Abstract
Glutaredoxins (GRX) are antioxidant enzymes that preferentially catalyze the reduction of protein-glutathione mixed disulfides. The formation of mixed disulfides with GSH is known as S-glutathionylation, a post-translational modification that is emerging as an important mode of redox signaling. Since asthma is a disease that is associated with increased oxidative stress and altered antioxidant defenses, we investigated the expression of GRX in a murine model of allergic airway disease. Sensitization and challenge of C57BL/6 mice with ovalbumin resulted in increased expression of GRX1 mRNA, as well as increased amounts of GRX1 protein and total GRX activity in the lung. Because GRX1 expression is prominent in bronchial epithelium, we isolated primary epithelial cells from mouse trachea to investigate the presence of GRX. Primary tracheal epithelial cells were found to express both GRX1 and 2 mRNA and detectable GRX activity. Treatment with IFN-γ increased the expression of GRX1 and overall GRX activity, resulting in attenuation of protein S-glutathionylation. In contrast, TGF-β1 caused decreased GRX1 expression and overall GRX activity, leading to markedly enhanced protein S-glutathionylation. GRX1 joins the cadre of antioxidant defenses known to be modulated during allergic airway inflammation.
Keywords: glutaredoxin, asthma, epithelium, IFN-γ, TGF-β
CLINICAL RELEVANCE
Glutaredoxin-1 can contribute to the disease pathology through alterations in protein S-glutathionylation.
Chronic inflammatory diseases of the lung, such as asthma, are accompanied by oxidative stress. The bulk of oxidant production has been attributed to inflammatory cells, which in human asthma consist of mainly eosinophils as well as neutrophils. In addition, during normal cellular respiration and aerobic metabolism as well as through activation of nonphagocytic NADPH oxidases (Nox) (1), resident epithelial cells can also generate oxidants. Finally, environmental exposures that cause asthma exacerbations increase the oxidative burden in the lungs.
Because oxidants can cause damage to macromolecules, lung tissue has evolved with a battery of antioxidant enzymes to protect against these oxidative insults and glutathione plays a major role herein. The importance of glutathione in protecting airspace epithelium against oxidant-mediated injury is underscored by the fact that its levels in epithelial lining fluid are ∼ 100-fold higher than in serum (2). As evidence of oxidative stress, oxidized glutathione levels (GSSG) were reported to be increased in BAL fluid, or induced sputum samples of patients with asthma compared with healthy control subjects (3, 4). Glutathione levels are regulated by a system of antioxidant enzymes that include glutathione peroxidases (GPx), glutathione reductase (GR), γ−glutamyl-cysteinyl synthase (γGCS), glutathione-S-transferase (GST), and glutaredoxins (GRX). Changes in some of these enzymes have been demonstrated in asthma, locally in lung tissue, as well as systemically. For instance, erythrocytes of patients with asthma display reduced GPx activity compared with control individuals (5), whereas levels of extracellular GPx levels were found to be increased in the lungs of individuals with asthma compared with control subjects (6). Polymorphisms in the GST-M1, GST-T1, and GST-P1 genes have also been associated with asthma (for review see Ref. 7). Decreases in activities of superoxide dismutases (8, 9) and catalase (10) in patients with asthma further contribute to enhanced oxidative stress.
As part of the antioxidant properties of glutathione, the tripeptide can conjugate with protein amino acid sulfhydryls through its proper thiol group to protect them directly against irreversible oxidations. The formation of protein mixed disulfides, also known as S-glutathionylation, has been demonstrated to occur in a number of proteins, like the transcription factors NF-κB (11) and AP-1 (12), under baseline conditions and is increased after oxidative stress. Since S-glutathionylation has been established to alter the function of these proteins, it is considered a post-translational modification through which oxidants can transduce signals, and serve as second messenger molecules. Mammalian GRX enzymes (thioltransferases) belong to the thioredoxin superfamily of enzymes and have been demonstrated to regulate this post-translational modification (13). GRXs are oxidoreductases that catalyze the reversible exchange of GSH with protein thiol groups. At high ratios of GSH/GSSG, as they occur in physiologic settings, the reduction of protein-glutathione mixed disulfides to restore the reduced sulfhydryl group is favored. This deglutathionylation reaction occurs through a monothiol mechanism (14) in which GRX itself is S-glutathionylated. The reduced state of GRX is restored by GSH coupled to GSSG reductase (15). Two mammalian GRX proteins have been identified to date. GRX1 is a cytosolic protein, whereas GRX2 contains a mitochondrial leader sequence but can also occur in the nucleus after alternative splicing (16, 17).
Studies on GRX enzymes in the lung are scant. To date GRX1 was found to be decreased in alveolar macrophages of patients with sarcoidosis and allergic alveolitis, but no difference was observed by immunohistochemistry between patients with interstitial pneumonia and control subjects (18). In addition, hyperoxia was found not to affect GRX expression (19). Given the importance of glutathione in maintaining the redox status of the lungs and the presence of oxidative stress in asthma, the goal of the current study was to investigate the expression of GRX in a mouse model of allergic airway disease. Furthermore, the modulation of GRX by cytokines that are relevant to allergic airway disease was examined in primary mouse tracheal epithelial cells.
MATERIALS AND METHODS
Animals
Six- to eight-week-old female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were housed in the University of Vermont Animal Facility. Mice were subjected to ovalbumin (OVA) sensitization and challenge as described elsewhere (20). The Institutional Animal Care and Use Committee granted approval for all studies.
Primary Cell Culture
Primary epithelial cells were isolated from C57BL/6 mice according to Wu and Smith (21) with minor modifications (22). For experiments, cells were plated on Collagen I–coated culture dishes or glass slides and switched to phenol red free DMEM/F12 media containing 2 mM l-Glutamine and P/S 24 h before initiation of experiments.
Semiquantitative PCR
Total RNA was isolated from lung using an RNeasy Mini Kit (Qiagen, Valencia, CA), DNase treated, and reverse transcribed into cDNA. Semiquantitative TaqMan PCR for GRX1 and 2 were performed (Applied Biosystems, Framingham, MA) and values were normalized to hypoxanthine guanine phosphoribosyl transferase (HPRT).
Immunohistochemistry
After mice were killed, lungs were instilled with 50% Tissue-Tek OCT Compound (Sakura Finetek Inc., Torrance, CA) in PBS and frozen in liquid nitrogen–chilled isopentane for the preparation of 10-μm frozen sections. Slides or cells were fixed with 4% paraformaldehyde (PFA), washed, and permeabilized with 1% Triton X-100 in PBS for 20 min. After blocking with 1% BSA in PBS (PBS/1% BSA), slides were incubated with rabbit anti-human GRX1 antibody (10 μg/ml; Labfrontier, Seoul, Korea) overnight at 4°C. After three washes in PBS/1% BSA, slides were incubated with goat anti-rabbit Cy3 secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) and counterstained with Sytox Green (Molecular Probes, Austin, TX) to label DNA. Slides were washed and coverslipped, and sections were scanned using an Olympus BX50 upright microscope configured to a Bio-Rad MRC 1024 confocal scanning laser microscope system (Bio-Rad, Hercules, CA) using a ×20 objective.
Western Blotting
Lung lysates were mixed with 2× Laemmli sample buffer, boiled, and loaded on polyacrylamide gels. Proteins were transferred to nitrocellulose, and Western blotting for GRX1 was performed using a GRX1 antibody (Labfrontier) as previously described (22).
GRX Activity Assay
GRX activity was assayed as previously described (23). Briefly, cells or lungs were lysed in buffer containing 137 mM Tris-HCl (pH 8.0), 130 mM NaCl, and 1% NP-40, cleared by centrifugation, and equalized for protein content. Before analysis of whole lung GRX activity, excess NADPH was removed from lung lysates through a Micro Bio-Spin 6 chromatography column (Bio-Rad). The lysates were incubated with reaction buffer containing 137 mM Tris-HCl (pH 8.0), 0.5 mM GSH, 1.2 units GSSG reductase (Roche, Branchburg, NJ), 0.35 mM NADPH, 1.5 mM EDTA (pH 8.0), and 2.5 mM Cys-SO3. The reaction was allowed to proceed at 30°C and NAPDH consumption was followed spectrophotometrically at 340 nm. Data are expressed as units, where 1 unit equals the oxidation of 1 μM NADPH/min/mg protein.
Assessment of GRX1-Catalyzed Cysteine Derivatization to Visualize S-Glutathionylation
Cells were grown on Collagen I–coated glass slides and exposed to test agents, and S-glutathionylation was assessed using GRX1-catalyzed cysteine derivatization. This newly developed assay (22), based on the biotin-switch procedure (24), uses a procedure that encompasses cell permeabilization and chemical blocking of reduced thiol groups using a buffer containing 25 mM Hepes, pH 7.7, 0.1 mM EDTA, 0.01 mM neocuproine, 20 mM N-ethylmaleimide, and 1% Triton X-100 for 30 min at 4°C, followed by GRX1-dependent reduction of protein-glutathione mixed disulfides using 27 μg/ml Escherichia coli or human GRX1 (American Diagnostica, Stamford, CT), 4 U/ml GSSG reductase (Roche), 1 mM GSH, 1 mM NADPH, and 1 mM EDTA in 50 mM Tris, pH 7.5, for 15 min at 37°C. Newly reduced sulfhydryl groups are labeled with 1 mM N-(3-maleimidylpropionyl) biocytin (MPB; Molecular Probes) for 1 h at room temperature and visualized using streptavidin–Alexa Fluor 568. Nuclei are counterstained with Sytox Green and slides were analyzed by confocal microscopy using a ×20 objective.
Statistical Analysis
In vivo data were expressed as mean ± SEM and compared by ANOVA. Differences were considered significant when P < 0.05. All experiments were repeated at least two times.
RESULTS
GRX Is Increased in Allergic Airway Disease
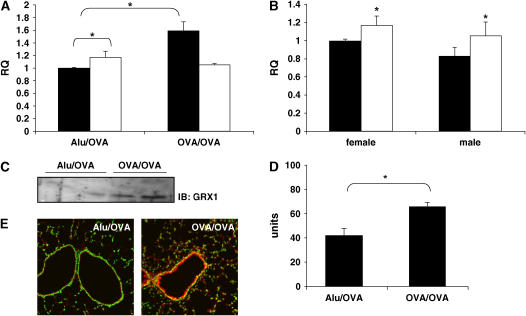
Immunization and challenge with OVA caused eosinophilic inflammation (25) and marked increases in levels of, among others, IL-4, -5, and −6; cytokine-induced neutrophil chemoattractant (KC); and monocyte chemotactic protein (MCP)1 in bronchoalveolar lavage fluid (N.L. Reynaert, unpublished observations). Total lung homogenates from mock-sensitized (Alu/OVA) mice expressed both GRX1 and GRX2 mRNA, in agreement with previous reports (18, 26). Basal expression of GRX2 mRNA in lung tissue was significantly higher then that of GRX1, which was also reported previously (26). Antigen sensitization and challenge (OVA/OVA) increased mRNA levels of GRX1, while not affecting GRX2 mRNA levels (Figure 1A). Since GRX1 mRNA expression was reported to be higher in the brain of female compared with male mice, and this was associated with protection against the induction of experimental Parkinson's disease (27), we investigated the differential expression of GRX in lungs of both sexes. Figure 1B demonstrates that in contrast to the brain, lungs of control male or female mice displayed no significant differences in the levels of either GRX1 or GRX2 mRNA, nor differences in GRX activity (data not shown). To corroborate increases in GRX1 mRNA expression in response to OVA, we performed Western blot analysis for GRX1. Results in Figure 1C demonstrate elevated levels of GRX1 protein in whole lung homogenates after sensitization and challenge with OVA when compared with mock-sensitized controls, which corresponded to increases in GRX activity (Figure 1D). Immunofluoresence analysis of lung sections revealed that in naïve mice GRX1 was localized to both airways and parenchyma, and increased predominantly in the airway epithelium in this experimental model of allergic airway disease (Figure 1E). Collectively, these data indicate that lung GRX1, but not GRX2, expression increases in allergic airway disease and that this corresponds to elevated levels of GRX activity.
Figure 1.
GRX is increased in allergic airway disease. (A) RNA was collected from lungs 48 h after the last challenge, reverse-transcribed, and analyzed for GRX1 (filled bars) and GRX2 (open bars) expression relative to HPRT by TaqMan PCR. Data are expressed as mean relative quantitation (RQ) from five mice per group (± SEM). *P < 0.05. (B) RNA was collected from lungs of mock-sensitized male and female mice 48 h after the last challenge and analyzed for GRX1 (filled bars) and GRX2 (open bars) expression relative to HPRT by TaqMan PCR. Data are expressed as mean RQ from five mice per group (± SEM). *P < 0.05 between GRX1 and GRX2. (C) Western blotting for GRX1 was performed on lung lysates. (D) GRX activity was assessed in lung lysates as described in Materials and Methods. Result is representative of three experiments and values are mean from three mice per group (± SEM). (E) Frozen sections were stained with an antibody directed against GRX1, followed by incubation with a Cy3-conjugated secondary antibody (red). Sytox Green was used as a nuclear counterstain, and sections were scanned by confocal microscopy. Images are representative of results from five to six mice per group.
Regulation of GRX1 and S-Glutathionylation by IFN-γ and TGF-β1 in vitro
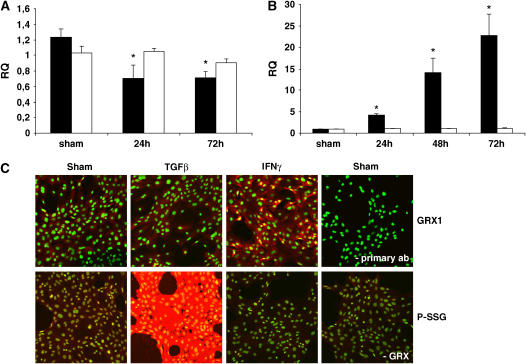
Since little information exists on the regulation of GRX expression and activity, we exposed primary mouse tracheal epithelial cells to cytokines relevant to asthma. We focused on tracheal epithelial cells because these cells demonstrated high levels of GRX expression (Figure 1E) and are known to play an important role in the defense against oxidants and the regulation of innate and adaptive immune responses (20). While no significant effects were observed in response to TNF-α on GRX activity, LPS, CpG DNA, IL-4, or TGF-β1 decreased GRX enzymatic activity (Table 1). In contrast, IL-13 and IFN-γ were the only mediators tested that caused an increase in GRX activity (Table 1). Since TGF-β1 and IFN-γ caused the strongest modulation of GRX activity, we further evaluated GRX expression profiles and protein S-glutathionylation in response to these cytokines. Consistent with decreases in GRX activity, TGF-β1 caused attenuated expression of GRX1 mRNA, while not affecting GRX2 mRNA levels (Figure 2A). Although we could not detect clear decreases in GRX1 protein levels in response to TGF-β1 compared with controls (Figure 2C, upper panels) via immunofluorescence, possibly due to the low level of staining present in control cells, TGF-β1 did result in marked increases in protein S-glutathionylation (Figure 2C, lower panels). Consistent with the increases in GRX activity in cells exposed to IFN-γ (Table 1), exposure to IFN-γ resulted in increased expression of GRX1 mRNA, whereas GRX2 mRNA levels were not affected (Figure 2B). Concomitant increases in GRX1 protein levels (Figure 2C, upper panels), and decreases in protein S-glutathionylation (Figure 2C, lower panels) were also detected by immunofluorescence in cells exposed to IFN-γ.
TABLE 1.
GLUTAREDOXIN ACTIVITY IN PRIMARY AIRWAY EPITHELIAL CELLS
| Fold Change | P Value | |
|---|---|---|
| Sham | 1 | |
| TNF-α | 0.76 ± 0.07 | 0.19 |
| LPS | 0.89 ± 0.07 | 0.03 |
| CpG DNA | 0.71 ± 0.003 | 0.03 |
| IL-13 | 1.42 ± 0.27 | 0.01 |
| IL-4 | 0.73 ± 0.07 | 0.03 |
| TGF-β1 | 0.60 ± 0.02 | 0.01 |
| IFN-γ | 1.93 ± 0.05 | 0.002 |
Cells were treated for 48 h with each agent at the following concentrations: 20 ng/ml TNF-α, 5 μg/ml LPS, 1 μg/ml CpG DNA, 10 pg/ml IL-13, 20 ng/ml IL-4, 10 ng/ml TGF-β1, or 20 pg/ml IFN-γ, and GRX activity was determined.
Figure 2.
Regulation of GRX1 and S-glutathionylation by IFN-γ and TGF-β1 in vitro. (A) Primary airway epithelial cells were treated with 5 ng/ml TGF-β1 or (B) 20 pg/ml IFN-γ; for the indicated time frames, mRNA was collected, reverse transcribed, and analyzed for GRX1 (filled bars) and GRX2 (open bars) expression relative to HPRT by TaqMan PCR. Data are expressed as mean (± SD) and *P < 0.05. (C) Primary airway epithelial cells were treated with 5 ng/ml TGF-β1 or 20 pg/ml IFN-γ for 48 h. Upper panels: Immunohistochemistry for GRX1 was performed (red) and nuclei were counterstained with Sytox Green (green). Lower panels: GRX1-reversible cysteine oxidation was performed (red) and nuclei were counterstained with Sytox Green (green). As reagent controls, either primary antibody (top) or GRX (-GRX, bottom) were omitted from the reaction mix.
DISCUSSION
In this study we identified GRX1 as part of the antioxidant defense system of the lungs that is up-regulated during allergic airway disease. Although basal expression levels of GRX2 mRNA were higher than GRX1, only increases in GRX1 mRNA were detected in lung tissue of mice with allergic airway disease (Figure 1). The two isoforms of GRX do not only differ in their intracellular localization (16, 17), but evidence is emerging that they might be differentially regulated as well. Recent studies have indicated that GRX2 is a redox sensor that is inactive as a dimer through the formation of an 2Fe-2S cluster. Upon oxidative stress, GRX2 is activated through oxidation of the 2Fe-2S cluster and its subsequent monomerization (28). Our present work demonstrates that GRX1 mRNA levels are increased after treatment with IFN-γ while conversely TGF-β1 treatment led to repressed expression of GRX1 mRNA (Figures 2B and 2A, respectively). These findings also suggest that GRX1 is regulated at the transcriptional level, whereas GRX2 may be regulated post-translationally. Such diverse regulatory mechanisms would allow for quick activation of GRX2 upon oxidative stress to confer rapid restoration of sulfhydryl groups, whereas GRX1 would provide delayed but sustained protection.
In the current study we report that IFN-γ enhanced GRX1 mRNA levels in primary tracheal epithelial cells. The antioxidant enzymes thioredoxin reductase (29) and MnSOD (30) have also been demonstrated to be positively regulated by IFN-γ. The promoter region of the human GRX1 gene, however, does not contain GAS elements (31), and IFN-γ is therefore likely to act in concert with other transcription factors to induce GRX1. Alternatively, given the late induction of GRX1 mRNA after IFN-γ stimulation, it is possible that intermediate IFN-γ–driven factors, such as IFN regulatory factors 1 and 2, are involved in inducing GRX1 transcription in a type 2 IFN response. Repression of GRX1 expression by TGF-β1 has been observed previously (18). Surprisingly, a repressive effect of TGF-β1 has also been reported toward multiple other antioxidant enzymes, like for instance catalase, MnSOD, Cu,ZnSOD (32), γ-glutamylcysteinyl synthetase (33). Although the mechanism and functional outcome of repression of antioxidant defenses by TGF-β1 remain elusive, several potential interactions between TGF-β1 and oxidants/antioxidants in the lung have been described, and it is thought that oxidants and TGF-β1 may co-operate to enhance fibrotic responses, for review (34). Altered levels of protein S-glutathionylation in response to TGF-β1 or IFN-γ could be the result of not only alterations in GRX1 expression and overall GRX activity, but could also arise from changes in total GSH and GSH/GSSG ratios. For instance, it has been determined in the past that TGF-β1 decreases GSH synthesis (33), which can lower GSH/GSSG and drive S-glutathionylation by GRX. To date, no clear data on the effects of IFN-γ on GSH are available, and further assessment of the contribution of altered GSH/GSSG levels or ratios induced by TGF-β1 or IFN-γ to changes in S-glutathionylation will be needed to clarify these issues.
Asthma or allergic airway disease is characterized by a Th-2 bias. GRX1 expression is elevated during allergic airway disease, but GRX1 appears to be negatively regulated by at least two Th2-associated cytokines, TGF-β1 and IL-4, whereas the Th1 cytokine IFN-γ positively impacted on GRX1 expression. While at first glance these results seem contradictory, Th1 co-development has been demonstrated to occur during allergic airway inflammation (35), and IFN-γ in particular has been found to be elevated in mouse models that use similar protocols to the one used in this current study (36). Increases in GRX1 and total GRX activity during allergic airway disease could be part of the protective antioxidant defense of the lung. Through its deglutathiolating activity, GRX1 could be responsible for liberating GSH from proteins to increase GSH levels to combat the oxidant stress associated with the inflammatory response. However, further evaluation of changes in GSH/GSSG ratios and quantitative assessment of protein S-glutathionylation in allergic airways disease models are needed to corroborate these possibilities. In addition, alterations in the kinetics and extent of protein S-glutathionylation that occur in association with alterations in GRX1 expression and activity could play an important role in the disease process by affecting cell signaling cascades. For instance, S-glutathionylation of the p50 and c-Jun subunit of the transcription factors NF-κB and AP-1 have been demonstrated to prevent DNA binding and consequent inflammatory gene transcription (11, 12). Alternatively, enhanced S-glutathionylation of protein tyrosine phosphatase 1B after NADPH oxidase activation in macrophages is critical to the activation of tyrosine kinases (37). Additional studies aimed at unraveling changes in S-glutathionylation of critical protein targets will need to be implemented to formally test these scenarios.
In vivo GRX1 expression appears to be mainly localized to airway epithelium (Figure 1E). Airway epithelium represents an important barrier of the lungs and confers antioxidant protection. It is therefore not surprising that GRX1 expression is prominently localized in epithelia under basal conditions, in addition to being up-regulated during allergic airway inflammation (Figure 1E). However, a previous study reported that human GRX1 was mainly localized in alveolar macrophages, and only weakly to bronchial epithelium (18), which might reflect differences in GRX expression among different species.
In summary, the present study provides the first evidence that expression of GRX is altered in mice with allergic airway inflammation. Given the emerging significance of GRX catalytic activity both in antioxidant defenses and cell signaling (13), it is plausible that this newly recognized enzyme could play a critical role in the etiology of allergic airway inflammation.
This work was supported by NIH RO1 HL60014, HL60812, Public Health Service P20 RL15557 (NCRR COBRE), PO1 HL67004, and a grant from the Dutch Asthma Foundation.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0259RC on September 15, 2006
Conflict of Interest Statement: N.L.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.F.M.W. is a member of the scientific advisory boards for GlaxoSmithKline (GSK), Boehringer Ingelheim, AstraZeneca, and Numico and received lecture fees from GSK, AstraZeneca, Boehringer Ingelheim. He received research grants between 2003 and 2005 from GSK, AstraZeneca, Boehringer Ingelheim, Centocor, and Numico. Y.M.W.J.-H. received $1,500 from Seprocor for a lecture given in the Research Triangle Park in January of 2005. A patent application that covers GRX dependent cysteine derivatization and subsequent detection of s-glutathionylated proteins is pending.
References
- 1.Forteza R, Salathe M, Miot F, Forteza R, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol 2005;32:462–469. [DOI] [PubMed] [Google Scholar]
- 2.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol 1987;63:152–157. [DOI] [PubMed] [Google Scholar]
- 3.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet 1999;354:482–483. [DOI] [PubMed] [Google Scholar]
- 4.Dauletbaev N, Rickmann J, Viel K, Buhl R, Wagner TO, Bargon J. Glutathione in induced sputum of healthy individuals and patients with asthma. Thorax 2001;56:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mak JC, Leung HC, Ho SP, Law BK, Lam WK, Tsang KW, Ip MS, Chan-Yeung M. Systemic oxidative and antioxidative status in Chinese patients with asthma. J Allergy Clin Immunol 2004;114:260–264. [DOI] [PubMed] [Google Scholar]
- 6.Comhair SA, Bhathena PR, Farver C, Thunnissen FB, Erzurum SC. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. FASEB J 2001;15:70–78. [DOI] [PubMed] [Google Scholar]
- 7.Malerba G, Pignatti PF. A review of asthma genetics: gene expression studies and recent candidates. J Appl Genet 2005;46:93–104. [PubMed] [Google Scholar]
- 8.Comhair SA, Xu W, Ghosh S, Thunnissen FB, Almasan A, Calhoun WJ, Janocha AJ, Zheng L, Hazen SL, Erzurum SC. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am J Pathol 2005;166:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Raeve HR, Thunnissen FB, Kaneko FT, Guo FH, Lewis M, Kavuru MS, Secic M, Thomassen MJ, Erzurum SC. Decreased Cu,Zn-SOD activity in asthmatic airway epithelium: correction by inhaled corticosteroid in vivo. Am J Physiol 1997;272:L148–L154. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, Zheng L, Kaveti S, Kinter M, Hazen SL, et al. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol 2006;176:5587–5597. [DOI] [PubMed] [Google Scholar]
- 11.Pineda-Molina E, Klatt P, Vazquez J, Marina A, Garcia de Lacoba M, Perez-Sala D, Lamas S. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 2001;40:14134–14142. [DOI] [PubMed] [Google Scholar]
- 12.Klatt P, Molina EP, Lamas S. Nitric oxide inhibits c-Jun DNA binding by specifically targeted S- glutathionylation. J Biol Chem 1999;274:15857–15864. [DOI] [PubMed] [Google Scholar]
- 13.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal 2005;7:348–366. [DOI] [PubMed] [Google Scholar]
- 14.Gravina SA, Mieyal JJ. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry 1993;32:3368–3376. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Jao S, Nanduri S, Starke DW, Mieyal JJ, Qin J. Reactivity of the human thioltransferase (glutaredoxin) C7S, C25S, C78S, C82S mutant and NMR solution structure of its glutathionyl mixed disulfide intermediate reflect catalytic specificity. Biochemistry 1998;37:17145–17156. [DOI] [PubMed] [Google Scholar]
- 16.Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV, Lou MF. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J Biol Chem 2001;276:30374–30380. [DOI] [PubMed] [Google Scholar]
- 17.Lundberg M, Johansson C, Chandra J, Enoksson M, Jacobsson G, Ljung J, Johansson M, Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J Biol Chem 2001;276:26269–26275. [DOI] [PubMed] [Google Scholar]
- 18.Peltoniemi M, Kaarteenaho-Wiik R, Saily M, Sormunen R, Paakko P, Holmgren A, Soini Y, Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum Pathol 2004;35:1000–1007. [DOI] [PubMed] [Google Scholar]
- 19.Barrios R, Shi ZZ, Kala SV, Wiseman AL, Welty SE, Kala G, Bahler AA, Ou CN, Lieberman MW. Oxygen-induced pulmonary injury in gamma-glutamyl transpeptidase-deficient mice. Lung 2001;179:319–330. [DOI] [PubMed] [Google Scholar]
- 20.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol 2002;160:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu R, Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 1982;18:800–812. [DOI] [PubMed] [Google Scholar]
- 22.Reynaert NL, Ckless K, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. In situ detection of S-glutathionylated proteins following glutaredoxin-1 catalyzed cysteine derivatization. Biochim Biophys Acta 2006;1760:180–187. [DOI] [PubMed] [Google Scholar]
- 23.Gan ZR, Wells WW. Purification and properties of thioltransferase. J Biol Chem 1986;261:996–1001. [PubMed] [Google Scholar]
- 24.Jaffrey SR, Snyder SH. The biotin switch method for the detection of s-nitrosylated proteins. Sci STKE 2001;2001:L1. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol 2001;281:L394–L402. [DOI] [PubMed] [Google Scholar]
- 26.Jurado J, Prieto-Alamo MJ, Madrid-Risquez J, Pueyo C. Absolute gene expression patterns of thioredoxin and glutaredoxin redox systems in mouse. J Biol Chem 2003;278:45546–45554. [DOI] [PubMed] [Google Scholar]
- 27.Kenchappa RS, Diwakar L, Annepu J, Ravindranath V. Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J 2004;18:1102–1104. [DOI] [PubMed] [Google Scholar]
- 28.Lillig CH, Berndt C, Vergnolle O, Lonn ME, Hudemann C, Bill E, Holmgren A. Characterization of human glutaredoxin 2 as iron-sulfur protein: a possible role as redox sensor. Proc Natl Acad Sci USA 2005;102:8168–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soderberg A, Sahaf B, Rosen A. Thioredoxin reductase, a redox-active selenoprotein, is secreted by normal and neoplastic cells: presence in human plasma. Cancer Res 2000;60:2281–2289. [PubMed] [Google Scholar]
- 30.Harris CA, Derbin KS, Hunte-McDonough B, Krauss MR, Chen KT, Smith DM, Epstein LB. Manganese superoxide dismutase is induced by IFN-gamma in multiple cell types. Synergistic induction by IFN-gamma and tumor necrosis factor or IL-1. J Immunol 1991;147:149–154. [PubMed] [Google Scholar]
- 31.Park JB, Levine M. The human glutaredoxin gene: determination of its organization, transcription start point, and promoter analysis. Gene 1997;197:189–193. [DOI] [PubMed] [Google Scholar]
- 32.Kayanoki Y, Fujii J, Suzuki K, Kawata S, Matsuzawa Y, Taniguchi N. Suppression of antioxidative enzyme expression by transforming growth factor-beta 1 in rat hepatocytes. J Biol Chem 1994;269:15488–15492. [PubMed] [Google Scholar]
- 33.Arsalane K, Dubois CM, Muanza T, Begin R, Boudreau F, Asselin C, Cantin AM. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol 1997;17:599–607. [DOI] [PubMed] [Google Scholar]
- 34.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med 2005;172:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randolph DA, Stephens R, Carruthers CJ, Chaplin DD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest 1999;104:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 2004;173:7003–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rinna A, Torres M, Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic Biol Med 2006;41:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]