Abstract
Angiogenesis is crucial for tumor biology. There are many mechanisms by which tumors induce angiogenesis. We hypothesize that each individual tumor develops a unique mechanism to induce angiogenesis, and that activation of a particular angiogenic pathway suppresses the evolution of alternative pathways. We characterized 168 human non–small cell lung cancer (NSCLC) specimens for levels of angiogenic factors (angiogenic CXC chemokines, basic fibroblast growth factor, and vascular endothelial growth factor). We also induced lung tumor formation in A/J mice by injecting the tobacco carcinogen NNK. We dissected individual lung tumors and measured expression of angiogenic factors from three distinct families using real-time PCR. Finally, we controlled the angiogenic milieu using in vivo models to determine the resultant phenotype of the angiogenic factors expressed by NSCLC cells. Human tumors displayed marked variation in the expression of angiogenic factors. Individual mouse tumors, even from within the same mouse, displayed variability in their pattern of expression of angiogenic factors. In a sponge model of angiogenesis using murine lung cancer cells, implanting LLC cells with an angiogenic factor suppressed the expression of other angiogenic factors in implanted sponges. This suppressive effect was not seen in vitro. We conclude that lung cancer tumors evolve a unique and dominant angiogenic phenotype. Once an angiogenic pathway is activated, it may allow for tumor growth to proceed in the absence of a selection pressure to activate a second pathway.
Keywords: angiogenesis, mouse model, chemokines, cytokines, carcinogen
CLINICAL RELEVANCE
This research demonstrates the diversity of mechanisms by which lung cancers induce angiogenesis and should change how the results of anti-angiogenic factors are viewed.
Angiogenesis is crucial for tumor growth and metastasis. The angiogenic switch refers to a phenotypic change that occurs early in a tumor's development that is necessary for growth beyond 2–3 mm in size. Hypoxia is the trigger for many of the mechanisms by which a tumor induces angiogenesis. For example, hypoxia induces the expression of hypoxia-inducible factor (HIF-1), which in turn up-regulates vascular endothelial growth factor (VEGF) (1). Another family of pro-angiogenic factors includes the CXC chemokines containing the glutamic acid-leucine-arginine (ELR) motif, which in humans includes CXCL1, CXCL3, CXCL5, and CXCL8 (2). We and others have described proangiogenic properties of this family of chemokines, which are produced by tumor cells and infiltrating stromal cells in lung cancer and other malignancies (3–6). These are largely produced by NF-κB transcriptional activation, and represent an alternative pathway for the angiogenic switch (7). Other important factors that promote tumor angiogenesis either directly or indirectly include basic fibroblast growth factor (bFGF), macrophage migration inhibitory factor (MIF), angiopoietin 1, and epidermal growth factor (EGF), among others.
Many studies describe the mechanism by which individual factors promote angiogenesis in vitro or in vivo. Studies of tumor-derived angiogenic factors have typically focused on one particular angiogenic pathway within a given tumor type. Likewise, many clinical trials employing anti-angiogenic therapies have focused primarily on blocking one particular anti-angiogenic strategy such as VEGF (8). Malignant tumors, however, demonstrate phenotypic variability even within the same histologic subtype. There is no reason to suspect that this variation should not also be present in the angiogenic switch. For example, we have shown that resected non-small cell lung cancer (NSCLC) specimens contain VEGF, as well as ELR+ CXC chemokines, and MIF. Based upon these human data, however, it is not clear whether such differences reflect the inherent variability that one might expect from the study of human tissues. The question of the variability and plasticity of the angiogenic switch requires further study in a more controlled experimental setting. This variability has important implications for the rational design of anti-angiogenic therapy.
Given these observations, we hypothesize that during tumorigenesis, initial selection pressures or genetic alterations lead to the development of one dominant angiogenic strategy. Furthermore, activation of a particular angiogenic pathway may suppress the expression of other angiogenic factors, as the angiogenic switch relieves the constraints imposed upon a developing tumor by hypoxia and/or nutrient deprivation. In this report, we support our hypothesis with data demonstrating that tumors from human lung cancer specimens express a pattern of angiogenic factors that suggests activation of one dominant factor, or “angiogenic signature” in each tumor. This angiogenic signature depends, in part, on the presence of mutant K-ras. Furthermore, we show that carcinogen-induced murine lung tumors in vivo, even from within the same mouse, vary in their angiogenic profiles. Finally, we show that the angiogenic “profile” of tumor cells has plasticity and can be altered in the short term in a sponge model of angiogenesis. This paradigm of variability and plasticity in the angiogenic switch has prognostic and therapeutic implications in NSCLC and other cancers.
MATERIALS AND METHODS
Reagents, Cell Lines, and Animals
Purified and biotinylated antibodies to human and murine VEGF, the human CXC Chemokines CXCL1, 3, 5, and 8, and the murine homologs CXCL-1, -2, and -5 were obtained from R&D Systems (Minneapolis, MN). Antibodies to Factor VIII and corresponding controls were obtained from DakoCytomation (Glostrup, Denmark). Semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) reagents were obtained from Applied Biosystems (Foster City, CA); Master Mix, Assay-On-Demand primers, and 96-well optical reaction plates. RT-PCR reagents (M-MLV reverse transcriptase, DTT 0.1M, Oligo-dT, dNTP mix) were obtained from Invitrogen (Carlsbad, CA). Polyvinyl alcohol sponge material was obtained from Rippey Corporation (El Dorado Hills, CA). Hypoxyprobe kit was obtained from Chemicon International (Temecula, CA). Murine Lewis Lung Carcinoma cells (LLC) were obtained from American Type Culture Collection (ATCC, Manassas, VA). C57BL6 and A/J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in specific pathogen–free conditions. CXCR2 knockout mice (C57B6) were also obtained from Jackson Labs.
Human Tumor Processing
Tissue specimens were obtained from consecutive individuals undergoing thoracotomy (n = 168) for suspected NSCLC. All of the patients provided informed consent, and tissue was obtained with approval of both the University of Michigan Tissue Procurement Core and Institutional Review Board. Standardized punch biopsy samples of tumor and normal lung distal to the tumor were homogenized and sonicated in Complete antiprotease buffer (Boehringer, Ingelheim, Germany) on recovery from the operating room. Specimens were centrifuged at 900 × g for 15 min, filtered through 0.45-μm Sterile Acrodiscs (Gelman Sciences, Inc., Ann Arbor, MI), and frozen at −70°C until thawed for assay by a specific enzyme-linked immunosorbent assay (ELISA). In addition, an adjacent portion of tumor was fixed in 10% zinc formalin and embedded in paraffin for histologic analysis. When size permitted, a portion of each tumor was homogenized in Trizol for extraction of mRNA using the manufacturer instructions (Invitrogen). University of Michigan Hospital pathologists determined the final histologic diagnosis. For each patient, smoking status was determined as current (smoking within the last 6 mo), former (quit for > 6 mo), or never (see Table 1).
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF THE 168 PATIENTS REPRESENTED IN OUR HUMAN TUMOR ANGIOGENIC PROFILES
| Stage | |
| I | 66.2 |
| II | 16.9 |
| IIIa | 8.7 |
| IIIb | 4.8 |
| IV | 3.4 |
| Male, % | 52 |
| Female, % | 48 |
| Smokers, % | |
| Current* | 11.5 |
| Ex | 81.3 |
| Never | 7.2 |
| Histology | |
| Adenocarcinoma | 48 |
| Squamous | 32 |
| NSCLC | 12 |
| Large Cell | 6 |
| BAC | 2 |
Definition of abbreviations: BAC, bronchioloalveolar cell carcinoma; NSCLC, non–small cell lung cancer, not otherwise specified by the pathologist.
All patients refrained from tobacco use for at least 4 wk before surgery. Ex-smokers are defined as no tobacco use for 6 mo before surgery.
ELISA
Aqueous tumor and normal lung extracts were subjected to specific ELISA according to our protocol published previously (9). All of the tissue samples were run in parallel for total protein content (Pierce, Rockford, IL), and results were standardized by expressing values as ng of cytokine per mg total protein. Because the angiogenic CXC chemokines are all of equivalent potency in terms of angiogenic activity and mediate their angiogenic effects via the same receptor (CXC receptor 2), we generated a combined “ELR score” for each of the human tumors (9), representing the sum of the levels (in ng of cytokine per mg of protein; ng/mg) of all of the measured CXC chemokines bearing the ELR motif (glu-leu-arg, shared by the angiogenic CXC chemokines)—that is, total levels of IL-8/CXCL8, ENA-78/CXCL5, GROα/CXCL1, and GROγ/CXCL3 for human tumors, and murine CXCL1, -2, and 5 for murine tumors.
Detection of K-ras Codon 12 Mutations
We used a modification of the method described by Kubrusly and coworkers (10) to detect activating mutations in codon 12 of the K-ras gene in cDNA from a subset of archived tumor specimens. We used the forward PCR primers 5′ ACT GAA TAT AAA CTT GTG GTA GTT GGA CCT-3′ (complimentary to bases 3–33 of the cDNA with a mismatch in the underlined base introducing a unique restriction site into the product when the sequence is wild-type). The reverse primer (corresponding to bases 139–150) was 5′CCA AGAGAC AGG TTT CTC CAT C 3′. The PCR reactions were performed in a reaction volume of 50 μl using the Promega Access PCR Kit (Catalog #A1250, Madison, WI) and 5.0 μl of tumor cDNA as the template (∼ 100 ng/sample), with the following parameters; 30 cycles of 96°C for 1 min for, 55°C for 1 min and 73°C for 30 s for extension. PCR products (10-μl aliquots) were subject to digestion with 4U BstNI (Roche Diagnostics, Mannheim Germany) for 90 min at 37°C, and resolved in 3% agarose gels containing ethidium bromide. Using these exonic primers, wild-type K-ras yields two bands of 127 and 30 bp, where tumors containing mutant K-ras showed an additional band of 157 bp corresponding to the noncutting mutant allele. Positive control cDNA was derived from A549 lung cancer cells, and negative control from normal human bronchial epithelial cells. Bands of selected samples were purified from the gel using Qiagen gel purification kits and submitted to the University of Michigan DNA sequencing core, where sequencing confirmed the identity of the products as homologous with mutant and wild-type K-ras.
Lung Tumor Induction
Female A/J mice (6–8 wk old) received intraperitoneal injections of 4-(methylnitrosamino)-1-(3-pyridyl)-butanone (NNK), 10 μmol (2 mg), as previously described (11). We killed animals at 6 mo after injection and removed the lungs for processing. With the use of a dissecting microscope, we removed individual lung tumors and homogenized them in Trizol (Invitrogen) for RNA analysis or Complete Lysis Buffer (Roche Diagnostics) for ELISA. A section of normal lung from an age-matched mouse not treated with NNK was removed and processed in identical fashion. This was used as the reference in real-time PCR reactions.
To generate larger tumors, we killed some mice 9 mo after NNK, and removed all visible tumors. We homogenized these tumors, then sonicated and filtered the aqueous extracts to perform ELISA for murine angiogenic cytokines. We were not able to generate a sufficient ELISA assay for murine bFGF, and these tumors were therefore only measured for their levels of mVEGF, as well as mCXCL1, mCXCL2, and mCXCL5, the functional murine equivalents of the human angiogenic CXC chemokines. Results were expressed as ng/mg, and the levels of CXC chemokines were summed to determine the “ELR score” as previously described (9).
RNA Processing and Semiquantitative Real-Time RT-PCR
We isolated RNA using manufacturer instructions provided with Trizol reagent (Invitrogen). Reverse transcription of mRNA to make cDNA copies was conducted using M-MLV reverse transcriptase (Invitrogen) and following manufacturer's protocols. Semi-quantitative real-time RT-PCR was performed on an ABI PRISM 7,000 Sequence Detection System apparatus (Applied Biosystems) using primers and probes as described above, and β-actin to normalize results. A section of normal lung from an untreated mouse served as the reference value to which all other measurements from tumors were normalized. The PCR reaction was performed in 25-μl volume containing 1 μl of cDNA per reaction. Data were analyzed with the sequence detection system software. Relative gene expression was calculated using the comparative cycle-threshold (CT) method.
Sponge Model of Angiogenesis
We used a sponge angiogenesis assay modified from the method described by Fife and colleagues (12). We shaved the flanks of 6-wk-old C57/B6 mice and anesthetized them with ketamine 100 mg/kg and xylazine 7.0 mg/kg. Immediately before implantation, we combined 106 LLC cells/ml with 2.5 μg/ml of the angiogenic substance of interest (mVEGF or mCXCL1) in serum-free media (Dulbecco's modified Eagle's medium). Two hundred microliters of the cell suspension was adsorbed onto to sterile 9-mm-diameter polyvinyl alcohol sponge discs (Rippey Corporation). Sponges containing LLC cells in media alone served as controls. In some experiments we used mice deficient in the receptor for angiogenic CXC chemokines, CXCR2 (CXCR2−/− mice). Under sterile conditions, a small incision was made and a sponge was inserted in each flank. After 72 h, we killed the animals and removed the sponges. Some sponges were placed immediately in Trizol and homogenized thoroughly; others were placed in 10% neutral buffered zinc-formalin; and a third group was homogenized in anti-protease buffer, filtered, and frozen for later analysis by ELISA. We briefly centrifuged the homogenate and filtered it (2.0 μm) to remove sponge debris. We used the supernatant to isolate mRNA, reverse transcribe to cDNA, and perform semiquantitative RT-PCR, as described above. The reference for all RT-PCR reactions was LLC cells cultured in vitro. Some of the sponges were homogenized in anti-protease buffer to perform ELISA.
Immunohistochemistry
After removal of mouse lungs, we fixed a portion of tumor in 10% zinc formalin. Paraffin-embedded tissue sections were dewaxed in xylene and rehydrated through graded concentrations of ethanol. Antigen retrieval was performed with 0.1% trypsin/CaCl2 for 30 min at 37°. Samples were then stained with appropriate primary antibodies for angiogenic factors, as previously described (13). Antigens were visualized with Vectastain ABC kits and diaminobenzidine (DAB) as the chromogen (Vector, Burlingame, CA) as suggested by the manufacturer. For all sections, we performed an appropriate control goat IgG.
We administered pimonidazole reagent, 60 mg/kg (Hypoxyprobe-1; Chemicon), to select animals 30 min before killing via tail vein injection. After killing, we removed the lungs and fixed/embedded them as described above. We performed immunohistochemistry according to the manufacturer's instructions, using Hypoxyprobe monoclonal antibody (Hypoxyprobe-1 kit).
Statistics
Graph Pad Prism 3.0 (San Diego, CA) was used for statistical analysis and plotting of results. Differences between groups were analyzed using Student's t test. For multiple comparisons, we used one-way ANOVA with Bonferroni's post-test analysis.
RESULTS
Human NSCLC Specimens Can Be Segregated on the Basis of the Angiogenic Factors They Express
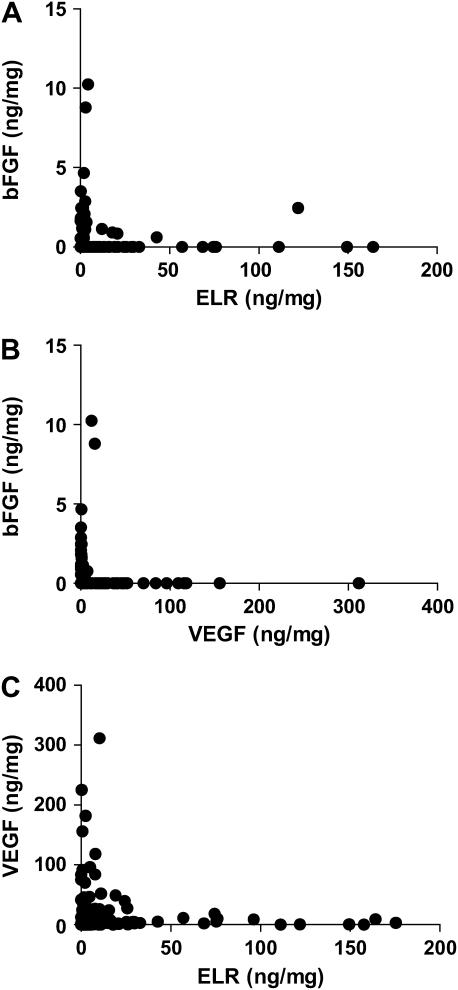
Using ELISA of tumor homogenate, we measured the tumor-associated levels of angiogenic factors from three different families: VEGF, bFGF, and the ELR-CXC chemokines (the ELR score; see Materials and Methods) (9). We chose these based upon previous studies demonstrating the importance of these pathways in mediating lung cancer angiogenesis (9, 14–19). Figure 1 compares the expression levels of these angiogenic factors in tumors from 168 patients with NSCLC resected at our institution (Demographics in Table 1). With the exception of 7 out of 168, tumors expressing bFGF did not express ELR-positive CXC chemokines (Figure 1A) or VEGF (Figure 1B, P < 0.0001 by χ2). Consistent with our previously published data (9), we did observe tumors with simultaneous expression of VEGF and the angiogenic CXC chemokines. However, when we divided tumors into groups based upon levels of cytokines above (high) and below (low) the median, the frequency of tumors in our cohort with both high VEGF levels and high ELR score (10.1%; Figure 1C), is less than would be expected by chance alone (25%; P = 0.013 by χ2). This supports our hypothesis that expression of a given angiogenic factor(s) reduces the likelihood of activation of a second pathway to promote angiogenesis. There was no correlation between the histology, stage, smoking status, or sex and the profile of angiogenic factors detected in any given tumor. These data suggest that tumors develop unique “angiogenic signatures” characterized by the expression of a dominant angiogenic factor.
Figure 1.
Comparison of levels of angiogenic factors measured by ELISA in homogenates of NSCLC tumors from 168 patients. (A) bFGF (ng/mg) compared with the ELR score (summation of ELR CXC chemokines CXCL1, CXCL3, CXCL5, and CXCL8 in ng of cytokine per mg of total protein). (B) Comparison of VEGF with bFGF. (C) Comparison of VEGF with the ELR score.
To determine if the expression of a dominant angiogenic factor(s) was associated with specific mutations, we determined the presence or absence of activating mutations on codon 12 of the K-ras gene in a subset of tumors, and compared the levels of chemokines (ELR score) and VEGF in groups defined by their K-ras status. Table 2 demonstrates that the presence of K-ras mutations was associated with lower levels of VEGF (P = 0.01), and a trend toward increased levels of the CXC chemokines (the ELR score; P = 0.08), suggesting that oncogenic K-ras favors the development of a chemokine predominant angiogenic signature.
TABLE 2.
THE PRESENCE OF MUTATIONS IN K-ras AND LEVELS OF VEGF AND CXC CHEMOKINES (ELR SCORE) IN THE CORRESPONDING TUMOR FROM A SUBSET OF TUMORS TAKEN FROM OUR DATABASE
| K-ras codon 12 | Mutant (n = 12) | Wild-Type (n = 22) | P Value |
|---|---|---|---|
| VEGF, ng/mg | 7.5 ± 3.8 | 41.3 ± 9.2 | 0.01 |
| ELR score, ng/mg | 134.6 ± 83.9 | 20.2 ± 11.3 | 0.08 |
Definition of abbreviations: ELR, glutamic acid-leucine-arginine; VEGF, vascular endothelial growth factor.
Tumors with K-ras codon 12 mutations are less likely to express high levels of VEGF, and demonstrate a trend toward increased levels of angiogenic CXC chemokines.
Murine Lung Tumors Develop Unique Angiogenic Strategies
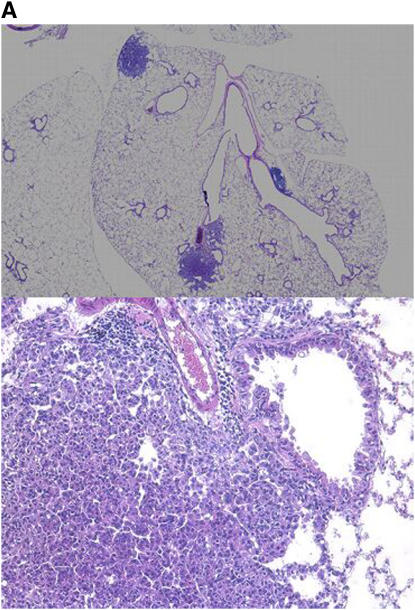
To provide further evidence for our hypothesis, we induced lung tumor formation in A/J mice by injection with the tobacco-derived carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-butanone (NNK), at a dose of 10 μmol (2 mg) (Figure 2A). Since hypoxia can be a primary stimulus for an angiogenic response, we first sought to determine whether NNK-induced lung adenomas manifested evidence of hypoxia. Hypoxyprobe, injected into mice shortly before killing, localized to the central areas of NNK-induced adenomas, where one would expect the presence of a hypoxic environment (Figure 2B). By contrast, normal murine lung was negative.
Figure 2.
(A) Photomicrographs (×40, top panel; ×200, bottom panel) of mouse lungs at 6 mo demonstrating development of adenomas in NNK-treated mice. (B) Hypoxia demonstrated with pimonidazole (top panel) in the central portion of an adenoma at 6 mo. Bottom panel is control antibody staining.
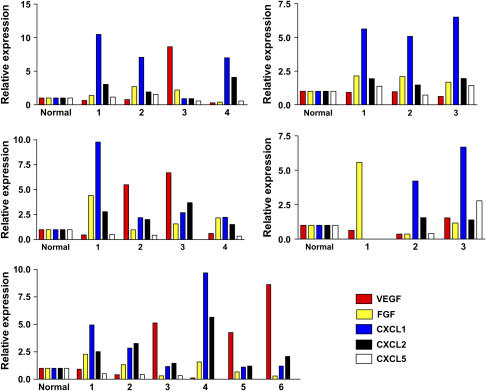
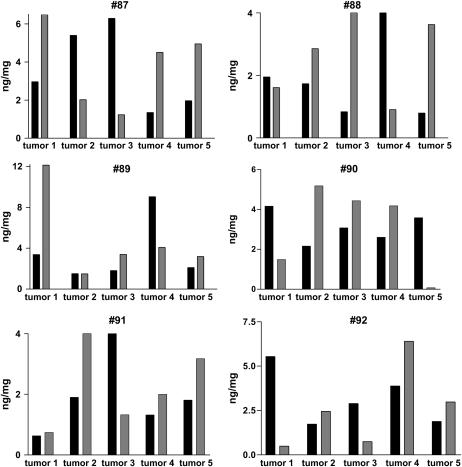
Because of the small size of these tumors, we used semiquantitative real-time PCR to measure levels of the following angiogenic substances: VEGF, bFGF, and the murine homologs of the ELR-CXC chemokines CXCL1, CXCL2, and CXCL5. Figure 3 shows the relative mRNA expression of the various angiogenic factors, from 20 individual lung tumors removed from five mice, 6 mo after NNK injection. Each graph represents a single animal, and each grouping of bars represents the angiogenic factor expression, or signature of a given tumor, with expression in normal lung from age matched mice not treated with NNK as the reference standard. To confirm the fidelity of the assay for the angiogenic “phenotype” of individual tumors, tumor mRNA specimens were run in repeated assays and the “intra-tumor” results were highly reproducible (data not shown). These data show that individual tumors, even from within the same mouse, express different angiogenic profiles. Figure 4 demonstrates the expression of angiogenic factors by ELISA, in larger tumors removed at 9 mo after NNK treatment.
Figure 3.
Angiogenic factor expression (angiogenic “signatures”) of 20 tumors from five mice, after 6 mo of NNK treatment. Each graph represents all visible tumors removed from the lungs of a single mouse (tumor #1, 2, 3, etc., with expression in normal lung from age-matched mice, not treated with NNK as the reference). We determined each tumor's phenotype with semiquantitative real-time RT-PCR using a normal mouse lung (age-matched, untreated with NNK) as a reference for relative expression levels.
Figure 4.
ELISA-based determination of expression of angiogenic CXC chemokines (expressed as ELR score, summing levels of CXCL1, CXCL2, and CXCL8 in ng/mg; shaded bars) and VEGF (ng/mg; solid bars) in Angiogenic factor expression (angiogenic “signatures”) of 30 tumors from six mice after 9 mo of NNK-induced tumor growth. Each graph represents all visible tumors removed from the lungs of a single mouse (tumor 1, 2, 3, etc.).
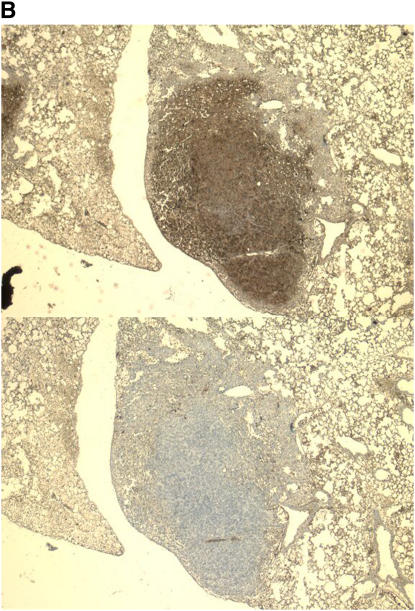
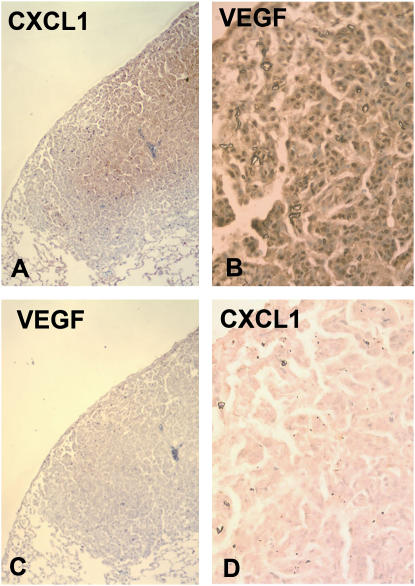
In further support of this, we performed immunohistochemistry of murine lung tumors. Figure 5 shows two representative tumors from the lung of a single mouse, both stained for expression of VEGF and CXCL1. Qualitative differences are present in the degree of immunostaining for CXCL1 (Figures 5A and 5D) and VEGF (Figures 5B and 5C). Staining of tumors with control IgG did not reveal any nonspecific staining (see Figures E5E–E5H in the online supplement).
Figure 5.
Photomicrographs of immunohistochemistry demonstrating expression of CXCL1 (A and D) or VEGF (B and C) in two tumors from within the same mouse. Tumor 1 (A and C; ×200) shows central staining for CXCL1 with no VEGF immunoreactivity, while tumor 2 (B and D; ×400) stains strongly for VEGF, but not for CXCL1.
The Angiogenic Switch Can Be Altered in the Short Term In Vivo
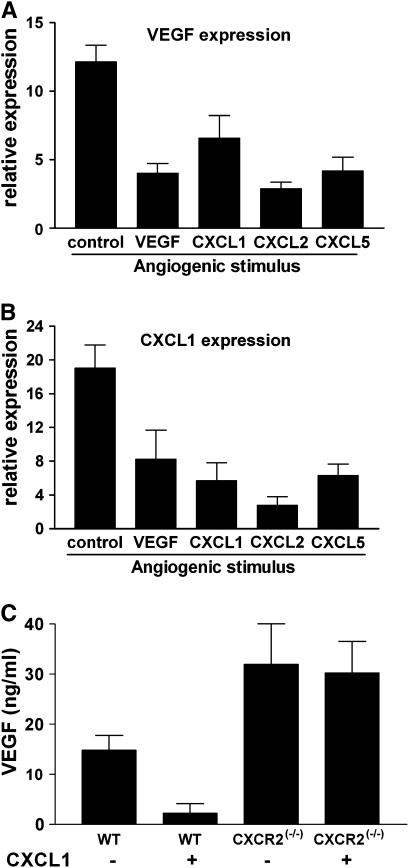
Tumors often develop resistance to cytotoxic therapy, during or after initially successful chemotherapy. If the angiogenic phenotype displayed similar plasticity, this would have implications for the design of anti-angiogenic therapy. We sought to determine whether the angiogenic phenotype of tumor cells could be altered in vivo. We employed a model that allowed us to influence the angiogenic milieu over a short period of time. LLC cells (1 × 106) were implanted in a polyvinyl alcohol sponge with the indicated angiogenic factor (“angiogenic stimulus” Figure 6; 500 ng/sponge) into subcutaneous pockets in C57BL6 mice. At 72 h sponges were removed, and total RNA was isolated (Trizol). We then determined mRNA expression of VEGF or CXCL1 in response to implantation in vivo. Results are expressed as the expression of cytokine mRNA by LLC cells in the sponge relative to the expression by LLC cells cultured in normoxic conditions in vitro. As expected, control LLC cells (in untreated sponges) demonstrated up-regulation of angiogenic factors CXCL1 and VEGF in vivo. As compared with control LLC cells (in untreated sponges), LLC cells co-implanted with angiogenic promoters, VEGF, or the murine ELR+CXC chemokines CXCL1, CXCL2, or CXCL5 expressed lower levels of VEGF or CXCL1 (Figures 6A and 6B, representative data from two experiments. P < 0.05 for all comparisons to control sponges). These data suggest that the presence of a pro-angiogenic factor in the microenvironment reduces the stimulus for production of angiogenic activity by the developing tumor, and supports our hypothesis that activation of an angiogenic switch may result in a unique and dominant angiogenic pathway in a given tumor. To further extend this, we implanted sponges containing LLC cells, with or without the angiogenic CXC chemokine CXCL1 using mice deficient for the receptor for CXCL1 (CXCR2−/− mice) or wild type (WT). Figure 6C demonstrates that VEGF levels (determined by ELISA) in sponges removed 72 h after implantation were significantly less in sponges co-implanted with CXCL1, as expected from our initial experiment (Figures 5A and 5B). However, in mice lacking CXCR2 (the receptor for angiogenic CXC chemokines), VEGF levels are not decreased in sponges implanted with LLC cells, with or without CXCL1 (P = 0.014 by ANOVA).
Figure 6.
Relative expression (by real-time RT-PCR) of VEGF (A) or CXCL1 (B) in LLC cells implanted with various angiogenic stimuli for 72 h in the flanks of C57B6 mice. Bars represent the mRNA expression of cytokine in LLC cells in sponges relative to LLC cells cultured in vitro in normoxic conditions. In each sponge implanted with the indicated angiogenic stimulus, the expression of the VEGF (A) or CXCL1 (B) was significantly reduced (P < 0.05). (C) VEGF levels (determined by ELISA) in LLC containing sponges co-implanted with CXCL1. In mice lacking CXCR2, VEGF levels are not affected by co-implantation with CXCL1 (P = 0.014 by ANOVA).
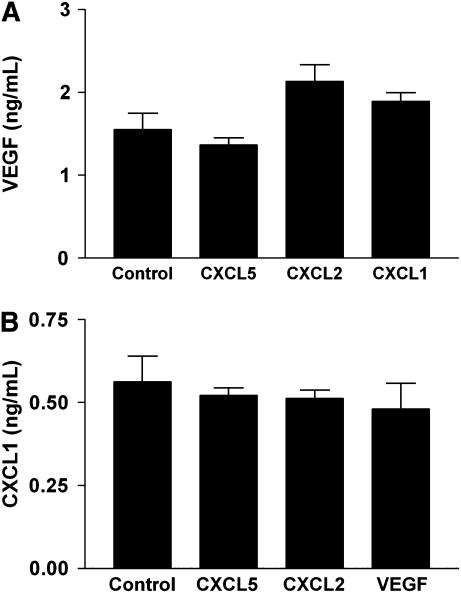
The above data demonstrate that the presence of an angiogenic factor in a sponge implant alters the expression of other angiogenic factors by LLC cells in vivo. To determine whether this was a direct effect on the LLC cells, we repeated the experiment by treating LLC cells in vitro with the indicated angiogenic factor(s), and measuring (by ELISA) the expression of angiogenic cytokines by cells cultured in hypoxia chambers in vitro. LLC cells treated with angiogenic factors in vitro under conditions of hypoxia do not demonstrate a reduction in expression of other angiogenic factors, further suggesting that the mechanism of suppression observed in vivo is indirect (Figure 7).
Figure 7.
Expression of VEGF (A) or CXCL1 (B) by hypoxic LLC cells in vitro in the presence of the indicated angiogenic factor (x axis labels) (P = NS for each comparison with control cells).
DISCUSSION
Here we describe the finding that non–small cell lung cancer tumors differ in their expression of angiogenic factors, or their angiogenic “signatures.” We base this finding upon data demonstrating that specimens of human non–small cell lung tumors vary in their expression of common angiogenic factors. In addition, murine lung tumors, even from within the same mouse, vary in their angiogenic profiles, as measured on the level of both mRNA and protein expression. Multiple investigations have shown the relationship between expression of angiogenic factors, tumor vascularity, and clinical outcomes in solid tumors including lung, esophageal, breast, ovarian, and colon cancers as well as melanoma among others (9, 20–29). To our knowledge, our observations are the first to characterize the diversity of the angiogenic switch in a carcinogen-induced model of tumorigenesis. In addition, we showed in vitro, using a sponge model of angiogenesis, that the angiogenic signal(s) generated by implanted lung cancer cells varies depending upon the microenvironment into which they are placed, suggesting that cancer cells can adapt their angiogenic profile to assure an angiogenic response from the surrounding tissue. The mechanism of this variability in angiogenic profile probably relates to the powerful selection pressure presented by hypoxia. Our data demonstrate that the murine lung tumors in this NNK model are indeed hypoxic, and that early vessel growth is induced in these tumors. While hypoxia leads to the activity of hypoxia-inducible factor (HIF)-1α, this is not the only mechanism by which hypoxia can stimulate an angiogenic response.
Our data are consistent with the recent findings of Mizukami and coworkers (7), who demonstrated that colon cancer cells induce angiogenic responses through multiple independent pathways. They employed a colon cancer cell line, in which HIF-1α was stably knocked down. DLD-1HIF-kd cells had predictably decreased levels of VEGF but increased levels of IL-8 (CXCL8). In tumor xenografts of HIF-1α knockdown cells, angiogenesis was stimulated by production and secretion of CXCL8 as a compensatory pathway. They further demonstrated that the mechanism of compensation involved hypoxia-induced production of hydrogen peroxide (H2O2), which subsequently activated NF-κB to induce CXCL8 expression. While hypoxia and H2O2 production may seem mutually exclusive, the production of H2O2 by NADPH-oxidases does not require high tensions of oxygen, and is actually activated in response to hypoxia (30). This H2O2-mediated activation of NF-κB was enhanced by the presence of mutant K-ras. Indeed, oncogenic K-ras transformation requires a functional NADPH-oxidase (31). Mizukami and colleagues concluded that K-ras mutations favored the expression of the angiogenic factor CXCL8, particularly in the setting of hypoxia (7). This is particularly relevant to our current study, in which the majority of mouse tumors developed an angiogenic signature characterized by predominant expression of angiogenic CXC chemokines, and in which the carcinogen (NNK) promotes tumors by induction of K-ras mutations (32). Our human tumor data suggest that while the presence of activating K-ras mutations does not exclusively predict the predominance of chemokine expression in the angiogenic mechanism of a tumor, there was a trend toward increased chemokine expression in K-ras mutant tumors compared with K-ras wild-type tumors. The presence of mutant K-ras did decrease the likelihood of VEGF being highly expressed in these tumors.
A limitation of our study is that we evaluated a limited number of angiogenic factors, relative to the number that are known to affect angiogenesis (33). However, since our purpose was to determine the diversity of the angiogenic switch, we limited our phenotyping of these tumors to these well-characterized angiogenic factors. Our observations support our hypothesis that tumors develop a unique angiogenic signature. As the availability of pharmacologic inhibitors of angiogenesis increases, it may be prudent for clinical trials of these newer agents to include stratification of patients based upon the angiogenic signatures determined by tissue taken from the tumor.
Results of experiments presented herein reflect events occurring during the carcinogen-induced development of a transformed tumor. These early stages of carcinogenesis are crucial in that the tumor is developing the angiogenic switch in response to hypoxia. The factor(s) promoting the angiogenic switch may be altered, as demonstrated by data from lung cancer cells implanted subcutaneously in the presence of various angiogenic factors. These data support a paradigm of tumor angiogenesis being a characteristic that is both variable from tumor to tumor, and adaptable within a given tumor. These findings have important clinical implications, as many current anti-angiogenic therapies target a single angiogenic factor or pathway. These therapies, while of some clinical utility, are not without their limitations and potential toxicities. A “one-size-fits-all” approach to anti-angiogenesis may be effective for only a limited proportion of patients. More targeted therapy, in which treatment is individualized to reflect the tumor's unique angiogenic phenotype, may benefit a larger percentage of patients. This clearly requires the development of additional pharmacologic anti-angiogenic agents that target a wider spectrum of angiogenic factors. Furthermore, the findings of this study, as well as the article by Mizukami and coworkers (7), argue for more vigorous development of therapies that are directly angiostatic, such as angiostatin/endostatin (34, 35), or the angiostatic chemokine CXCL10 (13, 36), as opposed to those which inhibit particular angiogenic factors.
Taken in aggregate, the results from our study and others suggest a redundancy, or multiplicity of independent mechanisms by which tumors induce angiogenesis, and different mechanisms by which tumor cells produce individual angiogenic factors. As recently observed by Kerbel and others, these findings have implications for the ongoing design of strategies aimed at inhibiting angiogenesis (37). Clinical studies have demonstrated some limited benefit of targeting VEGF (Bevacizumab), but not to the degree one would expect given the impressive pre-clinical data. In summary, we have demonstrated the inherent variability in angiogenic phenotypes of lung tumors. To our knowledge, ours is the first demonstration of this variability in a model of carcinogen-induced lung tumorigenesis.
This study was supported by NIH R01-CA94121 (D.A.A.), as well as a Sidney Kimmel foundation grant (D.A.A.), and the Flight Attendants Medical Research Institute (FAMRI; D.A.A.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2006-0311OC on November 1, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996;16:4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 1995;270:27348–27357. [DOI] [PubMed] [Google Scholar]
- 3.Arenberg DA, Polverini PJ, Kunkel SL, Shanafelt A, Hesselgesser J, Horuk R, Strieter RM. The role of CXC chemokines in the regulation of angiogenesis in non-small cell lung cancer. J Leukoc Biol 1997;62:554–562. [DOI] [PubMed] [Google Scholar]
- 4.Anderson IC, Mari SE, Broderick RJ, Mari BP, Shipp MA. The angiogenic factor interleukin 8 is induced in non-small cell lung cancer/pulmonary fibroblast cocultures. Cancer Res 2000;60:269–272. [PubMed] [Google Scholar]
- 5.White ES, Strom SRB, Wys NL, Arenberg DA. Non-small cell lung cancer cells induce monocytes to increase expression of angiogenic activity.J Immunol 2001;166:7549–7555. [DOI] [PubMed] [Google Scholar]
- 6.Moore BB, Arenberg DA, Stoy K, Morgan T, Addison CL, Morris SB, Glass M, Wilke C, Xue YY, Sitterding S, et al. Distinct CXC chemokines mediate tumorigenicity of prostate cancer cells. Am J Pathol 1999;154:1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med 2005;11:992–997. [DOI] [PubMed] [Google Scholar]
- 8.Sridhar SS, Shepherd FA. Targeting angiogenesis: a review of angiogenesis inhibitors in the treatment of lung cancer. Lung Cancer 2003;42:S81–S91. [DOI] [PubMed] [Google Scholar]
- 9.White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res 2003;9:853–860. [PubMed] [Google Scholar]
- 10.Kubrusly MS, Cunha JE, Bacchella T, Abdo EE, Jukemura J, Penteado S, Morioka CY, de Souza LJ, Machado MC. Detection of K-ras point mutation at codon 12 in pancreatic diseases: a study in a Brazilian casuistic. JOP 2002;3:144–151. [PubMed] [Google Scholar]
- 11.Hecht SS, Adams JD, Numoto S, Hoffmann D. Induction of respiratory tract tumors in Syrian golden hamsters by a single dose of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and the effect of smoke inhalation. Carcinogenesis 1983;4:1287–1290. [DOI] [PubMed] [Google Scholar]
- 12.Fife RS, Sledge GW Jr, Sissons S, Zerler B. Effects of tetracyclines on angiogenesis in vitro. Cancer Lett 2000;153:75–78. [DOI] [PubMed] [Google Scholar]
- 13.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass M, Taub DT, Iannetoni MD, Whyte RI, Strieter RM. Interferon-γ-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med 1996;184:981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta Y, Endo Y, Tanaka M, Shimizu J, Oda M, Hayashi Y, Watanabe Y, Sasaki T. Significance of vascular endothelial growth factor messenger RNA expression in primary lung cancer. Clin Cancer Res 1996;2:1411–1416. [PubMed] [Google Scholar]
- 15.Brattstrom D, Bergqvist M, Larsson A, Holmertz J, Hesselius P, Rosenberg L, Brodin O, Wagenius G. Basic fibroblast growth factor and vascular endothelial growth factor in sera from non-small cell lung cancer patients. Anticancer Res 1998;18:1123–1127. [PubMed] [Google Scholar]
- 16.Iwasaki A, Kuwahara M, Yoshinaga Y, Shirakusa T. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) levels, as prognostic indicators in NSCLC. Eur J Cardiothorac Surg 2004;25:443–448. [DOI] [PubMed] [Google Scholar]
- 17.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest 1998;102:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest 1996;97:2792–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 1994;179:1409–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularization to metastasis of non-small cell lung cancer. Lancet 1992;340:145–146. [DOI] [PubMed] [Google Scholar]
- 21.Hoef MEHMV, Knox WF, Dhesi SS, Howell A, Schor AM. Assessment of tumor vascularity as a prognostic factor in lymph node negative breast cancer. Eur J Cancer 1993;29A:1141–1145. [DOI] [PubMed] [Google Scholar]
- 22.Toi M, Hoshina S, Takayanagi T, Tominaga T. Association of vascular endothelial growth factor expression with tumor angiogenesis and with early relapse in primary breast cancer. Jpn J Cancer Res 1994;85:1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 24.Saito S, Tsuno N, Nagawa H, Sunami E, Zhengxi J, Osada T, Kitayama J, Shibata Y, Tsuruo T, Muto T. Expression of platelet-derived endothelial cell growth factor correlates with good prognosis in patients with colorectal carcinoma. Cancer 2000;88:42–49. [PubMed] [Google Scholar]
- 25.Masuya D, Huang C, Liu D, Kameyama K, Hayashi E, Yamauchi A, Kobayashi S, Haba R, Yokomise H. The intratumoral expression of vascular endothelial growth factor and interleukin-8 associated with angiogenesis in nonsmall cell lung carcinoma patients. Cancer 2001;92:2628–2638. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K, Kamada M, Slaton JW, Fukata S, Yoshikawa C, Tamboli P, Dinney CP, Shuin T. The prognostic value of angiogenesis and metastasis-related genes for progression of transitional cell carcinoma of the renal pelvis and ureter. Clin Cancer Res 2002;8:1863–1870. [PubMed] [Google Scholar]
- 27.Mukherjee T, Kumar A, Mathur M, Chattopadhyay TK, Ralhan R. Ets-1 and VEGF expression correlates with tumor angiogenesis, lymph node metastasis, and patient survival in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2003;8:8. [DOI] [PubMed] [Google Scholar]
- 28.Hata K, Nakayama K, Fujiwaki R, Katabuchi H, Okamura H, Miyazaki K. Expression of the angopoietin-1, angopoietin-2, Tie2, and vascular endothelial growth factor gene in epithelial ovarian cancer. Gynecol Oncol 2004;93:215–222. [DOI] [PubMed] [Google Scholar]
- 29.Koukourakis MI, Giatromanolaki A, Kakolyris S, O'Byrne KJ, Apostolikas N, Skarlatos J, Gatter KC, Harris AL. Different patterns of stromal and cancer cell thymidine phosphorylase reactivity in non-small-cell lung cancer: impact on tumour neoangiogenesis and survival. Br J Cancer 1998;77:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 1996;15:633–644. [DOI] [PubMed] [Google Scholar]
- 31.Mitsushita J, Lambeth JD, Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res 2004;64:3580–3585. [DOI] [PubMed] [Google Scholar]
- 32.Belinsky SA, Devereux TR, Maronpot RR, Stoner GD, Anderson MW. Relationship between the formation of promutagenic adducts and the activation of the K-ras protooncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res 1989;49:5305–5311. [PubMed] [Google Scholar]
- 33.Arenberg DA, Strieter RM. Angiogenesis. In: Gallin J, Snyderman R, editors. Inflammation: basic principles and clinical correlates, 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 851–864.
- 34.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997;88:277–285. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 1996;2:689–692. [DOI] [PubMed] [Google Scholar]
- 36.Arenberg DA, White ES, Burdick MD, Strom SRB, Strieter RM. Improved survival in tumor bearing SCID mice treated with interferon-g-inducible protein 10 (IP-10/CXCL10). Cancer Immunol Immunother 2001;50:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerbel RS, Yu J, Tran J, Man S, Viloria-Petit A, Klement G, Coomber BL, Rak J. Possible mechanisms of acquired resistance to anti-angiogenic drugs: implications for the use of combination therapy approaches. Cancer Metastasis Rev 2001;20:79–86. [DOI] [PubMed] [Google Scholar]