Abstract
Rationale: Human data suggest that the incidence of acute lung injury is reduced in patients with type II diabetes mellitus. However, the mechanisms by which diabetes confers protection from lung injury are unknown.
Objectives: To determine whether leptin resistance, which is seen in humans with diabetes, protects mice from hyperoxic lung injury.
Methods: Wild-type (leptin responsive) and db/db (leptin resistant) mice were used in these studies. Mice were exposed to hyperoxia (100% O2) for 84 hours to induce lung injury and up to 168 hours for survival studies. Alveolar fluid clearance was measured in vivo.
Measurements and Main Results: Lung leptin levels were increased both in wild-type and leptin receptor–defective db/db mice after hyperoxia. Hyperoxia-induced lung injury was decreased in db/db compared with wild-type mice. Hyperoxia increased lung permeability in wild-type mice but not in db/db mice. Compared with wild-type control animals, db/db mice were resistant to hyperoxia-induced mortality (lethal dose for 50% of mice, 152 vs. 108 h). Intratracheal instillation of leptin at a dose that was observed in the bronchoalveolar lavage fluid during hyperoxia caused lung injury in wild-type but not in db/db mice. Intratracheal pretreatment with a leptin receptor inhibitor attenuated leptin-induced lung edema. The hyperoxia-induced release of proinflammatory cytokines was attenuated in db/db mice. Despite resistance to lung injury, db/db mice had diminished alveolar fluid clearance and reduced Na,K-ATPase function compared with wild-type mice.
Conclusions: These results indicate that leptin can induce and that resistance to leptin attenuates hyperoxia-induced lung injury and hyperoxia-induced inflammatory cytokines in the lung.
Keywords: alveolar fluid clearance; pulmonary edema; Na,K-ATPase; diabetes mellitus; oxygen
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Human data suggest that patients with diabetes mellitus are less likely to develop acute lung injury/acute respiratory distress syndrome (ALI/ARDS); however, the mechanism by which diabetes mellitus confers this protection is unknown.
What This Study Adds to the Field
Leptin may play a role in the pathogenesis of ALI/ARDS and resistance to its effects may attenuate ALI/ARDS.
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common, devastating clinical syndromes that affect almost 200,000 people in the United States every year, leading to 74,500 deaths and 3.6 million hospital days (1). Despite several decades of research, there are few effective therapies, and the mortality from ALI remains unacceptably high.
Diabetes mellitus (DM) is associated with a 42 to 67% reduction in the risk of developing ALI (2–4). The mechanisms that underlie this surprising clinical finding have not been elucidated. It has been speculated that DM may alter the incidence of ALI because of its association with abnormalities in several aspects of the pathogenetic cascade of ARDS, including proinflammatory signaling (i.e., decreased activation and recruitment of circulating neutrophils into the lung parenchyma) (2, 4, 5).
DM is associated with increased levels of leptin, which is a 16-kD nonglycosylated protein encoded by the obese gene located on human chromosome 7 and on mouse chromosome 6 (6, 7). Leptin resistance is present in more than 90% of obese patients with type II diabetes and is believed to result from receptor down-regulation (8). Although classically considered a hormone because it regulates the balance between food intake and energy expenditure, leptin is also a cytokine of the type I cytokine family (7, 9). The leptin receptor is a member of the class I cytokine receptor family (10). Leptin is significantly increased in response to acute infection and sepsis and exerts direct effects on T-lymphocyte proliferation, macrophage phagocytosis, and secretion of other inflammatory cytokines (11).
We sought to test the hypothesis that leptin is important in ALI and that resistance to leptin may protect mice from ALI. We found that lung leptin levels were increased during ALI. Intratracheal instillation of leptin to mice caused lung edema and injury at 24 hours. We also found that mice with leptin resistance had less lung edema and injury and improved survival in response to hyperoxia compared with wild-type mice. Consistent with an immunomodulatory effect of leptin, mice with leptin resistance generated fewer proinflammatory cytokines and chemokines in the lung in response to hyperoxia. The improvement in lung edema and injury observed was not due to improved alveolar fluid clearance, because mice with leptin resistance had rates of alveolar fluid clearance lower than those in wild-type mice. Collectively, our findings provide a novel potential link between DM and ARDS, raising the possibility that leptin resistance induced by type II diabetes and/or obesity might provide protection against the development of ALI. Some of the results of these studies have been previously reported in the form of an abstract (12).
METHODS
Animals and Induction of ALI
The protocol for the use of mice was approved by the Animal Care and Use Committee at Northwestern University and Evanston Northwestern Healthcare Research Institute. Our animal protocol was approved for survival studies with death as an endpoint for up to 7 days at which point the remaining mice were euthanized. We purchased 6- to 8-week-old (20–25 g) male C57BL/6 (wild-type) mice and db/db mice (mice with leptin resistance due to defective leptin receptor) from Jackson Laboratories (Bar Harbor, ME) to use in the experiments. The db/db mice were obese (30–35 g), hyperphagic, hypometabolic, and hyperglycemic (13). All mice had free access to food and water during experiments. Mice were exposed to hyperoxia (100% normobaric O2) in two sets of experiments. In the first set of experiments, mice were exposed to hyperoxia for 84 hours before measurement of proinflammatory cytokines/chemokines and cell count in the bronchoalveolar lavage (BAL) fluid, and evaluation of lung permeability and total lung water content (indices of injury) as previously described (n = 4 mice/group) (14). In the second set, survival studies were conducted by exposure to hyperoxia for up to 168 hours. Surviving animals were counted at 12-hour intervals (n = 8 mice/group).
Intratracheal Administration of Leptin
Mice were anesthetized with pentobarbital (50 mg/kg body weight, intraperitoneally; Abbott Laboratories, Chicago, IL). After adequate sedation was achieved, animals were orally intubated under direct visualization using a 20-gauge plastic intravenous catheter (Angiocath; Becton-Dickenson, Sandy, UT) trimmed to approximately 2 cm. Endotracheal catheter placement was confirmed if occlusion of the catheter caused paradoxical thoracoabdominal respiratory efforts.
While breathing spontaneously, sterile phosphate-buffered saline (PBS) or leptin (4,500 or 9,000 pg; Sigma, St. Louis, MO) in sterile PBS was administered in two 25-μl aliquots via the endotracheal tube, 1 minute apart. Twenty-four hours after intratracheal instillation of leptin with or without the mouse-specific leptin inhibitor (R&D Systems, Minneapolis, MN), the lungs were harvested for histologic analysis.
Histology and Calculation of Lung Injury Scores
Preparation of the lungs was performed as described in the online supplement. The lung injury scores were calculated using the method described by Matute-Bello and colleagues and are described in detail in the online supplement (15).
Total Cell Membrane Isolation and Western Analysis
Membrane proteins were obtained by homogenizing lung tissue collected from the peripheral 1 to 2 mm of each lobe, as previously described (14). For Western analysis, 10 μg of whole cell membrane protein was separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electrophoretically transferred to nitrocellulose and probed with a rabbit anti-mouse leptin receptor antibody (1:2,000 dilution; Abcam, Cambridge, MA).
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded, longitudinal sections (5 μm) of mouse lungs using a rabbit anti-mouse leptin receptor antibody (1:400 dilution; Abcam) and the Vectastain kit (Vector Laboratories, Newcastle upon Tyne, UK). This antibody is raised against the receptor itself and therefore is highly specific in recognizing both the short and the long forms of the leptin receptor. The method is described in detail in the online supplement.
BAL Analysis and Measurement of Leptin Levels
BAL was performed through a 20-gauge angiocatheter ligated into the trachea. A 1.0-ml aliquot of PBS was instilled into mouse lungs and then carefully removed three times. A 200-μl aliquot of the BAL fluid was placed in a cytospin apparatus and centrifuged at 1,200 rpm for 5 minutes. The glass slides were Wright stained and subjected to a blinded manual cell count and differential. For each slide, the number of macrophages, neutrophils, lymphocytes, and other leukocytes per high-power field (500× magnification) was counted in 15 fields and the average was reported. The supernatant was used for the measurement of BAL protein (Bradford Assay; Bio-Rad Labs, Hercules, CA) and leptin analysis. Leptin levels were measured in undiluted BAL samples based on the instructions for the Quantikine mouse leptin ELISA kit (R&D Systems).
Measurement of Cytokines/Chemokines in BAL
Separate BAL samples were collected for cytokine/chemokine measurements. We used the BD Cytometric Bead Array (BD Biosciences, San Jose, CA) to measure BAL levels of cytokines/chemokines. BAL samples were analyzed in triplicates using the Mouse Inflammation Kit (BD Biosciences), which detects IL-6, IL-10, and IL-12; monocyte chemoattractant protein-1; IFN-γ; and tumor necrosis factor-α according to the instructions provided.
Assessment of Lung Permeability
Mice were anesthetized and a 20-gauge angiocatheter was sutured into the trachea. A total of 125 μl of a 16-mg/ml solution of fluorescein isothiocyanate (FITC)–labeled 5% albumin (Sigma-Aldrich) was injected retroorbitally into the venous system. After 60 minutes, BAL fluid was collected as described above. Relative lung permeability was estimated from the fluorescence in the BAL fluid measured by using a microplate reader (excitation 488 = nm, emission = 530 nm), as previously described (16).
Alveolar Fluid Clearance Measurements in Live Mice
The method of measurement was described previously (14, 17) and is also described in detail in the online supplement. Clearance is expressed as a percentage of total instilled volume cleared over 30 minutes.
Na,K-ATPase Function (Inorganic Phosphate Liberation from ATP) in the Distal Lung
The method of measurement has been described before and is described in detail in the online supplement (14, 18). Na,K-ATPase activity was quantified by comparing the amount of inorganic phosphate (Pi) liberated from ATP over 1 hour by 20 μg of basolateral cell membrane protein isolated from the peripheral lung in the presence and absence of the Na,K-ATPase inhibitor ouabain, as previously described (14, 18).
Statistical Analysis
Data are expressed as mean ± SEM. Data were analyzed using one-way analysis of variance. When analysis of variance indicated a significant difference, we explored individual differences with the Student's t test using Bonferroni correction for multiple comparisons. The lung injury scores were compared using a nonparametric test (Mann-Whitney test). Comparison of survival among groups exposed to hyperoxia was performed with using the Kaplan-Meier method to determine the lethal dose for 50% of mice (LD50). Statistical significance in all experiments was defined as p < 0.05.
RESULTS
Leptin Receptors Are Present in Distal Mouse Lungs
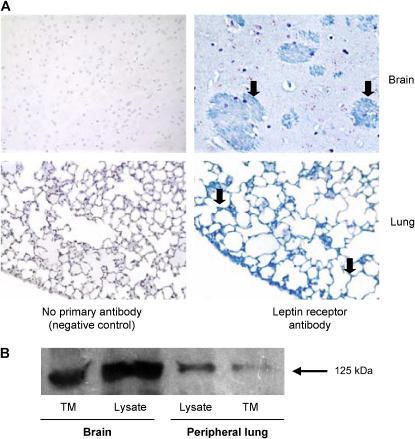
To determine whether leptin receptors are present in distal lung, we performed immunoblotting on peripheral mouse lung lysates and immunohistochemistry on fixed lung sections using an antibody for the leptin receptors. Immunohistochemistry showed the presence of leptin receptors both in the alveolar (Figure 1A) and bronchial epithelium (Figure E1A of the online supplement). Examination of high-power-field images of alveoli showed a circumferential pattern of immunostaining consistent with the presence of the receptors in both alveolar type 1 and type 2 epithelial cells (Figure E1B). Diffuse staining of the alveolar epithelia is consistent with the ubiquitous distribution of leptin receptors and the consequently diverse range of physiologic functions of leptin as reported in the literature (19, 20). Western blots from membranes isolated from distal lung tissue confirmed the presence of leptin receptors in distal lung (Figure 1B).
Figure 1.
Leptin receptors in distal mouse lungs. (A) Immunostaining of brain and whole lungs from wild-type mice using an anti-mouse leptin receptor antibody showing the presence of leptin receptors (arrows) in mouse brain and alveolar epithelium. Photomicrographs are of 5-μm paraffin-embedded sections (original magnification, ×200). Brain is shown as a positive control. (B) Representative Western blot of whole cell membrane fractions from lysates from peripheral lungs and brain (positive control) of wild-type mice. TM = total membrane.
Leptin Levels Are Increased in BAL during Hyperoxic ALI in Mice
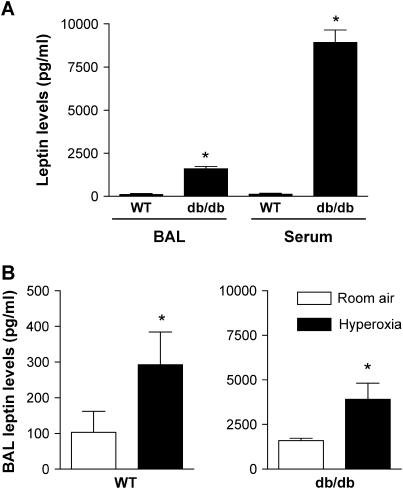
We used hyperoxia as a model to induce ALI because it affects primarily alveoli and therefore is well suited for the study of alveolar epithelium (14). We measured leptin levels in BAL fluid from animals at baseline and 84 hours after exposure to hyperoxia. This time point was chosen to measure the effects of hyperoxia on lung injury and leptin levels based on previous data from our laboratory (14) and others (21, 22) showing an LD50 of 108 to 120 hours for wild-type mice. Consistent with leptin resistance, both serum and BAL leptin levels were increased at baseline in db/db mice compared with wild-type mice (serum: 118.9 ± 60.2 vs. 8922 ± 722.7 pg/ml, n = 5, p < 0.05; BAL: 103.3 ± 58.5 vs. 1,595.0 ± 130.2 pg/ml, n = 4, p < 0.05) (Figure 2A). Exposure to hyperoxia caused an almost threefold increase in BAL leptin levels in wild-type mice (103.3 ± 58.5 vs. 292.6 ± 91.2 pg/ml, n = 4, p < 0.05) (Figure 2B). Similarly, hyperoxia caused a twofold increase in BAL leptin levels in db/db mice (1,595.0 ± 130.2 vs. 3,914 ± 895.4 pg/ml, n = 5, p < 0.05) (Figure 2C). These results are consistent with a role for leptin in development of ALI.
Figure 2.
Serum and bronchoalveolar lavage (BAL) leptin levels in mice. (A) Baseline serum and BAL fluid leptin levels in wild-type (WT) mice and mice with leptin resistance (db/db mice). (B) BAL fluid leptin levels in wild-type and db/db mice after exposure to hyperoxia (normobaric, 100% O2) for 84 hours (n ≥ 4 mice/group); * p < 0.05.
Severity of Hyperoxia-induced ALI Is Attenuated in Mice with Leptin Resistance
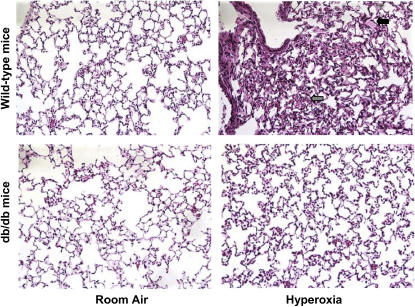
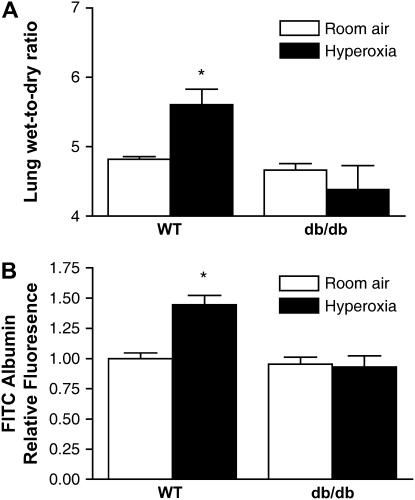
To determine if leptin affects the severity of ALI, we exposed wild-type and db/db mice to hyperoxia for 84 hours before BAL fluid was collected and lungs were excised for evaluation of total lung water content, alveolocapillary permeability, and histopathology. Histologic evaluation revealed only mild interstitial edema in db/db mice, whereas there was more interstitial edema as well as some areas with alveolar edema in wild-type mice (Figure 3). The median lung injury score was also lower in db/db mice compared with wild-type animals (1.3 vs. 1.8, p < 0.05). To further assess lung injury, we measured lung water content and lung alveolocapillary permeability using FITC-labeled albumin after 84 hours of exposure to hyperoxia. In accord with the histologic findings, there was less lung edema in db/db mice compared with wild-type mice (wet-to-dry ratio: 4.38 ± 0.34 vs. 5.61 ± 0.22, n = 4, p < 0.05) (Figure 4A). The increase in lung permeability observed in wild-type mice after exposure to hyperoxia was not seen in db/db mice (Figure 4B). Collectively, these results suggest that mice with leptin resistance have less pulmonary edema and histologic evidence of lung injury in response to hyperoxia.
Figure 3.
Effect of leptin receptor function on lung histology during hyperoxia-induced acute lung injury. Photomicrographs of hematoxylin-and-eosin–stained lungs from uninjured (room air) and injured (hyperoxic) wild-type and db/db mice exposed to hyperoxia for 84 hours. Arrows depict interstitial edema (gray) and alveolar edema (black). (Original magnification, ×200.)
Figure 4.
Effect of leptin receptor function on lung water content and lung permeability during hyperoxia-induced acute lung injury. (A) Lung wet-to-dry ratios. (B) Lung permeability measured using BAL fluid fluorescein isothiocyanate (FITC) levels 1 hour after venous injection from wild-type (WT) and mice with leptin resistance (db/db mice) exposed to hyperoxia for 84 hours (n = 4 mice/group); *p < 0.05.
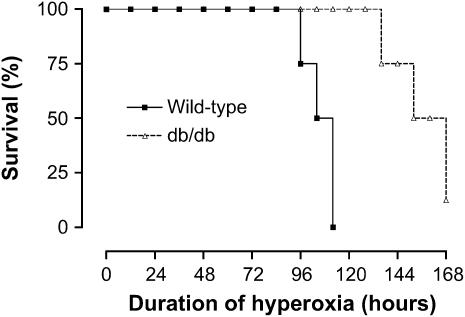
Mice with Leptin Resistance Have Improved Survival from Hyperoxia-induced ALI
To determine the effect of leptin on survival from ALI, wild-type and db/db mice were exposed to normobaric hyperoxia (100 O2), which is a well-established lung injury model for measurement of survival. Compared with wild-type mice, db/db mice lived significantly longer (LD50 of 108 h vs. 152 h, n = 8 mice/group, p < 0.05) (Figure 5). One of the db/db mice was still alive at the end of survival study, which was limited to 168 hours according to our animal protocol. These results indicate that leptin resistance confers improved survival from hyperoxic ALI.
Figure 5.
Effect of leptin receptor function on survival from hyperoxia-induced acute lung injury. Kaplan-Meier plot of survival of wild-type mice and mice with leptin resistance (db/db mice). The lethal dose for 50% of mice (LD50) for each group is as follows: wild-type mice (108 h), db/db mice (152 h) (n = 8 mice/group); p < 0.05.
Development of Hyperoxia-induced ALI Is Delayed in Mice with Leptin Resistance
To determine the effects of hyperoxia on db/db mouse lungs, we looked at the histologic changes and lung water content in these mice at a later time point (120 h) before the occurrence of death based on our survival studies. Examination of the lungs of db/db mice at 120 hours showed significant lung edema demonstrated by an elevated wet-to-dry ratio (6.4 ± 0.3, n = 4) as well as histologic evidence of severe ALI (Figure E2). These results suggest that the survival benefit associated with leptin resistance is due to a delay in the development of hyperoxia-induced ALI.
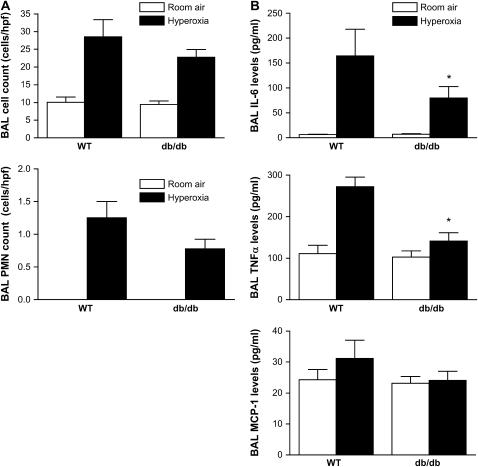
Lung Inflammatory Response to Hyperoxia Is Attenuated in Mice with Leptin Resistance
To ascertain if the lung inflammatory response during hyperoxia is altered in db/db mice compared with wild-type mice, we measured BAL cell count with differential as well as cytokine levels. Although BAL cell counts were lower in db/db mice compared with wild-type mice (28.6 ± 4.8 vs. 22.7 ± 2.2 cells/high-power field, n = 8, p = 0.27), this did not reach statistical significance (Figure 6A). Similarly, the number of neutrophils detected in the BAL from db/db mice was lower than that from wild-type mice, although this was not statistically significant. Hyperoxia-induced increases in BAL IL-6 (p = 0.047), tumor necrosis factor-α (p = 0.005), and monocyte chemoattractant protein-1 (p = 0.07) levels were attenuated in db/db mice in comparison to wild-type mice (Figure 6B). There was no significant difference in levels of IL-10, IL-12, and IFN-γ between wild-type and db/db mice (data not shown). These results suggest that protective effects of leptin resistance on hyperoxia-induced ALI may be mediated by attenuation of the inflammatory response to hyperoxia in the lung.
Figure 6.
Effect of leptin receptor function on inflammatory cells and mediators in the lung during hyperoxia-induced acute lung injury. (A) Cell and neutrophil counts (n = 8 mice/group) and (B) cytokine/chemokine levels in BAL collected from wild-type (WT) and db/db mice exposed to hyperoxia for 84 hours (n = 5 mice/group); *p < 0.05).
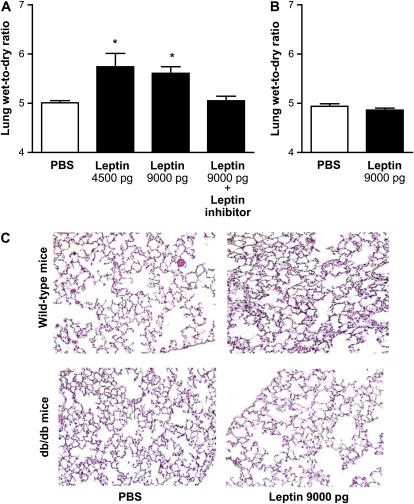
Tracheal Instillation of Leptin Causes ALI in Wild-type (Leptin Responsive) Mice But Not in db/db (Leptin Resistant) Mice
To confirm whether inhibition of the function of leptin is the cause of improved survival and lessened ALI observed in db/db mice, we treated wild-type and db/db mice with intratracheal instillation of leptin at the dose that we observed in the BAL fluid of wild-type mice exposed to hyperoxia for 84 hours (4,500 or 9,000 pg). These doses were calculated based on a 10- to 100-fold dilution factor introduced by the BAL into our measurements and are similar to doses used in previous in vitro studies (23). Control animals were treated with sterile PBS. Animals were killed for excision of the lungs for evaluation of lung water content and histology 24 hours after intratracheal instillation of leptin. Total water content was increased in leptin (9,000 pg/mice)-treated wild-type mice compared with PBS-treated control mice (5.0 ± 0.04 vs. 5.61 ± 0.13, n = 5–13, p < 0.05) (Figure 7A). Pretreatment with intratracheal administration of leptin inhibitor attenuated the leptin-mediated lung edema at 24 hours (5.05 ± 0.09). Leptin administration had no effect on total lung water content in db/db mice with leptin resistance (Figure 7B). Histologically, wild-type mice treated with leptin showed evidence of very mild interstitial edema and inflammation, whereas lung histology was normal in PBS-treated wild-type mice and PBS- and leptin-treated db/db mice (Figure 7C). Similarly, the median lung injury score was higher in wild-type mice compared with db/db mice (0.66 vs. 0). These results suggest that intratracheal administration of leptin causes lung edema in wild-type mice, an effect that is blocked by pharmacologic inhibition of leptin receptor or in the absence of functional leptin receptor.
Figure 7.
Effect of intratracheal administration of leptin on lung water content and histology. Lung wet-to-dry ratios from (A) wild-type mice and (B) mice with leptin resistance (db/db mice) 24 hours after intratracheal instillation of leptin with or without mouse-specific leptin inhibitor. Control mice received phosphate-buffered saline (PBS). (C) Photomicrographs of hematoxylin-and-eosin–stained lungs from wild-type and db/db mice exposed to leptin for 24 hours (original magnification, ×200) (n = 5–13 mice/group); *p < 0.05.
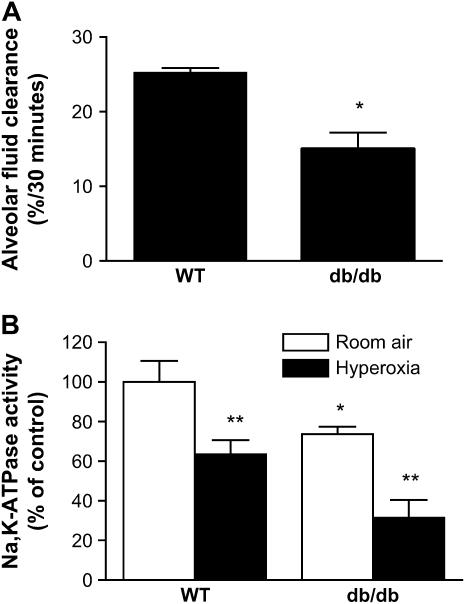
Alveolar Fluid Clearance Is Diminished in Mice with Leptin Resistance
To determine if the attenuation of lung edema and injury and improvement in survival from hyperoxic ALI in db/db mice resulted from increased alveolar epithelial Na+ transport, we measured alveolar fluid clearance in wild-type and db/db mice. Alveolar fluid clearance was approximately 40% lower in db/db mice compared with wild-type mice (15.0 ± 2.2 vs. 25.2 ± 0.7%/30 min) (Figure 8A). Alveolar epithelial Na+ transport assessed by measurement of Na,K-ATPase activity from peripheral lung tissue also showed a 36% reduction Na,K-ATPase activity in db/db mice relative to wild-type mice (Figure 8B). Exposure to hyperoxia caused an approximately 30% reduction in Na,K-ATPase activity both in wild-type and db/db mice. These results suggest that the attenuation of hyperoxia-induced lung edema and injury and improved survival we observed in db/db mice are not due to improved alveolar epithelial active Na+ transport.
Figure 8.
Alveolar fluid clearance and alveolar epithelial Na+ transport in mice. (A) Alveolar fluid clearance in wild-type (WT) mice and mice with leptin resistance (db/db mice). (B) Na,K-ATPase activity (ouabain-sensitive liberation of inorganic phosphate from ATP) in basolateral cell membranes isolated from the peripheral lung of untreated WT and db/db mice and after exposure to normobaric hyperoxia for 84 hours. Data are expressed as percentage of Na,K-ATPase activity in untreated WT mice (n = 5 mice/group); *p < 0.05, db/db mice compared with untreated WT control mice; **p < 0.05, after exposure to hyperoxia for 84 hours compared with untreated control mice.
DISCUSSION
These experiments demonstrate that mice with leptin resistance develop less lung edema and injury in response to hyperoxia, and have improved survival compared with mice with functional leptin receptors. Specific confirmation of the importance of leptin in the development of ALI comes from the measurements of lung leptin levels during ALI and from experiments demonstrating lung edema and ALI induced by the intratracheal instillation of leptin into mouse lungs. Although systemic levels of leptin have been reported to be elevated during sepsis and ALI (24, 25), our studies are the first to show an increase in lung leptin levels during ALI. Intratracheal instillation of leptin at the same concentrations observed in hyperoxia-exposed wild-type mice increased lung water content and caused histologic evidence of ALI within 24 hours compared with PBS-treated animals. This effect was not observed in mice with leptin resistance and was partially attenuated by pretreatment with leptin receptor inhibitor. Together, these findings suggest that leptin receptor activation is important in development of ALI and that leptin resistance protects mice from ALI and related mortality.
The findings of the current study corroborate the clinical data that showed a decreased incidence and mortality in patients with diabetes. In a cohort of 113 patients at risk for ARDS, Moss and colleagues (2) found a significant 67% reduction in incidence of ARDS in patients with diabetes compared with nondiabetic patients. Similarly, in a cohort of 688 patients at risk for ARDS, Gong and colleagues reported DM as a negative predictor for ARDS (3). In the study by Moss and colleagues, the blood glucose level on admission (a marker of how well DM is controlled) did not affect the incidence of ARDS in patients with diabetes (2). Furthermore, acute hyperglycemia on admission was not associated with change in incidence of ARDS in nondiabetic subjects, suggesting that, whereas hyperglycemia is one of the most obvious abnormalities in patients with diabetes, it may not be the only factor influencing the development of ARDS (4).
One potential mechanism by which leptin resistance protects mice from hyperoxic ALI is by eliminating the known proinflammatory effects of leptin, delaying the development of ALI. Consistent with this hypothesis, we demonstrated that the direct administration of leptin into mouse lungs was sufficient to induce a mild lung injury. Although hyperoxia caused ALI in mice with leptin resistance and wild-type mice, this response was substantially delayed in the leptin-resistant animals. Leptin resistance was associated with a less pronounced (although not statistically significant) elevation in white blood cells in the lungs and an attenuation of the production of the proinflammatory cytokines in response to hyperoxia. On the basis of our findings, we speculate that this attenuation of the inflammatory response delayed the development of hyperoxia-induced ALI and its related mortality. The attenuation of the inflammatory response in the leptin-resistant mice might have indirectly preserved alveolocapillary membrane integrity during hyperoxia. Alternatively, leptin resistance might have directly altered the susceptibility of the alveolar epithelial or endothelial cells to hyperoxic injury. Our observation that the leptin receptor is present in the alveolar space is consistent with this possibility. Identifying the molecular targets of leptin that are responsible for these responses is an important area of future investigation.
Our findings that hyperoxia-induced proinflammatory cytokine response is attenuated in leptin-resistant mice differ from those recently reported by Lu and colleagues. They showed increased release of systemic cytokines after exposure to ozone in db/db mice compared with wild-type mice (26). The discrepancy between our findings and recently published data from the ozone model in the same mice suggests that the effect of leptin resistance may differ based on the type of oxidant stimulus. This observation might offer a useful tool to further investigate the mechanisms by which leptin resistance may attenuate or aggravate oxidant-mediated inflammation in the lung.
There has been only one study that investigated the role of leptin during ALI (25). The results of this study were inconclusive. Similar to our findings, lung weight in response to hyperoxia was decreased in mice without functional leptin (ob/ob mice); however, in contrast to our findings, systemic administration of leptin or the leptin receptor inhibitor failed to modulate ALI in wild-type mice. The differences between our findings and this study are most likely due to different routes used to administer leptin and leptin receptor inhibitor. These investigators administered leptin and the leptin receptor inhibitors intraperitoneally, a strategy that is likely to expose the alveolar endothelium to high concentrations of leptin. By contrast, we administered leptin and the leptin receptor inhibitor intratracheally. This route of administration is likely to expose alveolar epithelial cells to relatively higher levels of leptin.
Our experiments suggest that loss of leptin receptor activation does not attenuate ALI by up-regulating alveolar fluid clearance or active Na+ transport. Mice with leptin resistance had lower alveolar fluid clearance rates at baseline due to diminished active Na+ transport evidenced by decreased activity of the Na,K-ATPase. However, this reduction in alveolar active Na+ transport did not affect baseline lung water content, which was similar both in db/db and control mice. Moreover, leptin resistance did not confer protection against ALI via attenuation of expected reduction in active Na+ transport during ALI (27, 28). Hyperoxia caused similar reductions in Na,K-ATPase activity in wild-type and db/db mice, providing further evidence against active Na+ transport as the mechanism of leptin resistance–mediated protection against ALI. How the loss of leptin signaling affects pathways downstream of the leptin receptor was not examined in these experiments.
The weight difference between wild-type and db/db mice might account for some of the observed protection against hyperoxic lung injury, because a “better” nutritional status might have allowed the continuation of intracellular anabolic functions in db/db mice during ALI (29). After 84 hours of exposure to hyperoxia, there was no significant change in weight in db/db mice. Although wild-type mice lost about 0.82 g (3.5% of their weight at 0 h), this was not statistically significant (Figure E3). Although we cannot completely exclude the possibility that this weight difference explained some of the protection conferred by leptin resistance, this seems less likely because other investigators have shown that the beneficial effects of higher weight or better nutritional status are attributable to improved alveolar epithelial active Na+ transport (i.e., Na,K-ATPase activity) (29, 30). The study by Factor and colleagues reported that continuous enteral feeding of rats with a formula enriched with glutamine (a precursor for gluthathione) reduced hyperoxia-induced ALI by providing metabolic precursors (i.e., ATP) necessary for cellular functions (i.e., Na,K-ATPase) (29). The levels of these precursors are not necessarily increased in leptin receptor–deficient animals despite their obesity. Indeed, we observed lower Na, K-ATPase activity at baseline and during hyperoxia in the db/db when compared with wild-type mice. These results highlight the disconnect between obesity and the nutritional state of the animal.
In summary, we conclude that leptin levels increase during hyperoxia-induced ALI and that leptin resistance delays hyperoxia-induced ALI. The administration of leptin in doses similar to those found in wild-type mice exposed to hyperoxia is sufficient to induce ALI. The protection conferred by leptin resistance is not the result of improved alveolar fluid clearance or active Na+ transport but may result from attenuation of leptin-mediated inflammatory response to hyperoxia.
Supplementary Material
Acknowledgments
The authors thank Dr. Anjana Yeldandi (Department of Pathology) for interpretation of immunohistochemistry, Dr. Thomas Corbridge for his suggestions on the manuscript, and Dr. Manu Jain for help with the statistical calculations.
Supported by the American Lung Association, the American Heart Association, and NIH grants HL067835 (G.R.S.B.), HL071643 (Project 5: principal investigator, D.A.D.), HL059956 (D.A.D.), ES015024 (G.M.M.), and ES013995 (G.R.S.B.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200603-312OC on December 21, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 2.Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, Hudson LD, Parsons PE. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med 2000;28:2187–2192. [DOI] [PubMed] [Google Scholar]
- 3.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med 2005;33:1191–1198. [DOI] [PubMed] [Google Scholar]
- 4.Frank JA, Nuckton TJ, Matthay MA. Diabetes mellitus: a negative predictor for the development of acute respiratory distress syndrome from septic shock. Crit Care Med 2000;28:2645–2646. [DOI] [PubMed] [Google Scholar]
- 5.Ward PA, Johnson KJ, Till GO. Animal models of oxidant lung injury. Respiration (Herrlisheim) 1986;50:5–12. [DOI] [PubMed] [Google Scholar]
- 6.Flier JS. Lowered leptin slims immune response. Nat Med 1998;4:1124–1125. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JM. Modern science versus the stigma of obesity. Nat Med 2004;10:563–569. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, et al. Crystal structure of the obese protein leptin-E100. Nature 1997;387:206–209. [DOI] [PubMed] [Google Scholar]
- 10.Tartaglia LA. The leptin receptor. J Biol Chem 1997;272:6093–6096. [DOI] [PubMed] [Google Scholar]
- 11.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, et al. Leptin regulates proinflammatory immune responses. FASEB J 1998;12:57–65. [PubMed] [Google Scholar]
- 12.Bellmeyer A, Budinger GR, Dean DA, Mutlu GM. Leptin is important in the pathogenesis of hyperoxic acute lung injury [abstract]. Proc Am Thorac Soc 2006;3:A838. [Google Scholar]
- 13.Leibel RL, Chung WK, Chua SC Jr. The molecular genetics of rodent single gene obesities. J Biol Chem 1997;272:31937–31940. [DOI] [PubMed] [Google Scholar]
- 14.Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, et al. Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling. Circ Res 2004;94:1091–1100. [DOI] [PubMed] [Google Scholar]
- 15.Matute-Bello G, Frevert CW, Kajikawa O, Skerrett SJ, Goodman RB, Park DR, Martin TR. Septic shock and acute lung injury in rabbits with peritonitis: failure of the neutrophil response to localized infection. Am J Respir Crit Care Med 2001;163:234–243. [DOI] [PubMed] [Google Scholar]
- 16.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bellmeyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY, et al. Proapoptotic Bid is required for pulmonary fibrosis. Proc Natl Acad Sci USA 2006; 103:4604–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutlu GM, Adir Y, Jameel M, Akhmedov AT, Welch L, Dumasius V, Meng FJ, Zabner J, Koenig C, Lewis ER, et al. Interdependency of beta-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res 2005;96:999–1005. [DOI] [PubMed] [Google Scholar]
- 18.Dumasius V, Sznajder JI, Azzam ZS, Boja J, Mutlu GM, Maron MB, Factor P. Beta(2)-adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ Res 2001;89:907–914. [DOI] [PubMed] [Google Scholar]
- 19.Fruhbeck G. A heliocentric view of leptin. Proc Nutr Soc 2001;60:301–318. [DOI] [PubMed] [Google Scholar]
- 20.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J 2006;393:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean JC, Liu Y, Brown LA, Marc RE, Klings E, Joyce-Brady M. Gamma-glutamyl transferase deficiency results in lung oxidant stress in normoxia. Am J Physiol Lung Cell Mol Physiol 2002;283:L766–L776. [DOI] [PubMed] [Google Scholar]
- 22.Morse D, Otterbein LE, Watkins S, Alber S, Zhou Z, Flavell RA, Davis RJ, Choi AM. Deficiency in the c-Jun NH2-terminal kinase signaling pathway confers susceptibility to hyperoxic lung injury in mice. Am J Physiol Lung Cell Mol Physiol 2003;285:L250–L257. [DOI] [PubMed] [Google Scholar]
- 23.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J 1999;13:1231–1238. [PubMed] [Google Scholar]
- 24.Bornstein SR, Preas HL, Chrousos GP, Suffredini AF. Circulating leptin levels during acute experimental endotoxemia and antiinflammatory therapy in humans. J Infect Dis 1998;178:887–890. [DOI] [PubMed] [Google Scholar]
- 25.Barazzone-Argiroffo C, Muzzin P, Donati YR, Kan CD, Aubert ML, Piguet PF. Hyperoxia increases leptin production: a mechanism mediated through endogenous elevation of corticosterone. Am J Physiol Lung Cell Mol Physiol 2001;281:L1150–L1156. [DOI] [PubMed] [Google Scholar]
- 26.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L856–L865. [DOI] [PubMed] [Google Scholar]
- 27.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376–1383. [DOI] [PubMed] [Google Scholar]
- 28.Olivera WG, Ridge KM, Sznajder JI. Lung liquid clearance and Na,K-ATPase during acute hyperoxia and recovery in rats. Am J Respir Crit Care Med 1995;152:1229–1234. [DOI] [PubMed] [Google Scholar]
- 29.Factor P, Ridge K, Alverdy J, Sznajder JI. Continuous enteral nutrition attenuates pulmonary edema in rats exposed to 100% oxygen. J Appl Physiol 2000;89:1759–1765. [DOI] [PubMed] [Google Scholar]
- 30.Sakuma T, Zhao Y, Sugita M, Sagawa M, Toga H, Ishibashi T, Nishio M, Matthay MA. Malnutrition impairs alveolar fluid clearance in rat lungs. Am J Physiol Lung Cell Mol Physiol 2004;286:L1268–L1274. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.