Abstract
Tyrosine nitration is a nitric oxide–derived post-translational modification of proteins. Elevated levels of specific plasma proteins modified by tyrosine nitration have been detected during acute and chronic inflammatory conditions, including acute lung injury (ALI). In the present study we examined whether circulating immunoglobulins against nitrated proteins are present in the plasma of subjects with clinically documented ALI. Affinity chromatography using covalently linked 3-nitrotyrosine was employed to identify plasma proteins that bind to this unusual amino acid. Western blotting and liquid chromatography-tandem mass spectrometry of in-gel digested protein bands revealed that the major proteins eluted from the affinity column were IgM and IgG. An enzyme-linked immunosorbent assay (ELISA) based on competition of horseradish peroxidase–derivatized 3-nitrotyrosine binding to plasma with unlabeled 3-nitrotyrosine was developed and validated. Using this ELISA, the levels of immunoglobulins that recognize 3-nitrotyrosine were significantly higher in the plasma of subjects with ALI compared with both normal control subjects and subjects with major trauma who did not develop ALI (0.36± 0.14 versus 0.03 ± 0.05, and 0.25 ± 0.15; P < 0.001 and P = 0.006, respectively). These data indicate that tyrosine-nitrated proteins induce the production of specific immunoglobulins during acute phase response and inflammation.
Keywords: tyrosine nitration, oxidative stress, immunoglobulins, affinity chromatography, biological markers
CLINICAL RELEVANCE
This study provides the first evidence that immunoglobulins against tyrosine-nitrated proteins are present in the plasma of subjects with acute lung injury (ALI). This finding may provide a novel biomarker to ascertain risk for the development of ALI.
Diffuse inflammation of the lung parenchyma and formation of reactive species during the initiation and progression of acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) have been documented (1). Activated inflammatory cells are significant sources of reactive species capable of oxidizing, chlorinating, and nitrating amino acids of proteins. Indeed, elevated levels of the modified amino acids, nitrotyrosine, chlorotyrosine, and ortho-tyrosine have been reported in the bronchoalveolar lavage fluid (BAL) and plasma of patients with ALI (2–5). Proteins modified by nitration on tyrosine residues were also immunohistochemically localized on lung cells in patients with ALI (2). In the setting of ALI, protein oxidation, via exposure to nitrating, and chlorinating oxidants have been linked with altered function of crucial proteins present in the alveolar space such as α1-proteinase inhibitor and surfactant protein-A (6) and of circulating plasma proteins such as ceruloplasmin and fibrinogen (7).
Post-translational modifications of proteins, including phosphorylation, methylation, and glycosylation, have been shown to induce immune responses, resulting in the generation of autoimmunity (8). Moreover, circulating antibodies directed against oxidatively modified macromolecules have been also detected in human subjects. For example, antibodies against oxidized phospholipids have been detected in patients with cardiovascular diseases and diabetic nephropathy (9–12). However, it is not known at present if nitration of tyrosine residues in proteins will induce immune responses in humans, despite studies in rabbits and mice indicating that several tyrosine nitrated proteins and peptides readily induce the generation of anti-nitrotyrosine antibodies (13–20).
Previous reports have also indicated increased production of immunoglobulins, specifically of IgE, in BAL and plasma of patients after traumatic injury and sepsis (21). Moreover, patients at risk for developing ARDS, including those from major trauma, were shown to have polyclonal autoantibodies to IL-8 in the BAL early during the initiation of injury (22–25). Elevated levels of the anti–IL-8 autoantibodies were associated with the development of ARDS as well as the outcome in patients with ALI (22–25). In addition, anti-phospholipid antibodies have been detected in the BAL and plasma of patients with ALI and ARDS (26, 27). These reports collectively suggest that humoral responses are induced rapidly after traumatic injury, sepsis, and ALI.
Therefore, we explored the possibility that the significant increases in protein tyrosine nitration in lung and plasma of patients with ALI after major trauma will induce the production of immunoglobulins that specifically recognize nitrated tyrosine residues.
MATERIALS AND METHODS
Affinity Purification of Plasma Proteins
An AminoLink Immobilization kit (Pierce, Rockford, IL) was used to affinity-purify proteins from human plasma that bind this unusual amino acid. Briefly, 3-nitrotyrosine was bound to the activated agarose at pH 10. Patient plasma (20 mg of total protein) was diluted in binding buffer (1.75 ml of 0.15 M NaCl, 0.1 M phosphate, pH 7.2), applied to the column, and incubated for 3 h at room temperature. Unbound protein fractions were eluted with binding buffer. Bound proteins were eluted with 0.1 M glycine-HCl, pH 2.5, and collected in tubes containing 1 M Tris-HCl, pH 9.0. Both unbound and bound fractions were concentrated with Centriprep YM-10 filters (Millipore Corp., Billerica, MA) by centrifugation at 2,750 × g. Fractions with low protein concentration were further concentrated by vacuum, after buffer exchange. The protein fractions were separated on 10% SDS-PAGE and transferred to PVDF membranes (Immobilon-P; Millipore). The blot was blocked with dry milk solution and then incubated with goat anti-human IgG and goat anti-human IgM (Sigma, St. Louis, MO), horseradish peroxidase (HRP)-conjugated antibodies (1:50,000). After antibody incubation, the blot was developed with ECL Western blotting detection reagent (Amersham Biosciences, Buckinghamshire, UK). To further validate that the bound fractions were immunoglobulins the eluted proteins were separated on 10% SDS-PAGE. The bands were excised and in-gel trypsin digestion was performed. The tryptic fragments were separated and analyzed using C18 reversed-phase capillary high-performance liquid chromatography (HPLC) in-line with an electrospray ion trap mass spectrometer (LC-MS/MS). The peptide MS/MS data collected in the mzData format was searched for protein identification using TurboSEQUEST and the indexed human RefSeq database.
Immunoglobulin Isolation
IgG was purified from plasma using Vivapure protein-A mini spin columns (VivaScience AG, Hannover, Germany) as recommended by the manufacturer.
Ligand Competition Enzyme-Linked Immunosorbent Assay
Each well of a 96-well microtiter plate (MAXI-SORP; Nunc, Roskilde, Denmark) was coated with 50 μl of serial dilution of human plasma (0.02–10 mg of protein/ml) in 50 mM sodium bicarbonate buffer, pH 9. For this ligand competition, enzyme-linked immunosorbent assay (ELISA) plasma that was thawed only once was used (repeated freezing and thawing of plasma is not recommended for this ELISA). After incubation overnight at 4°C, the plates were washed three times with Tris-buffered saline containing 0.05% tween-20 (TBS-T), and then blocked with 100 μl of filtered (0.45 μm) 1% BSA in TBS-T for 1 h at room temperature. After a wash with TBS-T, 100 μl of peroxidase-conjugated 3-nitrotyrosine (1:2,000) alone or in the presence of nitrotyrosine (2.5 mM) were added to each well, in 0.1% BSA in TBS-T, and incubated for 1 h at 37°C. The peroxidase-conjugated nitrotyrosine was prepared following the one-step procedure (28). Three series of washes with TBS-T were performed and the peroxidase activity bound to each well was determined with the 3,3′,5,5′ tetramethylbenzidine/H2O2 reagents (TMB; Pierce Biotechnology, Inc., Rockford, IL). Absorbance was measured at 450 nm on a microplate reader (Spectra-Max 250; Molecular Devices Corp., Sunnyvale, CA). A polyclonal rabbit anti-nitrotyrosine IgG antibody (40–0.078 μg of protein/ml) was run each day along with the plasma samples as positive control.
Human Subjects
The subjects for this study were participants in a prospective cohort study to determine the biological risk factors for ALI after major trauma. Subjects in this cohort study met the following inclusion criteria: (1) they were admitted to the Hospital of the University of Pennsylvania surgical intensive care unit (SICU) as a result of acute trauma directly from the hospital's emergency department; (2) they had an Injury Severity Score (ISS) ⩾ 16 as calculated on the basis of information available during their first 24 h of hospitalization (29). Exclusion criteria were death or discharge from the SICU in the first 24 h, age less than 14 yr, current or prior congestive heart failure or recent myocardial infarction, severe chronic respiratory disease, morbid obesity, burns over body surface area of 30% or more, lung transplantation, and bone marrow transplantation.
To qualify as an ALI case, subjects had to meet all ALI criteria within the first 5 d after major trauma. We used the standard criteria of the American European Consensus Conference (AECC) definition: acute onset; bilateral pulmonary infiltrates on chest radiograph consistent with pulmonary edema; absence of evidence of left atrial hypertension; and a ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2) ⩽ 300 (30). Study subjects were screened daily from the time of admission, and two physician investigators who were unaware of the status of plasma antibodies reviewed the arterial blood gases and chest radiographs for classification of ALI. From this cohort, 30 ALI cases (those who developed ALI after the first day of admission), and 30 at-risk control subjects were chosen who did not get ALI after major trauma. In addition to trauma at-risk control subjects, we included 30 normal nonsmoking volunteer control subjects (obtained from Cleveland Clinic Foundation) to compare with our critically ill patients. Based on the preliminary data during the development of the ELISA in trauma control subjects, we anticipated that 30 patients in each group would provide 95% power at an α level of 0.05 to detect a difference of one standard deviation between the groups, given an anticipated mean in non-ALI control subjects of 0.25 with a standard deviation of 0.15. Therefore we selected the first 30 sequential ALI cases and sequential control subjects without ALI from this cohort study. The characteristics of the study populations are presented in Table 1. Blood samples from residual EDTA collection tubes otherwise drawn for clinical purposes were collected in the first 24 h of admission, before development of ALI. Samples were centrifuged and plasma aliquoted and stored at −80°C. The institutional review board of the University of Pennsylvania approved the study protocol with waiver of informed consent.
TABLE 1.
CLINICAL CHARACTERISTICS OF THE STUDY POPULATION
| Clinical Variable | ALI (n = 30) | No ALI (n = 30) | Control (n = 30) | P Value† |
|---|---|---|---|---|
| Mean age, yr* | 44.1 ± 19.1 | 27.2 ± 10.0 | 54 ± 8 | 0.001 |
| Mean APACHE III* | 44.2 ± 11.8 | 40.3 ± 10.4 | NA | 0.180 |
| Mean ISS* | 27.4 ± 9.9 | 24.8 ± 6.4 | NA | 0.229 |
| Blunt trauma | 76.7% | 54.8% | NA | 0.073 |
| Female sex | 16.7% | 3.3% | 50% | 0.078 |
| African American race | 53.3% | 67.7% | 0.250 |
Value represents the mean ± standard deviation, n = 30 for all groups.
P values are derived from ANOVA for continuous data and χ2 tests for count data when three groups are available (age and sex), or by t tests and χ2 tests when data between two groups wee available (APACHE III, ISS, blunt trauma, race).
The significant difference in the ELISA values between subjects with ALI and at-risk subjects or normal control subjects were assessed by t test. To assess the effects of potentially confounding clinical variables in the critically ill control subjects, we used multivariable logistic regression methods. Clinical variables included in the models were: age, race, sex, mechanism of trauma (categorized as blunt or penetrating), ISS, and APCHE III score (31). For the purposes of these analyses, the APACHE III score was calculated without the blood gas measures, since these may be related to ALI. All statistical comparisons were conducted using STATA version 8.0 (STATA Data Corp., College Station, TX).
RESULTS
Affinity Purification of Circulating Immunoglobulins
The presence of circulating proteins that recognize the unusual amino acid 3-nitrotyrosine was explored by affinity capturing. Plasma from patients with documented ALI was applied to an affinity column in which the free amino acid 3-nitrotyrosine was coupled to the solid matrix via its amine group. The column was extensively washed of the unbound material, and the bound protein fraction was subsequently eluted and the fractions analyzed by SDS-PAGE chromatography. Silver staining of the gels from the bound fraction showed a major band with apparent molecular mass of 64 kD, and a minor band of nearly 50 kD (Figure 1A). To confirm the identity of the affinity purified proteins, the proteins were transferred to PVDF membranes and probed with either a goat anti-human IgM antibody or a goat anti-human IgG antibody. The anti-human IgM antibody recognized the 64-kD band consistent with the presence of the IgM heavy chain (μ-chain), whereas the anti-human IgG antibody specifically recognized the 50-kD corresponding to the heavy chain of IgG (γ-chain) (Figure 1B). As a control for the affinity purification, plasma was applied to an affinity column of activated agarose, without coupling of 3-nitrotyrosine, and the column was washed and bound fractions were eluted as described above. Silver staining of the gels showed that bound protein from this column was removed during the washing steps with no detectable protein eluting after application of 0.1 M glycine-HCl, pH 2.5 (Figure 1C).
Figure 1.
Affinity purification of anti-nitrotyrosine immunoglobulins from plasma of patients with ALI. (A) Representative silver stained 10% SDS-PAGE gel of fractions from the 3-nitrotyrosine affinity-purification of plasma. (B) Proteins were transferred to PVDF membranes and blotted with polyclonal anti-human IgM and polyclonal anti-human IgG antibodies. (C) Representative silver stained 10% SDS-PAGE gel of fractions from the affinity-purification of plasma from an activated agarose column without coupling of 3-nitrotyrosine. The data represent a single purification of three pooled plasma samples. The data were repeated five different times using three pooled plasma samples each time. Lane assignments for both blots: lane 1, unfractionated input plasma; lane 2, unbound fraction; lanes 3–10, several washes with binding buffer; lane 11, bound fraction eluted with 0.1 M Glycine-HCl, pH 2.5. The arrow indicates the band that was further analyzed by mass spectrometry.
In another set of experiments the bound fraction of the proteins eluted from the 3-nitrotyrosine columns were digested with trypsin and the tryptic fragments were analyzed by LC/MS/MS. The sequencing of the peptides in the 64-kD band indicated the presence of IgM (11 peptides identified with a coverage of 24% by amino acid count). These findings indicate that in the plasma of patients with ALI, primarily IgM immunoglobulin was specifically bound to 3-nitrotyrosine.
Development and Validation of Ligand Competition ELISA for Circulating Immunoglobulins that Recognize 3-Nitroyrosine
To screen and quantify the plasma levels of immunoglobulins that recognize 3-nitrotyrosine, a competition ELISA was developed. Increasing plasma protein concentrations (0.002–10 mg of protein/ml) were allowed to coat ELISA plates. After washing, the plates were incubated with the specific ligand 3-nitrotyrosine conjugated with HRP (NT-HRP), and allowed to react for 1 h at room temperature. The amount of bound ligand was measured by the HRP-catalyzed oxidation of the TMB substrate by monitoring the absorbance of the oxidized product at 450 nm. Typical binding curves of serially diluted plasma sample from patients with ALI are depicted in Figure 2. The changes in absorbance plotted against the log of plasma dilution revealed a sigmoidal curve typical of antibody binding. The specificity of ligand (NT-HRP) binding was studied by the inclusion of unlabeled 3-nitrotyrosine (2.5 mM) to compete the binding of the NT-HPR ligand. As shown in Figure 2A, incubation with unlabeled 3-nitoryrosine eliminated the binding of the labeled 3-nitrotyrosine. Therefore, in all assays performed the absorbance measured in the presence of the unlabeled 3-nitrotyrosine was subtracted from the binding curve of the NT-HRP ligand. No signal was obtained with the substitution of NT-HRP by cold HRP (data not shown). Moreover, competition of NT-HRP binding with unlabeled tyrosine or dopamine did not reduce the binding indicating specificity for 3-nitrotyrosine (Figure 2B). Further validation of the ELISA was provided by demonstrating that partial depletion of IgG from plasma significantly reduced binding to 3-nitrotyrosine by 75% as compared with the immunoglobulin-enriched fraction or the same plasma without immunoglobulin depletion at the same dilutions.
Figure 2.
ELISA screening for circulating antibodies that recognize 3-nitrotyrosine. (A) Representative semi-log plot of absorbance at 450 nm versus the dilution of plasma reveals a typical antibody-antigen binding relationship. Plates coated with increasing protein concentrations (0.002–10 mg of protein/ml) from patient plasma with clinically documented ALI were incubated with the specific ligand NT-HRP (75 nM) in the absence (solid circles), or the presence of 2.5 mM unlabeled NT (open circles) and developed with TMB. The difference in absorbance between the two conditions (solid diamonds) indicates the specific binding of the ligand. (B) The binding of NT-HRP (solid circles) was competed with 2.5 mM 3-nitrotyrosine (open circles) but not by 2.5 mM tyrosine (solid diamonds), or dopamine (solid triangles).
Plasma Levels of Immunoglobulins that Recognize 3-Nitroyrosine in ALI
Employing the competitive ELISA assay, we tested plasma from individuals with ALI. The demographic characteristics of the subject population are presented in Table 1. To confirm that these subjects had tyrosine-nitrated plasma proteins, the systemic levels of 3-nitrotyrosine were quantified by HPLC with on-line electrospray ionization tandem mass spectrometry using stable isotope dilution methodology (32). The levels of tyrosine-nitrated plasma proteins were 41.5 ± 4.5 μmol 3-nitrotyrosine/mol tyrosine and 35.7 ± 4.4 μmol 3-nitrotyrosine/mol tyrosine in subjects with trauma who failed to develop ALI and those who developed ALI, respectively. The levels in control subjects were 6.1 μmol 3-nitrotyrosine/mol tyrosine, as reported previously (32).
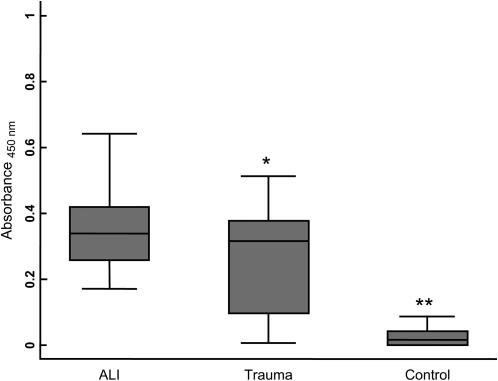
The mean immunoglobulin concentration that specifically recognized 3-nitrotyroisne was 0.36 ± 0.14 (corrected absorbance using 10 mg of plasma protein/ml) in the plasma of subjects with ALI (Figure 3). For comparison, the mean immunoglobulin concentration that specifically recognized 3-nitrotyrosine in healthy control subjects was 0.03 ± 0.05 (P < 0.001) and 0.25 ± 0.15 in subjects with major trauma who did not develop ALI (P = 0.006). Furthermore, a multivariable model indicated that this association of anti-nitrotyrosine immunoglobulins with ALI was independent of clinical variables, including imbalances in age and injury severity between the ALI and at-risk trauma populations. The unadjusted odds ratio (OR) for each 0.1 unit of absorption of anti-nitrotyrosine immunoglobulins was 1.76 (95% confidence intervals [95% CI], 1.14 and 2.72). The adjusted OR remained significant when simultaneously adjusted for age, sex, race, mechanism of trauma, APACHE III score, and ISS (2.06 [95% CI, 1.14 and 3.72], P = 0.017).
Figure 3.
Quantification of immunoglobulins that recognize 3-nitrotyrosine in subjects with major trauma that subsequently developed ALI, subjects at-risk after major trauma that did not develop ALI, and control subjects. Box-whisker plots of immunoglobulin levels encompass the 25th and 75th percentiles, and lines within boxes represent median values. Bars represent the 2.5th and 97.5th percentiles. Statistically significant differences in immunoglobulin content were found between ALI and control subjects (*P = 0.006 versus at-risk trauma subjects and **P < 0.001 versus healthy control subjects).
DISCUSSION
The data in this report provide the initial description of immunoglobulins that recognize the unusual amino acid 3-nitrotyrosine in human plasma. The specificity of the circulating immunoglobulins was examined and validated by affinity capturing and by a competitive ELISA. The affinity capturing employed immobilized 3-nitrotyrosine onto a solid matrix to enrich for proteins that bind this unusual amino acid. The major protein captured as revealed by Western blotting, and LC/ESI/MS/MS analysis of in-gel digested protein band was IgM. The bound fraction but not the unbound fraction from the 3-nitrotyrosine-affinity column recognized tyrosine-nitrated proteins, providing further validation for the specificity of the immunoglobulins. Furthermore, plasma depleted of most circulating immunoglobulins showed diminished response to nitrated protein antigens. By using an ELISA in which unlabeled 3-nitrotyrosine competed with HRP-derivatized 3-nitrotyrosine, elevated levels of the circulating immunoglobulins were measured in patients with ALI as compared with both normal and at-risk trauma control subjects. Patients with higher levels of immunoglobulins against 3-nitrotyrosine had a higher risk for ALI after major trauma, even after adjustment multiple clinical variables. The observation that both major trauma patients who subsequently developed ALI as well as those who did not develop ALI had similar levels of 3-nitrotyrosine is not surprising, as both groups had similar degree of injury (Table 1). On the other hand, the finding that the subset of individuals that develop ALI may develop immune responses against the modified proteins, despite the same overall burden of nitrated proteins, may indicate a differential reactivity against this post-translational protein modification as a result of the pathogenic processes leading to the development of ALI.
Nitrated proteins have been detected in humans and most mammals under normal physiologic conditions, and the levels are significantly increased in most inflammatory conditions, including ALI (2–7). The presence of nitrated proteins therefore raises the possibility that nitrated peptides could serve as immunogens generating specific antibodies. Indeed, studies in rabbits and mice indicate that nitrated proteins and peptides readily elicit production of antibodies (13–19). For example, immunization of rabbits with the synthetic octapeptide Cys-Gly-NTyr-Gly-Gly-Gly-NTyr-Gly (NTyr indicates 3-nitrotyrosine) conjugated to KLH by the cysteine residue resulted in the generation of specific anti-nitrotyrosine polyclonal antibodies (16, 17). Monoclonal antibodies that recognize only nitrated α-synuclein but not the unmodified protein or other tyrosine-nitrated proteins have been also generated (18). Moreover, clones of these anti–nitrated-α-synuclein antibodies specifically recognized a single nitrated tyrosine residue (one of the four nitrated tyrosine residues in α-synuclein) (18).
The molecular mechanisms that may govern the immunologic responses to tyrosine-nitrated proteins and peptides have been explored in recent publications. A robust immune response in transgenic mice that were genetically modified to express pigeon/moth cytochrome c, and thus do not mount a response to this protein, was elicited by immunization with tyrosine-nitrated cytochrome c peptides (19). Similarly, another study showed that nitrated peptides from hen egg-white lysozyme elicited production of monoclonal antibodies in transgenic mice that express hen egg-white lysozyme, where the protein behaves as a self-protein and thus does not mount an immune response (20). The same study showed that the tyrosine-nitrated peptides derived from hen egg-white lysozyme were selected for presentation to unique CD4 T cells. The presence of CD4 T cells that specifically responded to the nitrated peptides indicated that these cells have escaped negative selection. Moreover, in the hen egg-white lysozyme transgenic mice, infection with Listeria monocytogenes prompted the generation of antigen-presenting cells that contain nitrated peptides, indicating that these cells are capable of generating nitrated peptides in vivo, which may significantly augment the immunogenic response to the infectious agent (20). Collectively, these findings suggested that protein nitration is capable of inducing immune responses to autologous proteins and thus it could profoundly influence immunologic responses in autoimmune and inflammatory diseases.
At this time we can only speculate whether or not this immune response is beneficial or harmful to the host. The response to self-antigens may have an important role in normal catabolic processes that remove undesired antigens; for instance, antibodies to denatured IgG may help in eliminating antigen–antibody complexes from circulation; and antibodies to oxidized low-density lipoprotein may help in eliminating a potentially toxic lipid (33). In contrast, the presence of auto-antibodies to IL-8 that have been found either free or in complex with IL-8 in the BAL fluid of patients at risk for ALI or during ALI were shown to have proinflammatory activity and were associated with unfavorable outcomes (25). Presently, it is unclear which pathways remove nitrated proteins from circulation. Previously, we showed that putative pathways such as chymotrypsin are capable of degrading nitrated proteins but at significantly slower rate than unmodified proteins (34). It is possible that immunoglobulins that recognize 3-nitrotyrosine by removing nitrated proteins from plasma could play a role during inflammatory processes.
This work was supported by grants from the National Institutes of Health P01 HL076491 (S.L.H.) and P50 HL60290 (J.C., P.N.L., and H.I.).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0288SM on October 5, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2003;167:1027–1035. [DOI] [PubMed] [Google Scholar]
- 2.Haddad IY, Pataki G, Hu P, Galliani C, Beckman JS, Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest 1994;94:2407–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb NJ, Gutteridge JM, Baker C, Evans TW, Quinlan GJ. Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: evidence for neutrophil-mediated hydroxylation, nitration, and chlorination. Crit Care Med 1999;27:1738–1744. [DOI] [PubMed] [Google Scholar]
- 4.Lamb NJ, Quinlan GJ, Westerman ST, Gutteridge JM, Evans TW. Nitration of proteins in bronchoalveolar lavage fluid from patients with acute respiratory distress syndrome receiving inhaled nitric oxide. Am J Respir Crit Care Med 1999;160:1031–1034. [DOI] [PubMed] [Google Scholar]
- 5.Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S. Increased levels of nitrate and surfactant protein A nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med 2001;163:166–172. [DOI] [PubMed] [Google Scholar]
- 6.Zhu S, Kachel DL, Martin WJ II, Matalon S. Nitrated SP-A does not enhance adherence of Pneumocystis carinii to alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 1998;275:L1031–L1039. [DOI] [PubMed] [Google Scholar]
- 7.Gole MD, Souza JM, Choi I, Hertkorn C, Malcolm S, Foust RF 3rd, Finkel B, Lanken PN, Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2000;278:L961–L967. [DOI] [PubMed] [Google Scholar]
- 8.Doyle HA, Mamula MJ. Posttranslational protein modifications: new flavors in the menu of autoantigens. Curr Opin Rheumatol 2002;14:244–249. [DOI] [PubMed] [Google Scholar]
- 9.Lopes-Virella MF, Virella G, Orchard TJ, Koskinen S, Evans RW, Becker DJ, Forrest KY. Antibodies to oxidized LDL and LDL-containing immune complexes as risk factors for coronary artery disease in diabetes mellitus. Clin Immunol 1999;90:165–172. [DOI] [PubMed] [Google Scholar]
- 10.Paiker JE, Raal FJ, von Arb M. Auto-antibodies against oxidized LDL as a marker of coronary artery disease in patients with familial hypercholesterolaemia. Ann Clin Biochem 2000;37:174–178. [DOI] [PubMed] [Google Scholar]
- 11.Sherer Y, Tenenbaum A, Blank M, Shemesh J, Harats D, Fisman EZ, Praprotnik S, Motro M, Shoenfeld Y. Autoantibodies to oxidized low-density lipoprotein in coronary artery disease. Am J Hypertens 2001;14:149–154. [DOI] [PubMed] [Google Scholar]
- 12.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med 2005;353:46–57. [DOI] [PubMed] [Google Scholar]
- 13.Beckman JS, Ye Y-Z, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosine in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler 1994;375:81–88. [DOI] [PubMed] [Google Scholar]
- 14.Girault I, Karu AE, Schaper M, Barcellos-Hoff MH, Hagen T, Vogel DS, Ames BN, Christen S, Shigenaga MK. Immunodetection of 3-nitrotyrosine in the liver of zymosan-treated rats with a new monoclonal antibody: comparison to analysis by HPLC. Free Radic Biol Med 2001;31:1375–1387. [DOI] [PubMed] [Google Scholar]
- 15.Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart: implications to dysfunctional mitochondria in diabetes. J Biol Chem 2003;278:35844–35849. [DOI] [PubMed] [Google Scholar]
- 16.Fries DM, Paxinou E, Themistocleous M, Swanberg E, Griendling KK, Salvemini D, Slot JW, Heijnen HF, Hazen SL, Ischiropoulos H. Expression of inducible nitric-oxide synthase and intracellular protein tyrosine nitration in vascular smooth muscle cells: role of reactive oxygen species. J Biol Chem 2003;278:22901–22907. [DOI] [PubMed] [Google Scholar]
- 17.Heijnen HFG, van Donselaar E, Slot JW, Fries DM, Blachard-Fillion B, Hodara R, Lightfoot R, Polydoro M, Spielberg D, Thomson L, et al. Subcellular localization of tyrosine-nitrated proteins is dictated by reactive oxygen species generating enzymes and by proximity to nitric oxide synthase. Free Radic Biol Med 2006;40:1903–1913. [DOI] [PubMed] [Google Scholar]
- 18.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 2000;290:985–989. [DOI] [PubMed] [Google Scholar]
- 19.Birnboim HC, Lemay AM, Lam DK, Goldstein R, Webb JR. MHC class II-restricted peptides containing the inflammation-associated marker 3-nitrotyrosine evade central tolerance and elicit a robust cell-mediated immune response. J Immunol 2003;171:528–532. [DOI] [PubMed] [Google Scholar]
- 20.Herzog J, Maekawa Y, Cirrito TP, Illian BS, Unanue ER. Activated antigen-presenting cells select and present chemically modified peptides recognized by unique CD4 T cells. Proc Natl Acad Sci USA 2005; 102:7928–7933. [DOI] [PMC free article] [PubMed]
- 21.DiPiro JT, Howdieshell TR, Hamilton RG, Mansberger AR Jr. Immunoglobulin E and eosinophil counts are increased after sepsis in trauma patients. Crit Care Med 1998;26:465–469. [DOI] [PubMed] [Google Scholar]
- 22.Krupa A, Kato H, Matthay MA, Kurdowska AK. Proinflammatory activity of anti-IL-8 autoantibody:IL-8 complexes in alveolar edema fluid from patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 2004;286:L1105–L1113. [DOI] [PubMed] [Google Scholar]
- 23.Kurdowska A, Noble JM, Grant IS, Robertson CR, Haslett C, Donnelly SC. Anti-interleukin-8 autoantibodies in patients at risk for acute respiratory distress syndrome. Crit Care Med 2002;30:2335–2337. [DOI] [PubMed] [Google Scholar]
- 24.Takasaki J, Ogawa Y. Anti-interleukin-8 autoantibody in the tracheobronchial aspirate of infants with chronic lung disease. Pediatr Int 2001;43:48–52. [DOI] [PubMed] [Google Scholar]
- 25.Kurdowska A, Noble JM, Steinberg KP, Ruzinski JT, Hudson LD, Martin TR. Anti-interleukin 8 autoantibody: interleukin 8 complexes in the acute respiratory distress syndrome. Relationship between the complexes and clinical disease activity. Am J Respir Crit Care Med 2001;163:463–468. [DOI] [PubMed] [Google Scholar]
- 26.Maneta-Peyret L, Kitsiouli E, Lekka M, Nakos G, Cassagne C. Autoantibodies to lipids in bronchoalveolar lavage fluids of patients with acute respiratory distress syndrome. Crit Care Med 2001;29:1950–1954. [DOI] [PubMed] [Google Scholar]
- 27.Wiedermann FJ, Lederer W, Mayr AJ, Sepp N, Herold M, Schobersberger W. Prospective observational study of antiphospholipid antibodies in acute lung injury and acute respiratory distress syndrome: comparison with catastrophic antiphospholipid syndrome. Lupus 2003;12:462–467. [DOI] [PubMed] [Google Scholar]
- 28.Inoue H, Hisamatsu K, Ando K, Ajisaka R, Kumagai N. Determination of nitrotyrosine and related compounds in biological specimens by competitive enzyme immunoassay. Nitric Oxide 2002;7:11–17. [DOI] [PubMed] [Google Scholar]
- 29.Civil ID, Schwab CW. The abbreviated injury scale. 1985 revision: a condensed chart for clinical use. J Trauma 1988;28:87–90. [DOI] [PubMed] [Google Scholar]
- 30.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American European consensus conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed] [Google Scholar]
- 31.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adult. Chest 1991;100:1619–1636. [DOI] [PubMed] [Google Scholar]
- 32.Zheng L, Nukuna B, Brennan M-L, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest 2004;114:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binder CJ, Chang M-K, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med 2002;8:1218–1226. [DOI] [PubMed] [Google Scholar]
- 34.Souza JM, Choi I, Chen Q, Weisse M, Daikhin E, Yudkoff M, Obin M. Ara J, Horwitz J, Ischiropoulos H. Proteolytic degradation of tyrosine nitrated proteins. Arch Biochem Biophys 2000;169:360–366. [DOI] [PubMed] [Google Scholar]