Abstract
Rationale: Although interferon (IFN)-γ release assays are approved for the diagnosis of latent tuberculosis infection (LTBI), limited data exist regarding their performance in HIV infection.
Objectives: To compare tuberculin skin test (TST) results to the commercial IFN-γ release assay QuantiFERON-TB Gold In-Tube (QFT) for the diagnosis of LTBI in HIV-infected adults.
Methods: A total of 294 HIV-infected subjects sampled from two San Francisco cohorts underwent TST, using 5 TU of purified protein derivative, and QFT, measuring IFN-γ response to Mycobacterium tuberculosis–specific RD-1 antigens.
Main Results: Of 294 participants, 205 (70%) returned for an evaluable TST. Concordance between QFT and TST was 89.3% (kappa = 0.37, p = 0.007). However, in subjects with positive test results by either TST or QFT, only 28% (8/29) had positive test results by both modalities. TST-positive/QFT-negative discordant results were found in 5.1% of subjects and TST-negative/QFT-positive discordance in 5.6%. Indeterminate QFT results occurred in 5.1%, all due to a failure to respond to the phytohemagglutinin-positive control. Subjects with a CD4+ count of less than 100 cells/mm3 had a relative risk of an indeterminate result of 4.24 (95% confidence interval, 1.55–11.61; p= 0.003) compared with those with a CD4+ count of 100 or more.
Conclusions: Overall concordance between QFT and TST in HIV infection was high, but agreement among subjects with positive tests by either modality was low.
Keywords: latent tuberculosis infection, human immunodeficiency virus, QuantiFERON, interferon-γ assay, tuberculin skin test
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Although interferon-γ assays are promising alternatives to the tuberculin skin test, there are limited data on the performance of these diagnostics in the HIV-infected population, in whom latent tuberculosis infection diagnosis and treatment is a priority.
What This Study Adds to the Field
Concordance between IFN-γ release assays in HIV-infected subjects is high, but agreement between positive results with different tests is relatively low.
Tuberculosis (TB) has become the most important coinfection in the HIV epidemic, with an estimated 13 million people worldwide currently infected with both HIV and TB (1). Diagnosis and treatment of latent TB infection (LTBI) are essential to TB control and elimination. LTBI detection is crucial in HIV-infected individuals because they have a higher rate of progression to active TB than HIV-uninfected persons, even with the effective use of antiretroviral therapy (ART) (2).
However, LTBI diagnosis traditionally relies on the tuberculin skin test (TST), which is limited by the need for two visits, an often dismal return rate for evaluation (3, 4), the subjective nature of test placement and interpretation, and false-positive reactions from bacille Calmette-Guérin (BCG) vaccination and nontuberculous mycobacterium exposure. In addition, HIV-infected patients have a higher rate of anergy, particularly with advanced immunosuppression (5, 6).
Recently developed interferon (IFN)-γ release assays (IGRAs) using the Mycobacterium tuberculosis–specific antigens early secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) have shown promise as alternative diagnostic tools for LTBI. Tests using these TB-specific antigens have the advantage of decreased cross-reactivity in individuals exposed to BCG, Mycobacterium avium, and most nontuberculous mycobacteria (7). In the QuantiFERON-TB Gold assay (Cellestis, Carnegie, Australia), antigens are incubated with whole blood, after which lymphocyte release of IFN-γ is quantified by ELISA. An alternative assay, the enzyme-linked immunospot (ELISPOT) T-SPOT.TB assay (Oxford Immunotec, Oxford, UK), uses purified lymphocytes incubated with TB antigens and measures the number of IFN-γ–releasing cells. Compared with TST, data on the current generation of IGRAs suggest higher specificity (8), fewer false-positive reactions due to BCG vaccination (9), and greater sensitivity given a known TB exposure in environments with low TB incidence (10).
However, there are limited data describing IGRA performance in HIV-infected individuals, whose immunologic impairment may affect the performance of these lymphocyte-based assays (11). To better understand IGRA use in HIV infection, we compared the performance of QuantiFERON-TB Gold In-Tube Test (QFT) with conventional TST for the diagnosis of LTBI in an urban HIV-infected population.
METHODS
Study Population
Subjects were recruited from two established San Francisco cohorts of chronically HIV-infected adults [SCOPE and REACH (12, 13); see online supplement]. Subjects were eligible if they were 18 years or older and HIV-antibody positive. Ongoing treatment for TB or LTBI, status as a current TB suspect, prior severe reaction to TST, and current pregnancy were exclusion criteria. Current ART usage, previous TST results, history of prior TB or LTBI diagnosis and therapy, BCG vaccination, and risk factors for TB infection were recorded on entry. TB risk factors were defined as birth in a high TB incidence county (⩾ 25 cases/100,000), self-reported contact with an active TB case, and living, working, or volunteering in a homeless shelter, hospital, prison, drug rehabilitation unit, or nursing home.
IGRA
Blood was collected in three QFT evacuated tubes: one containing TB antigens (ESAT-6, CFP-10, and TB7.7), a positive control tube containing phytohemagglutinin, and a negative control tube. ELISA for IFN-γ was performed according to the manufacturer's specifications. A positive result was defined as TB antigen minus negative control IFN-γ of 0.35 IU/ml or more and 25% or more of negative control IFN-γ value. Indeterminate results were defined as either (1) a negative control IFN-γ level of more than 8.0 IU/ml or (2) a positive control IFN-γ response of 0.5 IU/ml or less with a TB antigen minus a negative control IFN-γ response of either less than 0.35 IU/ml or less than 25% of the negative control value (14). Because the IFN-γ ELISA cannot accurately quantify values of more than 10 IU/ml, such values were treated as 10 IU/ml during analysis.
QFT packaging states that positive results can be confirmed when TB infection is not suspected by retesting the original samples in duplicate and considering an individual to have tested QFT positive if one or both of the replicate tests are positive (14). Samples initially classified as positive QFT results were retested in duplicate to evaluate how this recommendation would affect positive test results. These results are reported; however, QFT test results were classified on the basis of the initial QFT test only, as the population studied was at risk for TB infection.
TST
One-step tuberculin skin testing with 5 TU of tuberculin (Tubersol; Aventis Pasteur, Toronto, ON, Canada) was performed in accordance with American Thoracic Society guidelines (15). TST results were interpreted 48 to 72 hours after placement, and maximum transverse diameter of the induration was recorded using blinded calipers. Positive TST results were defined as an induration of 5 mm or larger (15).
All participants gave informed consent. Research was approved by the institutional review board.
Statistical Analyses
TST and QFT results were analyzed as continuous variables and as dichotomized binary values. A chi-square test was used to compare proportions, and median values were compared using the Mann-Whitney rank sum test. Trend analysis was performed using a nonparametric test for trend across ordered groups. Concordance between dichotomized TST and QFT results was evaluated using agreement and kappa statistics. Correlation calculations were performed using Spearman correlation.
RESULTS
Study Population
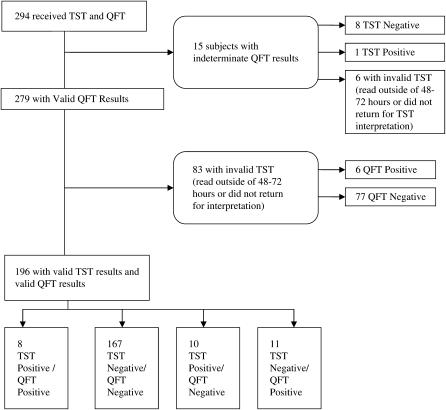
A total of 294 subjects were enrolled (Figure 1). The median age was 46 years (interquartile range [IQR], 42–51), and 229 (78%) were men. The median CD4+ count was 363 cells/mm3 (IQR, 214–581), and median CD4+ nadir was 132 cells/mm3 (IQR, 60–273). Current ART use was reported in 69% of subjects. HIV RNA level was less than 75 copies/ml in 45% of subjects; in addition, 16% of subjects reported a history of prior positive TST results, and 55% reported one or more risk factors for TB infection (Table 1).
Figure 1.
Flow diagram for 294 HIV-infected subjects who underwent QuantiFERON-TB Gold In-Tube (QFT) and tuberculin skin testing (TST). Agreement, 89.3%; kappa = 0.37, p < 0.001.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF HIV-INFECTED SUBJECTS UNDERGOING TUBERCULIN SKIN TESTING AND QuantiFERON TESTING
| All Subjects (n = 294) | TST Positive* (n = 19) | QFT Positive† (n = 25) | |
|---|---|---|---|
| Demographics | |||
| Age, median, yr (IQR) | 46 (42–51) | 47 (42–51) | 47.5 (44–50) |
| Male | 229 (78%) | 13 (68%) | 18 (72%) |
| Ethnicity | |||
| White | 86 (29%) | 4 (21%) | 4 (16%) |
| Black | 139 (47%) | 11 (58%) | 11 (44%) |
| Latino | 26 (9%) | 2 (10.5%) | 6 (24%) |
| Asian/Pacific Islander | 12 (4%) | 2 (10.5%) | 1 (4%) |
| Other | 31 (11%) | 0 | 3 (12%) |
| Homeless in past year | 52 (18%) | 4 (21%) | 4 (16%) |
| History of injection drug use (lifetime) | 108 (37%) | 6 (32%) | 8 (32%) |
| HIV-related factors | |||
| Median CD4+ cell count, at enrollment (IQR) | 363 (214–581) | 551 (296–768)‡ | 438 (351–596) |
| CD4+ cell count by strata | |||
| < 100 cells/mm3 | 31 (10.5%) | 0 | 0 |
| 100–350 cells/mm3 | 111 (37.8%) | 7 (37%) | 6 (24%) |
| > 350 cells/mm3 | 152 (51.7%) | 12 (63%) | 19 (76%) |
| Median CD4+ cell count nadir (IQR) | 132 (60–273) | 209 (156–428)§ | 186 (92–273) |
| Median log10 HIV RNA | 2.42 (2.03–4.37) | 2.47 (2.03–3.76) | 2.2 (2.03–3.56) |
| HIV RNA < 75 copies/ml | 131 (45%) | 7 (58%) | 12 (48%) |
| On ART, at enrollment | 202 (69%) | 9 (75%) | 15 (60%) |
| TB history | |||
| Previous TST | 273 (93%) | 18 (95%) | 23 (92%) |
| Previous positive TST | 44 (15%) | 12 (63%)§ | 11 (44%)‡ |
| Prior LTBI treatment | 36 (12%) | 12 (63%)§ | 9 (36%)§ |
| Prior active TB | 4 (1%) | 1 (5%) | 3 (12%)‡ |
| Self-reported BCG vaccination | 18 (6%) | 1 (5%) | 0 |
| Risk factors for TB infection | |||
| Lived or worked in homeless shelter, prison, hospital, or drug rehabilitation unit | 142 (48%) | 14 (74%)‡ | 11 (44%) |
| Birth in country with high TB incidence‖ | 13 (4%) | 1 (5%) | 3 (12%) |
| Self-reported contact with active TB case | 37 (13%) | 1 (5%) | 2 (8%) |
| Any of the above risk factors for TB infection | 162 (55%) | 14 (74%) | 14 (56%) |
Definition of abbreviations: ART = antiretroviral therapy; BCG = bacille Calmette-Guérin; IQR = interquartile range; LTBI = latent tuberculosis infection; QFT = QuantiFERON Gold In-Tube; TB = tuberculosis; TST = tuberculin skin test.
p values indicate statistically significant difference between TST positive and negative.
p values indicate statistically significant difference between QFT positive and negative.
p value < 0.05.
p value < 0.01.
Defined as incidence ⩾ 25/100,000.
TST
A total of 276 of 294 (94%) enrollees returned for TST evaluation, and 205 (70%) had TSTs read within 48 to 72 hours from placement. Subjects who did not return for TST evaluation within 48 to 72 hours were similar to those who did return in terms of TB risk factors and demographics, including CD4+ count and HIV RNA. Of those with valid TST results, 19 subjects (9.3%) had positive skin test results, with a median induration of 9 mm (IQR, 7–15 mm). Of subjects with CD4+ cell counts of more than 350 cells/mm3, 11.9% (12/101) had positive TST results, compared with 8.4% (7/83) with CD4+ counts of 100 to 350 cells/mm3 and no positive TST results with CD4+ counts of less than 100 cells/mm3 (p = 0.098, test for trend; Table 2).
TABLE 2.
QuantiFERON AND TUBERCULIN SKIN TEST RESULTS BY CD4+ CELL COUNT STRATA
| CD4+ Strata (cells/mm3)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| < 100 | 100–350 | > 350 | Total | |||||
| QFT Results by CD4+ Cell Count Strata (n = 294)* | ||||||||
| QFT positive | 0 | 6 (5.4%) | 19 (12.5%) | 25 (8.5%) | ||||
| QFT negative | 26 (83.9%) | 101 (91%) | 127 (83.6%) | 254 (86.4%) | ||||
| Indeterminate | 5 (16.1%) | 4 (3.6%) | 6 (3.9%) | 15 (5.1%) | ||||
| Total | 31 | 111 | 152 | 294 | ||||
| TST Results by CD4 Strata (n = 205)† | ||||||||
| TST positive | 0 | 7 (8.4%) | 12 (11.9%) | 19 (9.3%) | ||||
| TST negative | 21 | 76 (91.6%) | 89 (88.1%) | 186 (90.7%) | ||||
| Total | 21 | 83 | 101 | 205 | ||||
Definition of abbreviations: QFT = QuantiFERON Gold In-Tube; TST = tuberculin skin test.
p = 0.007, Fisher's exact test.
p = 0.098, test for trend.
In unadjusted analysis, the following were significant predictors of TST positivity: higher CD4+ nadir (per 100 cells/mm3; odds ratio [OR], 1.32; 95% confidence interval [CI], 1.09–1.60; p = 0.004), prior reactive TST results (OR, 7.72; 95% CI, 4.75–41.28; p < 0.001), and exposure to a shelter, prison, or drug rehabilitation unit (OR, 3.19; 95% CI, 1.10–9.21; p = 0.032). When controlled for current CD4+ cell count and CD4+ nadir, prior positive TST and exposure to a shelter, prison, or drug rehabilitation unit remained significant predictors of current reactive TST results (OR, 19.39; 95% CI, 5.59–67.09; and OR, 5.15; 95% CI, 1.32–20.07, respectively).
IGRA
QFT testing yielded positive results in 8.5% (25/294) of subjects, negative results in 86.4% (254/294) of subjects, and indeterminate results in 5.1% (15/294) of subjects (Table 2). Duplicate testing of positive QFT samples resulted in 20% (5/25) of samples with two negative tests, 12% (3/25) with one positive/one negative test, and 68% (17/25) with two positive tests. All indeterminate results were due to a failure to respond adequately to the positive control. Of those with a CD4+ cell count of less than 100 cells/mm3, 16.1% (5/31) had indeterminate QFT results, compared with 3.6% (4/111) and 3.9% (6/152) in the CD4+ strata of 100 to 350 or more than 350 cells/mm3, respectively (p = 0.007). Subjects with a CD4+ count of less than 100 cells/mm3 had a relative risk of an indeterminate result of 4.24 (95% CI, 1.55–11.61; p = 0.003) compared with those with a CD4+ count of 100 cells/mm3 or more.
Excluding indeterminate QFT results, unadjusted analysis revealed the following predictors of QFT positivity: CD4+ count of more than 350 cells/mm3 (OR, 3.17; 95% CI, 1.22–8.19; p = 0.02), prior positive TST (OR, 5.91; 95% CI, 2.40–14.57; p < 0.001), birth in a country with a high TB incidence rate (OR, 3.17; 95% CI, 1.15–8.73; p = 0.03), and Latino ethnicity (OR, 5.85; 95% CI, 1.51–22.73; p = 0.011). Controlling for current CD4+ count and ethnicity, prior positive TST and birth in a country with high TB incidence remained predictive of QFT positivity (Table 3).
TABLE 3.
MULTIVARIATE ANALYSIS OF RISK FACTORS FOR QuantiFERON POSITIVITY
| Adjusted OR (95% CI) | p Value | |
|---|---|---|
| CD4+ cell count > 350 cells/mm3 | 2.24 (0.79–6.38) | 0.13 |
| Birth in country with high TB incidence* | 7.09 (1.88–26.70) | 0.004 |
| Prior positive tuberculin skin test | 9.01 (3.26–24.91) | < 0.001 |
| Ethnicity | ||
| White | 1.00 | — |
| Black | 1.87 (0.52–6.77) | 0.34 |
| Latino | 2.01 (0.38–10.71) | 0.41 |
| Other | 1.96 (0.36–10.75) | 0.44 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; TB = tuberculosis.
Defined as TB incidence of ⩾ 25 cases/ 100,000.
Higher CD4+ cell count was correlated with higher IFN-γ release in response to TB antigens (rho = 0.12, p = 0.046) and with higher IFN-γ response to positive control (rho = 0.31, p ⩽ 0.001). Median IFN-γ response to TB antigens was 0.12 IU/ml, 0.14 IU/ml, and 0.16 IU/ml at CD4+ cell counts of less than 100, 100 to 350, and more than 350 cells/mm3, respectively (p = 0.008, test for trend). TB antigen–induced IFN-γ release was correlated with TST induration (rho = 0.2, p = 0.004).
Concordance between TST and IGRA
Excluding those with indeterminate QFTs and/or TSTs read outside 48 to 72 hours, 196 subjects had valid TST and QFT results. Of these, 167 (85.2%) were concordant in their negative results and 8 (4.1%) were concordant in their positive results, for an overall concordance of 89.3% (kappa = 0.37, p ⩽ 0.001). Of subjects without valid TST results, 6.7% (6/89) had positive QFT results, and 6.7% (6/89) had indeterminate QFT results.
TST+/QFT− discordant results were found in 5.1% (10/196) of subjects and TST−/QFT+ results in 5.6% (11/196) of subjects. Of TST−/QFT+ subjects, 45% (5/11) reported prior reactive TSTs, including 3 of 11 with previous isoniazid (INH) treatment. Sixty percent (6/10) of the TST+/QFT− subjects reported prior reactive TSTs, all with subsequent INH therapy. Given that individuals with reactive TST results are generally not serially skin tested, this discordance would decrease to 2.4% (4/165) for TST+/QFT− and 3.6% (6/165) for TST−/QFT+, if all subjects reporting prior positive TSTs were excluded.
In unadjusted analysis, TST+/QFT− discordance was predicted by prior positive TST (OR, 13.4; 95% CI, 3.39–52.90; p < 0.001), higher CD4+ nadir (per 100 cells/mm3; OR, 1.33; 95% CI, 1.06–1.68; p = 0.013), lower HIV RNA log10 copies (OR, 0.40; 95% CI, 0.18–0.90; p = 0.027), Asian/Pacific Islander ethnicity (OR, 13; 95% CI, 1.42–118.29; p = 0.023), and exposure to a shelter, prison, or drug rehabilitation unit (OR, 4.91; 95% CI, 1.01–23.81; p = 0.048). TST−/QFT+ discordance was predicted by prior positive TST (OR, 7.44; 95% CI, 2.03–27.35; p = 0.002), CD4+ count of more than 350 cells/mm3 (OR, 11.67; 95% CI, 1.46–93.37; p = 0.02), and birthplace in a county with high TB incidence rate (OR, 5.32; 95% CI, 1.23–22.92; p = 0.025).
DISCUSSION
Overall agreement between TST and QFT was 89.3%, excluding indeterminate QFT results. Concordance between RD-1 antigen–based QFT and TST has been reported to be from 53 to 94% in studies of immunocompetent populations screened for LTBI or evaluated in contact investigations (8, 16–22). This wide range of concordance has been attributed to variation in the amount and type of tuberculin solution used as well as heterogeneity in terms of BCG vaccination, local TB incidence, and estimated time since LTBI infection in the populations studied (21). Concordance in the current study falls within the upper range seen in other low TB incidence environments.
However, the discordance among subjects with a positive test using either modality raises concerns about the use of QFT in the HIV-infected population. Only 8 of 29 (28%) patients with a positive result on either QFT or TST had a positive result on both tests. Of the TST-positive results, 10 of 18 (56%) occurred in QFT-negative subjects; these positive TST results would have been missed if QFT were used alone.
TST−/QFT+ results were found in 5.6% of subjects, which may be attributable to either false-positive QFT results or to improved sensitivity of QFT over TST. Given the absence of a diagnostic gold standard, it must be noted that the terms specificity and sensitivity in reference to LTBI are only estimations. In populations with low TB risk, estimated specificity of QFT for LTBI detection has been high, ranging from 96 to 99.8% (9, 10, 14, 23, 24). In the current study, subjects with TST−/QFT+ results were more likely to have been born in countries with high TB incidence rates (OR, 5.1; 95% CI, 1.2–21.7; p = 0.03) than those with both negative QFT and TST results, which suggests increased sensitivity of QFT testing over TST, but which would require confirmation in a larger study.
The 5.1% of subjects with TST+/QFT− discordant results raises concern about the recent Centers for Disease Control and Prevention guidelines recommending use of QFT testing “in all circumstances in which the TST is currently used”(25). If QFT were used as the sole diagnostic assay, these TST-positive results would no longer be captured, leaving the essential question, Do these individuals have true TB infection or false-positive TST results? False-positive TSTs occur with exposure to atypical mycobacteria, which cannot be excluded, and with previous BCG vaccination, which were reported by only 1 of 10 of these discordant subjects. Alternatively, these discordant results may indicate a diminished sensitivity of QFT compared with TST for LTBI diagnosis. However, two contact investigations using QFT found a strong relationship between QFT positivity and level of exposure, suggesting sensitivity equivalent to TST (16) or superior to TST (22). Alternatively, these TST+/QFT− results may be explained by QFT reverting from positive to negative after LTBI therapy. Sixty percent of the TST+/QFT− subjects reported prior reactive TSTs, all with subsequent INH therapy. IGRA response to therapy remains an area of controversy, because some studies have demonstrated declining IFN-γ responses after LTBI or active TB treatment (26–28), whereas others have shown unchanged or increasing IFN-γ responses (24, 29, 30).
The intraassay variability encountered with repeat testing of the positive QFT samples raises concerns about QFT reproducibility in general. Twenty percent of the current study's positive QFT results could have been reclassified as negative with repeated testing of the initial specimen, and 32% (8/25) of subjects had at least one negative test result with duplicate testing. All result changes were due to small variations in the IFN-γ response. This may reflect the imposition of a binary cutoff on a continuous laboratory measurement with some inherent measurement variability. However, further investigation of QFT intraassay variability, including variability in indeterminate and negative results as well, is merited. A South African study has also raised this issue of assay variability, finding a surprisingly low 75% concordance between QuantiFERON-TB Gold and the QuantiFERON-TB Gold In-Tube results, which have different methods of collection but use the same TB antigens and thus would be expected to yield very similar test results (21).
Similar to TST (5, 31, 32), QFT may be less sensitive in advanced HIV disease. Lower CD4+ cell count was associated with weaker IFN-γ response to both TB antigens and to the positive control. Indeterminate results were more frequent in subjects with CD4+ counts of less than 100 cells/mm3 (16.1%) but did not differ in those with CD4+ counts of 100 to 350 versus counts of more than 350 cells/mm3 (3.6 vs. 3.9%, respectively). Danish HIV-infected subjects similarly demonstrated an elevated QFT indeterminate rate of 24% in those with CD4+ cell counts of less than 100 cells/mm3, compared with 2.8% in those with CD4+ counts of more than 100 cells/mm3 (11).
This study does not resolve the question of whether QFT testing should be done simultaneously with or in place of TST testing in HIV-infected patients. On the one hand, the significant risk of reactivation LTBI in HIV-infected subjects argues for simultaneous screening with both TST and QFT to minimize false-negative tests and missed opportunities for LTBI therapy. More than half of the TST-positive results occurred in QFT-negative subjects (10/18), and 40% (4/10) of TST+/QFT− subjects had new TST-positive results that would otherwise have been missed with QFT testing alone. Using the ELISpot IGRA, a Gambian study of child contacts to active TB cases found discordance at both ends of the exposure spectrum: increased TST+/ IGRA− at high-level exposure and increased TST−/IGRA+ at low-level exposure (33). These results also suggest that, for maximal LTBI diagnosis, both IGRA and TST would have to be used simultaneously.
On the other hand, simultaneous QFT and TST may be unrealistic in many settings. In the United States, TST limitations, which include poor performance in HIV-infected populations (5, 6) and the personnel requirements for appropriate placement and interpretation, make a one-visit blood test attractive for LTBI diagnosis. In addition, the current study's high 70% return rate for TST interpretation is atypical; return rates are often in the range of 30% (3, 4), which further reduce TST sensitivity and can increase the cost of LTBI screening. Although TST might capture potential LTBI cases that QFT testing misses, the inconvenience and low completion rate that historically accompany TSTs may limit the practical application of simultaneous TST and QFT testing.
This study is limited by its cross-sectional design, which precludes prospective evaluation of TB incidence. In addition, the small number of subjects with CD4+ counts of less than 100 cells/mm3 hinders examination of QFT in advanced HIV. The low rate of positive TST and QFT tests limits evaluation of risk factors associated with test positivity and with discordance between the tests. However, the 8 to 9% rate of LTBI suggested by TST and QFT testing in this study is consistent with that seen in other industrialized populations with low TB prevalence (34–36), where LTBI treatment is a clinical priority and where QFT testing will be feasible.
In conclusion, for diagnosis of LTBI in HIV-infected subjects, overall concordance between QFT and TST was high and similar to that seen in an immunocompetent population. However, among subjects with positive test results on either assay, the concordance was low (28%), which raises concern over implementation of QFT as a sole diagnostic for LTBI in this population. Until further data are available on the implication of discordant TST and QFT results, a strategy of simultaneous TST and QFT testing, where feasible, would maximize potential LTBI diagnoses in HIV-infected subjects. Duplicate testing of QFT-positive results as suggested by the manufacturer could have changed QFT results in 20% of subjects, which merits further evaluation of intraassay variability for all QFT results. Finally, QFT testing may be limited by an elevated rate of indeterminate results in subjects with CD4+ cell counts of less than 100 cells/mm3.
Acknowledgments
The authors thank the SCOPE and REACH teams for their exceptional organization and support and the study participants who made this research possible. They acknowledge Charles Daley (National Jewish Medical and Research Center) for his assistance in protocol development and Puneet Dewan (Centers for Disease Control and Prevention) for his critical reviews of the manuscript.
Supported by a grant (A.F.L.) from the California AIDS Research Institute, which is funded by the Universitywide AIDS Research Program, CC99-SF-001, and by the National Institutes of Health, UCSF-GIVI Center for AIDS Research, 5 P30 AI 27763. Additional funding provided by the Center for AIDS Prevention Studies T32 MH-19105-16 (A.F.L.), and the National Institutes of Health K24-AI51982 (D.V.H.) and RO-1 MH54908 (D.R.B.). Blood collection tubes, kits, and reagents were supplied by Cellestis, Inc. (Carnegie, Australia).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200608-1088OC on January 11, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.World Health Organization. Two diseases: one patient. Report of the Third Global TB/HIV Working Group meeting. Geneva, Switzerland: World Health Organization; 2003.
- 2.Lawn SD, Bekker LG, Wood R. How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS 2005;19:1113–1124. [DOI] [PubMed] [Google Scholar]
- 3.Chaisson RE, Keruly JC, McAvinue S, Gallant JE, Moore RD. Effects of an incentive and education program on return rates for PPD test reading in patients with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 1996;11:455–459. [DOI] [PubMed] [Google Scholar]
- 4.Malotte CK, Rhodes F, Mais KE. Tuberculosis screening and compliance with return for skin test reading among active drug users. Am J Public Health 1998;88:792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caiaffa WT, Graham NM, Galai N, Rizzo RT, Nelson KE, Vlahov D. Instability of delayed-type hypersensitivity skin test anergy in human immunodeficiency virus infection. Arch Intern Med 1995;155:2111–2117. [PubMed] [Google Scholar]
- 6.Duncan LE, Elliott AM, Hayes RJ, Hira SK, Tembo G, Mumba GT, Ebrahim SH, Quigley M, Pobee JO, McAdam KP. Tuberculin sensitivity and HIV-1 status of patients attending a sexually transmitted diseases clinic in Lusaka, Zambia: a cross-sectional study. Trans R Soc Trop Med Hyg 1995;89:37–40. [DOI] [PubMed] [Google Scholar]
- 7.Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000;356:1099–1104. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Choi HJ, Park IN, Hong SB, Oh YM, Lim CM, Lee SD, Koh Y, Kim WS, Kim DS, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J 2006;28:24–30. [DOI] [PubMed] [Google Scholar]
- 9.Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, Yim JJ. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA 2005;293:2756–2761. [DOI] [PubMed] [Google Scholar]
- 10.Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: part I. Latent tuberculosis. Expert Rev Mol Diagn 2006;6:413–422. [DOI] [PubMed] [Google Scholar]
- 11.Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P. Latent tuberculosis in HIV positive, diagnosed by the M. tuberculosis specific interferon gamma test. Respir Res 2006;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeks SG, Martin JN, Sinclair E, Harris J, Neilands TB, Maecker HT, Hagos E, Wrin T, Petropoulos CJ, Bredt B, et al. Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral-treated individuals with drug-resistant human immunodeficiency virus type 1. J Infect Dis 2004;189:312–321. [DOI] [PubMed] [Google Scholar]
- 13.Robertson MJ, Clark RA, Charlebois ED, Tulsky J, Long HL, Bangsberg DR, Moss AR. HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health 2004;94:1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.QuantiFERON-TB Gold In-Tube [package insert]. Available from: http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023 (accessed December 2005).
- 15.American Thoracic Society; Centers for Disease Control and Prevention. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376–1395. [DOI] [PubMed] [Google Scholar]
- 16.Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 2004;170:65–69. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, Bergamini BM, D'Amico R, Marchegiano P, Rumpianesi F, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med 2005;172:631–635. [DOI] [PubMed] [Google Scholar]
- 18.Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, Narang P, Daley CL, Granich RM, Mazurek GH, et al. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA 2005;293:2746–2755. [DOI] [PubMed] [Google Scholar]
- 19.Dogra S, Narang P, Mendiratta DK, Chaturvedi P, Reingold AL, Colford JM Jr, Riley LW, Pai M. Comparison of a whole blood interferon-γ assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. J Infect 2007;54:267–276. [DOI] [PubMed] [Google Scholar]
- 20.Porsa E, Cheng L, Seale MM, Delclos GL, Ma X, Reich R, Musser JM, Graviss EA. Comparison of a new ESAT-6/CFP-10 peptide-based gamma interferon assay and a tuberculin skin test for tuberculosis screening in a moderate-risk population. Clin Vaccine Immunol 2006;13:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahomed H, Hughes EJ, Hawkridge T, Minnies D, Simon E, Little F, Hanekom WA, Geiter L, Hussey GD. Comparison of mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int J Tuberc Lung Dis 2006;10:310–316. [PubMed] [Google Scholar]
- 22.Arend SM, Thijsen SFT, Leyten EMS, Bouwman JJM, Franken WPJ, Koster BFP, Cobelens FGJ, van Houte A-J, Bossink AWJ. Comparison of two interferon-γ assays and tuberculin skin test for tracing tuberculosis contacts. Am J Respir Crit Care Med 2007;175:618–627. [DOI] [PubMed] [Google Scholar]
- 23.Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, Shigeto E, Harada N, Mitarai S, Okada M, et al. Specific detection of tuberculosis infection: an interferon-γ-based assay using new antigens. Am J Respir Crit Care Med 2004;170:59–64. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson KA, Kon OM, Newton SM, Meintjes G, Davidson RN, Pasvol G, Wilkinson RJ. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J Infect Dis 2006;193:354–359. [DOI] [PubMed] [Google Scholar]
- 25.Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005;54(RR-15):49–55. [PubMed] [Google Scholar]
- 26.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis 2004;38:754–756. [DOI] [PubMed] [Google Scholar]
- 27.Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, Lalvani A. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am J Respir Crit Care Med 2006;174:831–839. [DOI] [PubMed] [Google Scholar]
- 28.Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, Hill AV, Lalvani A. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol 2001;167:5217–5225. [DOI] [PubMed] [Google Scholar]
- 29.Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Dheda K, Kalantri S. Persistently elevated T cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India: a preliminary report. J Occup Med Toxicol 2006;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrand RA, Bothamley GH, Whelan A, Dockrell HM. Interferon-gamma responses to ESAT-6 in tuberculosis patients early into and after anti-tuberculosis treatment. Int J Tuberc Lung Dis 2005;9:1034–1039. [PubMed] [Google Scholar]
- 31.Fisk TL, Hon HM, Lennox JL, Fordham von Reyn C, Horsburgh CR Jr. Detection of latent tuberculosis among HIV-infected patients after initiation of highly active antiretroviral therapy. AIDS 2003;17:1102–1104. [DOI] [PubMed] [Google Scholar]
- 32.Jones-Lopez EC, Okwera A, Mayanja-Kizza H, Ellner JJ, Mugerwa RD, Whalen CC. Delayed-type hypersensitivity skin test reactivity and survival in HIV-infected patients in Uganda: should anergy be a criterion to start antiretroviral therapy in low-income countries? Am J Trop Med Hyg 2006;74:154–161. [PubMed] [Google Scholar]
- 33.Hill PC, Brookes RH, Adetifa IM, Fox A, Jackson-Sillah D, Lugos MD, Donkor SA, Marshall RJ, Howie SR, Corrah T, et al. Comparison of enzyme-linked immunospot assay and tuberculin skin test in healthy children exposed to Mycobacterium tuberculosis. Pediatrics 2006;117:1542–1548. [DOI] [PubMed] [Google Scholar]
- 34.Brassard P, Bruneau J, Schwartzman K, Senecal M, Menzies D. Yield of tuberculin screening among injection drug users. Int J Tuberc Lung Dis 2004;8:988–993. [PubMed] [Google Scholar]
- 35.Smith B, Ryan MA, Gray GC, Polonsky JM, Trump DH. Tuberculosis infection among young adults enlisting in the United States Navy. Int J Epidemiol 2002;31:934–939. [DOI] [PubMed] [Google Scholar]
- 36.Janis EM, Allen DW, Glesby MJ, Carey LA, Mundy LM, Gopalan R, Chaisson RE. Tuberculin skin test reactivity, anergy, and HIV infection in hospitalized patients. Longcope Firm of the Osler Medical Housestaff. Am J Med 1996;100:186–192. [DOI] [PubMed] [Google Scholar]