Abstract
M-CSF induces PI 3-kinase activation, resulting in reactive oxygen species (ROS) production. Previously, we reported that ROS mediate macrophage colony-stimulating factor (M-CSF)–induced extracellular regulated kinase (Erk) activation and monocyte survival. In this work, we hypothesized that M-CSF–stimulated ROS products modulated Akt1 and p38 activation. Furthermore, we sought to clarify the source of these ROS and the role of ROS and Akt in monocyte/macrophage survival. Macrophages from p47phox−/− mice, lacking a key component of the NADPH oxidase complex required for ROS generation, had reduced cell survival and Akt1 and p38 mitogen-activated protein kinase (MAPK) phosphorylation compared with wild-type macrophages in response to M-CSF stimulation, but had no difference in M-CSF–stimulated Erk. To understand how ROS affected monocyte survival and signaling, we observed that NAC and DPI decreased cell survival and Akt1 and p38 MAPK phosphorylation. Using bone marrow–derived macrophages from mice expressing constitutively activated Akt1 (Myr-Akt1) or transfecting Myr-Akt1 constructs into human peripheral monocytes, we concluded that Akt is a positive regulator of monocyte survival. Moreover, the p38 MAPK inhibitor, SB203580, inhibited p38 activity and M-CSF–induced monocyte survival. These findings demonstrate that ROS generated from the NADPH oxidase complex contribute to monocyte/macrophage survival induced by M-CSF via regulation of Akt and p38 MAPK.
Keywords: Akt, macrophage/monocyte, p47phox, p38 MAP, ROS
CLINICAL RELEVANCE
This study provides insight into mechanisms underlying ROS-regulated cellular survival in normal monocytes and macrophages. It also provides supportive evidence for ROS-related cancer and inflammatory diseases therapy.
Monocytes are produced in the bone marrow, and in the absence of serum or survival factors they die via apoptosis in 24–48 h. To evade apoptosis, monocytes depend on activation by growth factors, such as macrophage colony-stimulating factor (M-CSF). We reported that the PI 3-kinase inhibitor LY294002 suppresses the survival of human monocytes and reduces Akt1 and extracellular regulated kinase (Erk) activation in response to M-CSF (1, 2). M-CSF-induced Erk activation is driven by the production of endogenous reactive oxygen species (ROS) and appears to be an important mediator of monocyte survival to M-CSF stimulation (2). However, the influence of M-CSF–induced ROS on Akt1 activity or other survival pathways in mononuclear phagocytes remains unknown.
Some proinflammatory cytokines, such as TNF-α, IL-1β, TGF-β, M-CSF, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and angiotensin II, induce cellular ROS production (3). While there are several oxidant-generating complexes in phagocytes, PI 3-kinase–initiated products can activate the NADPH oxidase system, a multi-component complex assembled at the membrane. Recruitment and the assembly of the cytosolic components, Rac2, p47phox, and p67phox to the membrane-bound gp91phox and p22phox, are PI 3-kinase dependent (4–6). Patients with autosomal recessive chronic granulomatous disease (CGD) can harbor mutations in which no membrane translocation of Rac2, p47phox, and p67phox occurs (7). The role of the NADPH oxidase system in Akt/protein kinase B activation is supported by studies in Rac2-deficient murine mast cells, which have reduced NADPH oxidase activity and ROS production as well as reduced Akt activation compared with wild-type cells (8). Rac2 is found in hematopoietic cells and is critical in the NADPH oxidase function (9). Data in this study demonstrate that ROS produced by the NADPH oxidase regulates mast cell survival through Akt activation.
There are three isoforms of Akt: Akt1, Akt2, and Akt3. While it is not clear if these isoforms have redundant or independent activity, Akt1 activity has been linked to survival events in both transformed and normal cells. To promote cellular survival, Akt is activated. Akt recognizes PI (3,4,5) P3 and PI (3,4) P2 via pleckstrin homology domains (see review in Ref. 10). Once membrane-localized, Akt is activated by phosphorylation on threonine-308 by the enzyme PDK1, promoting autophosphorylation of Akt on serine residue 473. Alternatively, some reports suggest that the serine 473 phosphorylation of Akt is mediated by PDK2/MapKK, PKC-β2, or integrin-linked kinase (ILK). For maximal activation, tyrosine phosphorylation of Akt by Src family kinases also appears important (see review in Ref. 11).
We reported that ROS mediate M-CSF–induced Erk activation and monocyte survival; however, the source of oxidant generation remained to be defined. Erk is a member of the mitogen-activated protein kinases (MAPKs). MAPKs consist of at least six major subfamily members, of which Erk, c-jun NH2-terminal kinase (JNK), and p38 MAPK are characterized. MAPKs regulate cell proliferation, differentiation, motility, and survival in response to a wide variety of stimuli, including growth factors and oxidative stress. The exact function of MAPKs on cellular survival and apoptosis are complex (6). p38 MAPK can promote either cellular survival or apoptosis (see review in Ref. 12). For example, IL-24–induced apoptosis and expression of growth arrest– and DNA damage (GADD)–inducible genes in melanoma cells are dependent on p38 MAPK. Similarly, cardiomyocytes and fibroblasts derived from p38 MAPK-α knockout mice are more resistant to apoptosis. In contrast, p38 MAPK activation protects neuronal PC12 cells from TNF-α–induced apoptosis and enhances osteoblastic SaOS-2 cell growth and chondrocytes differentiation. Other investigators reported that p38 MAPK play no role in cell survival, as reported in thymocytes derived from mice lacking either MMK3 or MMK6, which are upstream of p38 MAPK activation. Thus, it appears that cell type and stimulus have a powerful influence on the role of p38 MAPK on cell life or cell death.
Increased phosphorylation of p38 MAPK is linked to ROS generation in neuronal AF5 cells with stimulation of neurotransmitter N-methyl-D-aspartate (NMDA) (13). Phorbol myristate acetate (PMA)-treated mast cell (HMC-1) was shown to stimulate IL-8 and TNF-α production in a p38 MAPK/NF-κB–dependent manner (14). Since a majority of the data examining the regulation of p38 MAPK activity by ROS production and p38 MAPK–mediated cell survival involve cultured cell lines, we evaluated whether ROS-mediated p38 MAPK activation contributed to the survival of primary human monocytes.
In this work, we evaluated the influence of M-CSF–stimulated ROS generation on Akt activity, p38 MAPK phosphorylation, and cell survival in primary human monocytes and murine macrophages. We found that ROS produced by M-CSF stimulation induced cellular survival by activating Akt and p38 MAPK in normal human monocytes and macrophages.
MATERIALS AND METHODS
Materials
Endotoxin-free RPMI 1640 and PBS (< 10 pg/ml) were purchased from BioWhittaker (Walkersville, MD). FBS was obtained from Hyclone Laboratories (Logan, UT). Recombinant M-CSF was purchased from R&D Systems (Minneapolis, MN). DPI, LY294002, SB203580, and SB202474 were obtained from Calbiochem (San Diego, CA). Antibodies for Western blot analysis were obtained from Santa Cruz Biotech (Santa Cruz, CA) or Cell Signaling (Beverly, MA). All other reagents were purchased from Sigma (St. Louis, MO) unless indicated otherwise.
Purification of Peripheral Blood Monocytes
Monocytes (66 ± 2.1% CD14+) were isolated as previously described from buffy coats obtained from the American Red Cross or healthy volunteers following informed consent. Briefly, whole blood was diluted 1:1 with 1× PBS and layered over histopaque-1077. The mononuclear layer was clumped in RPMI 1640 medium supplemented with 10% FBS at 4°C for 1 h. Cells were layered over FBS for 20 min and monocytes were then collected.
Monocytes were also isolated using the Monocyte Isolation Kit from Miltenyi Biotech (Auburn, CA) according to the manufacturer's protocol. Antibody cocktail and magnetic beads coupled to an anti-hapten monoclonal antibody were added to the peripheral blood mononuclear cells (PBMC). PBMC were placed through a magnetic column to separate monocytes from other cell types (86 ± 3.3% CD14+).
After isolation, monocytes were resuspended at 1–10 × 106 cells/ml in RPMI 1640 medium supplemented with 10% FBS and 10 μg/ml polymyxin B for 1 h. Then the nonadherent cells were removed and cells were washed with warm RPMI 1640 medium. The monocytes were incubated in RPMI 1640 medium with 10 μg/ml polymyxin B for another hour before being subjected to inhibitors or M-CSF stimulation.
Isolation and Culturing of Bone Marrow–Derived Macrophages
Femoral and tibia bone marrow were isolated from p47phox-/-, myristoylated (Myr)-Akt1 or wild-type littermate mice. p47phox-/- mice were kindly provided by Dr. Steve Holland (NIH, Bethesda, MD). Constitutively active Myr-Akt1 transgenic mice were described previously and provided by Dr. Michael Ostrowski (The Ohio State University, Columbus, OH) (15).
The bone marrow progenitor cells were plated in RPMI supplemented with 10% FBS, 1% PSA (penicillin G sodium, streptomycin sulfate, and amphotericin B), 10 μg/ml of polymyxin B, and 20 ng/ml of M-CSF. Cells were cultured in 37°C incubators with 5% CO2 for 5 d with the addition of M-CSF each day. Differentiated bone marrow–derived macrophages (BMM) were serum-starved for 1 d at 37°C before being re-stimulated with M-CSF.
Electron Paramagnetic Resonance Spectroscopy
Human monocytes (1 × 106/condition) were isolated and incubated with specific inhibitors or the dimethylsulfoxide (DMSO) control, then were left unstimulated or stimulated with M-CSF (100 ng/ml) for an additional 20 min in the presence of the spin trap DMPO (5,5-dimethyl-1-pyrroline-N-oxide). The EPR spectra were collected at the indicated time points on a Varian E-9 EPR spectrometer (Palo Alto, CA). Typical instrument settings were microwave power: 20 mW, and modulation amplitude: 0.8 G, as previously described (2).
Annexin V and Propidium Iodide Staining
Cell apoptosis was measured using an Annexin V–FITC apoptosis detection kit according to manufacturer's protocol (BD PharMingen, San Diego, CA). Briefly, human monocytes (1 × 106) or murine BMM (5 × 105) were removed from culture dish by Accutase (eBioscience, San Diego, CA) and stained with Annexin V–FITC and propidium iodide (PI) according to instructions. Samples were analyzed by flow cytometry (FACSCalibur; BD PharMingen). Early stage of apoptosis was defined as Annexin V–positive PI-negative staining, and late stage of apoptosis and necrosis were defined as Annexin V and PI double-positive staining. In this study, we combined Annexin V–positive staining and Annexin V/PI double-positive staining in our statistics.
Immunoprecipitation and Immunoblotting
Monocytes (10 × 106 cell/condition) were isolated, stimulated, and lysed on ice for 15 min in 1× lysis buffer (Cell Signaling) containing protease inhibitor cocktail III (Calbiochem). Lysates were cleared of insoluble material then protein concentration determined (BioRad, Hercules, CA). The samples were separated by SDS-PAGE, transferred to a nitrocellulose membrane, probed with the indicated antibodies, and detected by ECL (Amersham Biosciences, Piscataway, NJ).
Transfection of Primary Human Monocytes
Monocytes were transfected using the Amaxa Nucleofector system (Amaxa, Cologne, Germany) with expression vectors containing cDNA corresponding to Myr-Akt1 (kindly provided by Dr. D. Stokoe, Cancer Research Institute, University of California). The monocytes (10 × 106) isolated from Buffy coats were resuspended in Monocyte Solution containing 0.5 μg of DNA and transfected with program Y-01, then immediately washed in X-VIVO 15 serum-free media (BioWhittaker, Walkersville, MD) and plated in 12-well plates.
In Vitro p38 MAPK Assays
In vitro p38 kinase activity was measured using the assay kit from Cell Signaling Technology (Beverly, MA) by following the manufacturer's protocol. In this method, equal amount of protein was immnuoprecipitated with immobilized p38 MAPK (Thr180/Tyr182) antibody. The kinase reaction was performed in the presence of ATF2 fusion protein and cold ATP. Phosphorylation of ATF2 was measured by Western blot using phospho-ATF2Thr71 antibody. Since SB203580 is a reversible p38 MAPK inhibitor, 100 nM of SB203580 was added in the cell lysis buffer and kinase buffer.
Subcellular Fractionation
Monocytes (20 × 10 (6) cells/condition) were transfected with cDNA and plated in 12-well plates. Cells were cultured at 37°C for 16–18 h and cells were removed from the plate and then cell membrane and cytosolic fraction were separated using the method described previously (16).
Statistical Analysis
Each experiment was performed in duplicate. All statistical analysis was performed using the mean ± SEM from individual donors. Difference between means was analyzed using ANOVA with Fisher's post hoc testing. P ⩽ 0.05 was defined as statistically significant in these studies.
RESULTS
DPI Reduces M-CSF–Induced ROS Production
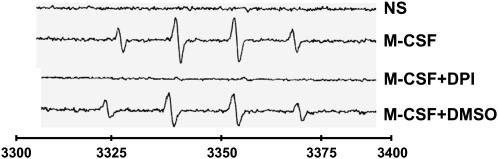
Previously we observed that production of ROS by M-CSF–stimulated monocytes is blocked by the PI 3-kinase inhibitor wortmannin (2). We also observed that ROS regulated Erk1/2 in M-CSF–stimulated human monocytes. Using electron spin resonance (ESR) with DMPO as a spin trap, which forms a relatively stable paramagnetic adduct when reacting with free radicals (17), we measured endogenous ROS produced by M-CSF–stimulated monocytes. The flavoprotein inhibitor DPI reduced ROS produced by M-CSF–stimulated monocytes to levels seen in unstimulated cells (Figure 1). In contrast, the vehicle control agent DMSO, a known antioxidant, slightly reduced the EPR signal in response to M-CSF. These data confirmed our previous observations that M-CSF promotes ROS production in human monocytes (2) and suggested that flavoprotein-containing oxidases such as NADPH oxidase were responsible.
Figure 1.
DPI inhibits endogenous ROS produced by M-CSF–stimulated monocytes. Human monocytes (1 × 106/condition) were incubated with DPI (10 μM) or DMSO control for 1 h and the cells were left unstimulated (NS) or stimulated with M-CSF (100 ng/ml) for an additional 20 min in the presence of the spin trap DMPO. EPR signal indicating ROS production was measured and recorded. Data shown are representative of three independent donors.
M-CSF–Mediated ROS Production Enhances Monocyte Survival
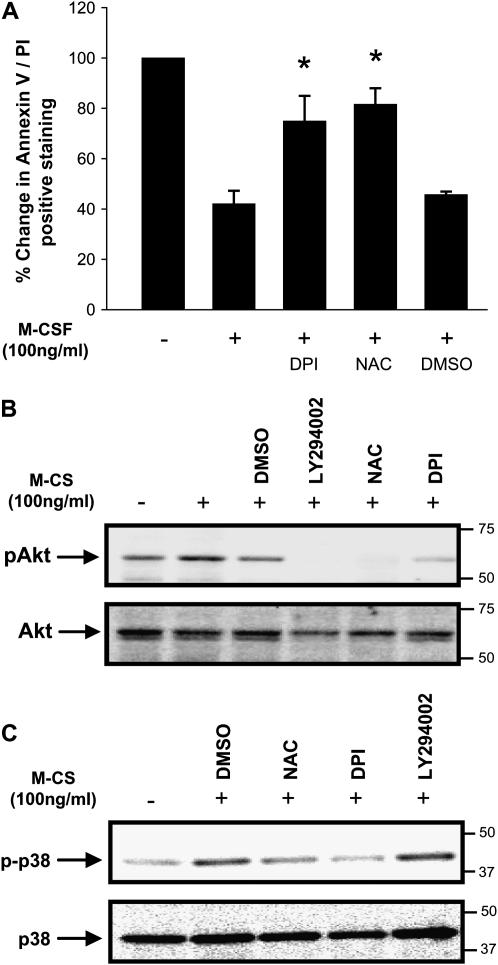
Since DPI and NAC block M-CSF–induced Erk activity and blocking MEK/Erk activity inhibits M-CSF–induced monocyte survival (2), we were interested in determining the role of ROS on monocyte survival. To determine whether inhibition of endogenous ROS reduced M-CSF–stimulated human cell survival, we stimulated human monocytes with M-CSF in the presence of DPI, NAC or the vehicle control DMSO (for DPI) and tested the cells for survival. The addition of DPI or NAC enhanced Annexin V/PI expression in M-CSF–stimulated monocytes compared to monocytes stimulated with M-CSF alone or with vehicle treatment. Importantly, M-CSF–stimulated cells treated with either DPI or NAC had Annexin V/PI staining to the level of apoptotic cells grown in the absence of M-CSF (Figure 2A).
Figure 2.
Inhibition of endogenous ROS by antioxidants reduces cell survival, phosphorylation of Akt and p38 MAPK in human monocytes. (A) Monocytes (1 × 106/condition) were isolated and incubated with DPI (10 μM), NAC (20 mM), or DMSO control for 1 h, then stimulated with (+) or without (−) M-CSF for an additional 24 h and stained with Annexin V/PI for flow cytometry. Unstimulated monocytes were used as the 100% Annexin V/PI control. Data are expressed as the mean ± SEM of six independent donors (*P < 0.01 compared with M-CSF alone control). (B) Monocytes (10 × 106/ condition) pretreated for 1 h with the indicated inhibitors were either unstimulated (−) or stimulated (+) with M-CSF (100 ng/ml) for 6 h, then lysed. An equal amount of protein was subjected to Western blot analysis using antibodies recognizing pAktThr308 and pAktSer473 or phospho-p38Thr180/Tyr182. (C) The membranes were stripped and reprobed for Akt1 or p38 MAPK. Shown in B and C is a representative blot from six independent donors.
Notably, even 35–40% of the M-CSF–stimulated cell population underwent apoptosis as defined by Annexin V/PI staining. This amount of cell death is attributed to purification procedure and mechanical removal from the culture dish and is similar to that of other reports (18). As an alternative method of measuring cell apoptosis, DNA fragmentation studies confirmed that DPI and NAC promoted oligonucleosomal DNA fragmentation in M-CSF–stimulated monocytes (data not shown). These observations demonstrate a critical role for ROS in mediating M-CSF–induced cell survival.
M-CSF–Mediated ROS Production Stimulate Akt and p38 MAPK Phosphorylation
Since monocyte survival is mediated in a PI 3-kinase–dependent manner (1), we next investigated whether Akt/protein kinase B activation by M-CSF was reduced in monocytes treated with NAC or DPI. The PI 3-kinase inhibitor LY294002 was used as an active inhibitor of Akt in M-CSF–stimulated cells. Akt activation was assessed at 7 h after stimulation because this time point corresponds to the initial activation of caspase-3 in serum-starved monocytes (19), an event preceding DNA fragmentation in monocyte apoptosis. As predicted, NAC, DPI and LY294002 all reduced the phosphorylation of Akt (pAkt) following M-CSF stimulation in monocytes (Figure 2B). This observation indicated that ROS functioned upstream of Akt.
Next, we wanted to investigate whether the inhibition of ROS production reduced the phosphorylation of p38 MAPK in M-CSF–stimulated monocytes. Phosphorylation of p38 MAPK was assessed at 7 h after stimulation with M-CSF. Similar to what we reported for Erk activation, p38 MAPK phosphorylation was attenuated by the presence of NAC and DPI in M-CSF–stimulated human monocytes (Figure 2C). Importantly, the PI 3-kinase inhibitor LY294002 did not reduce p38 MAPK phosphorylation (Figure 2C). These data demonstrated that reduced production or action of ROS suppressed the activity of Akt and p38 MAPK in M-CSF–stimulated human monocytes.
NADPH Oxidase Plays a Selective Role in M-CSF–Induced Akt and p38 Activity
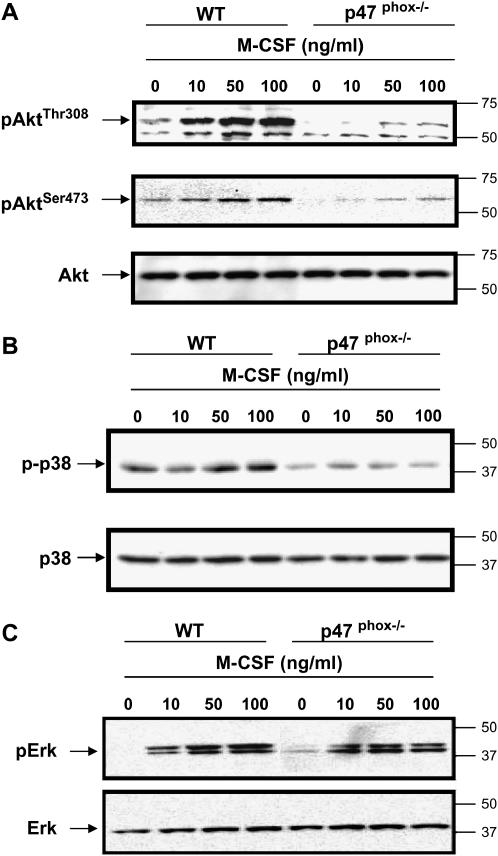
To define the source of ROS influencing Akt, p38 MAPK, and Erk activation in M-CSF–stimulated monocytes, bone marrow macrophages were derived from p47phox-/- or wild-type mice. These mice lack NADPH oxidase function and have significantly reduced ROS production (20). Similar to Rac2-null mice mast cells, BMM from p47phox-/- mice had reduced Akt1 phosphorylation compared with wild-type littermates in response to M-CSF stimulation (Figure 3A). Interestingly, p38 MAPK phosphorylation was also reduced after M-CSF stimulation (Figure 3B) in the cells from p47phox-/- mice, which was seen in all mice tested. Consistent with our finding, other investigators found that p38 MAPK kinase activation is attenuated in vascular smooth muscle cells from p47phox-/- mice compared with the wild-type mice in response to thrombin-activated ROS generation (21). Taken together, those observations indicate that ROS produced from the NADPH complex function upstream of p38 MAPK and Akt1.
Figure 3.
Macrophages from p47phox-/- mice have decreased Akt1 and p38 MAPK activation, but not Erk activation. BMM were derived from p47phox-/- or wild-type littermate mice (WT) by growth in the presence of M-CSF (20 ng/ml) for 5 d. The cells were serum-starved overnight, then stimulated with M-CSF (10, 50, or 100 ng/ml) for 5 min or left unstimulated. (A) Akt1 activation was assessed using equal amount of protein by Western blotting with antibodies to pAktThr308 (upper panel) and pAktSer473 (middle panel). Membranes were reblotted for total Akt1 (lower panel). (B) Western blot analysis using phospho-p38 MAPK antibody and equal loading was confirmed by p38 MAPK antibody. (C) Erk phosphorylation was detected using pErk 42/44 antibody. Equal loading is shown using Erk2 antibody. Shown are representative data from macrophages obtained from six independent mice.
Since we reported that M-CSF-induced ROS modulated Erk activity (2), we examined the effect of p47phox deficiency on M-CSF–stimulated Erk activity in macrophages. Our data showed no significant change in M-CSF–induced phosphorylation of Erk in p47phox-/- murine macrophages compared with wild-type cells (Figure 3C), as confirmed by densitometry analyses (data not shown). These observations suggest that ROS produced by the NADPH oxidase complex did not regulate Erk in M-CSF–stimulated macrophages. These data support a more direct role for ROS produced from the NADPH oxidase complex mediating Akt1 or p38 MAPK activation in M-CSF–stimulated mononuclear phagocytes, perhaps due to the cell membrane location of this oxidant-producing complex.
ROS from the NADPH Complex Affect Cellular Viability
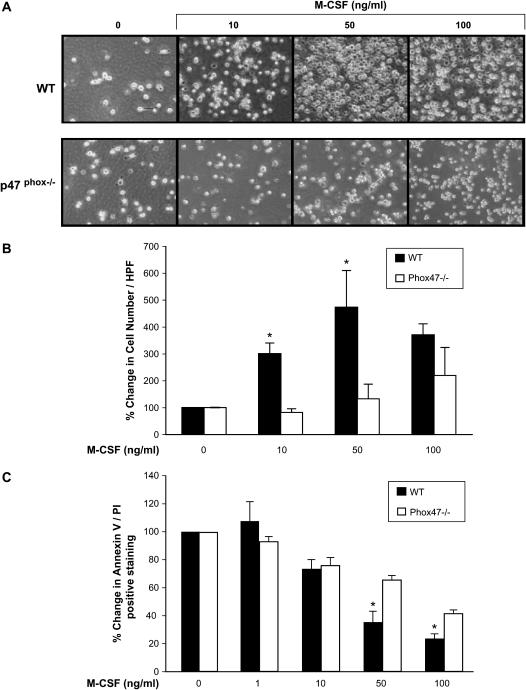
We next determined if reduced Akt1 activity in p47phox-/- murine macrophages played a role in cellular survival. Notably, p47phox−deficient mice are not phenotypically different than wild-type mice. Analysis of peripheral blood demonstrated normal numbers of circulating blood monocytes and other myeloid and lymphoid lineages in p47phox-/- and wild-type animals (data not shown), suggesting that NADPH oxidase function did not play a critical role in native cellular survival or proliferation. In contrast, the number of BMM produced by incubating the cells with M-CSF was reduced in cells from p47phox-/- mice compared with the wild-type mice at 0, 10, and 50 ng/ml of M-CSF (Figures 4A and 4B). Interestingly, staining for Annexin V/PI demonstrated enhanced survival only at M-CSF doses of 50 and 100 ng/ml in p47phox-/- cells, suggesting that other pathways compensated for the loss of these ROS at basal levels of M-CSF (Figure 4C). These data indicated the NADPH oxidase complex was involved in cellular survival in M-CSF–stimulated macrophages.
Figure 4.
Macrophages from p47phox-/- mice have reduced survival in response to M-CSF. BMM were derived from p47phox-/- or WT mice. (A) Cultured BMM (5 × 105/condition) were analyzed for total cell number in response to M-CSF using an Olympus 1X50 inverted microscope (Olympus, Center Valley, PA) equipped with a ×40 objective and Nikon camera. (B) Percent change in total cell number of M-CSF treated p47phox-/- and wild-type BMM compared with unstimulated BMM (0). Cell numbers were significantly decreased in the p47phox-/- mice BMM compared with wild-type BMM at M-CSF concentrations of 10 and 50 ng/ml (*P < 0.05). Data from A and B represent SEM of values obtained from macrophages obtained from two p47phox-/- and two wild-type mice. (C) BMM (5 × 105/condition) were collected and stained with Annexin V/PI. Data are expressed as the mean ± SEM from macrophages from nine independent mice (*P < 0.04 compared with BMM not stimulated with M-CSF).
Macrophages from Myr-Akt1 Mice Have Enhanced Survival
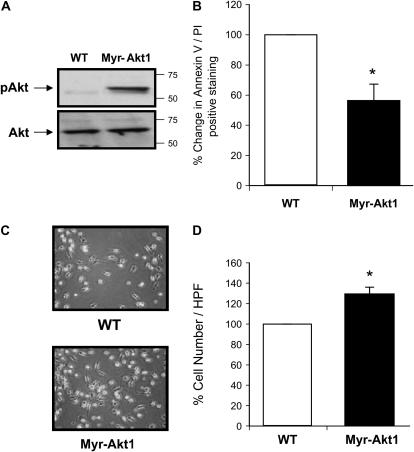
Since macrophages from the p47phox-/- mice had reduced Akt1 activity and cell survival in response to M-CSF stimulation, we next directly assessed the role of Akt1 in regulating mononuclear phagocyte survival. We examined macrophages from mice expressing a Myr-Akt1 isoform expressed in mononuclear phagocytes controlled by a c-Fms promoter. The myristoylation tag on Akt1 targets the protein to the membrane in close proximity to the PDK1 enzyme, rendering it constitutively active. Bone marrow from Myr-Akt1 and wild-type mice was isolated, differentiated, and cultured in serum-free media to examine differences in cell survival and differentiation. As expected, in the absence of stimulation, Akt1 was constitutively phosphorylated in Myr-Akt1 BMM, but not in wild-type BMM (Figure 5A). To assess the involvement of Akt in cell survival, BMM cells were differentiated over 5 d in M-CSF (20 ng/ml) and then serum-starved for 3 d. The Myr-Akt1 macrophages were resistant to basal cell death as demonstrated by less positive Annexin V/PI staining compared with wild-type cells (Figure 5B). As anticipated, after serum deprivation there were more live cells from Myr-Akt1 mice compared with cells from wild-type mice (Figures 5C and 5D). Collectively, these observations demonstrate the importance of Akt1 in regulating macrophage survival.
Figure 5.
Myr-Akt1–expressing macrophages have prolonged survival. BMM were generated from Myr-Akt1 or WT littermate mice by growth in M-CSF (20 ng/ml) for 5 d. The cells were serum starved for 24 h and (A) Akt1 activation was assessed by Western blotting with antibodies recognizing pAktThr308 and pAktSer473. The membranes then were reprobed for total Akt1. (B) The cells were incubated in RPMI 1640 medium for an additional 2 d to evaluate cell survival. BMM (5 × 105/condition) were removed from the culture plates by Accutase, then stained with Annexin V/PI and analyzed by flow cytometry. Data shown represent macrophages from six Myr-Akt and six wild-type mice (*P < 0.05 when comparing Myr-Akt1 cell numbers versus wild-type cells). (C) The pictures were taken using ×40 objective from Olympus IX50 inverted microscope equipped with a digital camera. (D) Cells were counted and there was a significant increase in the Myr-Akt1 mice BMM (*P < 0.05 compared with the WT control).
The Effect of Myr-Akt1 Expression on Human Monocyte Survival
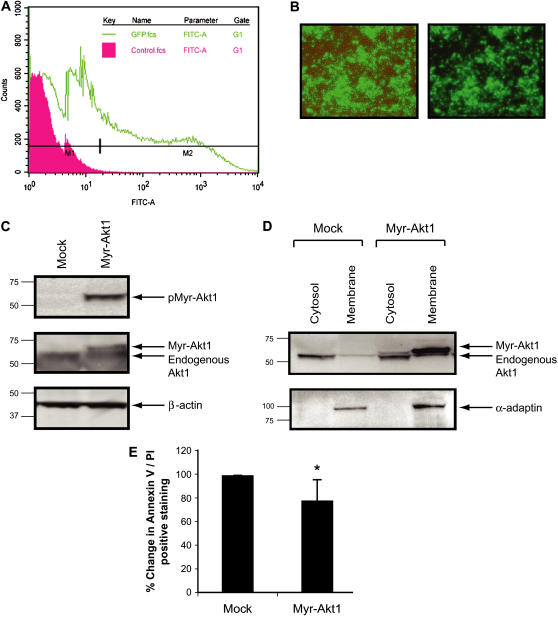
Since Myr-Akt1 expression in BMM increased cell survival in the absence of M-CSF, we next wanted to directly assess the role of Akt1 in human mononuclear phagocyte survival. First, we examined whether active Akt1 promoted the survival of human monocytes. To address this issue, we expressed Myr-Akt1 cDNA constructs in human monocytes. For these studies, we used the nucleofector system, which directly delivers DNA to the nucleus. We optimized this procedure to minimize cell death and maintain high efficient transfer of recombinant DNA. As shown in Figures 6A and 6B, primary human monocytes were transfected with ∼ 40% transfection efficiency using GFP plasmid control. Western blotting with phospho-Akt antibody indicated that transfection of Myr-Akt1 resulted in the constitutive activation of Akt1 without any stimulation (Figure 6C, upper panel). Expression of the transfected Myr-Akt1 in monocytes was confirmed (Figure 6C, middle panel) as well as equal protein loading with β-actin (Figure 6C, lower panel). Since the Myr tag on Akt1 targets the protein to the membrane, we separated membrane and cytosolic fractions and verified that Myr-Akt1 was targeted to the cell membrane after transfection (Figure 6D). To examine the role of the transfected Myr-Akt on survival in primary human monocytes, we incubated the transfected monocytes in serum-free medium devoid of growth factors for 7 h, stained the cells with Annexin V/PI, and analyzed them by flow cytometry. As shown in Figure 6E, Myr-Akt1–transfected cells had lower levels of Annexin V/PI staining compared to mock-transfected cells (P < 0.05). These data agree with other studies showing that constitutively activated Akt1 is vital for the survival of in vitro differentiated macrophages from human monocytes (22).
Figure 6.
Regulation of Akt1 in primary human monocytes influences cellular survival. Monocytes (10 × 106/condition) were isolated from buffy coat and transiently transfected with cDNA expressing eGFP or Myr-Akt1 using the Amaxa nucleofection. After washing, cells were plated in RPMI medium supplemented with 10% FBS and polymyxin B (10 μg/ml) and incubated at 37°C in a 5% CO2 incubator for 7 h. The monocytes were analyzed by (A) flow cytometry for transfection efficiency and (B) photography using Olympus 1X50 inverted fluorescence microscope. Images shown are phase contrast, brightfield (left panel), and fluorescence alone (right panel). (C) Cells were lysed 7 h after transfection and subjected to Western blot analysis with phospho-Akt1 antibody (upper panel). The membrane was subsequently blotted with total Akt1 antibody (middle panel) and then blotted with anti–β-actin antibody to ensure equal loading (lower panel). (D) After transfection, membrane and cytosolic fraction of the cells were also analyzed by Western blotting with anti-Akt1 antibody to show expression of Myr-Akt1 protein (upper panel). The same membranes were reprobed with the membrane-specific anti-α adaptin antibody to show the purity of the membrane fraction (lower panel). (E) Cells were stained with Annexin V/PI and analyzed by flow cytometry. Data are expressed as the mean ± SEM from six independent donors. (*P < 0.05 compared with the mock transfected samples).
p38 MAPK Regulates Human Monocyte Survival
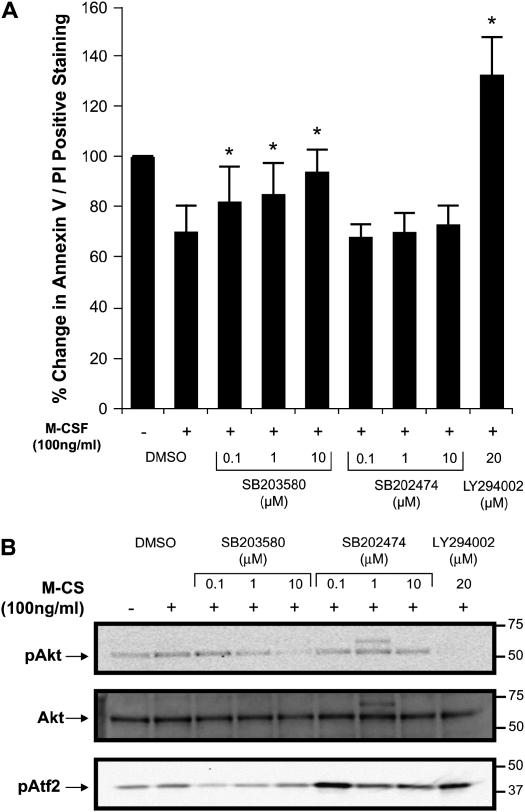
We next examined the role of p38 MAPK in M-CSF–stimulated monocyte survival. Annexin V/PI staining of cells was assessed at 7 and 24 h in the presence or absence of the p38 MAPK inhibitor SB203580 or its inactive form SB202474 after the addition of M-CSF. We did not observe significant changes in cellular survival when cells were treated with SB203580 for 7 h (data not shown). However, doses of SB203580 (100 nM–10 μM) decreased M-CSF–regulated monocyte survival after 24 h of incubation (Figure 7A). Since several investigators reported that SB203580 inhibited insulin- (23) and IL-2–induced Akt1 phosphorylation (24), we next investigated whether SB203580 interfered with Akt1 activation. Not surprisingly, we found that SB203580 at concentration of 100 nM did not affect Akt1 phosphorylation, while concentrations exceeding 1 μM of SB203580 or addition of the PI 3-kinase inhibitor LY294002 as a control abolished the phosphorylation of Akt in response to M-CSF (Figure 7B). We reasoned that higher dosage of SB203580 reduced monocyte survival due to the specific inhibition of p38 MAPK and nonspecific inhibition of Akt1, resulting in apoptosis. To show that SB203580 specifically inactivated p38 MAP kinase activity, we performed a kinase assay using the ATF2 fusion protein as a substrate. As shown in Figure 7B, the active p38 MAPK inhibitor SB203580, but not its inactive analogue SB202474, inhibited p38 MAP kinase activity across a dose range of the inhibitor (100 nM–10 μM) (Figure 7B). These observations suggest that Akt played an essential role in monocytes survival in response to M-CSF.
Figure 7.
Inhibition of p38 MAPK activation reduces human monocyte survival. (A) Monocytes (1 × 106/condition) were isolated and plated in RPMI 1640 medium and incubated with SB203580 or SB202474 (100 nM–10 μM), LY294002 (20 μM), or DMSO control for 1 h and then stimulated with 100 ng/ml of M-CSF and incubated for 24 h. The cells were then stained with Annexin V/PI and analyzed by flow cytometry. Data are expressed as the mean ± SEM from three different donors (*P < 0.05 compared with M-CSF–stimulated cells). (B) Monocytes (10 × 106/condition) were incubated in RPMI 1640 medium supplemented with polymyxin B (10 μg/ml), M-CSF (20 ng/ml) overnight. The cells were serum starved for 2 h and incubated with SB203580, SB202474, LY294002, or DMSO control for 1 h before stimulating with 100 ng/ml of M-CSF for 5 min. Whole cell lysates were immunoblotted with anti–phospho-Akt1 antibody (upper panel). The membrane was reblotted with total Akt1 antibody (middle panel). p38 kinase assay was performed with ATF2 fusion protein (lower panel). Data shown are representative of six independent donors.
DISCUSSION
ROS-mediated cell survival is dependent on the level and source of ROS, and the phase of the cell cycle (25). Here, we demonstrate the selective influence of NADPH oxidase complex on Akt1 and p38 MAPK activation and their subsequent role on cellular survival in M-CSF–stimulated mononuclear phagocytes. While Erk1/2 is at least partially regulated by ROS in M-CSF–stimulated monocytes and plays a role in monocyte survival (2), our data suggest that the NADPH oxidase complex is not the source of ROS-stimulating Erk activation. ROS from the NADPH oxidase complex appeared to regulate Akt1 and p38 MAPK activation in M-CSF–stimulated mononuclear phagocytes.
It is possible that PI 3-kinase-associated products selectively regulate the NADPH oxidase complex and target Akt1 and p38 MAPK but not Erk1/2. It has been reported that the phox homology (PX) domain of p47phox binds PtdIns (3,4) P2 resulting in p47phox translocation to the cell membrane and the subsequent activation of the NADPH oxidase system (26). Interestingly, in vascular smooth muscle cells, p47phox associates with the actin cytoskeleton (27). Disruption of this interaction is reported to reduce angiotension II–induced ROS production and decrease the phosphorylation of Akt and p38 MAPK, but does not affect the activation of Erk1/2. This observation is consistent with our findings that in M-CSF–stimulated murine macrophages, Akt1 and p38 MAPK, but not Erk, activation was reduced in the absence of p47phox.
While our data showed that macrophages from p47phox-/- animals had less phosphorylation of Akt1 and p38 MAPK and reduced cell survival compared with wild-type cells, the biological role of the NADPH oxidase complex in inflammatory diseases involving macrophages is not clear. Some reports show that cross-breeding p47phox-/- with apolipoprotein E−/− mice results in less atherosclerosis than in cross-bred wild-type C57Bl/6 and apolipoprotein E−/− mice (28), whereas others find no effect (29). These discrepant findings may relate to the impact of the NADPH oxidase complex on cell survival signals in growth factor–stimulated monocytes and also may be selective to the specific growth factors present in the environment. For example, in the presence of M-CSF, we surmise that ROS produced from both the NADPH oxidase complex, activating Akt1 and p38, and ROS from an alternate source, activating Erk, are needed to maximally facilitate monocyte survival. This hypothesis is supported by our current and previous findings (2) that both NAC and DPI significantly reduced M-CSF–induced monocyte survival and both Akt1 and Erk activation are reduced by these agents.
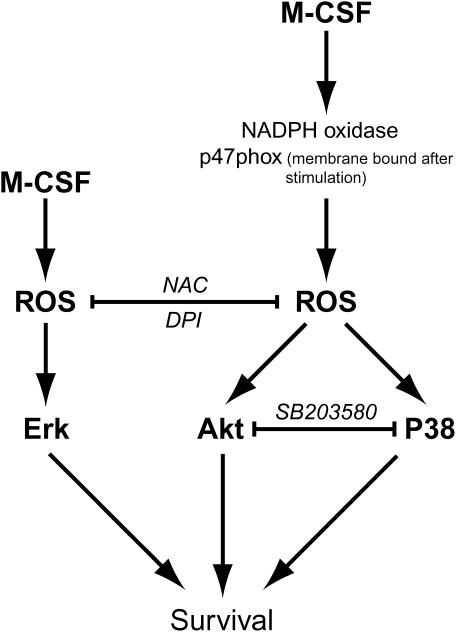
Since NAC and DPI reduced M-CSF–stimulated human monocyte survival and p38 MAPK phosphorylation, we reasoned that p38 activity was also important in M-CSF–induced cell survival. Using human monocytes, we observed that the p38 MAPK inhibitor SB203580 at the concentration of 100 nM efficiently inactivated p38 kinase activity, but did not reduce Akt1 phosphorylation. Importantly, this dose of the p38 inhibitor also reduced M-CSF–stimulated cell survival after 24 h of incubation. Collectively our data indicated that Akt1 and p38 MAPK are positive regulators of monocyte survival in response to M-CSF stimulation. Figure 8 illustrates the proposed pathway that Akt and p38 MAPK pathways play in ROS and M-CSF–induced cellular survival.
Figure 8.
Proposed simplified model for the NADPH oxidase system and M-CSF–induced human monocyte/macrophage survival involving Akt and p38 MAPK pathways.
Zhu and colleagues reported that NAC blocks G-CSF–induced Akt phosphorylation but not Erk1/2 or p38 MAPK phosphorylation in hematopoietic cell lines (5). However, in our studies using primary cells (2), we found that ROS inhibitors NAC and DPI blocked M-CSF–induced phosphorylation of Akt, Erk1/2, and p38 MAPK. Therefore, cellular response to ROS appears to differ between primary cells and cell lines. Furthermore, we found that both Akt1 and p38 MAPK phosphorylation were decreased in BMM from p47phox-/- mice and, therefore ROS generation appears to be upsteam of Akt1 and p38 MAPK in M-CSF–induced cellular signaling. In accordance with our data, Gao and coworkers proposed that ROS are upstream of Akt in leukemia cells (30). Although specific targets regulated by ROS in M-CSF–stimulated human monocytes are not clear, proteins containing redox-sensitive cysteine or methionine groups are susceptible to oxidation. Proteins important in cellular homeostasis fall into this category and include caspases, phosphatases, and transcription factors. Signaling molecules important in cellular survival, such as Akt1, caspase-3, and transcription factors including NF-κB, are modifiable by reactive oxygen or reactive nitrogen species (see review in Ref. 31).
It has been shown that cancer cells and normal cells differ in their ability to respond to ROS (32). In this article, we provided evidence that M-CSF–induced ROS production cooperates with Akt and p38 MAPK to promote monocyte and macrophage survival. Similarly, low concentrations of H2O2 accelerate wound healing in p47phox-/- mice by facilitating wound angiogenesis in vivo (33). In contrast, Mochizuki and colleagues reported that depletion of ROS by DPI or siNox4RNAs to inhibit NADPH oxidase 4 from human pancreatic adenocarcinoma cell line induces apoptosis and reduces Akt-Ask1 activity (34). Studies have demonstrated that increasing ROS production and downregulating Akt through chemotherapeutic drugs are effective to induce apoptosis in leukemia cells but not normal bone marrow cells (30, 35).
In summary, we provide several lines of evidence supporting an important role for the NADPH oxidase complex in influencing Akt1 and p38 MAPK activation. Furthermore, we show that Akt1 and p38 MAPK are important in monocyte/macrophage survival regulated by M-CSF. Understanding the influence of NADPH oxidase complex and ROS on monocyte survival and the function of Akt1 and p38 MAPK activation may provide new opportunities to influence cellular inflammation and cancer therapy.
Acknowledgments
The authors thank Drs. Chris Baran and Judy Opalek for their insightful discussions and technical assistance. The authors are grateful for the assistance provided by the Flow Cytometry core facility at the HLRI of the Ohio State University.
This work was supported by NIH grants HL63800, HL66108, HL67176, GM69589, American Lung Association Johnie Walker Murphy Career Investigator Award, and the Kelly Clark Foundation; G.L. was a fellow of the Stanley J. Sarnoff Endowment for Cardiovascular Science during the time of his research at The Ohio State University.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0165OC on August 24, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kelley TW, Graham MM, Doseff AI, Pomerantz RW, Lau SM, Ostrowski MC, Franke TF, Marsh CB. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem 1999;274:26393–26398. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt NY, Kelley TW, Khramtsov VV, Wang Y, Lam GK, Clanton TL, Marsh CB. Macrophage-colony-stimulating factor-induced activation of extracellular-regulated kinase involves phosphatidylinositol 3-kinase and reactive oxygen species in human monocytes. J Immunol 2002;169:6427–6434. [DOI] [PubMed] [Google Scholar]
- 3.Baran CP, Zeigler MM, Tridandapani S, Marsh CB. The role of ROS and RNS in regulating life and death of blood monocytes. Curr Pharm Des 2004;10:855–866. [DOI] [PubMed] [Google Scholar]
- 4.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci 2003;28:502–508. [DOI] [PubMed] [Google Scholar]
- 5.Zhu QS, Xia L, Mills GB, Lowell CA, Touw IP, Corey SJ. G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood 2006;107:1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 2002;90:1205–1213. [DOI] [PubMed] [Google Scholar]
- 7.Noack D, Rae J, Cross AR, Ellis BA, Newburger PE, Curnutte JT, Heyworth PG. Autosomal recessive chronic granulomatous disease caused by defects in NCF-1, the gene encoding the phagocyte p47-phox: mutations not arising in the NCF-1 pseudogenes. Blood 2001;97: 305–311. [DOI] [PubMed] [Google Scholar]
- 8.Yang FC, Kapur R, King AJ, Tao W, Kim C, Borneo J, Breese R, Marshall M, Dinauer MC, Williams DA. Rac2 stimulates Akt activation affecting BAD/Bcl-XL expression while mediating survival and actin function in primary mast cells. Immunity 2000;12:557–568. [DOI] [PubMed] [Google Scholar]
- 9.Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol 2001;2:211–215. [DOI] [PubMed] [Google Scholar]
- 10.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene 2003;22:8983–8998. [DOI] [PubMed] [Google Scholar]
- 11.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 2005;9:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 2004;68:320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yosimichi G, Nakanishi T, Nishida T, Hattori T, Takano-Yamamoto T, Takigawa M. CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK). Eur J Biochem 2001;268:6058–6065. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Ro JY. Signal pathway of cytokines produced by reactive oxygen species generated from phorbol myristate acetate-stimulated HMC-1 cells. Scand J Immunol 2005;62:25–35. [DOI] [PubMed] [Google Scholar]
- 15.Ganesan LP, Wei G, Pengal RA, Moldovan L, Moldovan N, Ostrowski MC, Tridandapani S. The serine/threonine kinase Akt Promotes Fc gamma receptor-mediated phagocytosis in murine macrophages through the activation of p70S6 kinase. J Biol Chem 2004;279:54416–54425. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Keogh RJ, Hunter MG, Mitchell CA, Frey RS, Javaid K, Malik AB, Schurmans S, Tridandapani S, Marsh CB. SHIP2 is recruited to the cell membrane upon macrophage colony-stimulating factor (M-CSF) stimulation and regulates M-CSF-induced signaling. J Immunol 2004;173:6820–6830. [DOI] [PubMed] [Google Scholar]
- 17.Roy S, Khanna S, Wallace WA, Lappalainen J, Rink C, Cardounel AJ, Zweier JL, Sen CK. Characterization of perceived hyperoxia in isolated primary cardiac fibroblasts and in the reoxygenated heart. J Biol Chem 2003;278:47129–47135. [DOI] [PubMed] [Google Scholar]
- 18.van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996;24:131–139. [DOI] [PubMed] [Google Scholar]
- 19.Fahy RJ, Doseff AI, Wewers MD. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol 1999;163:1755–1762. [PubMed] [Google Scholar]
- 20.Nowicki PT, Flavahan S, Hassanain H, Mitra S, Holland S, Goldschmidt-Clermont PJ, Flavahan NA. Redox signaling of the arteriolar myogenic response. Circ Res 2001;89:114–116. [DOI] [PubMed] [Google Scholar]
- 21.Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med 2002;32:1116–1122. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J Exp Med 2001;194:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talukdar I, Szeszel-Fedorowicz W, Salati LM. Arachidonic acid inhibits the insulin induction of glucose-6-phosphate dehydrogenase via p38 MAP kinase. J Biol Chem 2005;280:40660–40667. [DOI] [PubMed] [Google Scholar]
- 24.Lali FV, Hunt AE, Turner SJ, Foxwell BM. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J Biol Chem 2000;275:7395–7402. [DOI] [PubMed] [Google Scholar]
- 25.Hoidal JR. Reactive oxygen species and cell signaling. Am J Respir Cell Mol Biol 2001;25:661–663. [DOI] [PubMed] [Google Scholar]
- 26.Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol 2001;3:675–678. [DOI] [PubMed] [Google Scholar]
- 27.Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol 2005;25:512–518. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama M, Inoue N, Kawashima S. Role of the vascular NADH/NADPH oxidase system in atherosclerosis. Ann N Y Acad Sci 2000; 902:241–247. [DOI] [PubMed] [Google Scholar]
- 29.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol 2000;20:1529–1535. [DOI] [PubMed] [Google Scholar]
- 30.Gao N, Rahmani M, Shi X, Dent P, Grant S. Synergistic antileukemic interactions between 2-medroxyestradiol (2-ME) and histone deacetylase inhibitors involve Akt down-regulation and oxidative stress. Blood 2006;107:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res 2000;87: 179–183. [DOI] [PubMed] [Google Scholar]
- 32.Ungerstedt JS, Sowa Y, Xu WS, Shao Y, Dokmanovic M, Perez G, Ngo L, Holmgren A, Jiang X, Marks PA. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci USA 2005;102:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther 2006;13:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 2006;25:3699–3707. [DOI] [PubMed] [Google Scholar]
- 35.Dasmahapatra G, Rahmani M, Dent P, Grant S. The tyrphostin adaphostin interacts synergistically with proteasome inhibitors to induce apoptosis in human leukemia cells through a reactive oxygen species (ROS)-dependent mechanism. Blood 2006;107:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]