Abstract
Rationale: In an observational cohort study, women who self-selected for frequent aspirin use developed less newly diagnosed asthma than women who did not take aspirin.
Objective: To explore whether low-dose aspirin decreased the risk of newly diagnosed asthma in a randomized, double-blind, placebo-controlled trial.
Methods: The Physicians' Health Study randomized 22,071 apparently healthy male physicians, aged 40–84 yr at baseline and tolerant of aspirin, over an 18-wk run-in period, to 325 mg aspirin or placebo on alternate days. The aspirin component was terminated after 4.9 yr due principally to the emergence of a statistically extreme 44% reduction in risk of first myocardial infarction among those randomly assigned to aspirin.
Measurements: Physicians could self-report an asthma diagnosis on questionnaires at baseline, 6 mo, and annually thereafter. Asthma was not an a priori endpoint of the trial.
Results: Among 22,040 physicians without reported asthma at randomization, there were 113 new asthma diagnoses in the aspirin group and 145 in the placebo group. The hazard ratio was 0.78 (95% confidence interval, 0.61–1.00; p = 0.045). This apparent 22% lower risk of newly diagnosed asthma among those assigned to aspirin was not modified by baseline characteristics including smoking, body mass index, or age.
Conclusions: Aspirin reduced the risk of newly diagnosed adult-onset asthma in a large, randomized clinical trial of apparently healthy, aspirin-tolerant men. This result requires replication in randomized trials designed a priori to test this hypothesis; it does not imply that aspirin improves symptoms in patients with asthma.
Keywords: asthma, aspirin, NSAIDs, analgesics, obstructive airways disease
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Aspirin is known to worsen symptoms among a minority of patients with asthma. Recent epidemiologic and bench studies, however, suggest that frequent aspirin use might reduce the risk of developing asthma among adults.
What This Study Adds to the Field
This secondary analysis of a large randomized, double-blind, placebo-controlled trial of aspirin-tolerant men showed that randomization to aspirin reduced the risk of adult-onset asthma.
Asthma prevalence has increased in the United States since the late 1970s, particularly among children (1), and now approaches that of high-prevalence countries such as the United Kingdom and Australia (2). The increase in the United States coincided with a substantial decline in the use of aspirin as an antipyretic and analgesic in the late 1970s and 1980s (3). The switch from aspirin to other over-the-counter analgesics (e.g., acetaminophen) was particularly pronounced among children due to reports of a link between aspirin and Reyes syndrome in the early 1980s (4, 5). Because aspirin might promote Th1 and inhibit Th2 phenotypes (6, 7), Varner and colleagues hypothesized that the reduction in pediatric aspirin use in the United States contributed, in part, to the increase in asthma prevalence (3).
In a large observational cohort study of analgesic use and adult-onset asthma among women, participants who self-selected to take aspirin frequently had a lower rate of newly diagnosed asthma than those who never took aspirin, even after adjustment for a large number of potential confounding variables (8). The multivariate hazard ratio for newly diagnosed asthma was 0.60 (95% confidence interval [CI], 0.40–0.81) for those who took aspirin 15 or more days a month compared with those who never took aspirin. This finding, however, might have resulted from confounding by unmeasured factors, given its modest magnitude and the observational design of that study. For small to moderate effects, randomized trials of sufficient size, dose, and duration represent the most valid design. The Physicians' Health Study (PHS), a large-scale randomized, double-blind, placebo-controlled 2 × 2 factorial trial of aspirin and β-carotene, provided a unique opportunity to test whether low-dose aspirin decreases the risk of newly diagnosed adult-onset asthma. Some results in this article were presented in abstract form at the 2005 meeting of the European Respiratory Society (9).
METHODS
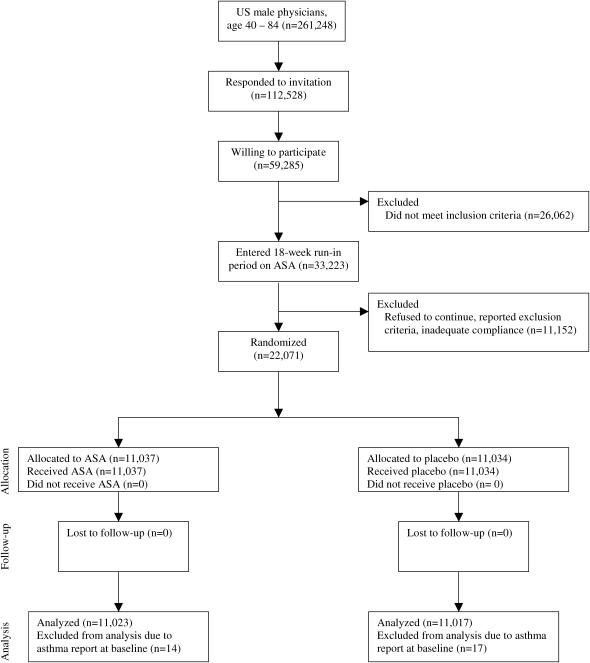
The PHS tested the risk and benefits of low-dose aspirin (325 mg on alternate days [Bufferin; Bristol-Myers Squibb, New Jersey]) and β-carotene (50 mg on alternate days [Lurotin; BASF, Ludwigshafen, Germany]) in the primary prevention of cardiovascular disease and cancer among 22,071 apparently healthy men aged 40–84 yr at entry in 1982. The design, methods, and results of the PHS have been described in detail (10). Participants were excluded if they had a previous diagnosis of myocardial infarction, stroke, transient ischemic attack, cancer (except nonmelanoma skin cancer), current renal or liver disease, peptic ulcer or gout, or if they reported current use of aspirin, vitamin A, or β-carotene supplements. A total of 33,223 willing and eligible male physicians aged 40 to 84 yr at baseline in 1982 were enrolled in a run-in phase during which all participants received active aspirin and β-carotene placebo (Figure 1). After 18 wk, participants were sent questionnaires asking about their health status, side effects, compliance, and willingness to continue in the trial. A total of 11,152 who had changed their minds, reported a reason for exclusion, or demonstrated inadequate compliance (defined as taking less than two-thirds of the study pills) were excluded from the trial, resulting in 22,071 willing and eligible male physicians who were then randomized to aspirin or β-carotene according to a factorial design. The aspirin arm was terminated early after an average follow-up of 4.9 yr on January 25, 1988, primarily because of the emergence of a statistically extreme (p < 0.00001) 44% reduction in risk of a first myocardial infarction in the aspirin group (10). Morbidity and mortality follow-up was more than 99% complete at the end of the randomized aspirin component. The β-carotene component of the trial continued to its scheduled end on December 31, 1995 (11).
Figure 1.
Flow diagram of the progress through the phases of the Physicians' Health Study. ASA = aspirin.
Ascertainment of Asthma
Baseline information was collected by mailed follow-up questionnaires that asked about many anthropometric, demographic, clinical diagnosis, and lifestyle variables. Twice in the first year and yearly thereafter, follow-up questionnaires were sent out asking about compliance with study medication, development of side effects, newly developed cardiovascular disease, newly developed cancer, dates of diagnoses, and personal characteristics and habits.
Participating physicians were asked about newly diagnosed diseases that required medical treatment, such as asthma, and their date of diagnosis on the baseline and each of the follow-up questionnaires. New diagnosis of asthma was not a prespecified endpoint of the PHS.
Statistical Analysis
We included all reports of asthma from enrollment until the unblinding of the aspirin component on January 25, 1988. We excluded 31 participants (14 in the aspirin group and 17 in the placebo group) who indicated an asthma diagnosis before randomization, leaving 22,040 participants without a report of asthma at baseline.
We compared continuous measurements using Student's t test and categorical variables using the χ2 test. The cumulative incidence of asthma over the period of the trial was compared across treatment arms, and analyzed according to the intention-to-treat principle. We used Kaplan-Meier plots and Cox proportional hazards models (12) to analyze the association between randomized aspirin assignment and time from randomization to date of new physician diagnosis of asthma. We also performed stratified analysis by smoking status (never, past, current), body mass index (< 25, 25–29.9, ⩾ 30 kg/m2), and age (in quartiles) and tested the significance of these interactions using model-based multiplicative terms.
RESULTS
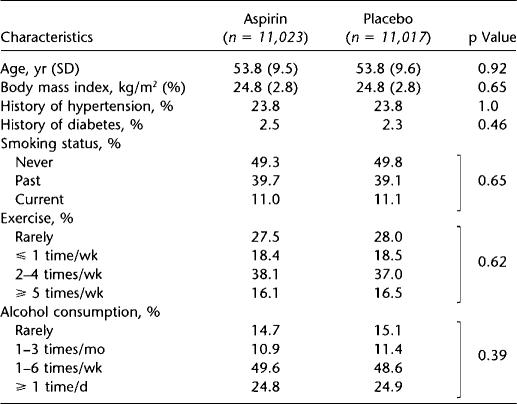
Table 1 summarizes the baseline characteristics of the 22,040 participants without reported asthma at baseline, according to randomized aspirin assignment. As expected in this large sample, the baseline characteristics were virtually identical in the aspirin and placebo groups.
TABLE 1.
BASELINE CHARACTERISTICS OF PARTICIPANTS WITHOUT REPORT OF ASTHMA DIAGNOSIS AT RANDOMIZATION (n = 22,040), ACCORDING TO RANDOMIZED ASPIRIN ASSIGNMENT
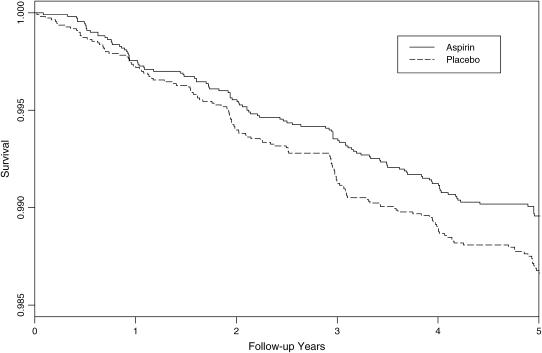
Over a mean follow-up of 4.9 yr (109,035 person-years), a total of 258 physicians reported new diagnoses of asthma, which corresponded to a cumulative incidence of adult-onset asthma of 1.2%. There were 113 new diagnoses of asthma reported in the aspirin group and 145 reported in the placebo group (Table 2). The hazard ratio of 0.78 (95% CI, 0.61–1.00; p = 0.045) for newly diagnosed asthma represented a 22% reduction due to aspirin. The cumulative incidence of asthma in the aspirin and placebo groups over follow-up is shown in Figure 2.
TABLE 2.
RANDOMIZED ASPIRIN ASSIGNMENT AND REPORT OF NEWLY DIAGNOSED ASTHMA IN THE PHYSICIANS' HEALTH STUDY
| Cases of Newly Diagnosed Asthma
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aspirin Group
|
Placebo Group
|
|||||||||
| (n = 11,023) 54,572 person-years | (n = 11,017) 54,462 person-years | Hazard Ratio | 95% CI | p Value | ||||||
| Overall, n | 113 | 145 | 0.78 | 0.61–1.00 | 0.045 | |||||
| Stratified Analyses | ||||||||||
| Smoking status, n | 0.83* | |||||||||
| Never | 48 | 70 | 0.69 | 0.48–1.00 | 0.049 | |||||
| Past | 59 | 62 | 0.93 | 0.65–1.33 | 0.71 | |||||
| Current | 6 | 13 | 0.47 | 0.18–1.23 | 0.12 | |||||
| Body mass index, kg/m2 | 0.89* | |||||||||
| < 25, n | 69 | 83 | 0.82 | 0.59–1.13 | 0.22 | |||||
| 25–29.9, n | 37 | 56 | 0.68 | 0.45–1.03 | 0.065 | |||||
| ⩾ 30, n | 7 | 6 | 1.13 | 0.38–3.37 | 0.82 | |||||
| Age, yr | 0.39* | |||||||||
| < 45.9, n | 19 | 38 | 0.49 | 0.28–0.85 | 0.01 | |||||
| 45.9–52.5, n | 31 | 25 | 1.25 | 0.74–2.12 | 0.40 | |||||
| 52.5–60.1, n | 23 | 36 | 0.65 | 0.38–1.09 | 0.10 | |||||
| > 60.1, n | 40 | 46 | 0.86 | 0.57–1.32 | 0.50 | |||||
Definition of abbreviation: CI = confidence interval.
p Value for interaction term of difference on the effect of aspirin on newly diagnosed asthma across subgroups of smoking status, body mass index, or age.
Figure 2.
Asthma-free survival during the randomized component of the Physicians' Health Study.
In stratified analyses, there were no statistically significant differences in the observed benefit of aspirin on adult-onset asthma by smoking status, body mass index, and age (Table 2). The magnitude of the reduction appeared to be greater among the subgroups who never smoked (p = 0.049) as well as among younger men (p = 0.01).
DISCUSSION
In this large randomized, double-blind, placebo-controlled trial among apparently healthy men, 325 mg of aspirin on alternate days statistically significantly reduced the incidence of newly diagnosed, adult-onset asthma by 22%. In subgroup analyses, there were no statistically significant modifications by smoking status, body mass index, and age, although the magnitude of the observed benefit appeared to be somewhat greater among younger men.
These randomized data among male physicians are consistent with prior findings from a large prospective cohort study of female registered nurses examining analgesic use and asthma, in which women who self-selected for frequent aspirin use had a lower rate of new physician diagnosis of asthma than women who did not take aspirin, even after adjustment for a large number of confounding variables (8). That study primarily examined acetaminophen use and newly diagnosed asthma; the observed inverse association of aspirin and newly diagnosed asthma, however, was independent of acetaminophen use and consistent across categories of other analgesic use.
No other large prospective studies have been published examining aspirin and risk of newly diagnosed asthma, to the best of our knowledge. A cross-sectional analysis of self-reported physician diagnosis of asthma did not show an association with aspirin use (13); however, that study was limited by its cross-sectional design and lack of randomized assignment of aspirin. An evaluation of asthma morbidity associated with another nonsteroidal antiinflammatory drug, ibuprofen, is available from a subgroup analysis of one large trial. In that randomized, double-blind trial, ibuprofen was compared with acetaminophen for pediatric febrile illness among 27,065 children aged 2 yr or less (14). Follow-up was too short to assess new-onset asthma but, in the subgroup of 1,879 children with asthma, asthma-related outpatient visits were significantly lower in the ibuprofen than in the acetaminophen arm (15). Asthma hospitalizations were also lower in the ibuprofen than the acetaminophen arm, although that result did not attain statistical significance, due possibly to the small number of children hospitalized. The trial did not include placebo control, so it is unclear whether ibuprofen decreased or acetaminophen increased asthma exacerbations.
Among individuals with asthma, aspirin is known to acutely precipitate bronchospasm in the subset of patients with aspirin-intolerant asthma (AIA). AIA affects a small minority of patients with asthma, approximately 4 to 11% in large population-based studies (16–18). The proportion is higher in adult referral populations and much lower—perhaps by three-quarters—among children (19). In patients with AIA, cyclooxygenase (COX)-1 inhibition is believed to increase the bronchoconstrictor cysteinyl leukotrienes (C4, D4, E4). Predisposition for AIA is associated with gene variants in leukotriene C4 synthase (20), cysteinyl leukotriene receptor 2 (21), thromboxane A2 (22), or prostaglandin E2 genes (23).
Most patients with asthma tolerate aspirin without problems and some patients with asthma improve when challenged with aspirin and other nonsteroidal antiinflammatory drugs (24–26). In aspirin-tolerant patients, the increase in cysteinyl leukotrienes and reduction in prostaglandin E2 may be counterbalanced or overwhelmed by reductions in the bronchoconstrictors prostaglandin D2, prostaglandin F2, and the thromboxanes (27) triggered by COX-1 inhibition.
In individuals without asthma, aspirin might reduce the risk of developing asthma via COX-dependent and COX-independent pathways. COX-1 inhibition blocks prostaglandin E2, which inhibits Th1 lymphocytes from releasing IFN-γ (28, 29) and may shift cytokine production from a Th2 phenotype toward a Th1 phenotype. Therapeutic doses of aspirin also inhibit IL-4– and IL-13–induced activation of signal transducer and activator of transcription 6 (STAT6) (6) and promote STAT1 activation in IFN-γ signaling via non–COX-dependent pathways (7), thereby potentially inhibiting Th2 immune responses while promoting Th1 responses. Additional COX-independent mechanisms include the inhibition of activation of nuclear factor-κB (30) and induction of lipoxin A4 and 15-epi-lipoxin A4, otherwise known as aspirin-triggered lipoxin (31). These products of arachadonic acid metabolism block airway hyperresponsiveness and pulmonary inflammation in murine models (32) and in vivo (33) and are currently seen as novel targets for asthma therapies. It is therefore mechanistically plausible that regular use of aspirin might reduce the risk of the development of asthma.
The strengths of this trial included a randomized, double-blind, placebo-controlled design, high follow-up and compliance, and physician participants who were likely to accurately report new diagnoses of asthma. This large trial had adequate size to detect a small to moderate benefit of aspirin on newly diagnosed adult-onset asthma but was not designed specifically to test this hypothesis.
The occurrence of newly diagnosed asthma was based on self-reports by physicians, who are unlikely to misreport their own medical diagnoses. Although there was likely underreporting of new asthma diagnoses to the general questionnaire item on new diagnoses, the randomized, double-blind, placebo-controlled design made it extremely unlikely that the underreports of asthma diagnoses depended on the assignment to the aspirin or placebo groups. Because any potential underreporting of the diagnosis was therefore likely random, such misclassification would reduce the observed estimate compared with a true benefit of aspirin on asthma and would bias our results toward the null.
The main analysis relied on reports of newly diagnosed asthma to general questionnaire items. We therefore performed a secondary analysis based on specific questionnaire items on physician-diagnosed asthma and date of diagnosis of physician-diagnosed asthma that were administered in 1997. This secondary analysis also yielded a statistically significant lower rate of newly diagnosed asthma in the aspirin group compared with the placebo group during the randomized phase of the trial, and the effect estimate was virtually identical (data not shown).
Chronic obstructive pulmonary disease can be misdiagnosed as adult-onset asthma, even in health professionals (34). In subgroup analyses, however, the observed benefit of aspirin on asthma was statistically significant among never-smokers and in the youngest age category. Furthermore, there were 11 more cases of chronic obstructive pulmonary disease in the aspirin group than in the placebo group, making it unlikely that the true benefit was really for chronic obstructive pulmonary disease. It was also very unlikely that the observed effect resulted from a reduction in “cardiac asthma” because only one physician experienced a myocardial infarction before reporting a new diagnosis of asthma.
Results of statistical tests for the main analysis yielded statistically significant results at standard thresholds of statistical significance, but not by large margins. It is therefore possible that our result is false-positive one. We doubt this, however, because the hypothesis was previously and independently formulated (3), the results from this randomized trial among men were consistent with those from a large observational prospective cohort study among women (8), and the mechanisms underlying the observed effect are biologically plausible.
Finally, although all participants were encouraged to use nonaspirin, non–nonsteroidal antiinflammatory drug pain medication, it is conceivable, although unlikely, that random assignment to low-dose aspirin decreased use of acetaminophen, which in turn might have contributed to fewer new diagnoses of asthma (8). We have no reason to believe that 325 mg of aspirin on alternate days did affect acetaminophen use, although data on acetaminophen use were not collected during the randomized phase of the PHS. Furthermore, data collected after the randomized phase of the PHS suggested that cumulative intake of acetaminophen did not increase asthma diagnoses in this cohort (35).
Our results were observed in apparently healthy men free from asthma at baseline. PHS participants were aged 40 to 84 yr at entry and thus belonged to a generation who were likely to have been exposed to aspirin in childhood. They all tolerated an 18-wk run-in period on aspirin before randomization (during which none, to our knowledge, reported aspirin-induced asthma), and none had an aspirin allergy before enrollment. These results may not generalize to children or aspirin-naive individuals and do not apply to patients with asthma.
In summary, aspirin reduced the risk of newly diagnosed, adult-onset asthma in this large randomized trial of apparently healthy male physicians. These results suggest that aspirin may reduce the risk of the development of asthma in adults. They do not imply that aspirin improves symptoms in patients with asthma; indeed, aspirin can cause severe bronchospasm in some patients with asthma. Because asthma was not the primary endpoint of the PHS, additional randomized trials would be helpful to confirm the apparent reduction in asthma incidence caused by aspirin.
Acknowledgments
The authors thank the participants in the Physicians' Health Study for their outstanding commitment and cooperation, the entire Physicians' Health Study staff for their expert and unfailing assistance, and Vadim Bubes, Ph.D., for programming assistance.
Supported by a Robert Wood Johnson Generalist Physician Faculty Scholar Award to R.G.B.; grants CA 34944 and CA 40360 from the National Cancer Institute, Bethesda, Maryland; and grants HL 26490 and HL 34595 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland.
Originally Published in Press as DOI: 10.1164/rccm.200603-411OC on October 26, 2006
Conflict of Interest Statement: R.G.B. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.K. has received research funding from Bayer AG ($50,000), McNeil Consumer & Specialty Pharmaceutical ($50,000), and Wyeth Consumer Healthcare ($50,000); he is a consultant to i3 Drug Safety, and received an honorarium from Organon for contributing to an expert panel. M.J.S. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E.B. has received research funding from Dow Corning Corporation, research support for pills and/or packaging from Bayer Health Care and the Natural Source Vitamin E Association, and $6,000 in honoraria from Bayer for speaking engagements. C.H.H. is funded by the Department of Biomedical Science and Center of Excellence at Florida Atlantic University (FAU); he is Principal Investigator or Co-Principal Investigator on two investigator-initiated research grants funded to FAU by Bayer ($1.5 million), testing the effects of aspirin dose on platelet biomarkers, inflammatory markers, nitric oxide formation, and endothelial function; he serves as consultant, including as Chair or Member on Data and Safety Monitoring Boards, for Actelion, Agatston Research Institute, Amgen, AstraZeneca, Bayer ($30,000/yr), Biovail, Bristol-Myers Squibb, Chattem, Delaco, the U.S. Food and Drug Administration, GlaxoSmithKline, Keryx, McNeil, Merck, Novartis, Pfizer, Reliant, TAP Pharmaceutical Products, United BioSource Corporation, and UpToDate; he serves on speakers' bureaus for AstraZeneca concerning lipids and heart failure, as well as Bristol-Myers Squibb, Reliant, and Pfizer concerning lipids; he receives royalties for authorship or editorship of three textbooks, as coinventor on patents concerning inflammatory markers and cardiovascular disease, which are held by Brigham and Women's Hospital; he has an investment management relationship with SunTrust Bank who has sole discretionary investment authority. J.M.G. has funding from McNeil Consumer Products ($45,682) and Pliva; he has received research support in the form of pills and or packaging from BASF, DMS Pharmaceuticals and Wyeth Pharmaceuticals; he has received honoraria from Bayer (€4,500 in 2005, €8,000 in 2006) and Pfizer for speaking engagements; he has served as a consultant for McNeil Consumer Products ($900 in 2006), Bayer ($50,000 in 2006), Edelman Publishing ($5,800 in 2005), and Wyeth Pharmaceuticals; he has served as an expert witness for Merck, Nutraquest, and GlaxoSmithKline.
References
- 1.Vollmer WM, Osborne ML, Buist AS. 20-year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. Am J Respir Crit Care Med 1998;157:1079–1084. [DOI] [PubMed] [Google Scholar]
- 2.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 1998;351:1225–1232. [PubMed] [Google Scholar]
- 3.Varner AE, Busse WW, Lemanske RFJ. Hypothesis: decreasing use of pediatric aspirin has contributed to the increasing prevalence of childhood asthma. Ann Allergy Asthma Immunol 1998;81:347–351. [DOI] [PubMed] [Google Scholar]
- 4.Halpin TJ, Holtzhauer FJ, Campbell RJ, Hall LJ, Correa-Villasenor A, Lanese R, Rice J, Hurwitz ES. Reye's syndrome and medication use. JAMA 1982;248:687–691. [PubMed] [Google Scholar]
- 5.Waldman RJ, Hall WN, McGee H, Van Amburg G. Aspirin as a risk factor in Reye's syndrome. JAMA 1982;247:3089–3094. [PubMed] [Google Scholar]
- 6.Perez GM, Melo M, Keegan AD, Zamorano J. Aspirin and salicylates inhibit the IL-4- and IL-13-induced activation of STAT6. J Immunol 2002;168:1428–1434. [DOI] [PubMed] [Google Scholar]
- 7.Chen LC, Kepka-Lenhart D, Wright TM, Morris SM Jr. Salicylate-enhanced activation of transcription factors induced by interferon-gamma. Biochem J 1999;342:503–507. [PMC free article] [PubMed] [Google Scholar]
- 8.Barr RG, Wentowski CC, Curhan GC, Somers SC, Stampfer MJ, Schwartz J, Speizer FE, Camargo CA Jr. Prospective study of acetaminophen use and risk of newly diagnosed asthma among women. Am J Respir Crit Care Med 2004;169:836–841. [DOI] [PubMed] [Google Scholar]
- 9.Barr RG, Kurth T, Stampfer MJ, Hennekens CH, Gaziano JM. Low dose aspirin and decreased adult-onset asthma: randomized comparisons from the Physicians' Health Study. Eur Respir J 2005;26:681s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med 1989;321:129–135. [DOI] [PubMed] [Google Scholar]
- 11.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians' Health Study II: a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol 2000; 10:125–134. [DOI] [PubMed]
- 12.Cox D. Regression models and life-tables. J R Stat Soc Ser B 1972;34:187–220. [Google Scholar]
- 13.McKeever TM, Lewis SA, Smit HA, Burney P, Britton JR, Cassano PA. The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. Am J Respir Crit Care Med 2005;171:966–971. [DOI] [PubMed] [Google Scholar]
- 14.Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics 1999;104:e39. [DOI] [PubMed] [Google Scholar]
- 15.Lesko SM, Louik C, Vezina RM, Mitchell AA. Asthma morbidity after the short-term use of ibuprofen in children. Pediatrics 2002;109:e20. [DOI] [PubMed] [Google Scholar]
- 16.Kasper L, Sladek K, Duplaga M, Bochenek G, Liebhart J, Gladysz U, Malolepszy J, Szczeklik A. Prevalence of asthma with aspirin hypersensitivity in the adult population of Poland. Allergy 2003;58:1064–1066. [DOI] [PubMed] [Google Scholar]
- 17.Hedman J, Kapiro J, Poussa T, Nieminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol 1999;28:717–722. [DOI] [PubMed] [Google Scholar]
- 18.Vally H, Taylor ML, Thompson PJ. The prevalence of aspirin intolerant asthma (AIA) in Australian asthmatic patients. Thorax 2002;57:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ 2004;328:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szczeklik A, Sanak M, Nizankowska-Mogilnicka E, Kielbasa B. Aspirin intolerance and the cyclooxygenase-leukotriene pathways. Curr Opin Pulm Med 2004;10:51–56. [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Chang HS, Park CS, Lee JH, Lee YM, Choi JH, Park HS, Kim LH, Park BL, Choi YH, et al. Association analysis of cysteinyl-leukotriene receptor 2 (CYSLTR2) polymorphisms with aspirin intolerance in asthmatics. Pharmacogenet Genomics 2005;15:483–492. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Choi JH, Park HS, Holloway JW, Lee SK, Park CS, Shin HD. Association of thromboxane A2 receptor gene polymorphism with the phenotype of acetyl salicylic acid-intolerant asthma. Clin Exp Allergy 2005;35:585–590. [DOI] [PubMed] [Google Scholar]
- 23.Jinnai N, Sakagami T, Sekigawa T, Kakihara M, Nakajima T, Yoshida K, Goto S, Hasegawa T, Koshino T, Hasegawa Y, et al. Polymorphisms in the prostaglandin E2 receptor subtype 2 gene confer susceptibility to aspirin-intolerant asthma: a candidate gene approach. Hum Mol Genet 2004;13:3203–3217. [DOI] [PubMed] [Google Scholar]
- 24.Szczeklik A, Gryglewski RJ, Nizankowska E. Asthma relieved by aspirin and by other cyclo-oxygenase inhibitors. Thorax 1978;33:664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kordansky D, Adkinson NF Jr, Norman PS, Rosenthal RR. Asthma improved by nonsteroidal anti-inflammatory drugs. Ann Intern Med 1978;88:508–511. [DOI] [PubMed] [Google Scholar]
- 26.Resta O, Foschino-Barbaro MP, Carnimeo N, Bavoso P, Picca V. Asthma relieved by acetylsalicylic acid and nonsteroid anti-inflammatory drugs. Respiration (Herrlisheim) 1984;46:121–127. [DOI] [PubMed] [Google Scholar]
- 27.Pang L, Pitt A, Petkova D, Knox AJ. The COX-1/COX-2 balance in asthma. Clin Exp Allergy 1998;28:1050–1058. [DOI] [PubMed] [Google Scholar]
- 28.Betz M, Fox BS. Prostaglandin E2 inhibits production of TH1 lymphokines but not TH2 lymphokines. J Immunol 1991;146:108–113. [PubMed] [Google Scholar]
- 29.Snijdewint FGM, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol 1993;150:5321–5329. [PubMed] [Google Scholar]
- 30.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994;265:956–959. [DOI] [PubMed] [Google Scholar]
- 31.Fiorucci S, Santucci L, Wallace JL, Sardina M, Romano M, del Soldato P, Morelli A. Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa. Proc Natl Acad Sci USA 2003;100:10937–10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4). Nat Med 2002;8:1018–1023. [DOI] [PubMed] [Google Scholar]
- 33.Bonnans C, Vachier I, Chavis C, Godard P, Bousquet J, Chanez P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med 2002;165:1531–1535. [DOI] [PubMed] [Google Scholar]
- 34.Barr RG, Herbstman J, Speizer FE, Camargo CA Jr. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol 2002;155:965–971. [DOI] [PubMed] [Google Scholar]
- 35.Kurth T, Gaziano JM. Cumulative analgesic use and risk of incident asthma in men [abstract]. Pharmacoepidemiol Drug Saf 2005;14:S134.