Abstract
Cigarette smoking is a major risk factor for chronic obstructive pulmonary disease (COPD). Recent reports of increased matrix metalloproteinase-2 (MMP-2) in lungs of patients with emphysema support the paradigm of proteinase/antiproteinase imbalance in the pathogenesis of COPD. We sought to define the signaling pathways activated by smoke and to identify molecules responsible for emphysema-associated MMP-2 expression. In this study, we show that cigarette smoke extract (CSE) induced MMP-2 protein expression and increased MMP-2 gelatinase activity of normal lung fibroblasts. We previously identified a transcription factor, early growth response 1 (EGR-1), with robust expression in the lung tissues of patients with COPD compared with control smokers. Here, the treatment of fibroblasts with CSE resulted in marked induction of EGR-1 mRNA and protein in a dose- and time-dependent manner, accompanied by increased EGR-1 binding activity. CSE-induced MMP-2 mRNA and protein expression and activity were significantly inhibited using EGR-1 small interfering RNA (siRNA) or in Egr-1–null−/− mouse fibroblasts. Furthermore, we observed induction of membrane type 1 matrix metalloproteinase (MT1-MMP), which has an EGR-1–binding site on its promoter, in CSE-treated primary normal lung fibroblasts. The concomitant MT1-MMP expression and MMP-2 activation by CSE are inhibited by EGR-1 siRNA. Rapid activation of mitogen-activated protein kinases was observed in CSE-treated fibroblasts. Chemical inhibitors of ERK1/2 MAPK, but not of p38 and JNK, decreased CSE-induced EGR-1 protein expression and MMP-2 activity of fibroblasts. The identification that induction of MMP-2 and MT1-MMP by CSE from lung fibroblasts is EGR-1–dependent reveals a molecular mechanism for matrix remodeling in cigarette smoke–related emphysema.
Keywords: chronic obstructive pulmonary disease, EGR-1, cigarette smoke extract, MMP-2, MT1-MMP
CLINICAL RELEVANCE
The study helps improve our knowledge of the pathogenesis of cigarette smoke–induced emphysema.
Chronic obstructive pulmonary disease (COPD), is the fourth leading cause of death in the United States and an important cause of morbidity and mortality worldwide, resulting in a substantial economic and social burden throughout the world (1). Currently, there are no effective drug therapies for COPD that are able to significantly alter disease progression, and little is known of the underlying molecular mechanisms that are responsible for its occurrence (2). The disease results mainly from interaction between individual genetic factors and environmental exposure to cigarette smoke. Cigarette smoke–induced emphysema is the major anatomic cause of COPD (1–3). To better understand the candidate genes involved in the development of COPD in smokers, we previously performed serial analysis of gene expression (SAGE) (4) and microarray analysis to analyze the global gene expression profiles of lung tissues from smokers who are at risk (GOLD-0) and who have developed moderate (GOLD-2) COPD (5). By analyzing the comprehensive data produced by SAGE and microarray, we have identified numerous classes of genes whose expression is altered in patients with COPD. Some of these genes have not been previously associated with COPD. Among the genes differentially expressed between moderate COPD and control smokers, early growth response 1 (EGR-1), a transcription factor, was identified with robust expression in the lung tissues of patients with COPD compared with control smokers. Further functional studies using lung fibroblasts from Egr-1−/− mice suggested that EGR-1 might play a key role in the development of cigarette smoke–related emphysema partly by regulating matrix metalloproteinase (MMP)-2 activity (5).
MMP-2 belongs to the family of MMPs that are highly conserved metal atom–dependent proteinases, which, collectively, are capable of degrading almost all extracellular matrix components (6, 7). Abnormal production and regulation of MMPs has been observed in a number of pathologic states, including emphysema (7, 8). It is generally known that the proteolytic breakdown of basement membranes leads to the loss of alveolar attachments, which is a key irreversible step in parenchymal destruction in emphysema development (9, 10). MMP-2 (gelatinase A) is one of the ECM-degrading proteases and was shown to cleave type I collagen, elastin, and degraded collagen that has been denatured into gelatin at normal body temperature; it has also been especially recognized as a major mediator of the degradation of basement membrane, which consists mainly of collagen type IV, laminin, and proteoglycans (11). Human studies revealed higher MMP-2 levels in human lungs with emphysema compared with control lungs, with localization in fibroblasts and alveolar macrophages (12). Cigarette smoke also increases MMP-2 content in mice bronchoalveolar lavage fluid (BAL) (13). In addition, MMP-2 in cascade with membrane type 1 matrix metalloproteinase (MT1-MMP) and tissue inhibitor of metalloproteinase (TIMP)2 is reported to control ECM degradation during epithelial branching and alveolization for normal mice lung morphogenesis (14). The above studies suggest that MMP-2 may play prominent roles in smoking-induced emphysema. However, the intracellular signaling mechanisms activated by tobacco smoke that mediate the MMP-2 function and subsequent matrix degradation in the lungs are not well understood. The present study was to characterize the role of EGR-1 in cigarette smoke–mediated induction and activation of MMP-2 in the structural pulmonary cells (lung fibroblasts). Primary fibroblasts from non-COPD lung were chosen to represent lung fibroblasts. Therefore, this model system was used to elucidate the early molecular responses activated by cigarette smoke that bring about MMP-2 activation associated with emphysema.
MATERIALS AND METHODS
Reagents
The monoclonal (anti) total MMP-2, active MMP-2, MT1-MMP, and TIMP2 antibodies were purchased from Abcam Inc. (Cambridge, MA). The polyclonal rabbit anti-EGR-1 antibody was from Santa Cruz Biotech., Inc. (Santa Cruz, CA). The (anti) phospho-p38 MAPK (Thr180/Tyr182), p38 MAPK, phospho-p44/42 MAPK (Thr202/Tyr204), p44/42 MAPK, phospho–stress-activated protein kinase (SAPK)/JNK (Thr183/Tyr185), and SAPK/JNK antibodies were purchased from Cell Signaling Technology, New England Biolabs (Beverly, MA). The specific inhibitor of p38 MAPK SB-203580, the mitogen/extracellular signal–regulated kinase (MEK) inhibitors U-0126, and SAPK/JNK MAPK inhibitor SP600125 were from Calbiochem (San Diego, CA).
Primary Lung Fibroblast Isolation and Culture
Primary human lung fibroblasts (NL9) were cultured from donor lung tissue obtained from the University of California. The donor (49-yr-old male) was a smoker (33 pk-yrs) and died from head trauma after a fall from a building. Primary mouse lung fibroblasts were cultured from lung tissues of Egr-1−/− and littermate control (Egr-1+/+) mice (gift of D. J. Pinsky, College of Physicians and Surgeons of Columbia University, New York). Fibroblast cell culture was performed as previously described (15) with a modification. Briefly, lung tissue was minced (1 mm) and pieces placed (15–20 pieces) into 100-mm culture dishes with a drop of serum onto each tissue piece. After 4 h incubation, the primary cultures were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C and 5% CO2 until confluence.
MTT Assay
Cellular toxicity and proliferation were measured by the MTT assay manufactured by Amresco (Solon, OH). Briefly, for each experimental condition, ∼ 2,000 cells were seeded in each well of a 96-well plate (eight repeats). Every group (n = 8 in every group) was treated with varying concentrations of cigarette smoke extract (CSE) for 24 h at 37°C. Then, 5 mg/ml MTT was added to each well and incubated for 4 h. The cells were lysed by adding 150 μl/well of dimethyl sulfoxide and read at 570 nm absorbance wavelength in microplate reader.
Preparation and Treatment of CSE
Nonfiltered research reference cigarettes (1R3F) were purchased from the University of Kentucky (Lexington, KY). CSE was prepared at a concentration of 1 cigarette/5 ml in serum-free DMEM with modifications to the methods described by Ishii and colleagues (16). This medium was defined as 100% CSE and can be used after adjusting to pH 7.4 and filtering through a 0.22-μm filter.
Synthesis of EGR-1 Small Interfering RNA and Transient Transfection of NL9 Cells
Double-stranded EGR-1 siRNA, targeting the coding region 1237–1258 relative to the start codon of EGR-1 gene, and a control scrambled duplex (sc EGR-1 small interfering RNA [siRNA]) (17), were synthesized by Dharmacon, Inc. (Lafayette, CO). NL9 cells were grown to 60–70% confluence in 6-well tissue culture plates before transfection with either EGR-1 siRNA or sc EGR-1 siRNA using TransIT-TKO transfection reagent (Mirus, Madison, WI), according to the manufacturer's instructions with minor modifications. Briefly, EGR-1 siRNA or sc EGR-1 siRNA (100 nM) was incubated in 200 μl Opti-MEMI containing 8 μl of TransIT-TKO transfection reagent for 20 min. The complexes were added to the NL9 cells in 1 ml DMEM containing 10% FBS and 1% penicillin-streptomycin and incubated at 37°C for 24 h. Then the medium was replaced and the cells were used for CSE exposure.
Real-Time Quantitative RT-PCR
Total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA), cleaned with the RNeasy Mini Kit (Qiagen, Valencia, CA) and the DNA-free kit (Ambion, Austin, TX) according to the manufacturer's protocols. Quantitative (Q)RT-PCR was performed as described (18, 19). Gene expressions were measured relative to the endogenous reference gene, human β-glucuronidase (β-GUS) or mouse β-actin, using the comparative CT method described previously (19). A calibrator RNA sample, human lung RNA or mouse lung RNA, was amplified in parallel to allow comparison of samples run at different times. The sequence of EGR1 QRT-PCR primers and probe is described in Ref. 5. MMP-2 and MT1-MMP PCR primers were purchased from Applied Biosystems (Foster City, CA) (MMP2: Hs00234422_m1, and Mm00439508_m1; MT1-MMP: Hs00237119_m1, Mm00485054_m1).
Western Blot Analysis
Total cellular extracts or concentrated media were obtained and Western blotting was performed as described previously (20). The antibodies used are rabbit anti-EGR1 antibody (1:500), phospho-specific and non–phospho-specific rabbit polyclonal antibodies to p38 MAPK, p44/42 MAPK, or SAPK/JNK (1:1,000), and mouse monoclonal antibodies to total or active specific MMP-2, TIMP2 (1:500), MT1-MMP (1:100), followed by incubation with horseradish peroxidase–conjugated anti-rabbit or anti-mouse antibody (1:2,000). All immunoblots were repeated in triplicate.
Gelatin Zymograghpy
Gelatin zymography for assaying MMP-2 was performed as described (21), with minor modifications. Briefly, fibroblasts were treated with CSE for 1–3 d. Culture media were concentrated using the Centricon-10 system (Amicon-Millipore, Bedford, MA). Concentrated medium (40 μl) was electrophoresed on 10% pre-cast polyacrylamide gel containing 2 mg/ml gelatin (Invitrogen). After re-naturing, the gel was incubated in 1× Zymogram Developing Buffer for 4 h at 37°C for maximum protease activity. All zymo-gels were repeated in triplicate.
Preparation of Nuclear Extracts and EGR-1 DNA Binding Activity Assay
Nuclear extracts were prepared from 4-h-CSE–treated NL9 cells using the Nuclear Extraction Kit (Panomics, Redwood City, CA), and EGR-1 DNA binding activity was measured using the BD Mercury TransFactor Kit (BD Biosciences, Palo Alto, CA) according to the manufacturer's instructions. Three independent experiments were performed, each with triplicate determinations. Statistical significance was determined by the Student's t test for paired data points comparing treated with control samples. P values < 0.05 were considered significant. Data are presented as mean ± SE of triplicate determinations.
RESULTS
CSE Induces MMP-2 Protein Production, and MMP-2 Activity in NL9 Fibroblasts
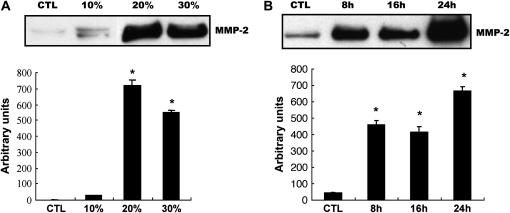
Similarly to other members of the metalloproteinase family, MMP-2 is secreted as a pro-enzyme, then activated by proteolytic cleavage extracellularly (6, 7). To study the effect of CSE on MMP-2 production, NL9 fibroblast cells were exposed to exogenous CSE. Media samples were collected and the production of MMP-2 was assessed by Western blot using the anti–MMP-2 monoclonal antibody. As shown in Figure 1A, CSE increased MMP-2 production in a dose-dependent manner. At a lower dose (10%), CSE stimulated MMP-2 production slightly, while 20% CSE-exposed NL9 cells produced significantly more MMP-2 than control. The effect of CSE on MMP-2 production was also time dependent (Figure 1B). Significant increases in MMP-2 production in concentrated media were seen after 24 h exposure to CSE (20%) compared with untreated controls. No significant differences in NL9 cell viability were observed as assessed by MTT assay after 24 h exposure to 20% CSE (1.16 ± 0.05) compared with control cells (1.2 ± 0.07). Decreased viability of cells were observed at the ceiling of dose–response at 50% CSE (0.49 ± 0.07).
Figure 1.
Effect of CSE on MMP-2 production and activation in NL9 culture media. Fibroblasts were incubated in the absence (CTL) or in the presence of CSE for the indicated concentrations and times, then the concentrated media were subjected to Western blot analysis using an anti–MMP-2 monoclonal antibody (A and B) or gelatin zymography (C). (A) Dose–response of MMP-2 production in NL9 culture media after 24 h of CSE exposure. (B) Time course of MMP-2 production in NL9 culture media treated with 20% CSE. (C) Gelatin zymography of concentrated NL9 media treated with 20% CSE to measure the induction of MMP-2 activity. The active form of MMP-2 was used as a positive control. Quantitation of Western blot immunoblots were performed by densitometric scanning of blots (A, B, and C, bottom). *P < 0.05.
We further measured MMP-2 activity in NL9 concentrated culture media using standard gelatin zymography. Zymography revealed three bands corresponding to the latent (72-kD), intermediary (64-kD), and active (62-kD) forms of MMP-2 (Figure 1C). Figure 1C shows that the activities of active MMP-2 were significantly increased in a time-dependent manner after 3 d of CSE exposure compared with untreated controls. The above data demonstrate that cigarette smoke can stimulate MMP-2 expression and activation of human lung fibroblasts.
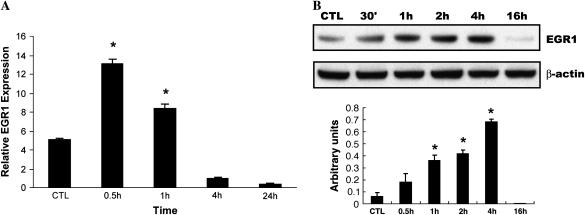
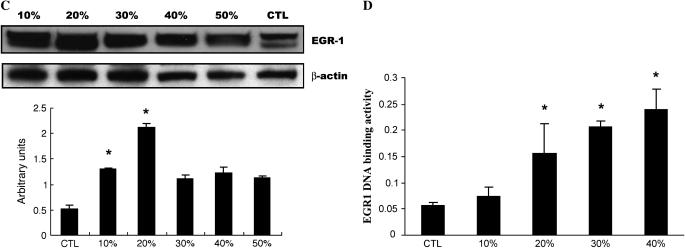
CSE Induces EGR-1 mRNA, Protein Expression, and EGR-1 DNA Binding Activity in NL9 Fibroblasts
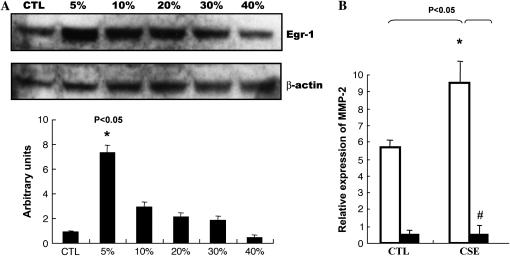
Our previous study (5) identified a ubiquitous transcription factor, early growth response (EGR-1), with more robust expression in lung tissues of patients with COPD than in control smokers using SAGE and microarray analysis. We also demonstrated EGR-1 involvement in cytomix (TNF-α, IL-1β, IFN-γ)-induced MMP-2 activity using lung fibroblasts from Egr1−/− and Egr1+/+ control mice. Here, human NL9 cells were exposed to CSE to determine whether cigarette smoke can induce EGR-1 expression. A QRT-PCR analysis showed that in response to 20% CSE, EGR-1 mRNA expression quickly reached its maximal level at 30 min, and then decreased after 4 h (Figure 2A). The increase of EGR-1 mRNA expression was reflected in increased EGR-1 protein production and EGR-1 transcriptional activity, respectively. Western blot analysis using polyclonal anti–EGR-1 antibodies shows that the increase in EGR-1 protein expression occurs in time-dependent (0.5–4 h) (Figure 2B) and dose-dependent manners (Figure 2C) in NL9 cells exposed to exogenous CSE. Nuclear extracts prepared from CSE-treated NL9 fibroblasts were assayed for EGR-1 transcriptional activity, as measured by the binding activity of EGR-1 to its consensus oligonucleotide sequence. In our previous work, we have reported a time-dependent increase in the binding activity of Egr-1 with its maximal level at 4 h (5). Here in Figure 2D, significant dose-dependent increases in EGR-1 transcriptional activity were observed in NL9 nuclear extracts after exposure to CSE compared with untreated controls.
Figure 2.
Induction of EGR-1 mRNA, protein expression, and EGR-1 DNA-binding activity by CSE in NL9 cells. Fibroblasts were incubated in the absence (CTL) or in the presence of CSE for the indicated concentrations and times. (A) Total RNA was extracted and subjected to QRT-PCR to measure the significant induction of EGR-1 mRNA by CSE (20%) (*P < 0.01). Whole cell lysates were subjected to Western blot analysis for dose response (B) and time course (C) of EGR-1 protein expression in NL9 cells treated with CSE. The same membrane was probed with an anti–β-actin antibody to assess equal loading of the gel. Quantitation of Western blot immunoblots were performed by densitometric scanning of blots. (B and C, bottom). Nuclear extracts were assayed for dose–response (D) of the activity of transcription factor EGR-1 by an ELISA-based TransFactor kit. There was significant increase in EGR-1 transcriptional activity in CSE-treated NL9 cells versus control cells. *P < 0.05.
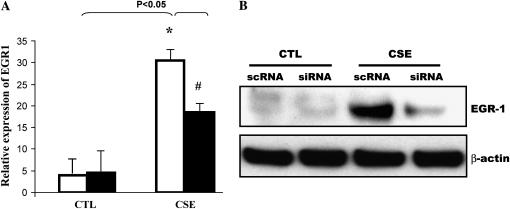
siRNA Duplexes for EGR-1 mRNA Block Cigarette Smoke–Stimulated MMP-2 Activation
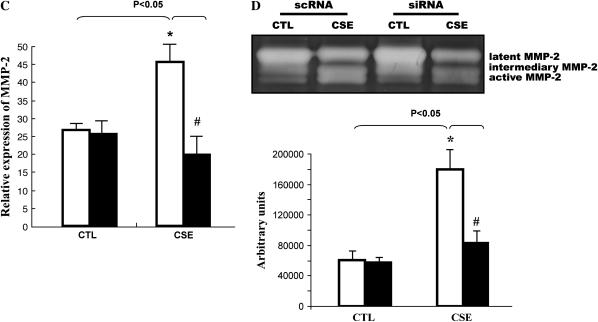
To evaluate the role of EGR-1 in cigarette smoke–mediated MMP-2 induction in NL9 cells, small interfering RNA duplexes (EGR-1 siRNA) were used to block EGR-1 protein production. The results presented in Figure 3 confirm that NL9 cells transfected with EGR-1 siRNA show a significant reduction in EGR-1 mRNA (Figure 3A) and protein expression (Figure 3B), compared with cells transfected with sc EGR-1 siRNA, in response to the 20% CSE treatment. Furthermore, the CSE (20%)-induced MMP-2 mRNA expression (Figure 3C) and activity (Figure 3D) were reduced in NL9 cells transfected with EGR-1 siRNA as assessed by QRT-PCR and standard gelatin zymography, respectively. The maximal reduction (65%) of MMP-2 activity was observed when NL9 cells were transfected with 100 nM EGR-1 siRNA (Figure 3D, bottom). The data in Figure 3D shows that transfection of NL9 cells with sc EGR-1 siRNA does not have any inhibitory effect on CSE-induced MMP-2 activity.
Figure 3.
The siRNA duplexes for EGR-1 (EGR-1 siRNA) suppress CSE-induced EGR-1 mRNA, protein expression and MMP-2 activity. NL9 cells were transfected with either 100 nM EGR-1 siRNA (si; solid bars) or scrambled EGR-1 siRNA (sc; open bars). After 24 h of transfection, cells were treated with CSE (20%); NL9 cells transfected with EGR-1 siRNA show a significant reduction in (A) EGR-1 mRNA, (B) EGR-1 protein, and (C) MMP-2 mRNA. (D) Gelatin zymography of concentrated media of NL9 cells treated with CSE (20%) for 2 d. Results of densitometry of active MMP-2 bands are shown in the bar graph at bottom of D.
CSE Cannot Induce MMP-2 Production and Activation in Lung Fibroblasts from Egr-1−/− Mice
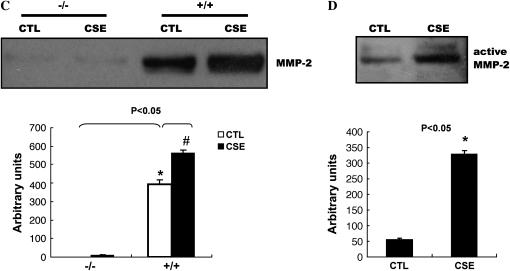
We used lung fibroblasts from mice in which the Egr-1 gene was deleted to further confirm whether CSE was modulating the MMP-2 response through Egr-1. Lung fibroblasts from Egr-1−/− and Egr-1+/+ mice were exposed to CSE; cells and culture media were collected for Western blot analysis. Figure 4A shows that CSE can induce Egr-1 expression in lung fibroblasts from Egr-1+/+ control mice in a dose-dependent manner. We observed markedly significant decreased MMP-2 mRNA expression in Egr-1−/− cells compared with Egr-1+/+ cells after CSE (Figure 4B). Western blots of media samples using (anti) total or active MMP-2 monoclonal antibodies shows that CSE could increase MMP-2 protein production and activation in culture media of lung fibroblasts from Egr-1+/+ mice (Figures 4C and 4D), whereas total MMP-2 were almost completely abolished in media of CSE-treated lung fibroblasts from Egr-1−/− mice (Figure 4C).
Figure 4.
CSE-induced total and active MMP-2 production in culture media of mouse lung Egr-1+/+ fibroblasts were suppressed in Egr-1−/− fibroblasts. (A) CSE induced Egr-1 expression in mouse lung Egr-1+/+ fibroblasts in a dose-dependent manner. Fibroblasts were incubated in the absence (CTL) or in the presence of CSE for the indicated doses for 1 h, and the whole cell lysates were subjected to Western blot analysis using an antibody against EGR-1. The same membrane was probed with an anti–β-actin antibody to assess equal loading of the gel. (B) MMP-2 mRNA levels in mouse lung Egr-1−/− (solid bars) and control Egr-1+/+ (open bars) fibroblasts after 4 h of 5% CSE treatment. (C) Total MMP-2 production in culture media of mouse lung Egr-1−/− and control Egr-1+/+ fibroblasts after 2 d of 5% CSE treatment. (D) Active MMP-2 in culture media of mouse lung Egr-1+/+ fibroblasts after 2 d of 5% CSE treatment. Quantitation of Western blot immunoblots were performed by densitometric scanning of blots (A, C, and D, bottom). *P < 0.05 vs. Egr-1+/+ CTL. *P < 0.05 vs. CSE treated Egr-1+/+ fibroblasts.
EGR-1 Regulates CSE-Induced MT1-MMP
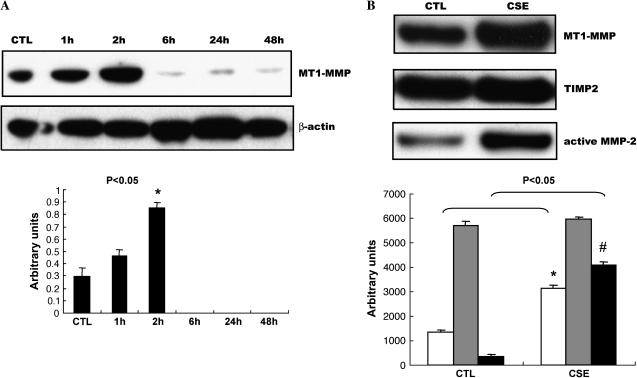
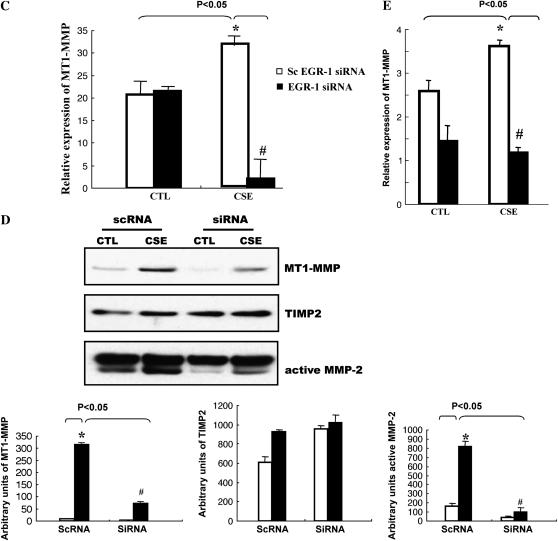
The aforementioned results suggest that transcription factor EGR-1 is involved in the CSE-induced MMP-2 activation. However, there is not a well-characterized EGR-1 regulatory element reported in the MMP-2 promoter. It has been established that pro–MMP-2's activation occurs mostly via a mechanism of trimolecular complex formation consisting of the zymogen MT1-MMP and TIMP2 (22), and the MT1-MMP promoter has a binding site for EGR-1 (23). To test the potential role of membrane-bound MT1-MMP in EGR-1–regulated CSE responses in cells, NL9 cells were exposed to 20% CSE and the cell lysates were used in Western blot analysis using monoclonal anti–MT1-MMP antibodies. Figure 5A shows that CSE (20%) can quickly induce MT1-MMP expression in NL9 cells. The peak MT1-MMP expression was observed at 2 h of treatment and disappeared after that time point. Meanwhile, we also measured a significant accumulation of MT1-MMP in concentrated media of NL9 after 2 d CSE exposure (Figure 5B). This increased accumulation of MT1-MMP is consistent with the CSE-increased active MMP-2 protein level in concentrated NL9 media as determined by Western blotting (Figure 5B). We also observed that CSE-induced MT1-MMP mRNA expression (Figure 5C) was reduced in NL9 cells transfected with EGR-1 siRNA as assessed by QRT-PCR. We observed markedly significant decreased MT1-MMP mRNA expression in Egr-1−/− cells compared with Egr-1+/+ cells after CSE (Figure 5E).
Figure 5.
EGR-1 mediated CSE-induced MT1-MMP production. (A) NL9 cells were exposed to 20% CSE and the cell lysates were subjected to Western blot to examine MT1-MMP expression. The same membrane was probed with an anti–β-actin antibody to assess equal loading of the gel. (B) Accumulation of MT1-MMP (open bars), TIMP2 (shaded bars), and active MMP-2 (solid bars) in concentrated media of NL9 cells after 2 d CSE exposure. The EGR-1 siRNA suppress CSE-induced MT1-MMP mRNA expression (C) and protein production (D). (Panel C: solid bars, EGR-1 siRNA; open bars, scEGR-1 siRNA.) Transfected NL9 cells treated with 20% CSE for 4 h were used for mRNA assay. The concentrated media of 2-d-CSE–treated transfected NL9 cells were used for MT1-MMP, TIMP2, and active MMP-2 assay. Quantitation of Western blot immunoblots were performed by densitometric scanning of blots (A, B, and D, bottom). (Bottom of panel D: open bars, CTL; solid bars, CSE.) (E) MT1-MMP mRNA levels in mouse lung Egr-1−/− (solid bars) and control Egr-1+/+ (open bars) fibroblasts after 4 h of 5% CSE treatment.
Subsequent analysis in concentrated media of CSE-treated NL9 cells that were transfected with EGR-1 siRNA shows that CSE-induced MT1-MMP protein production is markedly reduced when EGR-1 protein production is knocked down in NL9 cells transfected with EGR-1 siRNA (100 nM), compared with sc EGR-1 siRNA as detected with Western blotting (Figure 5D). Figure 5D also shows that this knockdown of EGR-1 protein attenuates active MMP-2 production in concentrated CSE-treated NL9 cell media. The activation of MMP-2 is also tightly controlled by its inhibitor TIMP2, the third component in the complex. Figure 5D shows that CSE (20%) has no apparent effect on TIMP2 protein level in concentrated media of NL9 cells. As seen in Figure 5D, TIMP2 expression did not change with transfection with either EGR-1 siRNA or sc EGR-1 siRNA in the presence of CSE (20%). Taken together, these findings suggest that MT1-MMP is involved in CSE-induced, EGR-1 regulated MMP-2 activation of NL9 cells.
ERK1/ERK2 MAP Kinase Pathway Is Involved in CSE-Induced MMP-2 Activation in NL9 Fibroblast Cells
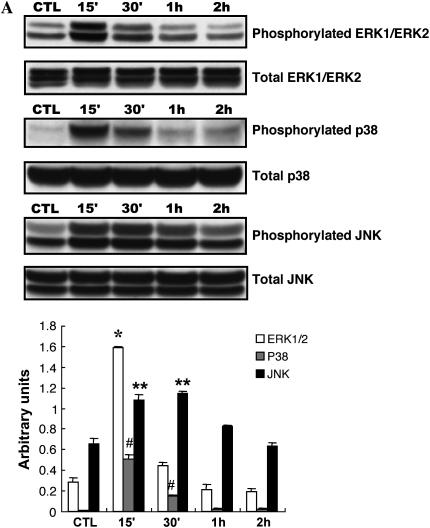
Recent studies have reported that CSE exerts its biological effects via the MAPK signaling pathway in several cell culture systems (24, 25). In addition, the mitogen-activated protein kinase (MAPK), extracellular signal–regulated protein kinase (ERK1/2), is involved in MMP expression (26, 27). To define MAPK intracellular signaling pathways mediating lung fibroblast MMP-2 induction by cigarette smoke, NL9 cells were exposed to 20% CSE to determine the activation of members of the MAPK superfamily. The levels of protein expression of ERK1/2, p38, and JNK in CSE (20%)-treated NL9 cells were assessed by Western blot analysis, using the corresponding phospho-specific and non–phospho-specific antibodies. The phospho-specific antibodies detect the phosphorylated forms of ERK1/2, p38, and JNK, whereas the corresponding non–phospho-specific antibodies detect total protein, independent of phosphorylation state. As shown in Figure 6A, 20% CSE caused significant increases in the phosphorylation of all three MAPK members within 15 min of exposure. The peak phosphorylation of ERK1/2 and p38 was observed at 15 min and declined at 30 min, whereas the maximal increase in phosphorylation of JNK occurred at 15–30 min and decreased after 1 h.
Figure 6.
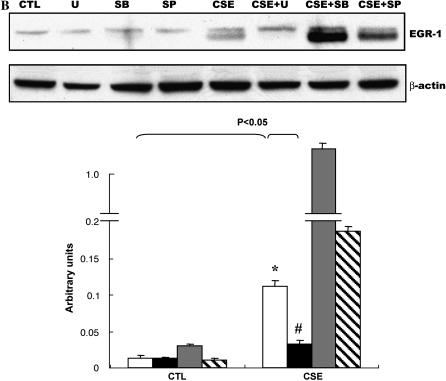
Effects of CSE on phosphorylation of the MAPKs in NL9 cells. Lysates from NL9 cells incubated in the absence (CTL) or in the presence of CSE (20%) for the indicated times were subjected to Western blot analysis using phospho-specific antibodies (A, top to bottom) to detect ERK1/2, p38 MAPK, or JNK. As controls, the same cell lysates were subjected to Western blot analysis using corresponding non–phospho-specific antibodies to detect total ERK1/2, total p38 MAPK, or total JNK. Quantitation of Western blot immunoblots were performed by densitometric scanning of blots (A, bottom) (*,#,**P < 0.05 versus each CTL). (B) Effects of MAPK inhibitors on CSE-induced EGR-1 protein expression in NL9 cells. NL9 cells were pretreated with or without MEK inhibitor U-0126 (U, 10 μM; solid bars), p38 MAPK inhibitor SB-203580 (SB, 10 μM; shaded bars), or JNK inhibitor SP600125 (SP, 10 μM; hatched bars) as indicated, and then incubated in the absence (CTL) or in the presence of CSE (20%) for 15 min to 1 h (open bars, dimethyl sulfoxide). Cells lysates were subjected to Western blot analysis using anti–EGR-1antibody. The same membrane was probed with an anti–β-actin antibody to assess equal loading of the gel. Quantitation of Western blot immunoblots were performed by densitometric scanning of blots (B, bottom). (C) Effects of MEK inhibitor U-0126 on CSE-induced MT1-MMP and TIMP2. (D) NL9 cells were pretreated with or without MEK inhibitor U-0126 (10 μM) for 1 h, and then incubated in the absence (CTL) or in the presence of CSE (20%) for 2 d. Concentrated media were subjected to gelatin zymography to measure MMP-2 activity. Results of densitometry of active MMP-2 bands are shown in the bar graph at bottom of D.
Since the major MAPKs were all activated by cigarette smoke, we used specific chemical inhibitors to pretreat NL9 cells before 20% CSE exposure to investigate whether CSE was modulating the functional response through one of the MAP kinase pathways. Ten micromolars of U-0126 (a selective inhibitor of MEK1/MEK2, the kinase upstream of ERK1/2) reduced CSE-mediated EGR-1 induction (Figure 6B). The p38 MAPK inhibitor SB 203580 (10 μM) and JNK MAPK inhibitor SP600125 (10 μM) had no effect on EGR-1 expression (Figure 6B). Based on these experiments using chemical inhibitors of p38 and JNK MAPK, these data indicate that p38 and JNK MAPK are not involved in CSE-induced EGR-1 expression in NL9 cells.
Furthermore, MT1-MMP production in concentrated media of CSE-exposed NL9 cells with or without U-0126 pretreatment was examined using Western blotting, and MMP-2 activity was detected using zymography. As shown in Figure 6, exposure with 20% CSE induced MT1-MMP protein level (Figure 6C) and MMP-2 activity (Figure 6D) in culture media from NL9 cells. These inductions were both significantly decreased (MMP-2 activity decreased 60% as shown in Figure 6D, bottom) by U-0126. It is noteworthy that these data support the role of ERK MAPK activation in response to cigarette smoke.
DISCUSSION
Cigarette smoke, a complex mixture of chemicals containing more than 4,000 different constituents including toxins, is the most important cause of COPD (28). Inhalation of toxins is thought to lead to a chronic inflammatory process that is quite widespread and extends to the whole airways and lung parenchyma, but principally centers around the net increase in proteolytic activity leading to the loss of alveoli function as a result of cigarette smoking. A variety of proteinases may be responsible, including MMPs, serine proteinases (such as neutrophil elastase), and cysteine proteinases (8). Correlative studies in human emphysematous lung tissue have demonstrated the presence of MMP-1, -2, -3, -8, -9, -12, -13, and MT1-MMP (29). Even though MMP-2 (gelatinase A) is one of the ECM-degrading proteases and was shown specifically to degrade basement membranes in capillary sprouting, epithelial branching, tissue remodeling, and tumor metastasis (14, 30, 31), the role of MMP-2 in cigarette-related emphysema development is not well known. There are reports of higher MMP-2 production in mice BAL fluid after cigarette smoke exposure (13), but no change in BAL fluid from subjects with emphysema and from healthy volunteers (32). One investigation using the immunolocalization of MMP-2 showed elevated levels of MMP-2 and MMP-14 in macrophages, as well as type II cells and fibroblasts in emphysematous lungs (12); however, another report observed unaffected MMP-2 activity in fibroblasts in collagen gel culture exposed to cigarette smoke (33). In our study, 20% CSE directly induced MMP-2 protein expression and increased the MMP-2 gelatinase activity of lung fibroblasts from smokers without COPD compared with those from untreated control subjects, without significant viability changes in these cells (data not shown). This observation is consistent with Kohno and colleagues' report that hyperoxia-activated MMP-2 played key roles in rat emphysematous changes (34). Our data suggest that cigarette smoke–activated MMP-2 is an important proteinase participating in the alveolar destruction that leads to emphysema. Furthermore, the intracellular signaling mechanism involved in this MMP-2 activation is also elucidated.
Besides the risk factors that are involved in airway obstruction of COPD such as smoke components, smoking manner, and passive smoke exposure (35), genetic predisposition is also considered a key factor that affects the pulmonary response to the inhalation of cigarette smoke and the production of airway obstruction (2, 36, 37). Here we found that EGR-1 may represent a key molecule that regulates downstream target genes in the pathogenesis of COPD.
EGR-1, a zinc finger transcription factor, has been termed an immediate-early response protein, and is rapidly activated in a wide variety of cells by multiple extracellular stimuli and environmental stresses (38, 39). As a major transcription factor, once activated, EGR-1 controls the expression of a diverse array of target genes encoding growth factors (platelet-derived growth factor, TGF-β1); cytokines (TNF-α); transcription factors (Id1); repair enzyme systems; cell cycle factors (p21, p53); apoptotic factors (Fas); proteases (MIT1-MMP); and stress response (HO-1) (39, 40). These target genes are involved in a variety of processes including proliferation, apoptosis, and differentiation. EGR-1 has been reported to be highly expressed in late-stage emphysema (41) and lung tissues of smokers with moderate COPD (5, 40). However, the potential function of EGR-1 involved in smoke-related COPD pathogenesis is poorly understood. Our data show that CSE can quickly alter EGR-1 expression and DNA-binding activity in human lung fibroblasts. CSE-induced MMP-2 activity was significantly inhibited using EGR-1 siRNA or in Egr-1−/− mouse fibroblasts. Interestingly, constitutive expression of MMP-2 was also significantly reduced in Egr-1−/− mouse fibroblasts, suggesting that Egr-1 also regulates basal expression of MMP-2 in fibroblasts. The present study strongly links the transcription factor EGR-1 to downstream effector MMP-2 mediating the alveolar destruction of emphysema.
Although there are no EGR-1–binding sites reported in the MMP-2 promoter, studies showed that the activation of MMP-2 correlates with the expression of MT1-MMP (42), whose promoter has a binding site for EGR-1 (23). This process is thought to involve a trimer formation of MT1-MMP, pro–MMP-2, and TIMP2. The concentrations of the two molecules: the free MT1-MMP and the trimer MT1-MMP·TIMP2·pro-MMP2 (22), are critical in determining the net level of MMP-2 activation. Sho and colleagues (42) reported that disproportional increase in MT1-MMP and TIMP2 by flow and shear stress contributed to MMP-2 activation. We observed that MMP-2, MT1-MMP, and TIMP2 are expressed constitutively in human lung fibroblasts. The concomitant MMP-2 activation and MT1-MMP synthesis by CSE which can be significantly inhibited using EGR-1 siRNA, in the absence of a corresponding increase in TIMP2 levels, could shift the balance in favor of increased production of catalytically active MMP-2 and is likely responsible for the alveolar destruction that leads to emphysema. The investigation of the role of EGR-1 in cigarette smoke–mediated MT1-MMP/TIMP2/pro–MMP-2 function would further our understanding of molecular pathways involved in the weakening lung parenchyma and the development of emphysema.
It has been shown that MAPK activation is involved in cigarette smoke–induced mucus production, airway inflammation, and MMP-1 expression seen in alveolar destruction (24, 43, 44). The ERK MAPK pathway is also implicated in EGR-1 activation associated with angiogenesis (45). The present study reveals a novel mechanism for matrix remodeling in COPD in which MMP-2 is induced by cigarette smoke in lung fibroblasts in an ERK-EGR1–dependent manner.
We used primary human lung fibroblasts in our studies. It is most likely that macrophages and neutrophils are the presumed in vivo source of injurious proteinases responsible for emphysema (46). Recent reports have shown that increased MMP-1 mRNA and protein levels and activation are directly induced by cigarette smoke in human small airway epithelial cells (24) and fetal lung fibroblasts (HFL-1) (25). All the information indicates that structural pulmonary cells as well as inflammatory cells are the source of enzymes that degrade lung tissue in emphysema, in addition to inflammatory cells (46). Our results demonstrate that in addition to its traditional function in maintenance of the extracellular matrix and in tissue repair after injury (47), the pulmonary fibroblast is much more than a target structural cell and can contribute directly to basement membrane disruption during cigarette-related COPD through MMP-2 production (48).
In summary, the present study demonstrates that cigarette smoke induces MMP-2 production and activation in the absence of a complete inflammatory program using human lung fibroblasts. These results support the paradigm of proteinase/antiproteinase imbalance in the pathogenesis of cigarette-related COPD (49). This increase in MMP-2 activation is due, at least in part, to a transient activation of signaling through ERK1/2 MAPK, transcription factor EGR-1, and MT1-MMP contributing to alveolar destruction. These acute effects of cigarette smoking on ECM degradation may have a role in the early stages of COPD development. The coordinated regulation of MMP-2 and MT1-MMP by EGR-1 may also contribute to the role that EGR-1 plays in the development of cigarette smoking–related COPD.
Acknowledgments
The authors thank D. J. Pinsky of the College of Physicians & Surgeons of Columbia University, New York, for his generous gift of Egr-1−/− and littermate Egr-1+/+ mice.
This work was supported by National Institutes of Health Grants R01 HL060234, R01 HL055330, and R01 HL079904, by PPG grant P01 HL070807 (Principal Investigator A.M.K.C.), and by National Natural Science Foundation of China 30671094 (Principal Investigator W.N.).
Originally Published in Press as DOI: 10.1165/rccm.2006-0106OC on November 10, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004;364:613–620. [DOI] [PubMed] [Google Scholar]
- 2.Molfino NA. Genetics of COPD. Chest 2004;125:1929–1940. [DOI] [PubMed] [Google Scholar]
- 3.Hogg JC, Senior RM. Chronic obstructive pulmonary disease - part 2: pathology and biochemistry of emphysema. Thorax 2002;57:830–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velculescu VE, Zhnag L, Vogelstein B, Kinzler KW. Serial analysis of gene expression. Science 1995;270:484–487. [DOI] [PubMed] [Google Scholar]
- 5.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, et al. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 2004;101:14895–14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matrisian LM. Matrix metalloproteinase gene expression. Ann N Y Acad Sci 1994;732:42–50. [DOI] [PubMed] [Google Scholar]
- 7.Belvisi MG, Bottomley KM. The role of matrix metalloproteinases (MMPs) in the pathophysiology of chronic obstructive pulmonary disease (COPD): a therapeutic role for inhibitors of MMPs? Inflamm Res 2003;52:95–100. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro SD. Proteinases in chronic obstructive pulmonary disease. Biochem Soc Trans 2002;30:98–102. [DOI] [PubMed] [Google Scholar]
- 9.Shiomi T, Okada Y, Foronjy R, Schiltz J, Jaenish R, Krane S, D'Armiento J. Emphysematous changes are caused by degradation of type III collagen in transgenic mice expressing MMP-1. Exp Lung Res 2003;29:1–15. [DOI] [PubMed] [Google Scholar]
- 10.Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, D'Armiento J. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med 2001;163:786–791. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Li L, Zielke HR, Cheng L, Xiao R, Crow MT, Stetler-Stevenson WG, Froehlich J, Lakatta EG. Increased expression of 72-kd type IV collagenase (MMP-2) in human aortic atherosclerotic lesions. Am J Pathol 1996;148:121–128. [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest 1998;78:1077–1087. [PubMed] [Google Scholar]
- 13.Seagrave J, Barr EB, March TH, Nikula KJ. Effects of cigarette smoke exposure and cessation on inflammatory cells and matrix metalloproteinase activity in mice. Exp Lung Res 2004;30:1–15. [DOI] [PubMed] [Google Scholar]
- 14.Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci 2002;115:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Tan FK, Xiong M, Milewicz DM, Feghali CA, Fritzler MJ, Reveille JD, Arnett FC. Systemic sclerosis (scleroderma): specific autoantigen genes are selectively overexpressed in scleroderma fibroblasts. J Immunol 2001;167:7126–7133. [DOI] [PubMed] [Google Scholar]
- 16.Ishii T, Matsuse T, Igarashi H, Masuda M, Teramoto S, Ouchi Y. Tobacco smoke reduces viability in human lung fibroblasts: protective effect of glutathione S-transferase P1. Am J Physiol 2001;280:L1189–L1195. [DOI] [PubMed] [Google Scholar]
- 17.Giri RK, Selvaraj SK, Kalra VK. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA dulexes for Early growth response-1 messenger RNA. J Immunol 2003;170:5281–5294. [DOI] [PubMed] [Google Scholar]
- 18.Ning W, Chu TJ, Li CJ, Choi AM, Peters DG. Genome-wide analysis of the endothelial transcriptome under short-term chronic hypoxia. Physiol Genomics 2004;18:70–78. [DOI] [PubMed] [Google Scholar]
- 19.Godfrey TE, Kim SH, Chavira M, Ruff DW, Warren RS, Gray JW, Jensen RH. Quantitative mRNA expression analysis from formalin-fixed, paraffin-embedded tissues using 5′ nuclease quantitative reverse transcription-polymerase chain reaction. J Mol Diagn 2000;2:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ning W, Song R, Li C, Park E, Mohsenin A, Choi AM, Choi ME. TGF-beta1 stimulates HO-1 via the p38 mitogen-activated protein kinase in A549 pulmonary epithelial cells. Am J Physiol 2002;283:L1094–L1102. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 1994;218:325–329. [DOI] [PubMed] [Google Scholar]
- 22.Karagiannis ED, Popel AS. A theoretical model of type I collagen proteolysis by matrix metalloproteinase (MMP) 2 and membrane type 1 MMP in thepresence of tissue inhibitor of metalloproteinase 2. J Biol Chem 2004;279:39105–39114. [DOI] [PubMed] [Google Scholar]
- 23.Haas TL, Stitelman D, Davis SJ, Apte SS, Madri JA. Egr-1 mediates extracellular matrix-driven transcription of membrane type 1 matrix metalloproteinase in endothelium. J Biol Chem 1999;274:22679–22685. [DOI] [PubMed] [Google Scholar]
- 24.Mercer BA, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem 2004;279:17690–17696. [DOI] [PubMed] [Google Scholar]
- 25.Rahman I. Oxidatives stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys 2005;43:167–188. [DOI] [PubMed] [Google Scholar]
- 26.Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem 1999;274:21491–21494. [DOI] [PubMed] [Google Scholar]
- 27.Ravanti L, Toriseva M, Penttinen R, Crombleholme T, Foschi M, Han J, Kahari VM. Expression of human collagenase-3 (MMP-13) by fetal skin fibroblasts is induced by transforming growth factor beta via p38 mitogen-activated protein kinase. FASEB J 2001;15:1098–1100. [PubMed] [Google Scholar]
- 28.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health 1997;50:307–364. [DOI] [PubMed] [Google Scholar]
- 29.Tetley TD. Macrophages and the pathogenesis of COPD. Chest 2002;121:156s–159s. [DOI] [PubMed] [Google Scholar]
- 30.Fan W, Karnovsky MJ. Increased MMP-2 expression in connective tissue growth factor over-expression vascular smooth muscle cells. J Biol Chem 2002;277:9800–9805. [DOI] [PubMed] [Google Scholar]
- 31.Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer 2000;36:1621–1630. [DOI] [PubMed] [Google Scholar]
- 32.Finlay GA, O'Driscoll LR, Russell KJ, D'Arcy EM, Masterson JB, FitzGerald MX, O'Connor CM. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med 1997;156:240–247. [DOI] [PubMed] [Google Scholar]
- 33.Zhu YK, Liu XD, Skold CM, Umino T, Wang HJ, Spurzem JR, Kohyama T, Ertl RF, Rennard SI. Synergistic neutrophil elastase-cytokine interaction degrades collagen in three-dimensional culture. Am J Physiol Lung Cell Mol Physiol 2001;281:L868–L878. [DOI] [PubMed] [Google Scholar]
- 34.Kohno M, Ishizaka A, Sawafuji M, Koh H, Hirayama Y, Ikeda E, Shiomi T, Ohashi A, Okada Y, Kobayash K. Hyperoxia-induced emphysematous changes in subacute phase of endotoxin-induced lung injury in rats. Am J Physiol Lung Cell Mol Physiol 2004;287:L184–L190. [DOI] [PubMed] [Google Scholar]
- 35.Speizer FE, Tager IB. Epidemiology of chronic mucus hypersecretion and obstructive airways disease. Epidemiol Rev 1979;1:124–142. [DOI] [PubMed] [Google Scholar]
- 36.Sethi JM, Rochester CL. Mechanical ventilation in chronic obstructive lung disease. Clin Chest Med 2000;21:67–86. [DOI] [PubMed] [Google Scholar]
- 37.Lomas DA, Silverman EK. The genetics of chronic obstructive pulmonary disease. Respir Res 2001;2:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan SF, Pinsky DJ, Mackman N, Stern DM. Egr-1: is it always immediate and early? J Clin Invest 2000;105:553–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svaren J, Sevetson BR, Golda T, Stanton JJ, Swirnoff AH, Milbrandt J. Novel mutants of NAB corepressors enhance activation by Egr transactivators. EMBO J 1998;17:6010–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petty TL. COPD in perspective. Chest 2002;121:116s–120s. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Yan SD, Zhu A, Zou YS, Williams M, Godman GC, Thomashow BM, Ginsburg ME, Stern DM, Yan SF. Expression of Egr-1 in late stage emphysema. Am J Pathol 2000;157:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sho E, Sho M, Singh TM, Nanjo H, Komatsu M, Xu C, Masuda H, Zarins CK. Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp Mol Pathol 2002;73:142–153. [DOI] [PubMed] [Google Scholar]
- 43.Cosio M, Guerassimov A. Chronic obstructive pulmonary disease. Inflammation of small airways and lung parenchyma. Am J Respir Crit Care Med 1999;160:S21–S25. [DOI] [PubMed] [Google Scholar]
- 44.Turato G, Zuin R, Miniati M, Baraldo S, Rea F, Beghe B, Monti S, Formichi B, Boschetto P, Harari S, et al. Airway inflammation in severe chronic obstructive pulmonary disease: relationship with lung function and radiologic emphysema. Am J Respir Crit Care Med 2002;166:105–110. [DOI] [PubMed] [Google Scholar]
- 45.Shi L, Kishore R, McMullen MR, Nagy LE. Lipopolysaccharide stimulation of ERK1/2 increases TNF-alpha production via Egr-1. Am J Physiol Cell Physiol 2002;282:C1205–C1211. [DOI] [PubMed] [Google Scholar]
- 46.Senior RM. Mechanisms of COPD: conference summary. Chest 2000;117:320s–323s. [DOI] [PubMed] [Google Scholar]
- 47.Knight D. Epithelium-fibroblast interactions in response to airway inflammation. Immunol Cell Biol 2001;79:160–164. [DOI] [PubMed] [Google Scholar]
- 48.Numanami H, Koyama S, Nelson DK, Hoyt JC, Freels JL, Habib MP, Amano J, Haniuda M, Sato E, Robbins RA. Serine protease inhibitors modulate smoke-induced chemokine release from human lung fibroblasts. Am J Respir Cell Mol Biol 2003;29:613–619. [DOI] [PubMed] [Google Scholar]
- 49.Voelkel NF, MacNee W. Chronic obstructive lung diseases. Hamilton, ON, Canada: BC Decker; 2002, pp. 90–113.