Abstract
Increased lung vascular permeability is an important contributor to respiratory failure in acute lung injury (ALI). We found that a function-blocking antibody against the integrin αvβ5 prevented development of lung vascular permeability in two different models of ALI: ischemia-reperfusion in rats (mediated by vascular endothelial growth factor [VEGF]) and ventilation-induced lung injury (VILI) in mice (mediated, at least in part, by transforming growth factor-β [TGF-β]). Knockout mice homozygous for a null mutation of the integrin β5 subunit were also protected from lung vascular permeability in VILI. In pulmonary endothelial cells, both the genetic absence and blocking of αvβ5 prevented increases in monolayer permeability induced by VEGF, TGF-β, and thrombin. Furthermore, actin stress fiber formation induced by each of these agonists was attenuated by blocking αvβ5, suggesting that αvβ5 regulates induced pulmonary endothelial permeability by facilitating interactions with the actin cytoskeleton. These results identify integrin αvβ5 as a central regulator of increased pulmonary vascular permeability and a potentially attractive therapeutic target in ALI.
Keywords: integrin αvβ5, lung vascular permeability, pulmonary endothelial barrier function
CLINICAL RELEVANCE
We describe a novel role for integrin αvβ5 in regulating lung vascular permeability and agonist-induced endothelial permeability. Furthermore, we suggest that αvβ5 regulation of the actin-cytoskeleton may be a mechanism responsible for these effects.
Acute lung injury (ALI) is a devastating clinical syndrome characterized by development of pulmonary edema and flooding of alveolar spaces leading to impaired gas exchange, arterial hypoxemia, and respiratory failure (1). While much progress has been made in understanding the pathogenesis of ALI, it is estimated that 190,600 cases of ALI occur every year in the United States alone; these are associated with 74,500 deaths and 3.6 million hospital days (2). Effective pharmacologic therapies are not currently available and the molecular mechanisms regulating ALI remain poorly understood.
Vascular permeability in the lung has long been considered a principal pathologic hallmark of ALI that is largely responsible for its characteristic pulmonary edema formation (3, 4). Recently, integrin αvβ5, a member of the integrin family of heterodimeric transmembrane cell surface receptors, was shown to specifically regulate increases in vascular permeability induced by vascular endothelial growth factor (VEGF) in the systemic circulation (5). Although regulation of permeability in the systemic and pulmonary circulations is often physiologically distinct, and the precise role of VEGF in ALI remains controversial, we hypothesized that αvβ5 could be an important regulator of vascular permeability in the lung. Therefore, we sought to determine whether αvβ5 could regulate lung vascular permeability in in vivo models of ALI.
In this report, we used two in vivo models of ALI to examine the role of αvβ5 in regulating lung vascular permeability: ischemia-reperfusion (IR)-induced and ventilator-induced lung injury (VILI). IR-induced lung injury is a significant clinical problem in cardiac surgery and, in particular, with lung transplantation (6). Although the pulmonary edema associated with lung transplantation is often mild and self-limiting, graft dysfunction attributed to IR can occur in up to 20% of patients, leading to prolonged post-transplant length of hospitalization and increased post-transplant mortality (7). Mechanical ventilation, while considered an essential tool for managing patients with respiratory failure, is now itself recognized, when administered at high tidal volumes, as an important contributing factor to the development of pulmonary edema (VILI) (1, 8, 9).
Our studies show that αvβ5 regulates lung vascular permeability in models of both IR and VILI. However, in the lung, as opposed to what has been described in the systemic vasculature (5), αvβ5 regulation of vascular permeability is not restricted to VEGF-induced effects alone; in pulmonary vascular endothelial cells, both genetic absence and blockade of αvβ5 prevented monolayer permeability induced by three very different edemagenic agonists—VEGF, TGF-β, and thrombin. Previous studies have identified the induction of actin stress fibers as an important step in regulating agonist-induced increases in endothelial paracellular permeability (10–16). Stress fiber formation induced by all three agonists was attenuated by blockade of αvβ5, suggesting a mechanism for how αvβ5 might regulate paracellular endothelial permeability in the lung downstream of multiple signaling pathways. Understanding how αvβ5 regulates pulmonary endothelial permeability could provide valuable insights into mechanisms regulating lung vascular permeability and could identify this integrin as a promising target for the treatment of ALI.
MATERIALS AND METHODS
Reagents and Antibodies
VEGF (R&D Systems, Minneapolis, MN), TGF-β (R&D Systems), thrombin (Amersham Biosciences, Piscataway, NJ), RhoA kinase (ROCK) inhibitor (Y-27632) (Calbiochem, San Diego, CA), VEGF receptor II-Ig chimera adenovirus (AdVEGFRII-Ig) (generous gift from Richard C. Mulligan, Harvard School of Medicine [17], Boston, MA), green fluorescent protein (GFP) adenovirus control (AdGFP) (generous gift from George Davis, Texas A&M University, College Station, TX), anti-integrin αvβ6 antibody (3G9) and TGF-β type II receptor IgG chimera (TGF-β-RII-Ig) (generous gifts from Paul Weinreb, Biogen Idec, Cambridge, MA), IgG2b isotype antibody: mouse anti-human low-density receptor (LDL) receptor antibody (CRL-1691, clone C7; American Type Culture Collection [ATCC], Manassas, VA), anti-integrin αvβ3 antibody (AP-3) (HB-242, ATCC), anti-integrin αvβ3 blocking antibody (LM609) (Chemicon, Temecula, CA), anti-integrin αvβ8 antibody (37E.1) (generous gift from Steve Nishimura, University of California, San Francisco, CA), collagenase A (Sigma, St. Louis, MO), heparin (Sigma), M-450 Dynabeads (Dynal, Carlsbad, CA), anti-Fcγ receptor II/III antibody (BD Pharmingen, San Jose, CA), anti-intracellular adhesion molecule (ICAM)-2 antibody (BD Pharmingen and Santa Cruz Biotechnology, Santa Cruz, CA), platelet endothelial cell adhesion molecule (PECAM, CD-31) (BioLegend, San Diego, CA), anti-vascular endothelial (VE)-cadherin antibody (Santa Cruz Biotechnology), anti-CD34 antibody (BD Pharmingen), 125I-labeled albumin (Jeanatope ISO-TEX Diagnostics, Friendswood, TX), 14C-bovine serum albumin (BSA) (Perkin-Elmer, Wellesley, MA).
β5 Subunit Knockout Mice (β5−/−)
129/svJae background β5 subunit knockout mice were generated and maintained in our laboratory as previously described (18).
Anti-αvβ5 Antibody
IgG2b isotype mouse monoclonal antibody against αvβ5 was raised and characterized as described in Results (Figure 1).
Figure 1.
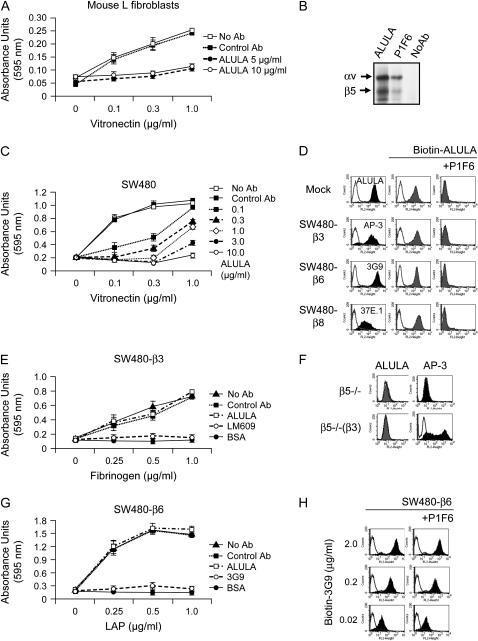
Characterization of anti-αvβ5 antibody (ALULA). (A) Mouse L fibroblast cell adhesion to a range of concentrations of vitronectin was determined in the presence of a range of concentrations of ALULA. Data shown are the means ± SE, n = 3. (B) SW480 cells were metabolically labeled with 35S-methionine, lysed in 1% Triton, and immunoprecipitated either without antibody, with ALULA, or with the commercially available mouse anti-human αvβ5 antibody, PIF6 (84). Precipitated proteins were separated by nonreducing SDS-PAGE and developed by autoradiography. (C) SW480 cell adhesion to a range of concentrations of vitronectin was determined in the presence of a range of concentrations of ALULA. Data shown are the means ± SE, n = 3. (D) Excess P1F6 completely inhibited binding of ALULA to mock-transfected SW480 cells (Mock), which express αvβ5 as their only αv-containing integrin. P1F6 competitively excludes ALULA from recognition binding sites in SW480 cells transfected with β3, β6, and β8 (SW480-β3, SW480-β6, SW480-β8). Cells were assessed by flow cytometry with antibodies specific for β3 (AP-3), β6 (3G9), or β8 (37E.1) (solid histograms). Biotinylated ALULA (Biotin-ALULA) antibody binding was assessed (shaded histograms) and reassessed with P1F6 competitive binding (100 μg/ml). Controls (no primary antibody) are shown as open histograms. (E) SW480-β3 cell adhesion to a range of concentrations of fibrinogen was determined in the presence of ALULA (10 μg/ml). αvβ3-specific blockade of adhesion was achieved using αvβ3 blocking antibody (LM609). Data shown are the means ± SE, n = 3. (F) β5 knockout mouse pulmonary endothelial cells (β5−/−) and β5 knockout mouse pulmonary endothelial cells expressing full-length human β3 (β5−/−[β3]) were assessed with flow cytometry using ALULA and anti-αvβ3 antibody (AP-3). (G) SW480-β6 cell adhesion to a range of concentrations of TGF-β1-latency-associated peptide (LAP) was determined in the presence of ALULA (10 μg/ml). αvβ6-specific blockade of adhesion was achieved using β6-blocking antibody (3G9). Data shown are the means ± SE, n = 3. (H) SW480-β6 cells were assessed with flow cytometry with biotinylated anti-αvβ6 antibody (Biotin-3G9) at different concentrations (2 μg/ml, 0.2 μg/ml, and 0.02 μg/ml) (solid histograms) and reassessed with P1F6 competitive binding (100 μg/ml). Controls (no primary antibody) are shown as open histograms.
IR Lung Injury Model
Sprague-Dawley rats (300–500 g) (Charles River Laboratories, Wilmington, MA) were transtracheally intubated and ventilated under isoflurane anesthesia with a tidal volume of 6 ml/kg, positive end-expiratory pressure (PEEP) of 10 cm H2O, and 100% oxygen (Model 683; Harvard Apparatus Co., Holliston, MA). A median sternotomy was performed, heparin (200 IU) was injected into the right ventricle, and cannulas were placed in the pulmonary artery and left ventricle. Unilateral lung IR was induced by right pulmonary artery ligation for 30 min followed by release and reperfusion for 3 h. Rats were treated with antibodies intravenously immediately before the experiment (4 mg/kg) or with adenovirus intramuscularly 7 d before the experiment (1010 pfu/kg). Previous studies have determined this to be the peak time for soluble VEGFRII secretion from the liver (19). Contralateral lungs served as nonischemic controls.
VILI Model
Mice were transtracheally intubated and ventilated with a high tidal volume of 20 ml/kg at a rate of 48 breaths/min (without PEEP) for 4 h using a mouse ventilator (Model 683; Harvard Apparatus Co). Animals were anesthetized using serial ketamine (37.5 mg/ml) and xylazine (250 mg/ml) intraperitoneal injections (100 μl/20 g) with equal volume injections of normal saline in matched animals. Matched nonventilated mice were administered equal volumes of anesthesia and saline to serve as baseline controls. Mice were administered antibodies by intraperitoneal injection 24 h before the experiment (4 mg/kg) or adenovirus intramuscularly 7 d before the experiment (1010 pfu/kg) (19). TGF-β-RII-Ig (25 μg in 100 μl sterile saline) was administered intravenously immediately before initiation of ventilation. Lungs were harvested immediately after 4 h of ventilation for lung vascular leak assay preparation.
Quantification of VEGF-Induced Vascular Leak
Vascular leak was studied 7 d after intramuscular administration of 1010 pfu/kg AdVEGFRII-Ig adenovirus (19) by measuring the extravasation of Evan's blue dye (30 mg/kg in 50 μl per mouse). After 5 min, vascular leak was induced by dermal injection of VEGF (100 ng in 10 μl normal saline) into mouse ears. After 1 h, 4 mm punch sections around the VEGF injection site were harvested and formamide-extracted dye was quantified as absorbance at 610 nm with a Spectra Max 190 Spectrophotometer (Molecular Devices, Sunnyvale, CA).
Lung Vascular Leak and Protein Permeability
0.5 μCi of 125I-labeled albumin in 300 μl sterile normal saline was administered intraperitoneally 4 h before lung harvest to ensure adequate distribution. After each experiment, a blood sample was obtained to measure the hemoglobin concentration and the water-to-dry weight ratio of blood for the extravascular plasma equivalents (EVPE) calculation. Lungs (left for IR, and bilateral for VILI) were homogenized and the extravascular lung water determined by calculating the water-to-dry weight ratio using the following equation: W/D = Qwet/Qdry, in which Qwet is the difference between the water content of the lung homogenate and the water content of the blood in the lung, and Qdry is the dry lung weight calculated as the weight of the lungs minus the blood and water volumes in the lung. Lung endothelial permeability to albumin, expressed as EVPE in ml, calculated using the following equation: EVPE = (CH – (CPend × QB))/CPave. CH represents the 125I counts/min/g in the homogenized lung, CPend represents the counts/min/g in plasma at the end of the experiment, and CPave represents the average counts/min/g in the plasma samples at the end of the experiment. QB is the blood volume in the lungs determined by the gravimetric method using weights from wet and dried lung homogenates (19, 20). Counts were measured on a Wizard γ counter (Perkin-Elmer). Control lungs included the contralateral nonischemic, nonreperfused right lung for IR, and lungs from nonventilated mice for VILI. Baseline lungs for IR were harvested from animals not subject to pulmonary artery ligation.
Cell Lines
Human pulmonary artery endothelial cells (HPAECs) (passages 3–9) (Clonetics, Walkersville, MD) were maintained in EBM-2 basal endothelial media supplemented with EGM-2 supplemental aliquots (Clonetics). Bovine pulmonary artery endothelial cells (BPAECs) (passages all < 10) (CCL-209, ATCC) and mouse pulmonary endothelial cells (see Isolation of Primary Mouse Endothelial Cells from β5 Subunit Knockout Mice below) were cultured in Dulbecco's minimal essential (DME)/F-12 medium supplemented with 20% fetal bovine serum (FBS), 50 mg/liter of endothelial mitogen (Biomedical Technologies, Stoughton, MA), and 10,000 U/liter of heparin. Cells were maintained on Corning polystyrene culture dishes (Fisher Scientific, Pittsburgh, PA) coated with type VI collagen (Sigma) and seeded onto surfaces pre-coated with vitronectin (Upstate Biotechnology, Charlottesville, VA), fibrinogen (Calbiochem), or recombinant TGF-β1 latency-associated peptide (LAP) (21) or onto collagen-coated transwells (Corning, Corning, NY) as required for individual experiments. Human SW480 cells (CCL-228, ATCC) were infected with a retrovirus to express full-length integrin β3 (pBABE-puro-β3) or transfected with the plasmid vector pcDNA1-neo-β6 to express full-length β6 (SW480-β3 and SW480-β6 cells). SW480-β8 cells were a generous gift from Steve Nishimura, University of California, San Francisco. SW480 cells were maintained in DMEM supplemented with 10% FBS and an appropriate selection marker (Geneticin [G418, Life Technologies, Inc., Carlsbad, CA] or puromycin [Calbiochem]).
Cell Adhesion Assay
Cells were allowed to adhere for 1 h to wells coated with a range of concentrations of specific ligand in the presence of control IgG antibody, saline, or the tested blocking antibody. Bovine serum albumin (BSA)-coated wells served as nonadhesion controls. Plates were then spun topside down at 40 × g to remove nonadherent cells, and the remaining cells were fixed with formalin, stained with crystal violet, and quantified by absorbance (595 nm).
Isolation of Primary Mouse Endothelial Cells from β5 Subunit Knockout Mice
Lung tissue was collected from β5 subunit knockout mice, pureed, digested with 0.1% collagenase A, filtered through 10-μm nylon mesh, centrifuged, and plated. At 16 h, negative selection was performed with M-450 Dynabeads pre-conjugated with anti-Fcγ receptor II/III antibody. Positive selection with Dynabeads pre-conjugated with anti-ICAM-2 antibody was performed on Days 3 and 7. To assess purity, cells were analyzed for expression of ICAM-2 and PECAM by flow cytometry (FACSort; Becton Dickinson, Franklin Lakes, NJ) and CD34 and VE-cadherin by immunocytochemistry.
Conditional Immortalization of β5 Subunit Knockout and Wild-Type Mouse Pulmonary Endothelial Cells and β5 Reconstitution
Primary endothelial cells were transfected with the tsA58 SV40 large and small T antigen genome (pUC18-tsA58 SV40) (generous gift from Jiyue Zhu, Penn State College of Medicine, Hershey, PA) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and repeatedly passaged for over 1 mo at 33°C to select for immortalized cells. Cells were incubated at 39°C for 24 h before all described experiments and before assessment for purity with endothelial cell markers (ICAM-2, VE-cadherin, PECAM, and CD-34). Immortalized cells were infected with retrovirus expressing full-length β5 (pWZL-β5) generated by sub-cloning β5 cDNA (Clone 5.1; ATCC) (22) into a pWZL-blast2 vector containing a blasticidin resistance gene.
Assay of Transendothelial Albumin Flux
Cells were seeded onto 6.5-mm collagen-coated PFTE membrane Costar Transwells (Fisher Scientific) at 75,000 cells per well and cultured to confluence. Cells were incubated with antibodies (10 μg/ml) for 1 h and then stimulated with VEGF (30 ng/ml), TGF-β (10 ng/ml), or thrombin (10 U/ml) for 1 h. 14C-BSA (0.005 μCi) (Perkin-Elmer) was applied to each upper compartment for 1 h at 37°C, after which contents from the lower compartment were collected and counted with an LS 6500 Multi-Purpose Scintillation Counter (Beckman, Fullerton, CA). Only monolayers retaining > 97% of tracer at baseline were studied.
Stress Fiber Visualization
Cells were grown on collagen-coated glass coverslips to confluence over 4 d. Serum-starved cells (12 h) were pre-treated with either control antibody or ALULA for 1 h, then stimulated with respective agonists (VEGF [30 ng/ml], TGF-β [10 ng/ml], or thrombin [10 U/ml]) for 10 min. Cells were then fixed with 3.7% paraformaldehyde for 10 min, permeabilized with 0.5% triton X-100, then stained with rhodamine phalloidin (Molecular Probes, Carlsbad, CA), mounted, and imaged using a Leica DM5000B microscope equipped for epifluorescence.
RhoA Activation Assay
Cells were grown on collagen-coated 100-mm culture dishes, serum-starved, stimulated, rinsed, and lysed per assay kit manufacturer's protocol (Upstate Biotechnology). After centrifugation, lysate supernatants were incubated with agarose beads conjugated with rhotekin Rho-binding domains (RBD) that recognize only GTP-bound active RhoA. RhoA was detected from pulled-down product by Western analysis using anti-RhoA antibodies (Upstate Biotechnology). GTPγS and GDP-spiked lysates served as positive and negative controls.
RESULTS
αvβ5 Antibody Characterization
To study the role of αvβ5 in regulating lung vascular permeability in rodent models of ALI, we needed an effective specific inhibitor of αvβ5 function that could recognize the specific rodent integrin. Therefore, we raised a monoclonal antibody against mouse αvβ5 (ALULA) by immunizing β5 subunit knockout mice with mouse L fibroblasts expressing αvβ5. We confirmed that ALULA functionally blocked mouse L cell adhesion to the αvβ5 ligand vitronectin (Figure 1A). ALULA has an identical immunoprecipitation profile to the previously validated anti-αvβ5 antibody P1F6 (23) (Figure 1B), and inhibits adhesion of human SW480 cells to vitronectin (Figure 1C). To exclude ALULA recognition of other αv-containing integrins, we performed binding site competition assays using the anti-αvβ5 antibody, P1F6. Excess P1F6 completely inhibited binding of ALULA to mock-transfected SW480 cells, which express αvβ5 as their only αv integrin (Figure 1D). ALULA binding to SW480 cells transfected with human β3, β6, or β8 (SW480-β3, -β6, and -β8) was similarly inhibited by P1F6 (Figure 1D). Furthermore, ALULA did not inhibit adhesion of SW480-β3 to the αvβ3 ligand fibrinogen (Figure 1E) and did not recognize αvβ3 in β5 knockout pulmonary endothelial cells transfected with full-length human β3 (Figure 1F). ALULA was also incapable of inhibiting adhesion of SW480-β6 cells to the β6 ligand TGF-β1 LAP (Figure 1G). Anti-αvβ6 antibody (3G9) binding to SW480-β6 cells was not affected by excess P1F6 at any dilution of 3G9 tested (Figure 1H). ALULA also did not affect SW480-β8 cell adhesion to TGF-β1-LAP, but this finding is not straightforward to interpret because of the lack of available αvβ8 antibodies that inhibit cell adhesion for use as a positive control (data not shown). In addition to mouse and human αvβ5, ALULA was shown to recognize bovine αvβ5 by flow cytometry using bovine pulmonary artery endothelial cells (BPAECs) (data not shown).
αvβ5 Regulates Lung Vascular Permeability in a Model of Lung IR-Induced ALI
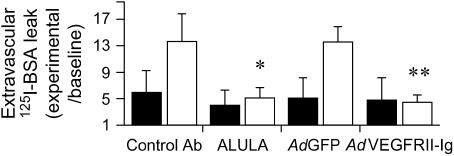
We initially chose to study IR-induced lung injury in rats (24) since VEGF had been implicated as a possible mediator of increased lung vascular permeability after IR (25, 26), and since αvβ5 had been shown to mediate VEGF-induced systemic vascular permeability (5). IR induced a robust increase in lung vascular permeability, as measured by parenchymal extravasation of an I125-albumin intravascular tracer, which was completely blocked by systemic administration of ALULA (Figure 2). To determine whether IR-induced lung vascular permeability was indeed dependent on VEGF, we used an adenovirus expressing a VEGF receptor II-IgG chimera (AdVEGFRII-Ig) that had previously been shown to be an effective blocker of VEGF effects in rodents (27). Administration of AdVEGFRII-Ig blocked IR-induced lung vascular permeability, whereas a control adenovirus expressing green fluorescent protein (AdGFP) did not (Figure 2).
Figure 2.
αvβ5 regulates lung vascular permeability in a rat model of IR-induced ALI. Rat lungs were subjected to unilateral left lung ischemia (30 min) followed by reperfusion (3 h) (open bars). Rats were treated with ALULA or an IgG2b isotype control antibody (Control Ab), VEGF receptor II-Ig chimera adenovirus (AdVEGFRII-Ig), or green fluorescent protein (GFP) adenovirus control (AdGFP). Lung vascular permeability is reported as extravascular I125-albumin leak normalized to leak measured from lungs harvested from animals not subject to pulmonary artery ligation or reperfusion (mock procedure) (Baseline). Contralateral (right) nonischemic, nonreperfused lung in each individual animal served as its control lung (Control; solid bars). Data shown are the means ± SE, n = 5. *P = 0.049 for rats treated with ALULA compared with those treated with Control Ab; **P = 0.002 for rats treated with AdVEGFRII-Ig compared with those treated with control AdGFP.
αvβ5 Regulates Lung Vascular Permeability in VILI
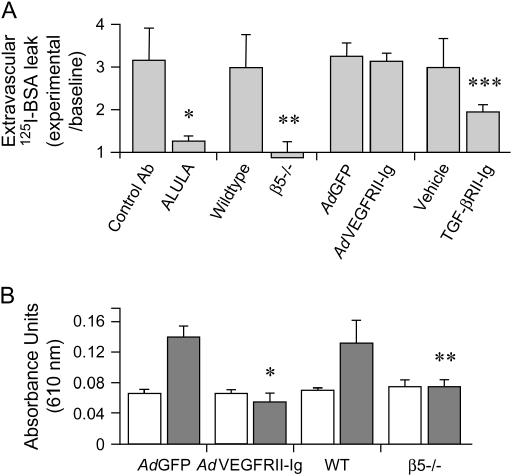
To determine whether the protective effect of blocking αvβ5 with ALULA could be generalized to other models of increased lung vascular permeability, and to take advantage of the availability of β5 knockout mice (18), we used a mouse model of VILI in which lung vascular permeability was induced by four hours of mechanical ventilation at a high tidal volume (20 ml/kg) (28). This model produced a robust increase in lung vascular permeability, which was completely blocked by ALULA (Figure 3A). β5 subunit knockout mice were also completely protected from increased lung vascular permeability (Figure 3A). However, in contrast to our results from the IR model, AdVEGFRII-Ig did not block lung vascular permeability induced by VILI. Adequate blockade of VEGF with AdVEGFRII-Ig was confirmed by the absence of dermal vascular leak after intradermal VEGF injection (Figure 3B). Since previous reports have associated the multifunctional cytokine TGF-β with VILI (29, 30), and our own previous work has shown TGF-β to be an important mediator of lung vascular permeability induced by other stimuli (including the anti-cancer drug bleomycin and bacterial lipopolysaccharide [31]), we examined the role of TGF-β in our VILI model. Administration of a recombinant TGF-β receptor II IgG chimera (TGF-βRII-Ig) significantly inhibited lung vascular permeability in VILI (Figure 3A).
Figure 3.
αvβ5 regulates lung vascular permeability in VILI. (A) Mice were treated with ALULA or isotype control antibody (Control Ab), VEGF receptor II-Ig chimera adenovirus (AdVEGFRII-Ig) or green fluorescent protein (GFP) adenovirus control (AdGFP), or TGF-β receptor II IgG chimera (TGF-βRII-Ig) or its vehicle control before ventilation at high tidal volume (20 ml/kg) for 4 h. Lung vascular permeability is reported as extravascular I125-labeled albumin leak normalized to baseline. Data shown are the means ± SE, n = 6. *P = 0.026 for mice treated with ALULA compared with those treated with Control Ab; **P = 0.021 for β5 knockout compared with wild-type (WT) control mice; ***P = 0.027 for mice treated with TGF-βRII-Ig compared with those treated with vehicle control. Baseline lung vascular permeability was measured from nonventilated mice. Baseline values were equivalent between WT and β5 knockout mice. (B) AdVEGFRII-Ig blocks systemic VEGF-induced vascular permeability. Blockade of VEGF-induced vascular permeability by AdVEGFRII-Ig was determined by measurement of extravasation of Evan's blue dye. β5 knockout or WT mice that had been infected with either AdVEGFRII-Ig or AdGFP were injected intravenously with Evan's blue dye. Vascular leak was induced by dermal injection of VEGF (solid bars). Open bars, saline. Total Evan's blue dye content in dermal biopsy sections was determined by formamide extraction and measurement of optical density (O.D.) absorbance at 610 nm. Data shown are the means ± SE, n = 6. *P = 0.001 for mice treated with AdVEGFRII-Ig compared with those treated with control AdGFP; **P = 0.037 for β5 knockout compared with WT control mice.
αvβ5 Regulates Pulmonary Artery Endothelial Permeability Induced by Diverse Mediators of ALI
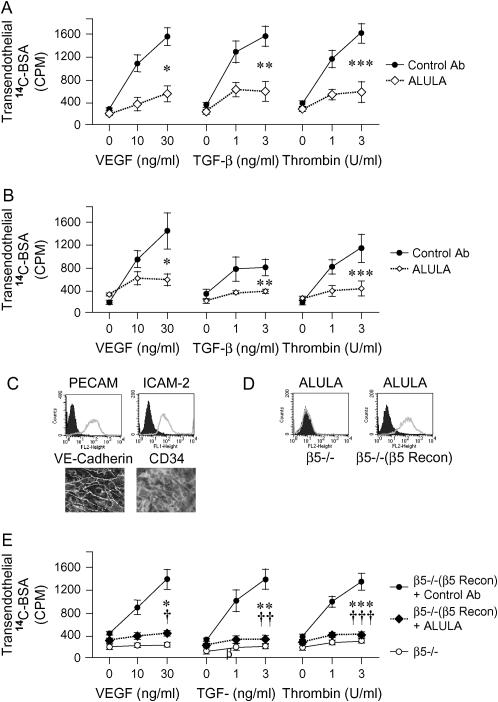
Our in vivo data suggested that the protective effects of blocking αvβ5 were not restricted to effects on VEGF-induced increases in vascular permeability. Since VEGF and TGF-β activate distinct families of receptors and trigger different initial signaling pathways, we hypothesized that αvβ5 might play a central regulatory role downstream of multiple agonist pathways. To test this hypothesis, we studied the effects of VEGF, TGF-β, and also the serine protease thrombin, on pulmonary endothelial cell barrier permeability using a C14-albumin flux assay. Thrombin has been extensively studied for its potent effects on increasing pulmonary endothelial permeability (13, 32–36). We stimulated both bovine pulmonary artery endothelial cell (BPAEC) (Figure 4A) and human pulmonary artery endothelial cell (HPAEC) (Figure 4B) monolayers with VEGF, TGF-β, and thrombin and found that dose-dependent increases in transendothelial C14-albumin flux in response to each agonist were blocked by ALULA.
Figure 4.
αvβ5 regulates permeability of pulmonary endothelial monolayers induced by diverse mediators of ALI. (A) Serum-starved confluent BPAEC monolayers on Transwells were incubated with ALULA or isotype control antibody (Control Ab) 1 h before stimulation with VEGF, TGF-β, or thrombin at the doses shown. Transendothelial leak was determined by application of a C14-albumin tracer to the apical well and subsequent collection and scintillation counting of basolateral well contents after 1 h. Data shown are the means ± SE, n = 3. *P = 0.013 for VEGF (30 ng/ml); **P = 0.018 for TGF-β (10 ng/ml); ***P = 0.022 for thrombin (10 U/ml) for cells treated with ALULA compared with those treated with Control Ab. (B) Serum-starved confluent HPAEC monolayers on Transwells were incubated with ALULA or Control Ab 1 h before stimulation with VEGF, TGF-β, or thrombin at the doses shown. Data shown are the means ± SE, n = 3. *P = 0.038 for VEGF (30 ng/ml); **P = 0.041 for TGF-β (10 ng/ml); ***P = 0.050 for thrombin (10 U/ml) for cells treated with ALULA compared with those treated with Control Ab. (C) β5 subunit knockout pulmonary endothelial cells (β5−/−) were assessed for endothelial markers by immunocytochemistry (vascular endothelial [VE]-cadherin and CD34) or flow cytometry (platelet endothelial cell adhesion molecule [PECAM] and intracellular adhesion molecule [ICAM]-2). Positive immunocytochemistry staining is shown in white. (D) β5 subunit knockout (β5−/−) and β5 reconstituted (β5−/−[β5 Recon]) mouse pulmonary endothelial cells were assessed for expression of β5 by flow cytometry with ALULA. Flow cytometry diagrams represent cells incubated with PBS and secondary antibody alone (solid histograms) or with selected primary antibodies (open histograms). (E) Serum-starved β5 knockout (β5−/−) and β5 reconstituted (β5−/−[β5 Recon]) pulmonary endothelial cell monolayers seeded onto Transwells were assessed for monolayer permeability. β5 reconstituted cells were incubated with ALULA or a Control Ab (10 μg/ml) 1 h before stimulation of both cell types with VEGF, TGF-β, or thrombin at the doses shown. Data shown are the means ± SE, n = 3. *P = 0.006 for VEGF (30 ng/ml); **P = 0.004 for TGF-β (10 ng/ml); ***P = 0.014 for thrombin 10 U/ml for β5−/−(β5 Recon) cells treated with Control Ab compared with β5−/− cells. †P = 0.009 for VEGF (30 ng/ml); ††P = 0.004 for TGF-β (10 ng/ml); †††P = 0.011 for thrombin (10 U/ml) for β5−/−(β5 Recon) treated with ALULA compared with those treated with Control Ab.
Genetic Absence of αvβ5 in Pulmonary Endothelial Cells Protects against Permeability Induced by Diverse Mediators of ALI
To confirm specificity of this effect to αvβ5, we used β5 knockout pulmonary endothelial cells isolated from β5 knockout mice. Primary mouse β5 knockout cells were conditionally immortalized by retroviral transfer of a temperature-sensitive SV40 large and small T antigen transgene. β5 reconstitution was performed by retroviral transfer of full-length human β5 into the immortalized β5 knockout cells. β5 expression and endothelial cell characteristics were confirmed by immunocytochemistry and flow cytometry (Figures 4C–4D). β5 knockout cells were completely resistant to increases in endothelial permeability induced by all three agonists (Figure 4E). As seen in both BPAECs and HPAECs, VEGF, TGF-β, and thrombin each induced dose-dependent increases in monolayer permeability in β5 reconstituted mouse pulmonary endothelial cells (Figure 4E). β5 reconstituted cells incubated with ALULA were resistant to agonist-induced permeability changes (Figure 4E).
αvβ5 Regulates Agonist-Induced Stress Fiber Formation in BPAECs
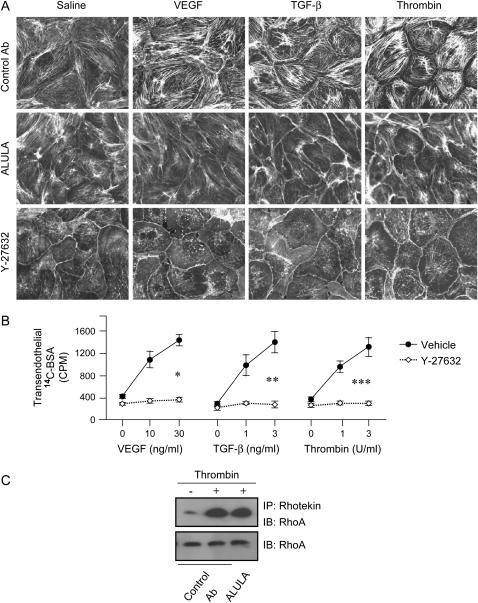
Filamentous (F)-actin can be induced to polymerize to form transcytoplasmic cables that generate tension between cell–cell junctions and focal adhesions on the extracellular matrix. Subsequent disruption of cell–cell junctions can lead to increased paracellular permeability. To test whether agonist-induced stress fiber formation could be regulated by αvβ5, we stimulated monolayers of BPAECs, treated with ALULA or control antibody, with the agonists VEGF, TGF-β, and thrombin. As has previously been reported (10–16), each of these agonists caused dramatic increases in actin stress fiber formation. Furthermore, in each case, this effect was markedly attenuated by αvβ5 blockade with ALULA compared with treatment with control antibody (Figure 5A).
Figure 5.
Agonist-induced stress fiber formation in bovine pulmonary artery endothelial cells is attenuated by αvβ5 blockade and ROCK inhibition, but αvβ5 blockade has no effect on RhoA activity. (A) Confluent monolayers of BPAECs were pretreated with either isotype control antibody (Control Ab) (10 μg/ml), ALULA (10 μg/ml), or ROCK inhibitor (Y-27632) (10 μM) for 1 h, then stimulated with agonists VEGF (30 ng/ml), TGF-β (10 ng/ml), and thrombin (10 U/ml) for 10 min. Cells were fixed, permeabilized, and stained with rhodamin-phalloidin. (B) Serum-starved confluent BPAEC monolayers on Transwells were incubated with ROCK inhibitor (Y-27632) (10 μM) or vehicle control for 1 h before stimulation with VEGF, TGF-β, or thrombin at the doses shown. Transendothelial leak was determined by application of a C14-albumin tracer to the apical well and subsequent collection and scintillation counting of basolateral well contents after 1 h. Data shown are the means ± SE, n = 3. *P = 0.001 for VEGF (30 ng/ml); **P = 0.005 for TGF-β (10 ng/ml); ***P = 0.003, for thrombin (10 U/ml) for cells treated with Y-27632 compared with those treated with vehicle. (C) Total cellular RhoA activation in was assessed using BPAECs pre-treated with either Control Ab (10 μg/ml) or ALULA (10 μg/ml), that were then stimulated for 2 min with thrombin (10 U/ml). Cell lysates were then incubated with agarose beads conjugated with rhotekin Rho-binding domains (RBD) that recognize only GTP-bound active RhoA (immunoprecipitation [IP]:Rhotekin). RhoA was detected from pulled-down product by immunoblot (IB) using anti-RhoA antibody (RhoA). Total lysate samples not incubated with rhotekin beads were immunoblotted with anti-RhoA antibody and served as total RhoA and loading controls.
Agonist-Induced Stress Fiber Formation and Permeability in BPAECs Are Blocked by RhoA Kinase Inhibition
The RhoA family of GTPases has been shown to be a critical regulator of actin stress fiber formation (37–39) as well as an important regulator of increased endothelial permeability (32, 33, 40–48). Each of the three agonists we used for these studies has previously been shown to activate RhoA (45, 49–59). We confirmed these findings by showing that inhibition of RhoA-activated kinase (ROCK) (immediate downstream effector of RhoA) prevents VEGF-, TGF-β-, and thrombin-induced stress fiber formation (Figure 5A). Furthermore, we confirmed that agonist-induced changes in permeability were completely blocked by ROCK inhibition (Figure 5B).
Agonist-Induced RhoA Activation in BPAECs Is Unaffected by αvβ5 Blockade
To determine whether αvβ5 might regulate agonist-induced stress fiber and permeability changes at the level of RhoA activation, we used a rhotekin RhoA-binding domain (RBD)-based active RhoA assay, to assess global agonist-induced increases in cellular RhoA activity. Previous studies have shown that RhoA is activated by thrombin, VEGF, and TGF-β (45, 49–59). We found robust RhoA activation by thrombin in BPAECs that was completely unaffected by αvβ5 blockade (Figure 5C). This finding suggests that αvβ5 contributes to RhoA and ROCK-mediated induction of stress fiber formation and increased endothelial permeability by acting downstream of RhoA.
DISCUSSION
Despite numerous advances in our understanding of the pathophysiology of ALI, specific molecular mechanisms responsible for this clinical syndrome remain poorly understood. In this report, we have characterized a novel and potentially critical role for integrin αvβ5 in the development of an important pathologic hallmark of ALI–increased lung vascular permeability. Furthermore, αvβ5 regulates increased permeability in human, bovine, and murine pulmonary endothelial cells induced by a range of different edemagenic agonists including VEGF, TGF-β, and thrombin. This is a particularly intriguing finding since a complex network of proinflammatory mediators, produced locally in the lung (by fibroblasts, inflammatory, epithelial, and endothelial cells), or derived from extrapulmonary sources, are thought to initiate and amplify the inflammatory response in ALI (31, 60, 61).
Our studies have also identified αvβ5 as a specific regulator of induction of actin stress fibers, a well-described contributor to induced increases in pulmonary endothelial permeability. This finding suggests that αvβ5 might regulate changes in vascular permeability by coordinating interactions with the actin cytoskeleton. Integrins are known to be principal components of focal adhesions—multimolecular structures that link the extracellular matrix (ECM) to the intracellular cytoskeleton. Several reports have linked focal adhesions and regulation of the actin cytoskeleton to endothelial barrier function, but the precise molecular basis for this regulation remains unclear (62–65). Butler and coworkers recently reported that isolated focal adhesion complexes can initiate actin polymerization of actin monomers de novo (66). Moreover, these investigators showed that actin polymerization is dependent on physical clustering of integrins to focal adhesion structures (66). Additional studies are required to identify the molecular mechanisms by which αvβ5 regulates stress fiber formation.
Physical passage of solutes through the endothelial barrier is thought to occur via paracellular pathways or through receptor-activated transcytosis (67, 68). The functional relevance, relative contribution, and molecular determinants of these distinct mechanisms remain incompletely understood, but it has been suggested that direct modification of the actin cytoskeleton in endothelial cells is important for increasing paracellular permeability. One frequently cited model describes paracellular gap formation as a consequence of imbalanced competition between cytoskeletal, adhesive cell–cell and cell–matrix forces (10, 16, 47, 69). In this model, F-actin polymerizes and bundles into morphologically distinct “stress fibers”. Actomyosin-mediated generation of tension leads to alteration of cell shape and formation of paracellular gaps. Stress fibers have been shown to form in endothelial cells stimulated by several vasoactive mediators (11–15, 70, 71), including VEGF, TGF-β, and thrombin (45, 49–59). Although our studies did not directly distinguish between paracellular and transcellular pathways, the parallel ability of αvβ5 to regulate both stress fiber formation and transendothelial flux suggests that in our system the paracellular pathway may be the more relevant.
Use of cells derived from proximal pulmonary macrovascular endothelium is a limitation to our studies. Microvascular endothelial cells are thought to be a more anatomically and physiologically relevant model of pulmonary capillary leak and many studies have detailed significant physiologic differences between lung cells from microvascular and macrovascular bed origins (72–78). Previously, the αvβ5-specific antibody P1F6 was shown to have no effect on ligand-induced increases in lung capillary hydraulic conductivity (79). Our studies are different because we have focused on agonist-induced permeability events, rather than on effects of integrin ligand binding alone. In fact, we found no effect of αvβ5 blockade on baseline permeability or on lung permeability in uninjured animals. However, future studies using pulmonary microvascular endothelial cells, pulmonary microvascular endothelial and alveolar epithelial cell co-culture systems (80), or perhaps capillary split-drop techniques (79, 81) would be necessary to address the important issue of what role αvβ5 might play in regulating capillary permeability.
The model of VILI we used uses relative tidal volumes substantially larger than any currently used for ventilation of people. Therefore, results of the current study cannot be directly extrapolated to suggest that αvβ5 blockade would diminish increased permeability induced by volutrauma in mechanically ventilated patients. Nonetheless, this model is widely used and likely does reflect the effects of excess stretch on alveolar units. Determination of the direct relevance of our findings to patients with VILI will need to await clinical studies with drugs designed to target this integrin.
Several important unanswered questions remain, including how actin stress fiber formation is regulated by αvβ5 ligation. Our observations that αvβ5 antibody produced identical results to both β5 knockout mice and β5 knockout cells strongly suggest that the antibody exerted its effect by specifically inhibiting αvβ5 function, rather than as a result of other antibody–integrin interactions. VEGF, TGF-β, and thrombin activate different families of receptors (tyrosine kinase, serine-threonine kinase, and G protein coupled, respectively) that initiate distinct proximal signaling pathways. It will be important to determine how these diverse pathways converge on αvβ5, and to identify common signaling intermediates. An example of such a signaling intermediate might be the RhoA small GTPase, which has been shown to be activated downstream to a variety of different agonist pathways (45, 49–59), and to be both a critical regulator of actin stress fiber formation (37–39) and increased endothelial permeability (32, 33, 40–48). Our findings in this report suggest that total cellular RhoA activation is not directly affected by αvβ5 blockade, implying that RhoA activation occurs upstream of αvβ5.
While we have focused on disruption of the pulmonary endothelial cell barrier as the main target of αvβ5 effects, there are alternative targets to consider. In vivo increases in lung vascular permeability in ALI involve complex interactions between multiple cell types, including leukocytes and epithelial cells, as well as endothelial cells (82). Since αvβ5 is widely expressed, it is possible that αvβ5-mediated effects on other cell types could contribute to the overall in vivo role of αvβ5 in regulating pulmonary edema formation. Our in vivo models were chosen primarily as models of increased lung vascular permeability. Relevance to ALI, and even to their specific clinical correlates may be questioned, for example, with experimental ischemia and reperfusion times (for IR) and brief relative ventilation periods and extreme tidal volume settings (for VILI). The complexities of ALI mandate that other models be tested. Ultimate proof of relevance will only come from clinical studies in patients at risk for or affected by ALI.
Finally, although our results demonstrate an important role for αvβ5 ligation in regulating pulmonary endothelial permeability, they do not identify the relevant in vivo ligand. Our cell culture studies were performed by seeding cells onto nonspecific collagen substrates in the presence of fetal calf serum, a rich source of vitronectin, and growing them in serum-enriched media over several days. This protocol allowed ample time for vitronectin to bind to the cells and substrate and for the cells to secrete additional ECM proteins. Our αvβ5 blocking antibody, ALULA, specifically recognizes αvβ5 and blocks adhesion to vitronectin in vitro. However, it is certainly plausible that, in vivo, other ligands are critical for the functions we have described. While vitronectin knockout mice have been observed to be viable and healthy (83), and therefore, would be a good model system to determine relevance of vitronectin, these studies might potentially be confounded by effects exerted by the integrin αvβ3, which shares vitronectin as a common ECM protein ligand.
Despite these gaps in our current understanding, the findings reported here have potential clinical relevance. Given the robust regulatory effects of blocking αvβ5 in two quite different in vivo models of increased lung vascular permeability and in the pulmonary endothelial permeability response to multiple biologically relevant agonists, αvβ5 appears to be an attractive therapeutic target for ALI, a substantial cause of morbidity and mortality that is currently largely untreatable.
This study was funded by HL53949, HL64353, HL56385 and Program in Genomics HL66600 (Baygenomics) from the NHLBI (to D.S.), HL074005 (SCCOR, Project 4) (to J.-F.P.), and UCTRDRP 12FT-0123 (to G.S.)
Originally Published in Press as DOI: 10.1165/rcmb.2006-0238OC on November 1, 2006
Conflict of Interest Statement: G.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. X.Z.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.K.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.A.M does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.S. is co-owner of a filed patent (pending) covering blockade of integrin αvβ5 for the treatment of acute lung injury. He also has had a sponsored research agreement with BiogenIdec to cover work on anti-integrin antibodies and acute lung injury for $150,000/year (total costs) since January 2002. J.-F.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med 2003;31:S276–S284. [DOI] [PubMed] [Google Scholar]
- 3.Groeneveld AB. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vascul Pharmacol 2002;39:247–256. [DOI] [PubMed] [Google Scholar]
- 4.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed] [Google Scholar]
- 5.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol 2002;157:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hertz MI, Taylor DO, Trulock EP, Boucek MM, Mohacsi PJ, Edwards LB, Keck BM. The registry of the international society for heart and lung transplantation: nineteenth official report-2002. J Heart Lung Transplant 2002;21:950–970. [DOI] [PubMed] [Google Scholar]
- 7.King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, Tribble CG, Kron IL. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg 2000;69:1681–1685. [DOI] [PubMed] [Google Scholar]
- 8.Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med 1993;21:131–143. [DOI] [PubMed] [Google Scholar]
- 9.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med 1994;20:225–232. [DOI] [PubMed] [Google Scholar]
- 10.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 1995;163:510–522. [DOI] [PubMed] [Google Scholar]
- 11.Joris I, Majno G, Ryan GB. Endothelial contraction in vivo: a study of the rat mesentery. Virchows Arch B Cell Pathol 1972;12:73–83. [DOI] [PubMed] [Google Scholar]
- 12.Joris I, Majno G, Corey EJ, Lewis RA. The mechanism of vascular leakage induced by leukotriene E4. Endothelial contraction. Am J Pathol 1987;126:19–24. [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW II, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol 1986;128:96–104. [DOI] [PubMed] [Google Scholar]
- 14.Wu NZ, Baldwin AL. Possible mechanism(s) for permeability recovery of venules during histamine application. Microvasc Res 1992;44:334–352. [DOI] [PubMed] [Google Scholar]
- 15.Wu NZ, Baldwin AL. Transient venular permeability increase and endothelial gap formation induced by histamine. Am J Physiol 1992;262:H1238–H1247. [DOI] [PubMed] [Google Scholar]
- 16.Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol 1994;267:L223–L241. [DOI] [PubMed] [Google Scholar]
- 17.Tseng JF, Farnebo FA, Kisker O, Becker CM, Kuo CJ, Folkman J, Mulligan RC. Adenovirus-mediated delivery of a soluble form of the VEGF receptor Flk1 delays the growth of murine and human pancreatic adenocarcinoma in mice. Surgery 2002;132:857–865. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Griffiths M, Wu J, Farese RV Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol 2000;20:755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank J, Wang Y, Osorio O, Matthay M. Beta-adrenergic agonist therapy accelerates the resoluation of hydrostatic pulmonary edema in sheep and rats. J Appl Physiol 2000;89:1255–1265. [DOI] [PubMed] [Google Scholar]
- 20.Pittet JF, Wiener-Kronish JP, McElroy MC, Folkesson HG, Matthay MA. Stimulation of lung epithelial liquid clearance by endogenous release of catecholamines in septic shock in anesthetized rats. J Clin Invest 1994;94:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 22.Ramaswamy H, Hemler ME. Cloning, primary structure and properties of a novel human integrin beta subunit. EMBO J 1990;9:1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wayner EA, Orlando RA, Cheresh DA. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol 1991;113:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang DM, Hsu K, Ding YA, Chiang CH. Interleukin-1 in ischemia-reperfusion acute lung injury. Am J Respir Crit Care Med 1997;156:1230–1234. [DOI] [PubMed] [Google Scholar]
- 25.Kazi AA, Lee WS, Wagner E, Becker PM. VEGF, fetal liver kinase-1, and permeability increase during unilateral lung ischemia. Am J Physiol Lung Cell Mol Physiol 2000;279:L460–L467. [DOI] [PubMed] [Google Scholar]
- 26.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med 2003;167:490–511. [DOI] [PubMed] [Google Scholar]
- 27.Takayama K, Ueno H, Nakanishi Y, Sakamoto T, Inoue K, Shimizu K, Oohashi H, Hara N. Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res 2000;60:2169–2177. [PubMed] [Google Scholar]
- 28.Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA. High tidal volume ventilation induces NOS2 and impairs cAMP- dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol 2003;284:L791–L798. [DOI] [PubMed] [Google Scholar]
- 29.Imanaka H, Shimaoka M, Matsuura N, Nishimura M, Ohta N, Kiyono H. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-beta 1 mRNA upregulation in rat lungs. Anesth Analg 2001;92:428–436. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto H, Teramoto H, Uetani K, Igawa K, Shimizu E. Cyclic stretch upregulates interleukin-8 and transforming growth factor-beta1 production through a protein kinase C-dependent pathway in alveolar epithelial cells. Respirology 2002;7:103–109. [DOI] [PubMed] [Google Scholar]
- 31.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, et al. TGF-beta is a critical mediator of acute lung injury. J Clin Invest 2001;107:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 2004;67:64–77. [DOI] [PubMed] [Google Scholar]
- 33.Bogatcheva NV, Garcia JG, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry (Mosc) 2002;67:75–84. [DOI] [PubMed] [Google Scholar]
- 34.Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol 1996;16:488–496. [DOI] [PubMed] [Google Scholar]
- 35.Konstantoulaki M, Kouklis P, Malik AB. Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am J Physiol Lung Cell Mol Physiol 2003;285:L434–L442. [DOI] [PubMed] [Google Scholar]
- 36.van Nieuw Amerongen GP, Natarajan K, Yin G, Hoefen RJ, Osawa M, Haendeler J, Ridley AJ, Fujiwara K, van Hinsbergh VW, Berk BC. GIT1 mediates thrombin signaling in endothelial cells: role in turnover of RhoA-type focal adhesions. Circ Res 2004;94:1041–1049. [DOI] [PubMed] [Google Scholar]
- 37.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992;70:389–399. [DOI] [PubMed] [Google Scholar]
- 38.Ridley AJ. GTPases: Rho. In: Hall A, editor. Frontiers in molecular biology. Oxford, UK: Oxford University Press; 2000. pp. 89–119.
- 39.Ridley AJ. Rho: theme and variations. Curr Biol 1996;6:1256–1264. [DOI] [PubMed] [Google Scholar]
- 40.Garcia JG, Verin AD, Schaphorst K, Siddiqui R, Patterson CE, Csortos C, Natarajan V. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60(src). Am J Physiol 1999;276:L989–L998. [DOI] [PubMed] [Google Scholar]
- 41.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 2001;114:1343–1355. [DOI] [PubMed] [Google Scholar]
- 42.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 2002;39:187–199. [DOI] [PubMed] [Google Scholar]
- 43.van Nieuw Amerongen GP, van Hinsbergh VW. Cytoskeletal effects of rho-like small guanine nucleotide-binding proteins in the vascular system. Arterioscler Thromb Vasc Biol 2001;21:300–311. [DOI] [PubMed] [Google Scholar]
- 44.van Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VW. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol 2000;20:E127–E133. [DOI] [PubMed] [Google Scholar]
- 45.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 2000;87:335–340. [DOI] [PubMed] [Google Scholar]
- 46.Adamson RH, Curry FE, Adamson G, Liu B, Jiang Y, Aktories K, Barth H, Daigeler A, Golenhofen N, Ness W, et al. Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol 2002;539:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 2001;91:1487–1500. [DOI] [PubMed] [Google Scholar]
- 48.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2003;284:L972–L980. [DOI] [PubMed] [Google Scholar]
- 49.Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 2005;288:L294–L306. [DOI] [PubMed] [Google Scholar]
- 50.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006;13:237–247. [DOI] [PubMed] [Google Scholar]
- 51.Zeng L, Xu H, Chew TL, Eng E, Sadeghi MM, Adler S, Kanwar YS, Danesh FR. HMG CoA reductase inhibition modulates VEGF-induced endothelial cell hyperpermeability by preventing RhoA activation and myosin regulatory light chain phosphorylation. FASEB J 2005;19:1845–1847. [DOI] [PubMed] [Google Scholar]
- 52.van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VW. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol 2003;23:211–217. [DOI] [PubMed] [Google Scholar]
- 53.Ohkawara H, Ishibashi T, Sakamoto T, Sugimoto K, Nagata K, Yokoyama K, Sakamoto N, Kamioka M, Matsuoka I, Fukuhara S, et al. Thrombin-induced rapid geranylgeranylation of RhoA as an essential process for RhoA activation in endothelial cells. J Biol Chem 2005;280:10182–10188. [DOI] [PubMed] [Google Scholar]
- 54.Anwar KN, Fazal F, Malik AB, Rahman A. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol 2004;173:6965–6972. [DOI] [PubMed] [Google Scholar]
- 55.Vouret-Craviari V, Grall D, Van Obberghen-Schilling E. Modulation of Rho GTPase activity in endothelial cells by selective proteinase-activated receptor (PAR) agonists. J Thromb Haemost 2003;1:1103–1111. [DOI] [PubMed] [Google Scholar]
- 56.Klarenbach SW, Chipiuk A, Nelson RC, Hollenberg MD, Murray AG. Differential actions of PAR2 and PAR1 in stimulating human endothelial cell exocytosis and permeability: the role of Rho-GTPases. Circ Res 2003;92:272–278. [DOI] [PubMed] [Google Scholar]
- 57.Vouret-Craviari V, Bourcier C, Boulter E, van Obberghen-Schilling E. Distinct signals via Rho GTPases and Src drive shape changes by thrombin and sphingosine-1-phosphate in endothelial cells. J Cell Sci 2002;115:2475–2484. [DOI] [PubMed] [Google Scholar]
- 58.Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-beta1-induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. J Appl Physiol 2006;101:375–384. [DOI] [PubMed] [Google Scholar]
- 59.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2006;290:L540–L548. [DOI] [PubMed] [Google Scholar]
- 60.Siflinger-Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003;284:L435–L451. [DOI] [PubMed] [Google Scholar]
- 61.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 2004;202:145–156. [DOI] [PubMed] [Google Scholar]
- 62.Wu MH. Endothelial focal adhesions and barrier function. J Physiol 2005;569:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 2003;19:677–695. [DOI] [PubMed] [Google Scholar]
- 64.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol 2002;4:E83–E90. [DOI] [PubMed] [Google Scholar]
- 65.Wiesner S, Lange A, Fassler R. Local call: from integrins to actin assembly. Trends Cell Biol 2006;16:327–329. [DOI] [PubMed] [Google Scholar]
- 66.Butler B, Gao C, Mersich AT, Blystone SD. Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr Biol 2006;16:242–251. [DOI] [PubMed] [Google Scholar]
- 67.Michel CC. The transport of albumin: a critique of the vesicular system in transendothelial transport. Am Rev Respir Dis 1992;146:S32–S36. [DOI] [PubMed] [Google Scholar]
- 68.Renkin EM. Capillary transport of macromolecules: pores and other endothelial pathways. J Appl Physiol 1985;58:315–325. [DOI] [PubMed] [Google Scholar]
- 69.Lum H, Malik AB. Mechanisms of increased endothelial permeability. Can J Physiol Pharmacol 1996;74:787–800. [DOI] [PubMed] [Google Scholar]
- 70.Cotran RS, Majno G. Studies on the intercellular junctions of mesothelium and endothelium. Protoplasma 1967;63:45–51. [PubMed] [Google Scholar]
- 71.Majno G, Joris I. Endothelium 1977: a review. Adv Exp Med Biol 1978;104:169–225, 481–526. [DOI] [PubMed] [Google Scholar]
- 72.Muth H, Maus U, Wygrecka M, Lohmeyer J, Grimminger F, Seeger W, Gunther A. Pro- and antifibrinolytic properties of human pulmonary microvascular versus artery endothelial cells: impact of endotoxin and tumor necrosis factor-alpha. Crit Care Med 2004;32:217–226. [DOI] [PubMed] [Google Scholar]
- 73.Beck GC, Yard BA, Breedijk AJ, Van Ackern K, Van Der Woude FJ. Release of CXC-chemokines by human lung microvascular endothelial cells (LMVEC) compared with macrovascular umbilical vein endothelial cells. Clin Exp Immunol 1999;118:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chetham PM, Babal P, Bridges JP, Moore TM, Stevens T. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol 1999;276:L41–L50. [DOI] [PubMed] [Google Scholar]
- 75.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol 1998;274:L810–L819. [DOI] [PubMed] [Google Scholar]
- 76.Moldobaeva A, Wagner EM. Heterogeneity of bronchial endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 2002;283:L520–L527. [DOI] [PubMed] [Google Scholar]
- 77.Irwin DC, Tissot van Patot MC, Tucker A, Bowen R. Direct ANP inhibition of hypoxia-induced inflammatory pathways in pulmonary microvascular and macrovascular endothelial monolayers. Am J Physiol Lung Cell Mol Physiol 2005;288:L849–L859. [DOI] [PubMed] [Google Scholar]
- 78.Qiao RL, Bhattacharya J. Segmental barrier properties of the pulmonary microvascular bed. J Appl Physiol 1991;71:2152–2159. [DOI] [PubMed] [Google Scholar]
- 79.Tsukada H, Ying X, Fu C, Ishikawa S, McKeown-Longo P, Albelda S, Bhattacharya S, Bray BA, Bhattacharya J. Ligation of endothelial alpha v beta 3 integrin increases capillary hydraulic conductivity of rat lung. Circ Res 1995;77:651–659. [DOI] [PubMed] [Google Scholar]
- 80.Hermanns MI, Unger RE, Kehe K, Peters K, Kirkpatrick CJ. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo-capillary barrier in vitro. Lab Invest 2004;84:736–752. [DOI] [PubMed] [Google Scholar]
- 81.Bhattacharya J. Hydraulic conductivity of lung venules determined by split-drop technique. J Appl Physiol 1988;64:2562–2567. [DOI] [PubMed] [Google Scholar]
- 82.Hasleton PS, Roberts TE. Adult respiratory distress syndrome - an update. Histopathology 1999;34:285–294. [DOI] [PubMed] [Google Scholar]
- 83.Zheng X, Saunders TL, Camper SA, Samuelson LC, Ginsburg D. Vitronectin is not essential for normal mammalian development and fertility. Proc Natl Acad Sci USA 1995;92:12426–12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cone RI, Weinacker A, Chen A, Sheppard D. Effects of beta subunit cytoplasmic domain deletions on the recruitment of the integrin alpha v beta 6 to focal contacts. Cell Adhes Commun 1994;2:101–113. [DOI] [PubMed] [Google Scholar]