Abstract
Most inhaled β2-adrenergic agonist and anticholinergic bronchodilators have low lipid solubility because of their transient or permanent positive net charge at physiologic pH. Airway absorption of these cationic drugs is incompletely understood. We examined carrier-mediated mechanisms of cationic drug uptake by human airway epithelia. Airway tissues and epithelial cells, obtained from lung donors without preexisting lung disease, were evaluated for organic cation transporter expression by quantitative RT-PCR and immunofluorescence. For in vitro functional studies on primary airway epithelial cells, uptake of the cationic fluorophore 4-[4-(dimethylamino)-styryl]-N-methylpyridinium (ASP+) was characterized. Quantitative RT-PCR analysis demonstrated high mRNA levels for two polyspecific organic cation/carnitine transporters, OCTN1 and OCTN2, in human airway epithelia. Immunofluorescence of human airway sections confirmed OCTN1/2 protein expression, with a predominant localization to the apical portion of epithelial cells. Primary airway epithelial cells showed a carrier-mediated, temperature-sensitive and saturable uptake of ASP+. Seventy-five to eighty percent of ASP+ uptake was inhibited by L-carnitine, an OCTN2-carried zwitterion. The uptake was pH dependent, with ∼ 3-fold lower rates at acidic (pH 5.7) than at alkaline (pH 8.2) extracellular pH. Albuterol and formoterol inhibited ASP+ uptake, suggesting that all these molecules are carried by the same transport mechanism. These findings demonstrate the existence and functional role of a pH-dependent organic cation uptake machinery, namely OCTN1 and OCTN2, in human airway epithelia. We suggest that epithelial OCTN1/2 are involved in the delivery of inhaled cationic bronchodilators to the airway tissue.
Keywords: airway epithelia, airway pH, bronchodilators, drug absorption, organic cation transporters
CLINICAL RELEVANCE
Airway absorption of bronchodilators with low lipid solubility is poorly understood. This study reveals a pH-dependent drug absorption machinery that is likely involved in the delivery of inhaled bronchodilators to the airway tissue.
The lungs provide a unique absorptive surface for drug delivery. The inhalation route allows exposure of airway tissues to high drug concentrations while minimizing systemic drug effects. The most common inhaled drugs are bronchodilators and anti-inflammatory agents used in the therapy of airway diseases such as asthma and chronic obstructive pulmonary disease. Effective airway absorption of these drugs is a prerequisite for local pharmacologic effects. Many inhaled drugs are rapidly absorbed into the airway because of their lipophilic chemical characteristics (1). However, the majority of the currently used bronchodilators have a transient or permanent positive charge at physiologic pH. These organic cations cannot freely diffuse across the cell membrane lipid bilayers of the airway epithelium to reach their targets in the airway tissue. In contrast to their well investigated receptor actions on smooth muscle and other cells in the airway (2, 3), the molecular mechanisms by which cationic drugs reach these receptors remain incompletely understood.
In recent years, a number of polyspecific uptake transporters have been identified that facilitate the passage of structurally diverse cationic drugs through membrane barriers (4). These include two distinct classes of the solute carrier (SLC22) transporter family (5): one driven by the transmembrane potential difference and the other by the transmembrane H+ gradient. Five human organic cation transporters have been cloned to date: the potential dependent organic cation transporters OCT1 (6), OCT2 (7), and OCT3 (8), and the pH-dependent organic cation/carnitine transporters OCTN1 (9) and OCTN2 (10). Among many critical biological functions such as intestinal absorption, placental transfer, and renal excretion of xenobiotics and endogenous substrates (5), these transporters seem to serve important airway functions. We have demonstrated the corticosteroid-sensitive uptake of norepineprine by OCT3 in vascular smooth muscle (11), an action that is likely influencing the sympathetic neurogenic tone of airway blood vessel (12). OCT1 and OCT2, on the other hand, may mediate acetylcholine release from epithelial cells to the airway lumen (13). Information on carrier-mediated cationic drug absorption into the human airway epithelium is lacking to date. With respect to drug absorption in monolayer cultures of airway epithelial cell lines, the cationic albuterol appears to be absorbed actively (14); however, others found no evidence of an active absorption mechanism for organic cations (15).
For inhaled bronchodilators, the airway epithelium is the major physicochemical barrier for reaching their receptors on airway smooth muscle. To identify the role of transporter-mediated mechanisms in the absorption of cationic bronchodilators, we investigated cationic drug uptake and transporter expression in primary human airway epithelia. Because measurements of drug absorption into human airway tissue are not feasible in vivo, we used donor lungs to isolate primary airway epithelial cells for functional studies.
MATERIALS AND METHODS
Materials
Unless otherwise stated all materials were obtained from Sigma Chemical Co. (St. Louis, MO).
Human Airway Tissues and Cells
Tracheas, large bronchi (⩾ third generation), and lung parenchyma were obtained from organ donors without preexisting lung disease whose lungs were rejected for transplantation because they failed to meet the standard selection criteria (16), and from patients with cystic fibrosis during lung transplantation. The studies were approved by the Institutional Review Board of the University of Miami, and the subjects or their surrogates gave informed consent. Primary airway epithelial cells were isolated as previously described (17). Freshly isolated cells were deposited onto human placental (type IV) collagen-coated glass coverslips at a density of 0.5 × 106 cells/cm2, and maintained ⩽ 36 h in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (Hyclone, Logan, UT), 100 U/ml penicillin, and 100 μg/ml streptomycin within a humidified atmosphere containing 5% CO2 at 37°C.
Cell Culture
Air–liquid interface (ALI) cultures of human airway epithelial cells were prepared as previously described (17).
Expression Profiling by Quantitative RT-PCR
Total RNA was extracted from abraded human tracheal epithelia and ALI cultures of airway epithelial cells using RNeasy Protect Mini Kit (Qiagen, Valencia, CA). RNA samples were treated with RNase-free DNase (Qiagen) and quantified spectrophotometrically at 260 nm. RNA integrity was confirmed using RNA 6,000 LabChip Kit (Agilent Technologies, Palo Alto, CA) and a bioanalyzer (model 2100, Agilent Technologies) provided by the University of Miami DNA Microarray Facility. Human placenta, liver, and kidney RNA samples were purchased from Ambion (Austin, TX). Total RNA (0.25 μg/reaction) was used for first-strand cDNA synthesis using Quantitect Reverse Transcription Kit (Qiagen).
To assess organic cation transporter mRNA expression levels, quantitative PCR was used with newly designed gene-specific primers (Table 1). Real-time PCR amplification reactions were performed with Quantitect SYBR Green PCR Kit (Qiagen). The cycling conditions comprised of 15 min polymerase activation at 95°C, and 45 cycles at 95°C for 15 s, 57°C for 30 s, and at 72°C for 30 s. Reactions were run on an iCycler IQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. Specificity of the amplificons were confirmed by purification on QiAquick PCR Purification Kit silica spin columns (Qiagen) and sequencing (DNA Core Laboratory, University of Miami).
TABLE 1.
CUSTOM-DESIGNED GENE-SPECIFIC PRIMERS FOR QUANTITATIVE PCR AMPLIFICATION OF THE MEMBRANE POTENTIAL SENSITIVE ORGANIC CATION TRANSPORTERS (OCT1, OCT2, AND OCT3), THE PH-DEPENDENT ORGANIC CATION/CARNITINE TRANSPORTERS (OCTN1 AND OCTN2), AND THE ENDOGENOUS CONTROL GENE GAPDH
| Forward Primer
|
Reverse Primer
|
||||||
|---|---|---|---|---|---|---|---|
| Name | GenBank Acc. No. | Gene Symbol | Sequence | Position | Sequence | Position | Product (bp) |
| OCT1 | NM_003057 | SLC22A1 | GCTGGCTACACCCTAATCACAGA* | 760–782 | GGCACACACCAGTAGTAGAGCA* | 953–932 | 194 |
| OCT2 | NM_003058 | SLC22A2 | CTTCAGCGCCTGAGACTTGAAG* | 1122–1143 | CACAGAGCTCGTGAACCAGTTG* | 1250–1229 | 129 |
| OCT3 | NM_021977 | SLC22A3 | TGCCTACTTCATCCCCAACTGG† | 807–828 | TTCCGAGTAATCAGCCAACGGG† | 923–902 | 117 |
| OCTN1 | NM_003059 | SLC22A4 | TCGTGACCGAGTGGAATCTGG* | 548–568 | CTGCCAAACCTGTCTGACAGC* | 671–651 | 124 |
| OCTN2 | NM_003060 | SLC22A5 | CCATTGTGACCGAGTGGAACC* | 601–621 | ACATTCTTCCGGCCAAACCTG* | 736–716 | 136 |
| GAPDH | NM_002046 | GAPDH | CCTCAAGATCATCAGCAATGCCT† | 531–553 | GATGGCATGGACTGTGGTCAT† | 645–625 | 115 |
Definition of abbreviations: bp, base pairs; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Primer spans an exon-intron boundary.
Forward and reverse primers localize on different exons.
The comparative threshold cycle (CT) method was used for relative quantification of PCR products (18). To establish PCR functionality of our custom-designed primers, amplification standard curves were generated for each gene by serial dilutions of placenta cDNA. The amplification efficiency (E) was calculated using data collected from the standard curve with the following formula: E = 10−1/slope − 1 (18). PCR efficiencies for all custom-designed primers were above 95%. Relative gene expression levels were calculated by normalizing the data to the control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and relative to the sample with the highest expression level of the respective gene (calibrator). Thus, data were first normalized to the control gene by: ΔCT = CTtarget gene − CTcontrol gene. Then, mRNA expression data were converted to relative levels using the following formula: Relative expressiontarget gene = 2−ΔΔCT , where ΔΔCT = ΔCTtarget gene − ΔCTcalibrator (18).
OCTN1/2 Immunolocalization in Human Airway Tissues and ALI Cultures of Airway Epithelial Cells
Tracheal rings and lung parenchyma were fixed in 4% paraformaldehyde and embedded for paraffin sectioning. Autofluorescence of deparaffinized sections was reduced by incubating sections in 5 mg/ml sodium borohydride in PBS for 30 min at room temperature. Sections were subjected to antigen retrieval by incubating in 10 mM citrate buffer (pH 6) for 15 min at 80°C followed by blocking in 1% BSA in PBS for 60 min at room temperature. Then, sections were incubated overnight with 5 μg/ml affinity-purified goat anti-human OCTN1 or OCTN2 IgG (Santa Cruz, Santa Cruz, CA), or nonimmune goat IgG (negative control) at 4°C. Alexa Fluor 488–conjugated rabbit anti-goat IgG (Molecular Probes, Eugene, OR) was used as secondary reagent. Sections were mounted with Prolong Gold antifade permanent mounting reagent containing 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (Molecular Probes).
To localize organic cation transporter proteins in airway epithelial cells cultured at the ALI, antibodies against OCTN1/2 and acetylated tubulin (to label cilia) were used. Culture inserts with airway epithelial cells were excised, and fixed and permeabilized with acetone-methanol (1:1) at −20°C. Autofluorescence reduction, blocking, and incubation with anti-OCTN1/2 antibodies or nonimmune IgG (negative control) and the secondary reagent were performed as described for tissue sections. Co-localization with cilia was examined by incubating the samples with a monoclonal anti-acetylated tubulin antibody for 60 min at room temperature followed by Alexa Fluor 555–conjugated goat anti-mouse F(ab')2 fragment (Molecular Probes) as a secondary reagent. After mounting with Prolong Gold (with DAPI), images were captured using a Zeiss LSM-510 confocal laser scanning microscope (Zeiss MicroImaging, Thornwood, NY), provided by the University of Miami Imaging Core Facility.
Organic Cation Uptake Studies
To measure organic cationic uptake, primary airway epithelial cells plated on collagen-coated coverslips were exposed to the fluorophore 4-[4-(dimethylamino)-styryl]-N-methylpyridinium (ASP+) in modified Hanks' Balanced Salt Solution (mM: 138 NaCl, 5.3 KCl, 4.2 NaHCO3, 1.3 CaCl2, 0.5 MgCl2, 0.4 Mg2SO4, 0.4 KH2PO4, 0.3 Na2HPO4, 5.6 glucose, 20 Hepes, pH 7.4) at 37°C or 4°C. Tetraethylammonium (TEA+), corticosterone, L-carnitine, or ergothioneine was also added in to assess their inhibitory potential. Na+ dependence of ASP+ uptake was investigated by changing the incubation solution to Na+-free solution, in which Na+ was replaced isotonically with N-methyl-D-glucamine. Reaction was stopped by washes with ice-cold PBS and the samples were mounted with Gel/Mount (Biomedia, Foster City, CA) for quantification using fluorescence microscopy.
Single-cell fluorescence was quantified as previously described (11, 19, 20) using ASP+-specific excitation (465–495 nm) and emission (595–615 nm) filters.
Statistics
Each experiment was repeated with cells from at least three different lungs. Results were expressed as mean ± SEM of at least three experiments. Two groups were compared using Student's unpaired t test, while the Tukey-Kramer test was used for comparison of more than two groups if an ANOVA indicated a statistically significant difference by a P < 0.05. Half-saturation constant (Km) and maximum uptake rate (Vmax) were calculated by fitting the uptake rate (v) to the following equation by means of nonlinear least squares regression analysis using Prism v4.0 (GraphPad, San Diego, CA): v = Vmax × [S] / (Km + [S]), where [S] was [ASP+].
RESULTS
Transporter mRNA Expression
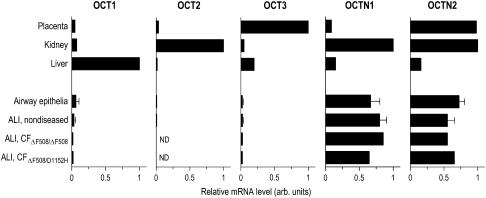
Relative mRNA expression levels for the membrane potential sensitive transporters OCT1, OCT2, and OCT3 were highest in human liver, kidney, and placenta, respectively (Figure 1). OCT1 and OCT3 expression in airway epithelia remained relatively low compared with the highest expressing tissues, while OCT2 mRNA expression was nearly undetectable. OCTN1 and OCTN2 relative mRNA expression levels were the highest in placenta and kidney. In contrast to low expression levels for the membrane potential sensitive transporters, mRNA expression levels of the pH-dependent organic cation transporters OCTN1 and OCTN2 were almost as high in airway epithelia as in placenta and kidney.
Figure 1.
Comparison of organic cation transporter mRNA expression levels in human tissues and cells. Relative mRNA expression levels of the membrane potential sensitive organic cation transporters (OCT1, OCT2, and OCT3) and the pH-dependent organic cation/carnitine transporters (OCTN1 and OCTN2) were measured using quantitative RT-PCR. Tracheas and large bronchi (⩾ third generation) were obtained from human donors without preexisting lung disease (n of donors = 4), and from patients with cystic fibrosis (ΔF508/ΔF508 homozygote and ΔF508/D1152H heterozygote) (n of donors = 2). RNA was extracted from scraped airway epithelium and/or cells cultured and re-differentiated at the ALI. Placenta, kidney, and liver RNA samples were obtained from a single donor subject. Expression data are indicated relative to the sample (calibrator) with the highest expression level of the respective gene: liver for OCT1, kidney for OCT2, OCTN1, and OCTN2, and placenta for OCT3, respectively. Bars indicate the average of triplicate quantitative RT-PCR measurements. Error bars indicate SEM for samples obtained from four different human donors. ND = not detectable level.
Airway epithelial cells cultured at the ALI for 6–8 wk expressed mRNA for these transporter molecules in nearly identical amounts compared with freshly harvested airway epithelial cells by scraping. This finding supports the concept that airway epithelial re-differentiation at the ALI provides a good model system for studying the function of these transporters. Transporter mRNA expression levels were also investigated in ALI cultures established from airway tissues of patients with cystic fibrosis, an inherited disease of apical chloride channel dysfunction caused by the mutations in the gene encoding of the cystic fibrosis transmembrane conductance regulator (CFTR). ALI cultures established from either homozygote ΔF508/ΔF508 or heterozygote ΔF508/D1152H patients indicated that high mRNA expression levels for pH-dependent drug transporters were not altered in these individuals. Although CFTR function and the clinical course of the disease are proposed to be influenced by “modifier genes,” such as epithelial drug efflux transporter downregulation (21), epithelial cationic drug uptake transporters are likely not involved in this process.
OCTN1/2 Immunolocalization in the Human Airway
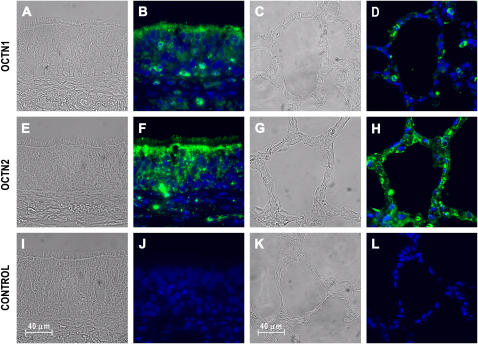
Since the mRNA expression levels for the pH-dependent organic cation transporters were high in airway epithelia, immunohistochemistry was used to localize OCTN1 and OCTN2 within human trachea and lung parenchyma (Figure 2). OCTN1 immunoreactivity was largely associated with the apical portion of epithelial cells in the trachea (Figure 2B), whereas lower levels of OCTN1 immunoreactivity were detectable in alveolar epithelia (Figure 2D). Interestingly, OCTN1-specific labeling was also seen with other, most likely inflammatory cells infiltrating airway epithelia (both in the airway and alveoli). This is consistent with the recently demonstrated OCTN1 expression in leukocytes positive for CD14+ and monocytes (22). OCTN2 immunoreactivity was also largely associated with the apical portion of airway epithelial cells (Figure 2F). In contrast to OCTN1, OCTN2 immunoreactivity was seen in the lung parenchyma, especially with the surface of the alveolar epithelia (Figure 2H).
Figure 2.
Immunofluorescence analysis reveals polarized expression of the pH-dependent organic cation transporter OCTN1 and OCTN2 in human airways. After autofluorescence reduction and antigen retrieval (see Materials and Methods), human trachea and lung parenchyma sections were incubated with 5 μg/ml affinity-purified goat IgG against human OCTN1 (A–D) and OCTN2 (E–H), or nonimmune goat IgG as a negative control (I–L) followed by AlexaFluor 488–coupled rabbit anti-goat IgG. Sections were mounted in a reagent containing DAPI to stain nuclei (blue). OCTN1 (green) is primarily localized to the apical surface of tracheal epithelia (B), whereas less staining is seen in alveolar epithelia (D). Inflammatory cells in the trachea and the alveoli reveal OCTN1 immunoreactivity. OCTN2 (green) is also localized to the apical surface of airway epithelial cells (F). OCTN2 immunoreactivity is largely associated with the surface of the alveolar epithelia in the lung parenchyma (G). Figure shows bright field (A, C, E, G, I, K) and fluorescence images (B, D, F, H, J, L).
OCTN1/2 Immunolocalization in ALI Cultures of Airway Epithelial Cells
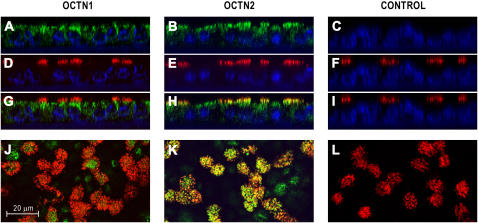
In addition to human airway sections, the cellular localization of OCTN1/2 was further examined in airway epithelial cells cultured at the ALI (Figure 3). Similar to the apically polarized expression in the airway epithelium in tissue sections, confocal imaging showed that OCTN1 and OCTN2 expression was largely associated with the apical/subapical portion of the cells (Figures 3A and 3B). Using antibodies against acetylated tubulin for co-localization studies revealed that OCTN2 is expressed in cilia (Figures 3H and 3K), whereas OCTN1 did not co-localize with cilia (Figures 3G and 3J).
Figure 3.
Immunolocalization of OCTN1 and OCTN2 proteins in human airway epithelial cells grown and re-differentiated in ALI cultures. To identify cellular localization of the pH-dependent organic cation transporters, epithelial cultures were double-labeled with antibodies to OCTN1, OCTN2, or nonimmune IgG (negative control) (green) and acetylated tubulin (red) for analysis with confocal laser scanning microscopy. Samples were mounted in a reagent containing DAPI to stain nuclei (blue). Images are z-axis reconstructions of OCTN1 (A), OCTN2 (B), and acetylated tubulin expression (D–F); overlay z-axis reconstructions (G–I); and overlay confocal images at the level of the cilia (J–L). Images demonstrate OCTN1 and OCTN2 expression in the apical/subapical portion of the cells. Overlay images reveal co-localization of OCTN2 and acetylated tubulin (H and K) indicating OCTN2 protein expression in cilia of airway epithelial cells.
ASP+ Uptake Characteristics in Human Airway Epithelial Cells
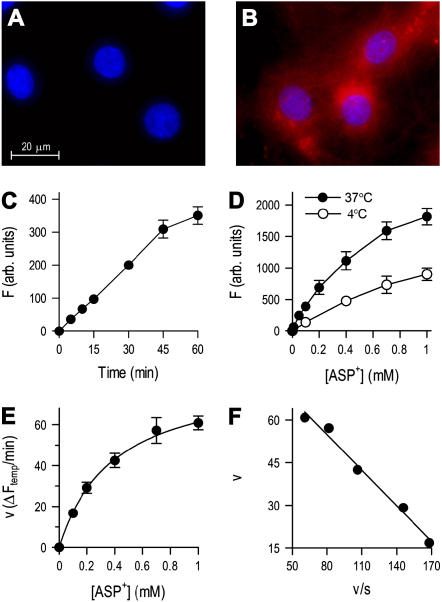
Single-cell transport studies were performed by measuring the differences in ASP+-specific fluorescence between cells exposed to incubation medium with or without ASP+ (Figures 4A and 4B). We first examined the time course of ASP+-specific fluorescence after exposing the cells to 10 μM ASP+ at 37°C. Cumulative fluorescence in epithelial cells increased in a time-dependent manner, and was linear with time up to at least 30 min (Figure 4C). To characterize the saturable uptake system in airway epithelial cells, temperature dependence of ASP+ uptake was examined (23) since membrane transport of cations/carnitine is reduced at low temperatures (e.g., activity of OCTN2 transport is reduced to 3% at 4°C compared with the uptake at 37°C) (10). In contrast, nonspecific binding of ASP+ to the cell surface (causing an overestimation of transport when measured by fluorescence) is nearly unchanged at reduced temperature (24). Figure 4D shows the temperature dependence of fluorescence after exposing the cells from 5 μM to 1 mM ASP+ for 15 min at 37°C or 4°C. ASP+ uptake, which was calculated as the difference in fluorescence between measurements at 37°C and 4°C, is shown in Figure 4E. The Eadie-Hofstee plot, a linear transformation of the classical Michaelis-Menten saturation kinetics equation, gave a single straight line (Figure 4F), suggesting a single functional transporter site with an estimated Km of 394.4 ± 15.4 μM. These data indicate that ASP+ uptake in human airway epithelial cells is accomplished by a saturable carrier mechanism.
Figure 4.
Uptake measurements with the cationic fluorophore ASP+ indicate a carrier-mediated mechanism for cationic drug uptake in human airway epithelial cells. Unexposed primary airway epithelial cells (A) and cells exposed to 10 μM ASP+ for 15 min at 37°C (B). Images show ASP+ (red) and DAPI nuclear stain fluorescence (blue). (C) Time course of fluorescence (F) after exposing the cells to 10 μM ASP+ at 37°C. (D) Effect of temperature on fluorescence after exposing the cells to ASP+ for 15 min (E) Temperature-sensitive (saturable) uptake of ASP+ was calculated as the difference between the fluorescence after exposure to ASP+ at 37°C and 4°C. (F) Eadie-Hofstee plot: the single straight line indicates that a single organic cation uptake system exists in airway epithelial cells. Results represent mean ± SEM (n = 3 or 4 experiments).
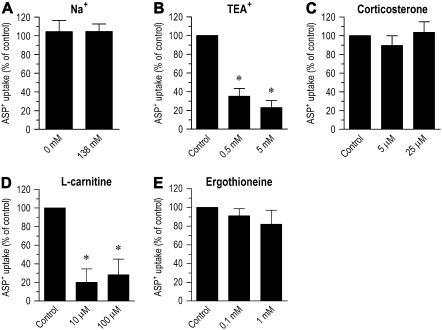
Pharmacologic Characterization of ASP+ Uptake
To investigate whether ASP+ uptake was a Na+-dependent process, we measured the effect of replacing Na+ in the incubation medium with N-methyl-D-glucamine. Figure 5A shows that the entry of ASP+ into airway epithelial cells is not dependent on sodium, since the uptake was nearly identical in the presence or absence of Na+ (P > 0.05). ASP+ uptake was also measured in the presence of various compounds known to inhibit organic cation/carnitine uptake in other systems. In the presence of the organic cation TEA+ (0.5 or 5 mM), ASP+ uptake was reduced to 35.2 ± 8.1% or 23.1 ± 7.5%, respectively (P < 0.05) (Figure 5B). Corticosterone was tested because of its ability to rapidly inhibit the organic cation transporter OCT1, OCT2, and OCT3 with IC50 values of 21.7 μM (25), 34 μM (13), and 0.2 μM (11), respectively. No significant inhibition of ASP+ uptake was obtained by 5 or 25 μM corticosterone (P > 0.05) (Figure 5C), indicating no significant functional role for the membrane potential dependent (steroid-sensitive) organic cation transporters in ASP+ uptake. L-Carnitine was also tested at two concentrations that preferentially inhibit OCTN2 (i.e., Km = 4.34 μM) (10) compared with OCTN1 (i.e., Km > 1 mM) (26). In the presence of 10 or 100 μM L-carnitine, ASP+ uptake was reduced to 20.1 ± 14.5% or 28 ± 16.9% of control, respectively (P < 0.05) (Figure 5D). Finally, we tested ergothioneine, a natural antioxidant amino acid exclusively transported by OCTN1 (Km = 21 μM) but not by OCTN2 (27). No significant inhibition of ASP+ uptake was seen by 0.1 or 1 mM ergothioneine (P > 0.05) (Figure 5E). These data suggest that organic cation transport is largely mediated by OCTN2 in human airway epithelia.
Figure 5.
Pharmacologic evidence that the ASP+ uptake mechanism carries both organic cations and L-carnitine into human airway epithelial cells. (A) Effect of extracellular Na+ on ASP+ uptake. For the Na+-free experiment, NaCl was replaced by an equimolar concentration N-methyl-D-glucamine. (B–D) Effect of organic cation transporter inhibitors, including tetraethylammonium (TEA+), corticosterone, L-carnitine, and ergothioneine on ASP+ uptake. Cells were incubated with 10 μM ASP+ for 15 min at 37°C in all experiments in the presence or absence of the inhibitors. Control was calculated as the temperature-sensitive (saturable) uptake of ASP+. Results represent mean ± SEM (n = 3 or 4 experiments). *P < 0.05 versus control.
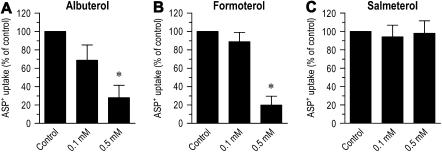
ASP+ Uptake Inhibition by Cationic Bronchodilators
To assess whether cationic β2-adrenergic agonist bronchodilators are substrates for cation uptake in airway epithelial cells, we measured the ability of albuterol and formoterol (AstraZeneca, Lund, Sweden) to inhibit the ASP+ uptake. After a 15-min incubation with 10 μM of ASP+ with 0.1 or 0.5 mM albuterol at 37°C, ASP+ uptake was reduced to 68.6 ± 16.7% (P > 0.05) or 28.1 ± 13.4% (P < 0.05) of control, respectively (Figure 6A). Likewise, addition of 0.1 or 0.5 mM formoterol to the incubation solution reduced ASP+ uptake to 88.8 ± 10.1% (P > 0.05) or 19.8 ± 9.6% (P < 0.05) of control, respectively (Figure 6B). In addition to cationic bronchodilators, we also investigated the effect of the lipophilic β2-adrenergic agonist bronchodilator salmeterol. No significant inhibition of ASP+ uptake was seen by 0.1 or 0.5 mM salmeterol (P > 0.05) (Figure 6C). These findings support the notion that cationic bronchodilators inhibit ASP+ uptake through competition for the same transport mechanism rather than β2-adrenergic receptor activation.
Figure 6.
Effects of albuterol, formoterol, and salmeterol on ASP+ uptake into human airway epithelial cells. ASP+ uptake was measured by exposing the cells to 10 μM ASP+ for 15 min in the presence or absence of 0.1 mM and 0.5 mM albuterol (A), formoterol (B), or salmeterol (C) at 37°C. Control was calculated as the temperature-sensitive (saturable) uptake of ASP+. Results represent mean ± SEM (n = 3 or 4 experiments). *P < 0.05 versus control.
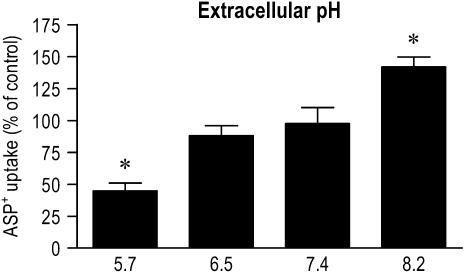
Extracellular pH Dependence of ASP+ Uptake
Extracellular pH dependence of ASP+ uptake is shown in Figure 7. When the bathing medium was adjusted to acidic pH (5.7), the saturable uptake of ASP+ by airway epithelial cells decreased to 44.9 ± 4.1% of that at neutral pH (P < 0.05). The uptake at alkaline pH (8.2) was increased to 141.9 ± 7.6% of that at neutral pH (P < 0.05).
Figure 7.
Effect of extracellular pH on organic cation uptake by human airway epithelial cells. ASP+ uptake was measured by exposing the cells to 10 μM ASP+ for 15 min at 37°C in an incubation medium adjusted to different pH levels. Control was calculated as the temperature-sensitive (saturable) uptake of ASP+. Results represent mean ± SEM (n = 3 or 4 experiments). *P < 0.05 versus control.
DISCUSSION
In the present study, we provide evidence for an active absorption mechanism for cationic drugs in the human airway epithelia, accomplished by apically polarized expression of the pH-dependent organic cation transporters OCTN1 and OCTN2. Our in vitro functional studies on primary airway epithelial cells isolated from donor lungs indicated that cationic drug absorption is mainly associated with OCTN2. Our findings also suggested that the OCTNs carry commonly used cationic bronchodilators, such as formoterol and albuterol. Finally, our studies suggest that the pH-dependent uptake demonstrated in vitro could limit the absorption of inhaled cationic bronchodilators in vivo in medical conditions associated with airway or airway surface liquid pH acidification (28).
Lipid solubility, molecule size, and pKa-dependent ionization are major factors in drug absorption (1). However, it is becoming increasingly clear that physicochemical properties cannot be considered as the only major factors of the extent of drug absorption. Many cells possess specialized membrane transport mechanisms for the entry of physiologically important molecules, such as sugars, amino acids, and neurotransmitters. The SLC22 transporter family has been identified to handle cellular influx of organic cations, a chemically diverse group of compounds. These include a large array of molecules of physiologic (e.g., acetylcholine, noradrenaline) and pharmacologic (e.g., chemotherapeutic agents, antihistaminics, antacids, antiarrhythmics) importance (29). The majority of β2-adrenergic agonist (e.g., albuterol and formoterol) and anticholinergic bronchodilators are also organic cations because of their permanent or transient positive charge at physiologic pH. Since carrier mechanisms facilitate the movement through cell membranes, the identification of pulmonary uptake transporters and their carried substrates is of clinical importance.
In our studies, airway epithelial cells were isolated from the proximal airway region (from the trachea to the segmental bronchi) of donor lungs to investigate organic cation transport mechanisms. Because the airway smooth muscle is located in the conducting zone of the lung (30), inhaled bronchodilators should be deposited here to achieve effective bronchodilation. This notion has been supported by the finding that targeting of an inhaled β2-adrenergic agonist to the proximal airway region is more important for bronchodilation than alveolar targeting (31, 32). To reach their receptors on airway smooth muscle, inhaled drugs must pass through the airway epithelium, a major physicochemical barrier. The delivery of inhaled cationic drugs to reach their receptors on airway smooth muscle depends on the concerted action of epithelial drug absorption and release mechanisms. Evidence supports the existence of multiple drug efflux systems with overlapping substrate specificity in the airway (33). Several members of the ATP-binding cassette (ABC) efflux transporter family, such as the multidrug resistance–associated protein 1 (34) and breast cancer resistance protein (35), are known to be expressed at the basolateral side of airway epithelia. Although their functions have largely been studied in the context of decreased intracellular concentrations of cytotoxic anti-cancer drugs (36), the basolateral transporters are likely involved in the transepithelial movements of aerosolized drugs previously absorbed into the airway epithelium. In contrast to efflux transporters, information is lacking on epithelial drug absorption mechanisms. Therefore, we measured cationic drug uptake by primary airway epithelial cells to identify the role of absorptive carrier mechanisms. Our single-cell microscopic measurements were performed with the permanently positively charged and nontoxic fluorophore ASP+. In prior studies, ASP+ has been successfully used to investigate organic cation uptake in vivo (37) and in various culture systems (38).
Airway epithelial transport through the peptide transporter PEPT2 has been proposed to have a major role in the absorption of inhaled peptides and peptidomimetic drugs (39). In the present study, we demonstrated that the uptake of organic cations is also carrier-mediated in airway epithelial cells by showing the transport's temperature sensitivity, saturable kinetics, and inhibition with the classical organic cation TEA+. The carnitine-sensitive characteristics of ASP+ uptake suggested that the organic cation transport is largely mediated by OCTN2, an organic cation/carnitine transporter whose major roles have recently been demonstrated in human heart (40), placenta (41), and skeletal muscle (42). Together with our studies that demonstrate OCTN2 co-localization with acetylated tubulin in cilia of epithelial cells, these studies suggest that transporter availability at the cell surface might be a major factor in determining the transporter's role in the cellular uptake of organic cations. Furthermore, because formoterol and albuterol inhibited ASP+ uptake, these drugs also are likely substrates for organic cation carrier mechanisms in airway epithelial cells. Our findings are supported by a recent study describing polarized (five to seven times greater apical-to-basolateral than basolateral-to-apical) transepithelial transport of the cationic albuterol in monolayer cultures of Calu-3 and 16HBE14o-airway epithelial cell lines (14). However, since others found no evidence of active organic cation transport in Calu-3 layers (15), inter-laboratory differences in culture methods seem to significantly influence organic cation permeability and/or transporter expression in cell culture models of the airway epithelial barrier.
Corticosteroids have the unique ability to inhibit the membrane potential sensitive organic cation transporters through an acute, nontranscriptional mechanism. We demonstrated this action of OCT3 in smooth muscle cells of airway blood vessels (11, 19), and proposed that this mechanism is responsible for the rapid airway vasoconstrictor action of inhaled corticosteroids seen in vivo in healthy subjects and in subjects with asthma (43). Since all steroid-sensitive transporters have been demonstrated to be expressed in the airway epithelium recently (11, 13, 14), this finding raises the concern that inhaled corticosteroids might inhibit the absorption of inhaled β2-adrenergic agonists when the two drugs are administered in combination. Our observations suggest that this may not be the case because we found low mRNA expression levels of, and no significant functional role for, steroid-sensitive transporters in cationic drug uptake by the airway epithelium.
In contrast to other tissues, such as airway vascular smooth muscle with high expression levels for the corticosteroid-sensitive OCT3 (11), airway epithelia predominantly express mRNA for the pH-dependent organic cation/carnitine transporter OCTN1 and OCTN2. From the viewpoint of the potential effect of airway pH on cationic drug absorption, we evaluated the effect of extracellular pH on ASP+ uptake by airway epithelial cells in vitro. Since an alkaline pH would increase the amount of uncharged weakly basic drug available for passive diffusion (44), we used the permanently positively charged ASP+ to assess pH dependence of active uptake mechanisms. Our studies demonstrated that uptake decreased by ∼ 3-fold when the pH was changed from 8.2 to 5.7 in the incubation medium. Together with our immunolocalization studies, showing apically polarized expression of OCTN1 and OCTN2 in the human airway epithelium, it is expected that pH of the airway lining fluid has a significant influence on the absorption of inhaled cationic drugs.
Airway pH homeostasis is poorly understood because of the difficulties of direct pH measurements in the airway lining fluid. Most data on human airways were obtained indirectly, by measuring pH of exhaled breath condensates. Exhaled breath condensate assays revealed acidification in a variety of airway diseases, including asthma (45). It has been proposed that acidification reflects pathology of airways (28); however, there are concerns that salivary acids and bases can influence measurements (46). Our in vitro studies suggest that alkaline pH in the airway lining fluid would favor the airway absorption of inhaled cationic drugs. In contrast, drug absorption may be significantly impaired in medical conditions associated with airway or airway surface liquid acidification. Nevertheless, to what extent airway pH might influence pharmacologic responses to inhaled cationic drugs remains to be evaluated.
This work was supported in part by an academic research grant from AstraZeneca and NHLBI grants HL-60644 and HL-66125. G.H. is a recipient of the Bolyai Fellowship of the Hungarian Academy of Sciences.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0230OC on August 17, 2006
Conflict of Interest Statement: G.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.A.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.E.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.W. received $89,000 in 2006 as a research grant from AstraZeneca.
References
- 1.Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc 2004;1:338–344. [DOI] [PubMed] [Google Scholar]
- 2.Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol 2006;117:18–24. [DOI] [PubMed] [Google Scholar]
- 3.Gross NJ. Anticholinergic agents in asthma and COPD. Eur J Pharmacol 2006;533:36–39. [DOI] [PubMed] [Google Scholar]
- 4.Kerb R. Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett 2006;234:4–33. [DOI] [PubMed] [Google Scholar]
- 5.Koepsell H. Polyspecific organic cation transporters: their functions and interactions with drugs. Trends Pharmacol Sci 2004;25:375–381. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol 1997;51:913–921. [DOI] [PubMed] [Google Scholar]
- 7.Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol 1997;16:871–881. [DOI] [PubMed] [Google Scholar]
- 8.Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci 1998;1:349–351. [DOI] [PubMed] [Google Scholar]
- 9.Tamai I, Yabuuchi H, Nezu J, Sai Y, Oku A, Shimane M, Tsuji A. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett 1997;419:107–111. [DOI] [PubMed] [Google Scholar]
- 10.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem 1998;273:20378–20382. [DOI] [PubMed] [Google Scholar]
- 11.Horvath G, Sutto Z, Torbati A, Conner GE, Salathe M, Wanner A. Norepinephrine transport by the extraneuronal monoamine transporter in human bronchial arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2003;285:L829–L837. [DOI] [PubMed] [Google Scholar]
- 12.Horvath G, Wanner A. Inhaled corticosteroids: effects on the airway vasculature in bronchial asthma. Eur Respir J 2006;27:172–187. [DOI] [PubMed] [Google Scholar]
- 13.Lips KS, Volk C, Schmitt BM, Pfeil U, Arndt P, Miska D, Ermert L, Kummer W, Koepsell H. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol 2005;33:79–88. [DOI] [PubMed] [Google Scholar]
- 14.Ehrhardt C, Kneuer C, Bies C, Lehr CM, Kim KJ, Bakowsky U. Salbutamol is actively absorbed across human bronchial epithelial cell layers. Pulm Pharmacol Ther 2005;18:165–170. [DOI] [PubMed] [Google Scholar]
- 15.Mathia NR, Timoszyk J, Stetsko PI, Megill JR, Smith RL, Wall DA. Permeability characteristics of calu-3 human bronchial epithelial cells: in vitro-in vivo correlation to predict lung absorption in rats. J Drug Target 2002;10:31–40. [DOI] [PubMed] [Google Scholar]
- 16.Frost AE. Donor criteria and evaluation. Clin Chest Med 1997;18:231–237. [DOI] [PubMed] [Google Scholar]
- 17.Nlend MC, Bookman RJ, Conner GE, Salathe M. Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. Am J Respir Cell Mol Biol 2002;27:436–445. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 19.Horvath G, Lieb T, Conner GE, Salathe M, Wanner A. Steroid sensitivity of norepinephrine uptake by human bronchial arterial and rabbit aortic smooth muscle cells. Am J Respir Cell Mol Biol 2001;25:500–506. [DOI] [PubMed] [Google Scholar]
- 20.Horvath G, Torbati A, Conner GE, Salathe M, Wanner A. Systemic ovalbumin sensitization downregulates norepinephrine uptake by rabbit aortic smooth muscle cells. Am J Respir Cell Mol Biol 2002;27: 746–751. [DOI] [PubMed] [Google Scholar]
- 21.Hurbain I, Sermet-Gaudelus I, Vallee B, Feuillet MN, Lenoir G, Bernaudin JF, Edelman A, Fajac A. Evaluation of MRP1–5 gene expression in cystic fibrosis patients homozygous for the delta F508 mutation. Pediatr Res 2003;54:627–634. [DOI] [PubMed] [Google Scholar]
- 22.Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T, Suzuki M, Nagasaki M, Ohtsuki M, Ono M, et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet 2003;35:341–348. [DOI] [PubMed] [Google Scholar]
- 23.Biermann J, Lang D, Gorboulev V, Koepsell H, Sindic A, Schroter R, Zvirbliene A, Pavenstadt H, Schlatter E, Ciarimboli G. Characterization of regulatory mechanisms and states of human organic cation transporter 2. Am J Physiol Cell Physiol 2006;290:C1521–C1531. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz JW, Blakely RD, DeFelice LJ. Binding and transport in norepinephrine transporters. Real-time, spatially resolved analysis in single cells using a fluorescent substrate. J Biol Chem 2003;278:9768–9777. [DOI] [PubMed] [Google Scholar]
- 25.Hayer-Zillgen M, Bruss M, Bonisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol 2002;136:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, Ganapathy V. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim Biophys Acta 2000;1466:315–327. [DOI] [PubMed] [Google Scholar]
- 27.Grundemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, Jung N, Rubbert A, Schomig E. Discovery of the ergothioneine transporter. Proc Natl Acad Sci USA 2005;102:5256–5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricciardolo FLM, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol 2004;113:610–619. [DOI] [PubMed] [Google Scholar]
- 29.Koepsell H, Schmitt BM, Gorboulev V. Organic cation transporters. Rev Physiol Biochem Pharmacol 2003;150:36–90. [DOI] [PubMed] [Google Scholar]
- 30.Ebina M, Yaegashi H, Chiba R, Takahashi T, Motomiya M, Tanemura M. Hyperreactive site in the airway tree of asthmatic patients revealed by thickening of bronchial muscles: a morphometric study. Am Rev Respir Dis 1990;141:1327–1332. [DOI] [PubMed] [Google Scholar]
- 31.Usmani OS, Biddiscombe MF, Nightingale JA, Underwood SR, Barnes PJ. Effects of bronchodilator particle size in asthmatic patients using monodisperse aerosols. J Appl Physiol 2003;95:2106–2112. [DOI] [PubMed] [Google Scholar]
- 32.Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med 2005;172:1497–1504. [DOI] [PubMed] [Google Scholar]
- 33.van der Deen M, de Vries EG, Timens W, Scheper RJ, Timmer-Bosscha H, Postma DS. ATP-binding cassette (ABC) transporters in normal and pathological lung. Respir Res 2005;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brechot J-M, Hurbain I, Fajac A, Daty N, Bernaudin J-F. Different pattern of MRP localization in ciliated and basal cells from human bronchial epithelium. J Histochem Cytochem 1998;46:513–518. [DOI] [PubMed] [Google Scholar]
- 35.Scheffer GL, Pijnenborg ACLM, Smit EF, Muller M, Postma DS, Timens W, van der Valk P, de Vries EGE, Scheper RJ. Multidrug resistance related molecules in human and murine lung. J Clin Pathol 2002;55: 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006;5:219–234. [DOI] [PubMed] [Google Scholar]
- 37.Pietruck F, Ullrich KJ. Transport interactions of different organic cations during their excretion by the intact rat kidney. Kidney Int 1995;47: 1647–1657. [DOI] [PubMed] [Google Scholar]
- 38.Mason JN, Farmer H, Tomlinson ID, Schwartz JW, Savchenko V, DeFelice LJ, Rosenthal SJ, Blakely RD. Novel fluorescence-based approaches for the study of biogenic amine transporter localization, activity, and regulation. J Neurosci Methods 2005;143:3–25. [DOI] [PubMed] [Google Scholar]
- 39.Groneberg DA, Nickolaus M, Springer J, Doring F, Daniel H, Fischer A. Localization of the peptide transporter PEPT2 in the lung: implications for pulmonary oligopeptide uptake. Am J Pathol 2001;158:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grube M, Schwabedissen H, Prager D, Haney J, Moritz KU, Meissner K, Rosskopf D, Eckel L, Bohm M, Jedlitschky G, et al. Uptake of cardiovascular drugs into the human heart - Expression, regulation, and function of the carnitine transporter OCTN2 (SLC22A5). Circulation 2006;113:1114–1122. [DOI] [PubMed] [Google Scholar]
- 41.Grube M, Schwabedissen HMZ, Draber K, Prager D, Moritz KU, Linnemann K, Fusch C, Jedlitschky G, Kroemer HK. Expression, localization, and function of the carnitine transporter OCTN2 (SLC22A5) in human placenta. Drug Metab Dispos 2005;33:31–37. [DOI] [PubMed] [Google Scholar]
- 42.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. Insulin stimulates L-carnitine accumulation in human skeletal muscle. FASEB J 2006;20:377–379. [DOI] [PubMed] [Google Scholar]
- 43.Kumar SD, Brieva JL, Danta I, Wanner A. Transient effect of inhaled fluticasone on airway mucosal blood flow in subjects with and without asthma. Am J Respir Crit Care Med 2000;161:918–921. [DOI] [PubMed] [Google Scholar]
- 44.Youdim KA, Avdeef A, Abbott NJ. In vitro trans-monolayer permeability calculations: often forgotten assumptions. Drug Discov Today 2003; 8:997–1003. [DOI] [PubMed] [Google Scholar]
- 45.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 2000;161:694–699. [DOI] [PubMed] [Google Scholar]
- 46.Effros RM, Casaburi R, Su J, Dunning M, Torday J, Biller J, Shaker R. The effects of volatile salivary acids and bases on exhaled breath condensate pH. Am J Respir Crit Care Med 2006;173:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]