Abstract
Rationale: Unloading the diaphragm via mechanical ventilation (MV) results in rapid diaphragmatic fiber atrophy. It is unknown whether the myonuclear domain (cytoplasmic myofiber volume/myonucleus) of diaphragm myofibers is altered during MV.
Objective: We tested the hypothesis that MV-induced diaphragmatic atrophy is associated with a loss of myonuclei via a caspase-3–mediated, apoptotic-like mechanism resulting in a constant myonuclear domain.
Methods: To test this postulate, Sprague-Dawley rats were randomly assigned to a control group or to experimental groups exposed to 6 or 12 h of MV with or without administration of a caspase-3 inhibitor.
Measurements and Main Results: After 12 h of MV, type I and type IIa diaphragm myofiber areas were decreased by 17 and 23%, respectively, and caspase-3 inhibition attenuated this decrease. Diaphragmatic myonuclear content decreased after 12 h of MV and resulted in the maintenance of a constant myonuclear domain in all fiber types. Both 6 and 12 h of MV resulted in caspase-3–dependent increases in apoptotic markers in the diaphragm (e.g., number of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling positive nuclei and DNA fragmentation). Caspase-3–dependent increases in apoptotic markers occurred after 6 h of MV, before the onset of myofiber atrophy.
Conclusions: Collectively, these data support the hypothesis that the myonuclear domain of diaphragm myofibers is maintained during prolonged MV and that caspase-3–mediated myonuclear apoptosis contributes to this process.
Keywords: muscle atrophy, respiratory muscle, apoptosis, ventilatory weaning
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Although mechanical ventilation–induced diaphragm inactivity results in fiber atrophy, it is unknown if prolonged mechanical ventilation is associated with alterations in myonuclear domain via apoptotic mechanisms.
What This Study Adds to the Field
Our results reveal that inhibiting caspase-3 activation and myonuclear loss during mechanical ventilation attenuates diaphragmatic muscle atrophy.
Mechanical ventilation (MV) is a clinical intervention for patients who are unable to maintain adequate alveolar ventilation. Recent evidence reveals that controlled MV results in a swift progression of diaphragmatic atrophy and weakness (1–6). It seems that this diaphragmatic atrophy and weakness contributes to difficulty in weaning patients from the ventilator (7). The mechanism(s) responsible for the rapid onset of diaphragmatic atrophy and weakness are not fully understood. Therefore, delineating these mechanisms is a prerequisite for the development of therapeutic strategies to circumvent weaning difficulties.
Mechanical ventilation–induced diaphragmatic atrophy and contractile dysfunction is characterized by oxidative stress and stress-related gene expression in myofibers that occurs within a matter of hours (7, 8). In addition to myofibrillar protein loss, extracellular matrix expansion, and metabolic enzyme alterations (9–11), prolonged disuse of skeletal muscle results in the selective loss of myonuclei (12–16). Myonuclear loss may be a strategy used by the fiber to maintain a constant ratio of cytoplasmic myofiber volume per myonucleus (i.e., myonuclear domain) (16). The biological significance of the myonuclear domain is based on the theory that a single myonucleus can sustain necessary gene expression for limited area of cytoplasm. This notion is supported by the observations that new nuclei are incorporated into locomotor skeletal muscle fibers during growth and that nuclei are lost during atrophy (12, 14, 16). Although it is known that controlled MV decreases protein synthesis and induces proteolysis and myofiber atrophy across all fiber types (6, 17, 18), it is unknown whether the myonuclear domain of diaphragm myofibers is altered during the fiber atrophy associated with MV.
Regulation of myonuclear loss in skeletal muscle could occur through extrinsic death receptor and intrinsic (sarcoplasmic reticulum and mitochondrial) mediated pathways (19). Caspase (cysteine-dependent, aspartate-directed protease) activation results in protein cleavage and functions in intrinsic and extrinsic pathways of apoptosis. Caspase-3 has been extensively characterized as a regulator of cellular DNA fragmentation in numerous models of skeletal muscle atrophy, including burn injury, heart failure cachexia, and hindlimb suspension (16). Numerous cellular mechanisms lead to caspase-3 activation, including mitochondrial-dependent (cytochrome c release) and mitochondrial-independent (e.g., Ca2+ activation of calpain) pathways resulting in the stimulation of DNases responsible for cellular DNA fragmentation and protein degradation (19). Oxidative stress occurs rapidly in the diaphragm during mechanical ventilation (18), and oxidant stress can promote cytochrome c release and cellular Ca2+ overload in cells (10, 20). This fact suggests a potential role for caspase-3 activation in the regulation of myonuclear number during the rapid atrophy that occurs in the diaphragm after prolonged MV.
Although it is clear that MV-induced diaphragm inactivity results in fiber atrophy (3, 5, 6), it is unknown if prolonged MV is associated with alterations in diaphragmatic myonuclei via apoptotic mechanisms. Based on reports investigating locomotor skeletal muscle atrophy (12, 14–16), we hypothesized that diaphragmatic inactivity during prolonged MV would result in myofiber atrophy and myonuclear loss, resulting in the maintenance of a constant myonuclear domain. Moreover, we postulated that the MV-induced loss of myonuclei occurs via caspase-3–regulated, apoptotic-like mechanisms. We also predicted that the initiation of myonuclear apoptosis is critically linked to MV-induced disuse atrophy. Our results support these hypotheses and establish that 12 h of MV promotes myofiber atrophy and a caspase-3–mediated proportional decrease in myonuclear number, thereby maintaining a constant myonuclear domain. Our data reveal that inhibiting caspase-3 activation and myonuclear loss in the diaphragm during MV attenuates myofiber atrophy, establishing a critical link between caspase-3 activation/myonuclear apoptosis and diaphragmatic atrophy that occurs during MV.
METHODS
Experimental Design
This experiment was approved by the University of Florida Animal Care and Use Committee. Adult female Sprague-Dawley rats were assigned to an acutely anesthetized control group (n = 6), a 12-h MV group (n = 6), and a 12-h MV group administered a specific caspase-3 inhibitor (Asp-Glu-Val-Asp-CHO [DEVD-CHO]) (n = 6). Based on our results from this initial study, to investigate the time course of MV-induced loss of myonuclei, we performed a second series of experiments and randomly assigned rats to one of three experimental groups: acute anesthesia control (n = 6), 6 h of MV (n = 6), and 6 h of MV with DEVD-CHO administration (n = 6).
Acutely Anesthetized Control Animals
Control animals were subjected to an acute plane of surgical anesthesia with an intraperitoneal injection of sodium pentobarbital (60 mg/kg body weight).
Mechanical Ventilation
All surgical procedures were performed using aseptic techniques. MV animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg body weight), tracheostomized, and mechanically ventilated using a volume-driven small animal ventilator (Harvard Apparatus, Cambridge, MA). An arterial catheter was inserted into the carotid artery for constant measurement of blood pressure and periodic blood sampling for analysis of arterial pH and blood gases. Upon completion of MV, segments of the costal diaphragm and the plantaris muscle were removed for analysis.
Myofiber Cross-sectional Area and Morphologic Analyses
Serial sections from frozen diaphragm and plantaris samples were cut at 10 μm using a cryotome (Shandon Inc., Pittsburgh, PA) and stained for dystrophin and myosin heavy chain (MHC) type I and MHC type IIa proteins for fiber cross-sectional area analysis. Myonuclear domain (μm3 cytoplasmic myofiber volume/myonucleus) was determined in the corresponding serial sections stained with hematoxylin and eosin.
In SituTdT-mediated dUTP Nick-End Labeling
The nuclei with DNA strand breaks were assessed using a fluorometric TdT-mediated dUTP nick-end labeling (TUNEL) detection kit (1684795; Roche Applied Science, Indianapolis, IN).
Genomic DNA Isolation
A section of the costal diaphragm was homogenized in 1 ml DNAzol reagent (Molecular Research Inc., Cincinnati, OH), and genomic DNA was isolated.
Ligation-mediated Polymerase Chain Reaction Ladder Assay
The detection of nucleosomal DNA ladders of apoptotic nuclei was performed using a ligation-mediated polymerase chain reaction ladder assay kit (Maxim Biotech, Inc., San Francisco, CA).
Cytosolic Protein Isolation
To assay the levels of caspase-3 activation in the diaphragm, a section of the ventral costal diaphragm was homogenized in Tris-HCl buffer and centrifuged for the isolation of cytosolic proteins.
Western Blot Analysis
Proteins (100 μg) from the cytosolic fraction were separated via polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and incubated with primary antibodies against procaspase-3 and cleaved active caspase-3. Membranes were exposed to horseradish peroxidase–conjugated secondary and chemiluminescence and were developed using autoradiography film.
Statistical Design
Comparisons between groups for each dependent variable measured were made by one-way analysis of variance. When significant differences were observed, a Tukey honestly significantly different test was implemented post hoc. Significance was established at p < 0.05.
RESULTS
12 h of MV
Systemic and biological response.
Initial and final body weights of the animals did not differ (p > 0.05) between treatment groups (data not shown). Cardiovascular dynamics during MV was monitored via measurement of heart rate and systolic blood pressure. Heart rate (365 ± 9 beats/min) and mean blood pressure (90 ± 4 mm Hg) homeostasis were maintained during MV across all treatment groups. Blood gas homeostasis and pH (data not shown) were maintained within physiologic levels during the experiment as reported previously (1, 4, 6, 18). Because sepsis is associated with diaphragmatic contractile dysfunction, strict aseptic techniques were followed throughout the experiments (1, 4, 6, 18). The colonic temperature of all MV animals remained constant at 37 ± 0.5°C during the experiments.
Myofiber Cross-sectional Area and Morphologic Analyses of Nuclear Content
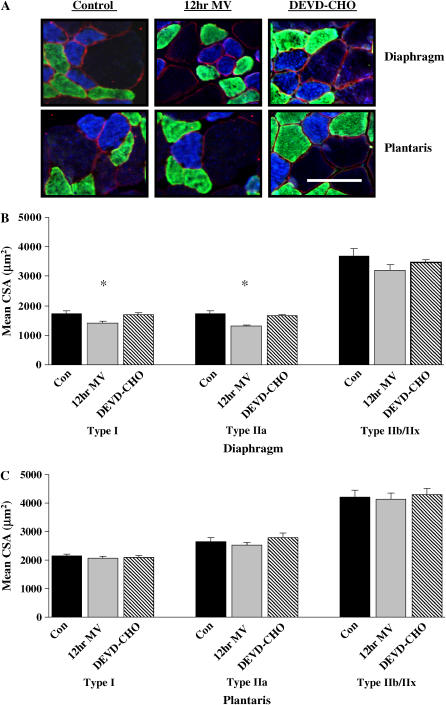
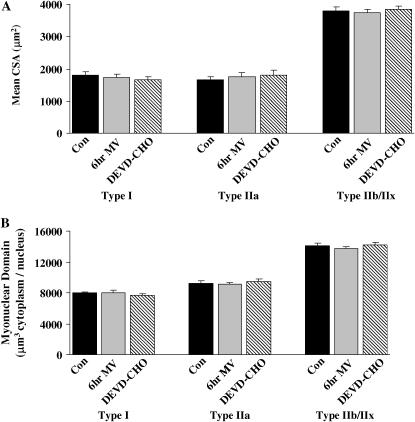
To characterize the response of diaphragm myofibers and myonuclei to MV, we chose a 12-h treatment period because we have previously demonstrated this duration as the early occurrence of contractile dysfunction in this model (4). Diaphragmatic myofiber cross-sectional area was determined for individual fiber types in cross sections obtained from 12 h MV and 12 h MV with caspase-3 inhibition via DEVD-CHO. Mechanical ventilation for 12h resulted in significant atrophy of type I (17%) and type IIa (23%) diaphragm myofibers (Figures 1A and 1B). DEVD-CHO administration attenuated atrophy of type I and type IIa diaphragm myofibers during 12 h of MV (Figure 1B). The plantaris muscle was analyzed to investigate the presence of the potential confounding effects of anesthesia on myonuclei loss in locomotor skeletal muscle. Muscle atrophy did not occur in type I, type IIa, or type IIb/IIx plantaris muscle myofibers after 12 h of MV with or without DEVD-CHO administration (Figure 1C).
Figure 1.
Fiber cross-sectional area (CSA) in diaphragm and plantaris skeletal muscle myofibers expressing myosin heavy chain (MHC) I (type I), MHC IIa (type IIa), and MHC IIb/IIx (type IIb/IIx). (A) Representative fluorescent staining of MHC I (DAPI filter/blue), MHC IIa (FITC filter/green), and dystrophin (Rhodamine filter/red) proteins in diaphragm and plantaris skeletal muscles. (B) Mean CSA (μm2) of type I, type IIa, and type IIB/IIX diaphragm skeletal muscle myofibers. (C) Mean CSA (μm2) of type I, type IIa, and type IIb/IIx plantaris skeletal muscle myofibers. Con = acutely anesthetized control group; 12hr MV = 12 h of mechanical ventilation; DEVD-CHO = 12 h of mechanical ventilation with caspase-3 inhibition. Values are means ± SE. *Significantly (p < 0.05) different from myofiber type matched controls. Bar = 30 μm.
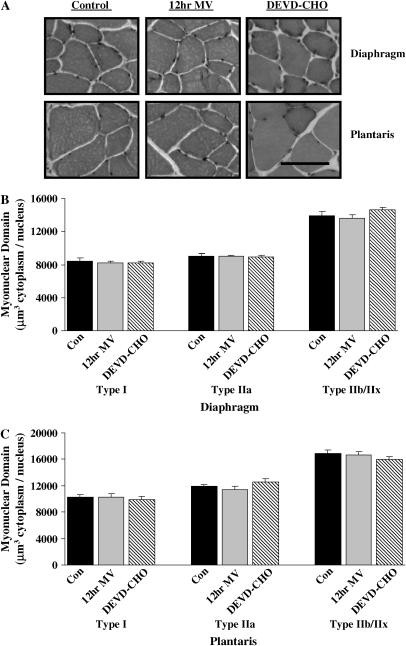
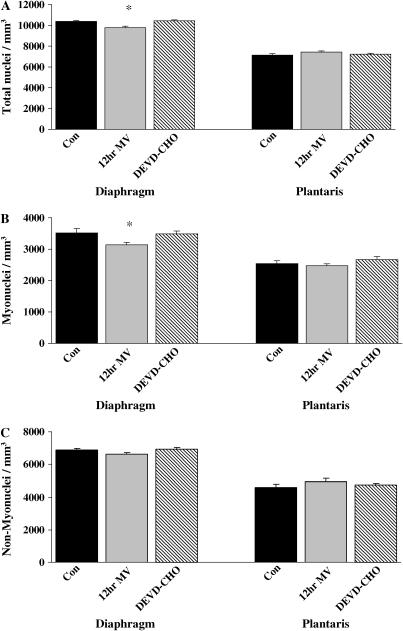
The nuclear content of the diaphragm and plantaris muscles was determined in cross-sections obtained from 12-h MV treatment groups (Figure 2A). Myonuclear domain (μm3 cytoplasm/nucleus) remained constant across all fiber types in diaphragm (Figure 2B) and plantaris (Figure 2C) muscle myofibers after 12 h of MV with or without DEVD-CHO administration. Nuclei were classified as myonuclei, nonmyonuclei (including all extracellular matrix nuclei), and total muscle nuclei (myonuclei and nonmyonuclei). Diaphragm muscle total nuclear content (total nuclei/mm3) significantly decreased by 6% with 12 h MV (Figure 3A). Myonuclear content was decreased 11% (myonuclei/mm3) with 12 h MV (Figure 3B). DEVD-CHO administration prevented the decrease in total nuclear and myonuclear content in the diaphragm associated with prolonged MV. There were no MV- or DEVD-CHO–associated alterations in plantaris muscle total nuclei, myonuclei, or nonmyonuclei content (Figures 3A–3C).
Figure 2.
Myonuclear domain size in diaphragm and plantaris skeletal muscle myofibers expressing heavy chain (MHC) I (type I), MHC IIa (type IIa), and MHC IIb/IIx (type IIb/IIx). (A) Representative hematoxylin–eosin staining of diaphragm and plantaris skeletal muscles. (B) Myonuclear domain size (μm3 cytoplasm/myonucleus) of type I, type IIa, and type IIb/IIx diaphragm skeletal muscle myofibers. (C) Myonuclear domain size of type I, type IIa, and type IIb/IIx plantaris skeletal muscle myofibers. Con = acutely anesthetized control group; 12 h MV = 12 h of mechanical ventilation; DEVD-CHO = 12 h of mechanical ventilation with caspase-3 inhibition. Values are means ± SE. Bar = 30 μm.
Figure 3.
Morphologic analysis of nuclear localization. The number of diaphragm and plantaris muscle total, myofiber-associated, and non–myofiber-associated (extracellular matrix and blood vessel–associated) nuclei (nuclei/mm3) in the acutely anesthetized control group (Con), the group receiving 12-h MV, and the group receiving 12-h MV with DEVD-CHO. (A) Total nuclei (nuclei/mm3). (B) Myonuclei (nuclei/mm3). (C) Non–myofiber nuclei (nuclei/mm3). Con = acutely anesthetized control group, 12hr MV = 12 h of mechanical ventilation, DEVD-CHO = 12-h of mechanical ventilation with caspase-3 inhibition. Values are means ± SE. *Significantly (p < 0.05) different from muscle-matched controls.
In SituCell Death (TUNEL) and Biochemical Markers of Apoptosis
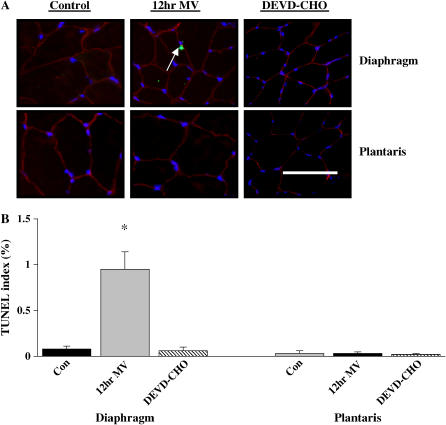
Nuclei with DNA strand breaks were assessed using fluorometric TUNEL (Figure 4A). Compared with control values (0.13 ± 0.06%), the ratio of TUNEL-positive total muscle nuclei to total muscle nuclei increased with MV (0.88 ± 0.13%) and was attenuated by DEVD-CHO administration (0.18 ± 0.03%). Twelve hours of MV increased the incidence of TUNEL-positive myonuclei in the diaphragm (Figure 4B), whereas DEVD-CHO administration returned the incidence of TUNEL-positive myonuclei baseline control values (Figure 4B). Twelve hours of MV with or without DEVD-CHO administration did not influence the incidence of TUNEL-positive myonuclei in the plantaris muscle (Figure 4B).
Figure 4.
Fluorescence labeling of diaphragm and plantaris skeletal muscle with dystrophin (Rhodamine filter/red), terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) (FITC filter/green), and nuclei (DAPI filter/blue). (A) Representative images of diaphragm and plantaris control (Con), 12-h MV, and 12-h MV with DEVD-CHO caspase-3 treatment skeletal muscle (DEVD-CHO). Arrow indicates a TUNEL-positive myonucleus. (B) TUNEL index (% TUNEL-labeled myonuclei). Values are means ± SE. *Significantly (p < 0.05) different from muscle matched controls. Bar = 30 μm.
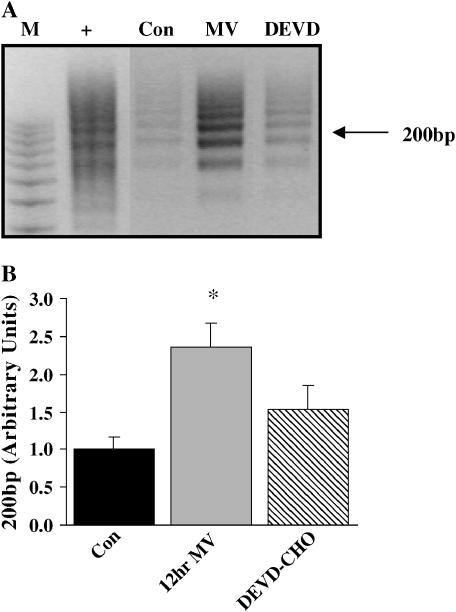
Further evidence of DNA fragmentation in the 12-h MV diaphragm was investigated by ethidium-bromide agarose gel electrophoresis. Figure 5A illustrates electrophoretic separation of the DNA fragments extracted from control (Lane 3), MV diaphragm (Lane 4), and MV-administered DEVD-CHO (Lane 5) diaphragms. Twelve hours of MV increased 200 kb DNA fragmentation by 136% above control values. DEVD-CHO administration decreased DNA fragmentation 35% from MV values alone.
Figure 5.
Nucleosomal DNA laddering in 12-h mechanically ventilated diaphragm skeletal muscle. (A) Agarose gel electrophoresis depicting nucleosomal DNA ladders after amplification by ligation-mediated polymerase chain reaction. M = molecular weight marker, 100-bp ladder; + = positive control DNA provided in the DNA Ladder Assay PCR kit (Roche Applied Science, Indianapolis, IN). Con = control; 12hr MV = 12 h of mechanical ventilation; DEVD = 12 h of mechanical ventilation with DEVD-CHO administration. (B) Fold change of 200-kbp laddered DNA. Values are means ± SE and normalized to controls. *Significantly (p < 0.05) different from controls.
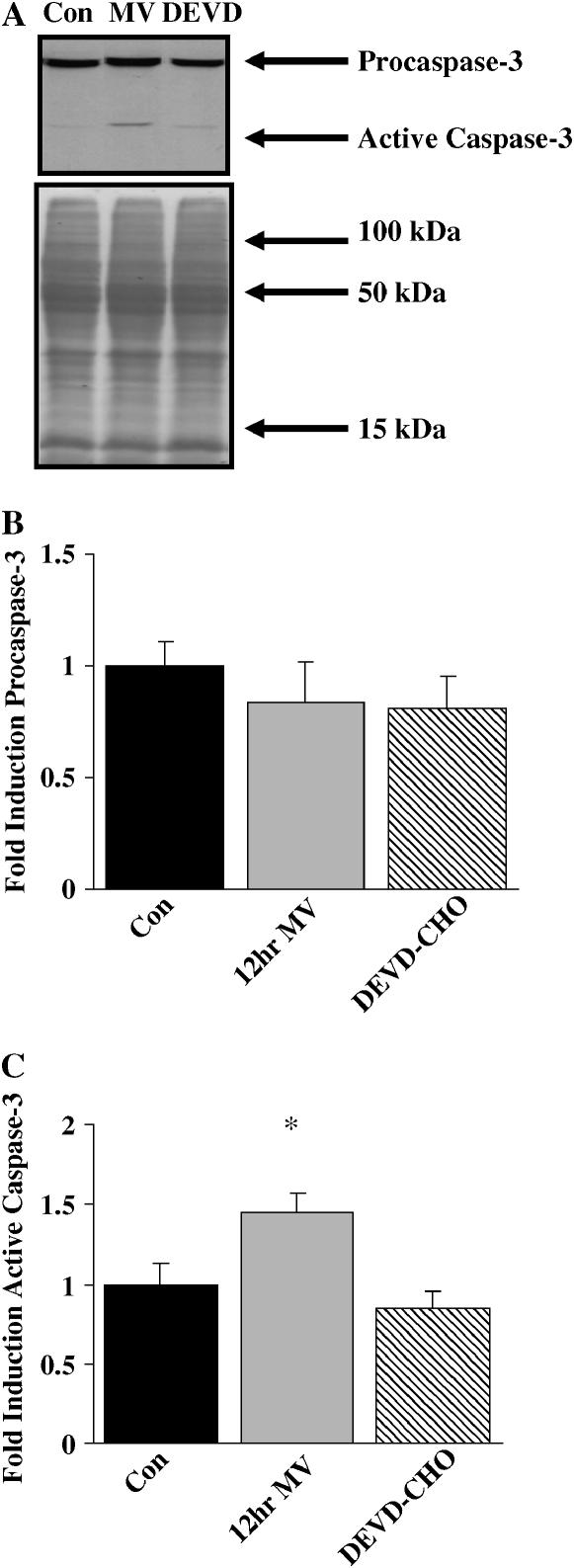
Western blot analysis was performed to determine the expression and activity levels of caspase-3 protein (Figure 6A). Twelve hours of MV did not alter the expression of procaspase-3 protein (Figure 6B). However, active caspase-3 significantly increased (45%) after 12 h of MV, and DEVD-CHO administration attenuated this increase (Figure 6C).
Figure 6.
Western blot analysis for procaspase-3 and active caspase-3 in 12 h mechanically ventilated diaphragm skeletal muscle. (A) Representative Western blot stained with total and active caspase-3 primary antibodies and representative Ponceau-stained nitrocellulose membrane. Con = control; 12hr MV = 12 h of mechanical ventilation; DEVD = 12 h of mechanical ventilation with DEVD-CHO administration.(B) Mean densitometry data for procaspase-3. (C) Mean densitometry data for active caspase-3. Values are means ± SE and normalized to controls. *Significantly different from controls.
6 h of MV
Myofiber and myonuclear response.
We investigated whether 6 h of MV altered myonuclear domain. Furthermore, we determined whether inhibition of caspase-3 activity at 6 h would alter the myonuclear response of the diaphragm to ventilation. We chose to investigate 6 h of MV because we have previously established that 6 h is the earliest time period that diaphragmatic oxidative injury can be detected after the initiation of MV (18). Six hours of MV with or without DEVD-CHO administration resulted in no diaphragmatic myofiber atrophy (Figure 7A) and no alterations in myonuclear domain (Figure 7B) of type I, type IIa, or type IIb/IIx diaphragm myofibers. In addition, compared with control, 6 h of MV did not promote alterations in total nuclear (control = 10,271 ± 207 nuclei/mm3 versus MV = 10,083 ± 205 nuclei/mm3), myonuclear (control = 3,529 ± 100 nuclei/mm3 versus MV = 3,445 ± 61 nuclei/mm3), or nonmyonuclear content (control = 6,742 ± 125 nuclei/mm3 versus MV = 6,638 ± 197 nuclei/mm3) in the diaphragm. Furthermore, compared with control and MV, 6 h of MV with DEVD-CHO administration did not alter total nuclear (9,993 ± 468 nuclei/mm3), myonuclear (3,426 ± 101 nuclei/mm3), or nonmyonuclear content (7,145 ± 223 nuclei/mm3) in the diaphragm.
Figure 7.
Fiber CSA and myonuclear domain in control (Con), 6 h mechanically ventilated (6hr MV), and 6 h MV with DEVD-CHO administration (DEVD-CHO) diaphragm skeletal muscle myofibers expressing MHC I (type I), MHC IIa (type IIa), and MHC IIb/IIx (type IIb/IIx). (A) Mean CSA (μm2) of type I, type IIa, and type IIb/IIx diaphragm skeletal muscle myofibers. (B) Myonuclear domain size (μm3 cytoplasm/myonucleus) of type I, type IIa, and type IIb/IIx diaphragm skeletal muscle myofibers. Values are means ± SE.
In situ cell death (TUNEL) and biochemical markers of apoptosis.
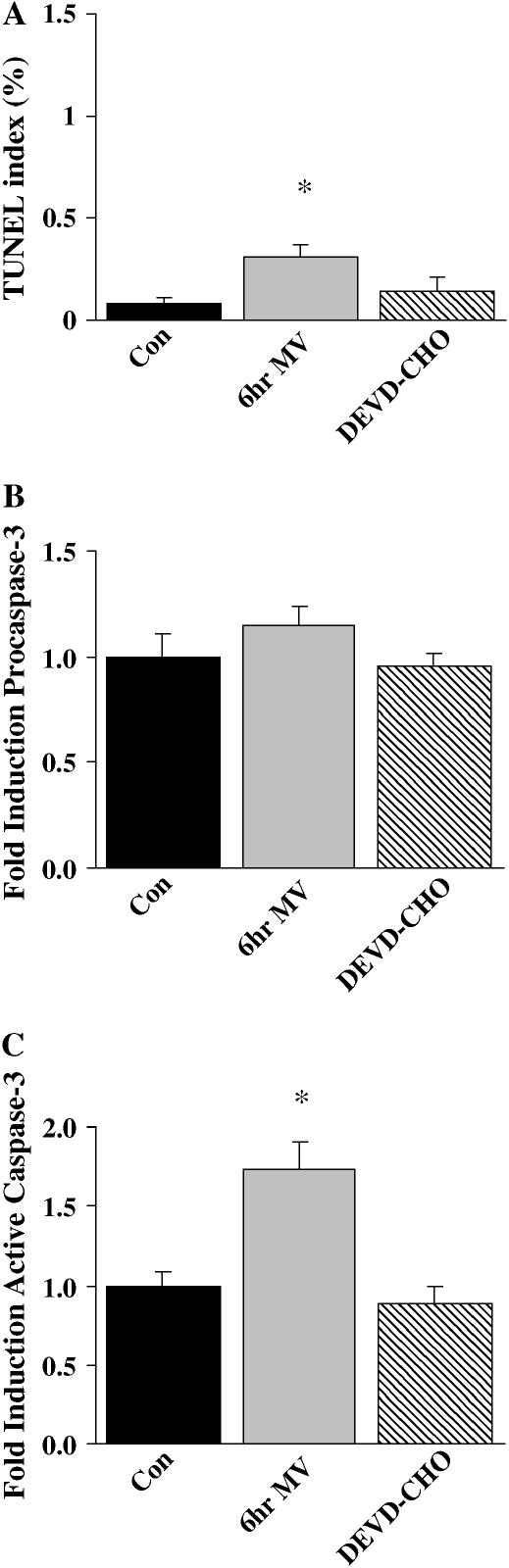
Compared with control, DNA strand breaks assessed using fluorometric TUNEL revealed an increased incidence of TUNEL-positive total nuclei in diaphragm muscle from animals exposed to 6 h of MV (control = 0.06 ± 0.07% vs. MV = 0.33 ± 0.04%), whereas this increase in MV-induced TUNEL-positive total nuclei was attenuated by DEVD-CHO administration (0.09 ± 0.03%). TUNEL-positive myonuclei increased in the diaphragm with 6 h of MV (Figure 8A), and DEVD-CHO administration attenuated this increase. Six hours of MV (1.77 ± 0.30 integrated optical density [IOD]) increased 200 kb DNA fragmentation 77% from control values (1.00 ± 0.21 IOD). Due to variation in the results, a DEVD-CHO–induced decrease (25%) in 200 kb DNA fragmentation (1.34 ± 0.13 IOD) as compared with 6-h MV values (p < 0.09) was not found to be significant. Compared with control values, 6 h of MV did not alter procaspase-3 protein expression (Figure 8B) but was associated with a 73.0% increase (p < 0.05) in cleaved caspase-3 activity (Figure 8C). DEVD-CHO administration attenuated the increase in caspase-3 activity.
Figure 8.
Biochemical markers of nuclear apoptosis in 6 h MV diaphragm skeletal muscle. (A) Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) (index: % TUNEL-labeled myonuclei). (B) Mean densitometry procaspase-3 protein. (C) Mean densitometry active caspase-3 protein. Con = acutely anesthetized control group; 6hr MV = 6 h of mechanical ventilation; and DEVD-CHO = 6 h of MV with DEVD-CHO administration. Values are means ± SE. Densitometry values normalized to control values. *Significantly (p < 0.05) different from controls.
DISCUSSION
Overview of Principal Findings
We have previously shown that diaphragmatic atrophy and contractile dysfunction occurs with as little as 18 h of controlled MV (4, 6). The current study expands on these previous findings and reveals that atrophy of diaphragm (MHC type I and MHC type IIa) myofibers occurs after 12 h of MV. These experiments demonstrate for the first time that the myonuclear domain of atrophying diaphragm myofibers expressing MHC type I and MHC type IIa is maintained during MV. Moreover, the MV-induced loss of diaphragmatic myonuclei occurred in parallel with the increased number of TUNEL-positive myonuclei and DNA fragmentation; this observation is consistent with the concept that apoptotic mechanisms are responsible for the loss of diaphragmatic myonuclei during prolonged MV. Our experiments provide new and mechanistic evidence that caspase-3 activation is a requirement for MV-induced diaphragmatic myofiber atrophy and myonuclear apoptosis. A detailed discussion of these findings is presented in the following sections.
MV-induced Alterations in Myonuclear Domain
Skeletal muscle is unique in that it is capable of modulating its size in response to alterations in functional demand. Skeletal muscle fibers are multinucleated cells, and each myonulei is thought to control a portion of the muscle fiber; a relationship known as the myonuclear domain (21). The ability of skeletal muscle to hypertrophy seems to be tightly coupled to the addition of myonuclei via satellite and myogenic cell fusion with existing myofibers (21). For example, the myonuclear domain of muscle fibers is maintained during muscle fiber hypertrophy due to postnatal growth (22–24), synergistic muscle ablation (25, 26), stretch (27), or exercise (28, 29). Muscle disuse atrophy due to a variety of experimental models results in the concurrent loss of myonuclei (14, 21, 30–34). An important finding in the current study is that MV results in the loss of diaphragm myonuclei in a manner that is proportional to the degree of type I and type IIa fiber atrophy, resulting in the maintenance of a constant myonuclear domain. This finding is consistent with previous studies investigating locomotor skeletal muscle atrophy during space flight–induced atrophy of type I and type II myofibers (14). In contrast, skeletal muscle myonuclear domain is decreased after spinal cord isolation (35) and unloading via hindlimb suspension (36). It has been theorized that differences in the response of skeletal muscle myonuclear domain size could be explained by differences in the degree of atrophy, neural influence, muscle fiber type, or the duration of the disuse intervention (37). In this context, the diaphragm is a mixed muscle including type I, IIa, IIb, and IIx fiber types that are not stimulated to contract during controlled MV (4). During MV, specific atrophy of type I and type IIa fiber types transpires in a matter of hours. In addition, type II diaphragm muscle myofibers atrophy by approximately 30% after 18 h of MV (6). Taken into context with the current study, these data suggest that type II fibers in the diaphragm predominantly atrophy between 12 and 18 h of MV. It is possible that longer periods of MV, resulting in greater total muscle myofiber atrophy, could result in proportionately greater cross-sectional area loss than myonuclear loss, thereby decreasing myonuclear domain. Therefore, the maintenance of myonuclear domain during MV may, at least in part, be a factor inherent to the unique rapid nature of this model. Additional experiments are required to determine the effects of longer duration MV on myonuclear domain.
MV-induced Myonuclear Apoptosis
Myonuclear apoptosis has been documented in a variety of muscle-wasting conditions, such as muscular dystrophy (38), denervation-induced atrophy (31), aging (39, 40), and hindlimb unloading (12, 13, 21), and could provide a mechanism for the maintenance of myonuclear domain during MV. Apoptosis results from the activation of endonucleases (e.g., caspase-3–mediated activation of nucleases) that cleave double-stranded DNA between nucleosomes. Detection of DNA fragments by measurement of nucleosomal DNA ladders or TUNEL are widely used and well-accepted techniques of apoptosis detection (41). In the current experiments, the presence of nucleosomal DNA ladders and a greater number of TUNEL-positive myonuclei suggests that myonuclear apoptosis occurred in the diaphragm during MV. In accordance with previous investigations, our findings may represent a modified form of “apoptosis” that results in death of myonuclei without ensuing myofiber death (12, 42). This form of apoptosis seems to be responsible for the elimination of myonuclei from diaphragm fibers during MV. Six hours of MV resulted in an increased number of TUNEL-positive myounuclei without corresponding alterations in myofiber area or myonuclear domain. This time point may represent the initiation of apoptotic-like mechanisms responsible for myonuclear loss and subsequent muscle atrophy.
Apoptosis of skeletal muscle myonuclei seems to occur in parallel with muscle wasting in several models of disuse atrophy. It is unknown whether myonuclear apoptosis is critically linked to skeletal muscle atrophy. In this regard, Dupont-Versteegden and colleagues (13) recently demonstrated that the early initiation of myonuclear apoptosis occurs before fiber atrophy in the soleus muscle during hindlimb unloading and suggested that this myonuclear apoptosis is a contributing mechanism to atrophy. In this article, we provide further evidence for the occurrence of myonuclear apoptosis before the onset of myofiber atrophy and demonstrate for the first time that the inhibition of signaling pathways involved in apoptosis (i.e., inhibition of caspase-3 activity) results in the attenuation of myofiber atrophy during disuse. Although numerous signaling pathways have been theorized to be important in the regulation of myonuclear apoptosis in skeletal muscle, the current study indicates that caspase-3 is the critical component of myonuclear apoptotic signaling in MV-induced diaphragmatic myonuclear apoptosis. In contrast, Dupont-Versteegden and colleagues (13) have reported a key role for endonuclease G in a caspase-independent pathway for myonuclear apoptosis in the soleus muscle during hindlimb suspension. The muscle wasting that occurs in the diaphragm during prolonged MV represents a highly accelerated form of muscle fiber atrophy as compared with other models of disuse skeletal muscle atrophy. It follows that the apoptotic signaling pathways active in the diaphragm during prolonged MV may differ from the apoptotic signaling pathways responsible for myonuclear apoptosis in locomotor skeletal muscle in a model of reduced-use muscle atrophy.
Consistent with previous reports (5, 6, 20), the current data indicate that MV results in significant atrophy of the costal diaphragm. Furthermore, atrophy of MHC type I and MHC type IIa fibers corresponds with previous data demonstrating deficits in force production after 12 h of MV (4). This rapid rate of MV-induced diaphragmatic atrophy greatly exceeds the time course of atrophy observed in locomotor muscles during periods of disuse (43). For example, it would take at least 96 h to achieve the same level of atrophy in unloaded locomotor muscle as observed in the diaphragm after 12 h of MV (44). Specifically, the 2 d required to induce a 17% decrease in soleus muscle myofiber cross-sectional area reported by Dupont-Versteegden and colleagues (13) occurs in diaphragm myofibers expressing type I MHC after only 12 h of mechanical ventilation. In fact, the diaphragm loses 16 to 23% of its mean cross-sectional area in only 6 h (between 6 and 12 h of ventilation). In addition, the rate of MV-induced atrophy exceeds that reported in the diaphragm with denervation (45), spinal cord hemisection (46), and corticosteroid administration (37). These facts suggest that the signaling associated with myofiber atrophy in this model may involve different or accelerated signaling mechanisms that are normally associated with slower models of myofiber wasting.
Caspase-3 represents a complex atrophic signaling protein in skeletal muscle. In addition to its role in mitochondrial-dependent and mitochondrial-independent mediated apoptosis, caspase-3 also serves as a protease involved in myofibrillar protein release, resulting in contractile protein proteolysis (10). Therefore, inhibition of caspase-3 during MV may result in the retardation of myofilament release associated with myofiber atrophy in this model. Moreover, our results reveal that administration of a caspase-3–specific inhibitor during the initial 6 h of mechanical ventilation results in the attenuation of myonuclear apoptosis at a time point when no measurable alterations in myofiber size are present. This finding suggests a specific role for caspase-3 signaling in myonuclear apoptosis independent of its potential role in myofilament release during ventilation-induced muscle atrophy.
Conclusions
This is the first study to demonstrate that myonuclear domain size is maintained during MV-induced diaphragmatic atrophy. The loss of myonuclei necessary for the maintenance of myonuclear domain during MV seems to be, at least in part, due to caspase-3 mediated myonuclear apoptosis. The current data provide the first evidence that caspase-3 activation is required for diaphragmatic fiber atrophy during the first 12 h of MV. Given that difficulty in weaning patients from MV is a significant clinical problem, it is important to develop therapeutic countermeasures to retard MV-induced diaphragmatic atrophy and weakness. The current investigation provides a theoretical basis for future translational clinical studies investigating caspase-3 inhibition in the diaphragm as a therapeutic countermeasure to retard MV-induced diaphragm atrophy and dysfunction.
Supplementary Material
Acknowledgments
The myosin heavy chain type I (A4.840) antibody developed by Helen M. Blau was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development (NICHD) and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA.
Supported by National Institutes of Health grant R01HL62361 (S.K.P.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200601-142OC on November 2, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Criswell DS, Shanely RA, Betters JJ, McKenzie MJ, Sellman JE, Van Gammeren DL, Powers SK. Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest 2003;124:2302–2308. [DOI] [PubMed] [Google Scholar]
- 2.Gayan-Ramirez G, de Paepe K, Cadot P, Decramer M. Detrimental effects of short-term mechanical ventilation on diaphragm function and IGF-I mRNA in rats. Intensive Care Med 2003;29:825–833. [DOI] [PubMed] [Google Scholar]
- 3.Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med 1994;149:1539–1544. [DOI] [PubMed] [Google Scholar]
- 4.Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol 2002;92:1851–1858. [DOI] [PubMed] [Google Scholar]
- 5.Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol 2002;92:2585–2595. [DOI] [PubMed] [Google Scholar]
- 6.Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 2002;166:1369–1374. [DOI] [PubMed] [Google Scholar]
- 7.DeRuisseau KC, Kavazis AN, Deering MA, Falk DJ, Van Gammeren D, Yimlamai T, Ordway GA, Powers SK. Mechanical ventilation induces alterations of the ubiquitin-proteasome pathway in the diaphragm. J Appl Physiol 2005;98:1314–1321. [DOI] [PubMed] [Google Scholar]
- 8.Deruisseau KC, Shanely RA, Akunuri N, Hamilton MT, Van Gammeren D, Zergeroglu AM, McKenzie M, Powers SK. Diaphragm unloading via controlled mechanical ventilation alters the gene expression profile. Am J Respir Crit Care Med 2005;172:1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diffee GM, Caiozzo VJ, Herrick RE, Baldwin KM. Contractile and biochemical properties of rat soleus and plantaris after hindlimb suspension. Am J Physiol 1991;260:C528–C534. [DOI] [PubMed] [Google Scholar]
- 10.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 2005;288:R337–R344. [DOI] [PubMed] [Google Scholar]
- 11.Tsika RW, Herrick RE, Baldwin KM. Time course adaptations in rat skeletal muscle isomyosins during compensatory growth and regression. J Appl Physiol 1987;63:2111–2121. [DOI] [PubMed] [Google Scholar]
- 12.Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol 1997;273:C579–C587. [DOI] [PubMed] [Google Scholar]
- 13.Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD, Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol 2006;291:R1730– R1740. [DOI] [PubMed] [Google Scholar]
- 14.Hikida RS, Van Nostran S, Murray JD, Staron RS, Gordon SE, Kraemer WJ. Myonuclear loss in atrophied soleus muscle fibers. Anat Rec 1997;247:350–354. [DOI] [PubMed] [Google Scholar]
- 15.Primeau AJ, Adhihetty PJ, Hood DA. Apoptosis in heart and skeletal muscle. Can J Appl Physiol 2002;27:349–395. [DOI] [PubMed] [Google Scholar]
- 16.Sandri M. Apoptotic signaling in skeletal muscle fibers during atrophy. Curr Opin Clin Nutr Metab Care 2002;5:249–253. [DOI] [PubMed] [Google Scholar]
- 17.Shanely RA, Van Gammeren D, Deruisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE, Powers SK. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med 2004;170:994–999. [DOI] [PubMed] [Google Scholar]
- 18.Zergeroglu MA, McKenzie MJ, Shanely RA, Van Gammeren D, DeRuisseau KC, Powers SK. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol 2003;95:1116–1124. [DOI] [PubMed] [Google Scholar]
- 19.Dupont-Versteegden EE. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 2005;40:473–481. [DOI] [PubMed] [Google Scholar]
- 20.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol 1998;275:C1–24. [DOI] [PubMed] [Google Scholar]
- 21.Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve 1999;22:1350–1360. [DOI] [PubMed] [Google Scholar]
- 22.Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol 1970;44:459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 1971;170:421–435. [DOI] [PubMed] [Google Scholar]
- 24.Wigmore PM, Baillie HS, Khan M, Morrison EH, Mayhew TM. Nuclear number during muscle development. Muscle Nerve 1992;15:1301–1302. [PubMed] [Google Scholar]
- 25.Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve 1994;17:608–613. [DOI] [PubMed] [Google Scholar]
- 26.Snow MH. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat Rec 1990;227:437–446. [DOI] [PubMed] [Google Scholar]
- 27.Winchester PK, Gonyea WJ. Regional injury and the terminal differentiation of satellite cells in stretched avian slow tonic muscle. Dev Biol 1992;151:459–472. [DOI] [PubMed] [Google Scholar]
- 28.Cabric M, James NT. Morphometric analyses on the muscles of exercise trained and untrained dogs. Am J Anat 1983;166:359–368. [DOI] [PubMed] [Google Scholar]
- 29.Darr KC, Schultz E. Exercise-induced satellite cell activation in growing and mature skeletal muscle. J Appl Physiol 1987;63:1816–1821. [DOI] [PubMed] [Google Scholar]
- 30.Adams V, Jiang H, Yu J, Mobius-Winkler S, Fiehn E, Linke A, Weigl C, Schuler G, Hambrecht R. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol 1999;33:959–965. [DOI] [PubMed] [Google Scholar]
- 31.Borisov AB, Carlson BM. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec 2000;258:305–318. [DOI] [PubMed] [Google Scholar]
- 32.Dupont-Versteegden EE, Murphy RJ, Houle JD, Gurley CM, Peterson CA. Activated satellite cells fail to restore myonuclear number in spinal cord transected and exercised rats. Am J Physiol 1999;277:C589–C597. [DOI] [PubMed] [Google Scholar]
- 33.Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE. Satellite cell regulation of muscle mass is altered at old age. J Appl Physiol 2004;97:1082–1090. [DOI] [PubMed] [Google Scholar]
- 34.Smith HK, Maxwell L, Martyn JA, Bass JJ. Nuclear DNA fragmentation and morphological alterations in adult rabbit skeletal muscle after short-term immobilization. Cell Tissue Res 2000;302:235–241. [DOI] [PubMed] [Google Scholar]
- 35.Roy RR, Zhong H, Siengthai B, Edgerton VR. Activity-dependent influences are greater for fibers in rat medial gastrocnemius than tibialis anterior muscle. Muscle Nerve 2005;32:473–482. [DOI] [PubMed] [Google Scholar]
- 36.Kasper CE, Xun L. Cytoplasm-to-myonucleus ratios in plantaris and soleus muscle fibres following hindlimb suspension. J Muscle Res Cell Motil 1996;17:603–610. [DOI] [PubMed] [Google Scholar]
- 37.Verheul AJ, Mantilla CB, Zhan WZ, Bernal M, Dekhuijzen PN, Sieck GC. Influence of corticosteroids on myonuclear domain size in the rat diaphragm muscle. J Appl Physiol 2004;97:1715–1722. [DOI] [PubMed] [Google Scholar]
- 38.Sandri M, Carraro U, Podhorska-Okolov M, Rizzi C, Arslan P, Monti D, Franceschi C. Apoptosis, DNA damage and ubiquitin expression in normal and mdx muscle fibers after exercise. FEBS Lett 1995;373:291–295. [DOI] [PubMed] [Google Scholar]
- 39.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol 2002;282:R519–R527. [DOI] [PubMed] [Google Scholar]
- 40.Strasser H, Tiefenthaler M, Steinlechner M, Eder I, Bartsch G, Konwalinka G. Age dependent apoptosis and loss of rhabdosphincter cells. J Urol 2000;164:1781–1785. [PubMed] [Google Scholar]
- 41.Adams V, Gielen S, Hambrecht R, Schuler G. Apoptosis in skeletal muscle. Front Biosci 2001;6:D1–D11. [DOI] [PubMed] [Google Scholar]
- 42.Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol 2005;288:C338–C349. [DOI] [PubMed] [Google Scholar]
- 43.Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol 2003;95:2185–2201. [DOI] [PubMed] [Google Scholar]
- 44.Thomason DB, Biggs RB, Booth FW. Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Physiol 1989;257:R300–R305. [DOI] [PubMed] [Google Scholar]
- 45.Geiger PC, Bailey JP, Zhan WZ, Mantilla CB, Sieck GC. Denervation-induced changes in myosin heavy chain expression in the rat diaphragm muscle. J Appl Physiol 2003;95:611–619. [DOI] [PubMed] [Google Scholar]
- 46.Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol 1997;82:1145–1153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.