatants were obtained from the culture medium of NCI-H292 cells that were incubated with 0–30 U/ml of FXa and were measured using the DuoSet ELISA Development System kit (R&D Systems, Minneapolis, MN).
Data Analysis
Data were analyzed using the Student's t test and are represented as the mean ± SEM. Analysis of variance, with Dunnett's test for multiple comparisons, was used for determining significance (Excel Statistics, version 5.0; Esumi Co. Ltd., Tokyo, Japan). A p value of less than 0.05 was considered statistically significant.
RESULTS
Increase of Coagulation FX mRNA Level in the Lungs of Asthmatic Mice
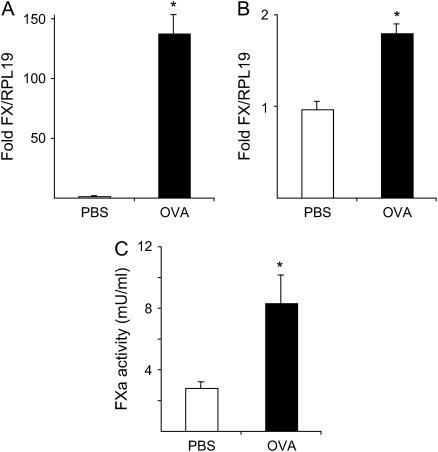
After mice were exposed to OVA for 16 wk, their lungs were removed, and total RNA was isolated. The mRNA levels of FX were compared with PBS-exposed mice (Figure 1A). FX transcripts were significantly elevated in OVA-exposed mice relative to PBS controls (137.26 ± 16.29 [n = 6] vs. 1.03 ± 0.09 [n = 6], fold change relative to RPL19, respectively; p = 0.0019). In BALF, FX message was only detected in macrophages, and its level was increased in OVA-challenged mice relative to PBS-challenged mice (1.79 ± 0.10 [n = 4] vs. 0.96 ± 0.09 [n = 4], fold change relative to RPL19, respectively; p = 0.0009) (Figure 1B). FX mRNA levels were undetectable in the human epithelial cell line NCI-H292, eosinophils from BALF, and T and B cells purified from OVA-challenged mouse lung lymph nodes (data not shown).
Figure 1.
(A) Quantitative reverse transcriptional–polymerase chain reaction (RT-PCR) analysis of factor X (FX) mRNA in lungs obtained from ovalbumin (OVA)-exposed mice (solid bar, n = 6) at 16 wk of treatment versus phosphate-buffered saline (PBS)-treated mice (open bar, n = 6). The values indicate the fold difference relative to RPL19 mRNA. Data represent the mean ± SEM. *p = 0.004 versus PBS-treated mice. (B) Quantitative RT-PCR analysis of FX mRNA in macrophages obtained from bronchoalveolar lavage fluid (BALF) of OVA-exposed mice (solid bar, n = 4) versus PBS-treated mice (open bar, n = 4). Values indicate the fold difference relative to the RPL19 housekeeping gene. Data represent the mean ± SEM. *p < 0.001 versus PBS-treated mice. (C) FXa activity in BALF from OVA-exposed mice (solid bar, n = 5) versus PBS-treated mice (open bar, n = 5) determined by a S-2222–based chromogenic assay. *p = 0.04 versus PBS-treated mice.
Increase of Coagulation FXa Activity in the BALF of Asthmatic Mice
FXa activity was measured in BALF from mice exposed to OVA for 12 wk, along with similarly treated PBS controls. The FXa activity was increased in OVA-challenged mice in comparison to PBS-challenged mice (8.30 ± 1.86 mU/ml [n = 5] vs. 2.80 ± 0.42 mU/ml [n = 5], respectively; p = 0.04) (Figure 1C). To confirm that FXa is the enzyme responsible for hydrolysis of S-2222 in BALF, tick anticoagulant peptide (TAP) (a generous gift from Dr. S. Krishnaswamy, Children's Hospital of the University of Pennsylvania), specific for inhibition of FXa, was added to the reaction mixture. Studies using purified human FXa and murine FXa (Hematologic Technologies, Burlington, VT) demonstrated an approximately 10-fold weaker inhibition of murine FXa relative to human FXa. In the interest of BALF sample stability, it was required that a concentration of TAP be used at which the inhibition of mouse FXa is immediate. Under these experimental requirements, at 10−4 M TAP, more than 80% of the S-2222 hydrolysis due to murine FXa was inhibited when mouse BALF, TAP, and S-2222 were added together (data not shown). Thus, at least 80% of the increased S-2222 activity in BALF was attributed to FXa.
Effect of FPX on Respiratory Function
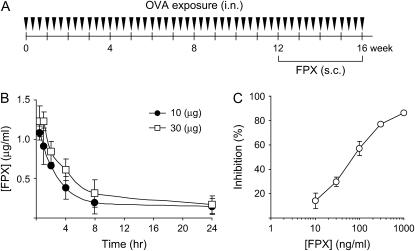
To investigate the effects of FXa inhibition on asthmatic responses, FPX, a selective FXa inhibitor, was administered subcutaneously to mice 6 d/wk for 4 wk during 12–16 wk of OVA challenge (Figure 2A). This time frame was chosen because eosinophilic inflammation, airway wall thickness, and airway hyperresponsiveness plateaued at these time points (10). Serum concentrations of FPX were monitored by the ability of test sera to inhibit FXa activity after a single injection of FPX (Figure 2B). The serum concentration of FPX was 0.913 and 1.229 μg/ml, at a dose of 10 μg or 30 μg, respectively, at 1 h after injection. FPX levels were detectable after 8 h (0.194 and 0.312 μg/ml, at a dose of 10 or 30 μg, respectively), and these values were higher than the IC50 value (0.20 μg/ml) for inhibiting FXa activity (Figure 2C). The FPX concentrations in the BALF obtained 1 h after its injection were 0 and 0.358 ± 0.002 ng/ml in vehicle (PBS)- and FPX-injected mice, respectively.
Figure 2.
(A) Timeline showing the protocols used for OVA exposure (instillation of OVA solution intranasally 3 d/wk for 16 wk). Fondaparinux (FPX) was administered at 10 and 30 μg by subcutaneous injection 6 d/wk from Week 13 to Week 16 after initiation of OVA challenge. Fluticasone was given at a dose of 10 μg, intranasally, 6 d/wk, from Week 13 to Week 16. Mice were killed 24 h after the final OVA challenge. (B) Serum FPX concentration in mice as a function of time after a single dose of 10 μg or 30 μg of FPX. (C) Factor Xa inhibitory activity of FPX as a function of FPX concentration. Data represent the mean ± SEM.
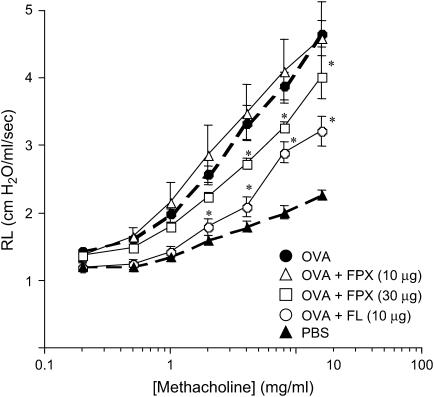
Respiratory function was determined 24 h after the final antigen provocation, and the lung resistance (Rl) was found to be significantly increased in OVA-challenged mice after 16 wk of challenge. Rl was determined by exposing the mice to increasing concentrations of aerosolized methacholine. FPX (30 μg) attenuated the increase of Rl on OVA-challenged mice, compared with OVA-challenged mice not receiving FPX, although this agent was not effective at lower doses of 10 μg (Figure 3). The effect of FPX at 30 μg was slightly less than fluticasone (10 μg), a glucocorticoid frequently used to treat asthma, but it still significantly attenuated airway hyperresponsiveness.
Figure 3.
Effects of FPX and the positive control, fluticasone (FL), on respiratory function in mice exposed to OVA for 16 wk. Airway hyperresponsiveness (AHR) was induced by methacholine inhalation (1–16 mg/ml). AHR was monitored 24 h after the final OVA challenge. Data represent the mean ± SEM (n = 6–9). *p < 0.05 versus OVA-challenged mice not treated with FPX.
Effect of FPX on Cell Numbers in BALF
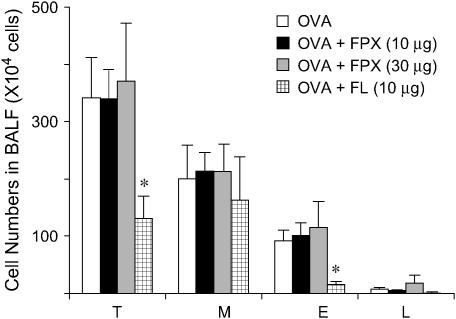
Inflammatory cells in BALF were increased after OVA exposure for 16 wk and were compared with PBS-treated mice. Among the inflammatory cells, macrophages, eosinophils, and lymphocytes were increased in OVA-challenged mice. FPX treatment for 4 wk, from Week 13 to Week 16 after initiation of the OVA challenge, did not affect the total cell numbers in the BALF (Figure 4). Specifically, eosinophils, macrophages, and lymphocytes were not affected by treatment with this drug. In contrast, fluticasone decreased the numbers of eosinophils and lymphocytes and the total number of cells in BALF.
Figure 4.
Effects of FPX on cell numbers in the BALF of OVA-exposed mice after 16 wk of OVA challenge. BAL was performed 24 h after the final challenge of OVA. BALF cells were counted and dispersed onto microscope slides using a cytospin. Cytospin preparations were stained with DiffQuick to obtain differential leukocyte counts. Total cell (T), macrophage (M), eosinophil (E), and lymphocyte (L) counts in BALF samples obtained from mice exposed to OVA for 16 wk. Data represent the mean ± SEM (n = 5–6). *p < 0.05 versus OVA-challenged mice not treated with FPX.
Effect of FPX on Goblet Cell Hyperplasia, Airway Wall Thickness, Collagen Deposition, and Mucus Production
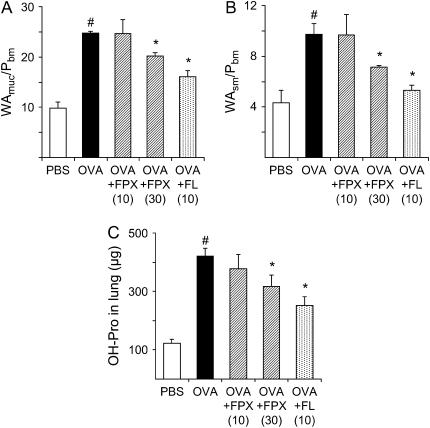
Periodic acid–Schiff staining (goblet cells) was increased in lung tissue from OVA-challenged mice compared with FPX-treated, OVA-challenged mice (Figures 5A and 5B). Mucus secretion was also observed in OVA-challenged mice, which was not present in FPX-treated, OVA-challenged mice (Figures 5A and 5B). In addition, collagen deposition (Figure 5C) and the presence of smooth muscle cells (Figure 5E) were increased in OVA-challenged mice relative to FPX-treated, OVA-challenged mice (Figures 5D and 5F, respectively). To quantify the thickness of the mucosal layer, cross-sectional areas were obtained using computer graphic analysis of Elastica van Gieson–stained sections as previously reported (10). The thickness of the mucosal layer was increased approximately 2.5× in 16-wk OVA-challenged mice in comparison to PBS-challenged mice (Figure 6A and Table E1 in the online supplement). The thickness of the smooth muscle layer was also increased by approximately 2.2× (Figure 6B and Table E1). FPX treatment of OVA-challenged mice, at a dose of 30 μg, significantly reduced the thickness of the mucosal layer compared with nontreated, OVA-challenged mice (Figures 6A and 6B and Table E1).
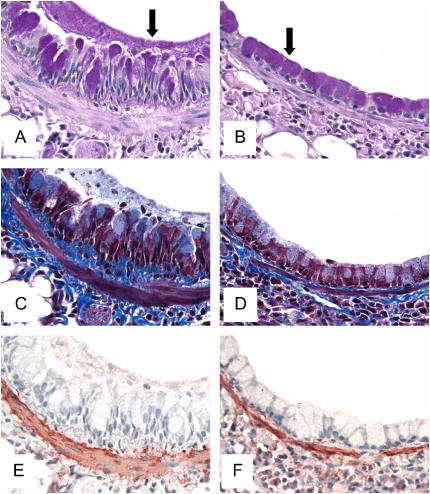
Figure 5.
Periodic acid–Schiff (A and B), Masson trichrome (C and D), and α-smooth muscle actin (E and F) staining of whole lung sections from mice challenged with OVA for 16 wk. The photomicrographs are representative of lungs removed from OVA-challenged controls (A, C, E) and FPX-treated, OVA-challenged mice (B, D, F). Goblet cell hyperplasia and mucus plugs in control mice were observed around the airways (arrow in A), but goblet cell hyperplasia was decreased and mucus plugs not evident in mice treated with 30 μg FPX (arrow in B). Enhanced collagen deposition (C, dark-blue areas) and smooth muscle cell hyperplasia (E, brown areas) were observed in control mice relative to FPX-treated, OVA-challenged mice (D and F, respectively). Original magnification: ×400.
Figure 6.
Effects of FPX and the positive control FL on airway wall thickness in bronchi in mice challenged with OVA for 16 wk. Values for cross-sectional areas of (A) mucous layer and (B) smooth muscle cell layer. Data represent the mean ± SEM (n = 3–4) for each group. #p < 0.05 versus PBS-treated mice; *p < 0.05 versus OVA-challenged mice not treated with FPX. (C) Hydroxyproline (OH-Pro) levels in lung tissue were measured and expressed as micrograms per lung. Data represent the mean ± SEM (n = 5–10). *p < 0.05 versus OVA-challenged mice not treated with FPX. #p < 0.05 versus PBS-treated mice.
Effect of FPX on Hydroxyproline Contents in the Lung
The hydroxyproline content in the lungs was determined as a marker of fibrosis. This value was significantly increased in OVA-challenged mice compared with PBS-challenged mice (421.8 ± 26.6 [n = 10] vs. 122.6 ± 12.9 [n = 6] μg/lung, respectively; p < 0.000001) (Figure 6C). FPX treatment of OVA-challenged mice significantly decreased the hydroxyproline content in the lungs in comparison to OVA-challenged mice (316.5 ± 39.9 [n = 10] vs. 421.8 ± 26.6 [n = 10] μg/lung, respectively; p = 0.04) (Figure 6C).
Effect of FPX on mRNA Levels of FX, PAR-1, and PAR-2 in the Lung
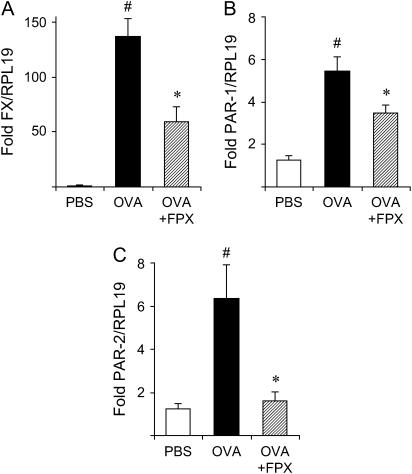
The mRNA levels of FX, PAR1, and PAR2 were analyzed in lung tissue by quantitative RT-PCR (Figure 7). The levels of FX mRNA were increased in 16-wk OVA-challenged mice in comparison to PBS-challenged mice (Figure 7A and Table E2). FPX treatment of OVA-challenged mice, at a dose of 30 μg for 4 wk, significantly decreased the FX mRNA levels relative to nontreated OVA-challenged mice (Figure 7A and Table E2). Furthermore, mRNA levels of PAR-1 and PAR-2 were increased in OVA-challenged mice in comparison to PBS-challenged mice (Figures 7B and 7C and Table E2). These levels were decreased after FPX treatment (Figures 7B and 7C and Table E2).
Figure 7.
Effects of FPX on mRNA levels of (A) FX, (B) PAR1, and (C) PAR2 in the lungs of mice challenged with OVA for 16 wk. The values indicate the fold difference relative to RPL19. Data represent the mean ± SEM (n = 5–6). #p < 0.05 versus PBS-treated mice; *p < 0.05 versus OVA-challenged mice not treated with FPX.
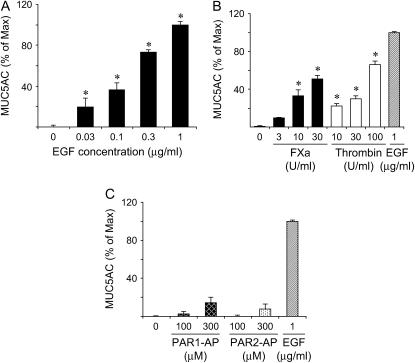
Effect of FXa, Thrombin, and PAR1-AP and PAR2-AP on Mucin Production in NCI-H292 Cells
NCI-H292 cells, a human mucin-producing cell line, were seeded in 96-well plates, and mucin production was investigated after stimulation with EGF or FXa. MUC5AC production was increased in a dose-dependent manner by the addition of EGF (Figure 8A). MUC5AC production was also significantly increased by stimulation with FXa in a dose-dependent manner (Figure 8B). In an additional set of experiments in which 30 μg of FPX and 12.8 μg/ml of antithrombin-III were added to the cells stimulated with 30 U/ml thrombin, mucin levels diminished from 51.35 ± 2.93% of maximum (n = 4) to 4.74 ± 1.70% of maximum (n = 4) (p < 0.00001). Thrombin also increased mucin production in a dose-dependent manner (Figure 8B), although activation of PAR1 or PAR2 did not seem to have a significant affect on mucin production (Figure 8C) as previously observed (16).
Figure 8.
MUC5AC production in NCI-H292 cells. NCI-H292 cells were cultured in 96-well plates and stimulated with (A) epidermal growth factor (EGF); (B) FXa, thrombin, or EGF; or (C) PAR1-activating peptide (PAR1-AP) or PAR2-AP at the indicated concentrations. MUC5AC production was determined as percentage relative to maximum expression (stimulation with EGF at 1 μg/ml).
Effect of FXa on Amphiregulin mRNA and Protein Levels in NCI-H292 Cells
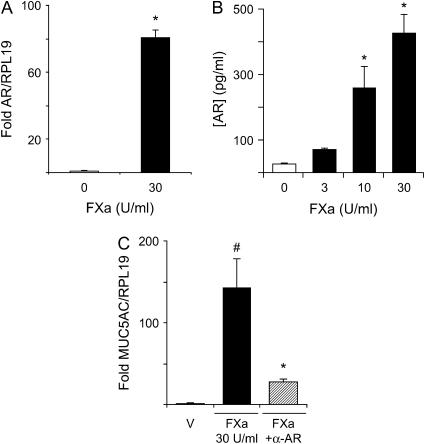
The mRNA levels of amphiregulin were significantly elevated in NCI-H292 cells after incubation with 30 U/ml of FXa relative to nonstimulated cells (80.78 ± 4.34–fold [n = 4] vs. 1.08 ± 0.24–fold [n = 4], relative to RPL19; p = 0.0003, respectively) (Figure 9A). In addition, protein levels in the supernatant increased in an FXa dose–dependent manner and were significantly higher than nonstimulated cells at 10 U/ml and 30 U/ml of FXa (p < 0.01) (Figure 9B). The increase in FXa-induced MUC5AC mRNA levels in NCI-H292 cells was decreased by incubating with an antiamphiregulin antibody (Figure 9C).
Figure 9.
(A) Amphiregulin (AR) mRNA and (B) protein levels in NCI-H292 cells stimulated with FXa. Amphiregulin mRNA levels were significantly increased after stimulation with 30 U/ml FXa (n = 4); *p = 0.0003 (A). This correlated with effects on protein levels, which increased in an FXa dose–dependent manner (n = 4); *p < 0.01 (B). Anti-amphiregulin antibody (α-AR) reduced the mRNA levels of FXa-induced MUC5AC in NCI-H292 cells (C).
DISCUSSION
The results from this study identify FXa as a novel participant in airway remodeling in asthma and link FXa with amphiregulin production for the first time. The observation that FX mRNA expression was present in the lung tissue and FXa activity in BALF in OVA-challenged mice supports a role for FXa in the asthmatic response. Further studies indicated that FX was highly expressed in purified alveolar macrophages from BALF. This is consistent with other studies in which FX was shown to be expressed in murine peritoneal macrophages (17). mRNA levels of FX were also elevated in lung tissue, most probably from type II alveolar epithelial cells and/or bronchial epithelial cells (18). FPX attenuated the airway hyperresponsiveness induced by OVA challenge, although it did not affect the recruitment of inflammatory cells in BALF. FPX treatment decreased the thickness of the airway wall and the hydroxyproline content, a measure of fibrosis, in the lungs. In experiments using NCI-H292, a human-derived mucus-producing cell line, FXa induced mucin production. These results indicate that FXa is associated with asthma by increasing airway wall thickness and exacerbating asthma.
In the present study, FPX attenuated the airway remodeling, thereby ameliorating the airway hyperresponsiveness in the chronic asthma model under conditions in which airway changes have already been established. Although FPX is less effective than fluticasone, which is one of the most efficient drugs for treating asthma, FPX ameliorated the airway remodeling process. FPX is a synthetic antithrombin-III (AT-III) binding pentasaccharide and shows a therapeutic advantage in preventing thromboembolism (19). The mechanism by which FPX inhibits FXa is by enhancing the reactivity of AT-III to FXa. FPX potentiates the neutralization of FXa by AT-III approximately 300× greater than heparin, but several reports indicate that it also inactivates other enzymes (20). However, the concentration of FPX for effective inhibition of FXa is approximately 10-fold less compared with that for other enzymes. Therefore, we propose that the effects we observed in this study are due primarily to inhibition of FXa, and this was confirmed by a similar use of another specific FXa inhibitor, TAP.
Several studies have demonstrated that FXa stimulates fibroblast proliferation and induces collagen production through the PAR1 pathway (21, 22). However, up to now, it has not been reported that FXa is associated with mucin production. In this study, mucin production (MUC5AC) was analyzed by using NCI-H292 cells that were derived from human mucoepidermoid carcinoma cells. Although there are several differences between this cell line and primary lung epithelial cells, this cell line has been used by other investigators to study mucin production, and EGF is a strong inducer of mucin in this cell line. MUC5AC production in NCI-H292 cells was up-regulated by FXa in a dose-dependent manner. Additional studies demonstrated that mucin production by NCI-H292 cells was induced by thrombin and FXa. It was therefore speculated that mucin production was mediated by PAR1 or PAR2 because both are expressed in the epithelial and smooth muscle cells of the respiratory system (23, 24). Indeed, studies have implicated coagulation FVIIa and FXa as possible activators of PAR2 in the airway (24). However, neither PAR1 nor PAR2 activating peptides stimulated mucin production. Recent studies have indicated that a trypsin-like protease (human airway trypsin-like protease [HAT]) can enhance mucin production through an amphiregulin/EGFR pathway, and activation of PAR2 is not sufficient for HAT-induced release of amphiregulin or induction of mucin production (16). In addition, it has been shown that FVIIa/tissue factor (TF) complex up-regulates mRNA levels of amphiregulin in a human keratinocyte cell line (25). Amphiregulin is a member of the EGF family and, like other members of this family, has been reported to play an important role in cell proliferation (26, 27), cell survival (28), and cell differentiation (26). Additional studies have demonstrated that amphiregulin increases mucin production in airway epithelial cells and increases expression in mast cells from patients with asthma, events that were correlated with goblet cell hyperplasia in the mucosa (29). In the current study, FXa significantly increased amphiregulin mRNA and protein levels in NCI-H292 cells, and the addition of a specific antibody to amphiregulin significantly attenuated FXa-mediated mucin production. Therefore, FXa may induce mucin production by regulating amphiregulin production directly or indirectly through activation/membrane release of HAT from NCI-H292 cells and potentially in vivo through a murine homolog of HAT (30)
Although this investigation demonstrated that activation of PAR1 and PAR2 did not seem to play a significant role in mucin production, these receptors may contribute to other events associated with airway remodeling. PAR comprises a unique family of G-protein–coupled receptors, which are cleaved at an activation site within the N-terminal exodomain by serine proteinases. After cleavage, the new N-terminal sequence functions as a tethered ligand that binds intramolecularly to activate the receptor (24, 31). Four PARs have been identified and several studies have shown that PAR1 and PAR2 are expressed in the airway epithelial cells (32–38). Activation of PAR2 in vivo leads to a proinflammatory responses, such as neutrophil and eosinophil infiltrations (36, 39), and bronchoconstriction (35). Serine proteases (e.g., trypsin, mast cell tryptase, and FVIIa and FXa) have been shown to activate PAR2 (40). In addition to PAR2, FXa has also been shown to activate PAR1 (41). In the present study, mRNA levels of PAR1 and PAR2 were increased in 16-wk–challenged mice, and these levels decreased after FPX treatment. Previous studies have identified a role for PAR1 in tissue fibrosis in lung (21, 22) and activation of these receptors, potentially by FXa, may contribute to airway remodeling by regulating fibroblast proliferation and collagen deposition. Therefore, FXa could regulate asthmatic events through its participation in a number of different pathways.
In conclusion, it has been demonstrated that FXa is involved in airway remodeling. The presence of FXa led to increased mucin production through increased amphiregulin, although FXa did not affect inflammatory cell infiltration into the lung in OVA-challenged mice. Other contributions by FXa during the remodeling phase of this disease may involve activation of PARs and resultant increased fibroblast proliferation and collagen production.
Supplementary Material
Acknowledgments
The authors thank Ms. Deborah Donahue for the animal work, Ms. Melanie DeFord for assistance with cell culture, and Ms. Mayra J. Sandoval-Cooper for assistance with histology.
Supported by grant HL73750 from the National Institutes of Health and the Kleiderer-Pezold endowed professorship (F.J.C.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200608-1097OC on November 2, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cho SH, Tam SW, Demissie-Sanders S, Filler SA, Oh CK. Production of plasminogen activator inhibitor-1 by human mast cells and its possible role in asthma. J Immunol 2000;165:3154–3161. [DOI] [PubMed] [Google Scholar]
- 2.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, Martin G, Panju M, Inman MD, Gauldie J. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol 2005;32:99–107. [DOI] [PubMed] [Google Scholar]
- 3.Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun 2002;294:1155–1160. [DOI] [PubMed] [Google Scholar]
- 4.Hataji O, Taguchi O, Gabazza EC, Yuda H, Fujimoto H, Suzuki K, Adachi Y. Activation of protein C pathway in the airways. Lung 2002;180:47–59. [DOI] [PubMed] [Google Scholar]
- 5.Yuda H, Adachi Y, Taguchi O, Gabazza EC, Hataji O, Fujimoto H, Tamaki S, Nishikubo K, Fukudome K, D'Alessandro-Gabazza CN, et al. Activated protein C inhibits bronchial hyperresponsiveness and Th2 cytokine expression in mice. Blood 2004;103:2196–2204. [DOI] [PubMed] [Google Scholar]
- 6.Wagers SS, Norton RJ, Rinaldi LM, Bates JH, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest 2004;114:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H, Kobayashi T, Hataji O, Urano H, Zhou H, et al. Thrombin in the airways of asthmatic patients. Lung 1999;177:253–262. [DOI] [PubMed] [Google Scholar]
- 8.Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, Gleich GJ, Dolovich J, Hargreave FE. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med 1996;154:308–317. [DOI] [PubMed] [Google Scholar]
- 9.Terada M, Kelly EA, Jarjour NN. Increased thrombin activity after allergen challenge: a potential link to airway remodeling? Am J Respir Crit Care Med 2004;169:373–377. [DOI] [PubMed] [Google Scholar]
- 10.Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med 2003;168:959–967. [DOI] [PubMed] [Google Scholar]
- 11.Singh B, Shinagawa K, Taube C, Gelfand EW, Pabst R. Strain-specific differences in perivascular inflammation in lungs in two murine models of allergic airway inflammation. Clin Exp Immunol 2005;141:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinagawa K, Ploplis VA, Castellino FJ. Involvement of coagulation Factor Xa (FXa) in a murine model of asthma [abstract]. Proc Am Thorac Soc 2006;3:A330. [Google Scholar]
- 13.Shinagawa K, Anderson GP. Rapid isolation of homogeneous murine bronchoalveolar lavage fluid eosinophils by differential lectin affinity interaction and negative selection. J Immunol Methods 2000;237:65–72. [DOI] [PubMed] [Google Scholar]
- 14.Iwaki T, Cruz DT, Martin JA, Castellino FJ. A cardioprotective role for the endothelial protein C receptor in lipopolysaccharide-induced endotoxemia in the mouse. Blood 2005;105:2364–2371. [DOI] [PubMed] [Google Scholar]
- 15.Iwaki T, Sandoval-Cooper MJ, Brechmann M, Ploplis VA, Castellino FJ. A fibrinogen deficiency accelerates the initiation of LDL-driven atherosclerosis via thrombin generation and platelet activation in genetically predisposed mice. Blood 2006;107:3883–3891. [DOI] [PubMed] [Google Scholar]
- 16.Chokki M, Yamamura S, Eguchi H, Masegi T, Horiuchi H, Tanabe H, Kamimura T, Yasuoka S. Human airway trypsin-like protease increases mucin gene expression in airway epithelial cells. Am J Respir Cell Mol Biol 2004;30:470–478. [DOI] [PubMed] [Google Scholar]
- 17.Pejler G, Lunderius C, Tomasini-Johansson B. Macrophages synthesize factor X and secrete factor X/Xa-containing prothrombinase activity into the surrounding medium. Thromb Haemost 2000;84:429–435. [PubMed] [Google Scholar]
- 18.Krupiczojc MA, Scotton CJ, Laurent GJ, Chambers RC. Local factor X expression is increased in the injured lung and induces myofibroblast differentiation in vitro. Proc Am Thorac Soc 2006;3:A767. [Google Scholar]
- 19.Klauss V, Spannagl M. Thrombin inhibitors and anti-factor Xa agents in the treatment of arterial occlusion. Curr Drug Targets 2006;7:1285–1290. [DOI] [PubMed] [Google Scholar]
- 20.Olson ST, Swanson R, Raub-Segall E, Bedsted T, Sadri M, Petitou M, Hérault JP, Herbert JM, Björk I. Accelerating ability of synthetic oligosaccharides on antithrombin inhibition of proteinases of the clotting and fibrinolytic systems: comparison with heparin and low-molecular-weight heparin. Thromb Haemost 2004;92:929–939. [DOI] [PubMed] [Google Scholar]
- 21.Blanc-Brude OP, Archer F, Leoni P, Derian C, Bolsover S, Laurent GJ, Chambers RC. Factor Xa stimulates fibroblast procollagen production, proliferation, and calcium signaling via PAR1 activation. Exp Cell Res 2005;304:16–27. [DOI] [PubMed] [Google Scholar]
- 22.Chambers RC, Laurent GJ. Coagulation cascade proteases and tissue fibrosis. Biochem Soc Trans 2002;30:194–200. [DOI] [PubMed] [Google Scholar]
- 23.Cocks TM, Moffatt JD. Protease-activated receptor-2 (PAR2) in the airways. Pulm Pharmacol Ther 2001;14:183–191. [DOI] [PubMed] [Google Scholar]
- 24.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev 2004;84:579–621. [DOI] [PubMed] [Google Scholar]
- 25.Camerer E, Gjernes E, Wiiger M, Pringle S, Prydz H. Binding of factor VIIa to tissue factor on keratinocytes induce gene expression. J Biol Chem 2000;275:6580–6585. [DOI] [PubMed] [Google Scholar]
- 26.Falk A, Frisen J. Amphiregulin is a mitogen for adult neural stem cells. J Neurosci Res 2002;69:757–762. [DOI] [PubMed] [Google Scholar]
- 27.Kato M, Inazu T, Kawai Y, Masamura K, Yoshida M, Tanaka N, Miyamoto K, Miyamori I. Amphiregulin is a potent mitogen for the vascular smooth muscle cell line, A7r5. Biochem Biophys Res Commun 2003;301:1109–1115. [DOI] [PubMed] [Google Scholar]
- 28.Hurbin A, Dubrez L, Coll JL, Favrot MC. Inhibition of apoptosis by amphiregulin via an insulin-like growth factor-1 receptor-dependent pathway in non-small cell lung cancer cell lines. J Biol Chem 2002;277:49127–49133. [DOI] [PubMed] [Google Scholar]
- 29.Okumura S, Sagara H, Fukuda T, Saito H, Okayama Y. Fcε RI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J Allergy Clin Immunol 2005;115:272–279. [DOI] [PubMed] [Google Scholar]
- 30.Hansen IA, Fassnacht M, Hahner S, Hammer F, Schammann M, Meyer SR, Bicknell AB, Allolio B. The adrenal secretory serine protease AsP is a short secretory isoform of the transmembrane airway trypsin-like protease. Endocrinology 2004;145:1898–1905. [DOI] [PubMed] [Google Scholar]
- 31.Hollenberg MD, Compton SJ. International Union of Pharmacology: XXVIII. Proteinase-activated receptors. Pharmacol Rev 2002;54:203–217. [DOI] [PubMed] [Google Scholar]
- 32.Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, Thompson PJ, Stewart GA. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol 2002;168:3577–3585. [DOI] [PubMed] [Google Scholar]
- 33.Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, Henry PJ, Carr MJ, Hamilton JR, Moffatt JD. A protective role for protease-activated receptors in the airways. Nature 1999;398:156–160. [DOI] [PubMed] [Google Scholar]
- 34.D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem 1998;46:157–164. [DOI] [PubMed] [Google Scholar]
- 35.Ricciardolo FL, Steinhoff M, Amadesi S, Guerrini R, Tognetto M, Trevisani M, Creminon C, Bertrand C, Bunnett NW, Fabbri LM, et al. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am J Respir Crit Care Med 2000;161:1672–1680. [DOI] [PubMed] [Google Scholar]
- 36.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol 2002;169:5315–5321. [DOI] [PubMed] [Google Scholar]
- 37.Vliagoftis H, Schwingshackl A, Milne CD, Duszyk M, Hollenberg MD, Wallace JL, Befus AD, Moqbel R. Proteinase-activated receptor-2-mediated matrix metalloproteinase-9 release from airway epithelial cells. J Allergy Clin Immunol 2000;106:537–545. [DOI] [PubMed] [Google Scholar]
- 38.Vliagoftis H, Befus AD, Hollenberg MD, Moqbel R. Airway epithelial cells release eosinophil survival-promoting factors (GM-CSF) after stimulation of proteinase-activated receptor 2. J Allergy Clin Immunol 2001;107:679–685. [DOI] [PubMed] [Google Scholar]
- 39.Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol 1999;163:5064–5069. [PubMed] [Google Scholar]
- 40.Riewald M, Kravchenko VV, Petrovan RJ, O'Brien PJ, Brass LF, Ulevitch RJ, Ruf W. Gene induction by coagulation factor Xa is mediated by activation of protease-activated receptor 1. Blood 2001;97:3109–3116. [DOI] [PubMed] [Google Scholar]
- 41.Ruf W, Dorfleutner A, Riewald M. Specificity of coagulation factor signaling. J Thromb Haemost 2003;1:1495–1503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.