Abstract
Intra-alveolar fibrin deposition is a common response to localized and diffuse lung infection and acute lung injury (ALI). We hypothesized that the alveolar epithelium modulates intra-alveolar fibrin deposition through activation of protein C. Our obejctives were to determine whether components of the protein C activation pathway are present in the alveolar compartment in ALI and whether alveolar epithelium is a potential source. In patients with ALI, pulmonary edema fluid levels of endothelial protein C receptor (EPCR) were higher than plasma, suggesting a source in the lung. To determine whether alveolar epithelial cells are a potential source, protein C activation by A549, small airway epithelial, and primary human alveolar epithelial type II cells was measured. All three cell types express thrombomodulin (TM) and EPCR, and activate protein C on the cell surface. Activation of protein C was inhibited by cytomix (TNF-α, IL-1β, and IFN-γ). Release of EPCR and TM into the conditioned medium was inhibited by the metalloproteinase inhibitors tumor necrosis factor protease inhibitor (TAPI) and GM6001, indicating that the shedding of EPCR and TM from the alveolar epithelium is mediated by a metalloproteinase. These findings provide new evidence that the alveolar epithelium can modulate the protein C pathway and thus could be an important determinant of alveolar fibrin deposition. Local fibrin deposition may be a fundamental mechanism for the lung to localize and confine injury, thus limiting the risk of dissemination of injury or infection to the systemic circulation.
Keywords: alveolar epithelium, endothelium, protein C, coagulation, acute lung injury
CLINICAL RELEVANCE
The finding that thrombomodulin and endothelial protein C receptor can be released from lung epithelium by cytokine-induced metalloproteolytic cleavage adds to our understanding of fibrin deposition in the airspaces and may lead to new treatments for acute lung injury.
The alveolar epithelium plays a fundamental role in the pulmonary response to acute lung injury (1). The critical functions of the alveolar epithelium include active ion transport for the removal of alveolar edema fluid (2); secretion of surfactant to maintain alveolar stability, promote gas exchange, and assist in host defense (3); and barrier properties that protect against alveolar flooding and prevent the translocation of cytokines and bacteria into the circulation. However, the role of the alveolar epithelium in regulating intra-alveolar coagulation has not been adequately appreciated. In patients with acute lung injury, intra-alveolar activation of the coagulation cascade with the deposition of fibrin along the injured alveolar surface (hyaline membranes) has been well described (4–6). However, the mechanisms that modulate intra-alveolar fibrin deposition are not well understood. Therefore, this study explores the possible contribution of the alveolar epithelium to modulation of intra-alveolar coagulation using both in vitro studies and clinical samples from patients with acute lung injury. Identifying the cellular and molecular mechanisms that regulate intra-alveolar fibrin deposition in acute lung injury and the role of the alveolar epithelium in this process may lead to a better understanding of the pathogenesis of hyaline membrane formation.
The protein C pathway is a central endogenous regulator of blood coagulation. Protein C is a vitamin K–dependent plasma glycoprotein that circulates as a biologically inactive zymogen (7). The zymogen is converted into activated protein C (APC) by the thrombomodulin (TM)–thrombin complex on the cell surface (8). Activation of protein C is further enhanced when protein C is bound to the endothelial protein C receptor (EPCR) (9). APC suppresses thrombin formation by proteolytically inactivating coagulation factors Va and VIIIa. TM and EPCR were originally described in endothelial cells (10, 11), but subsequently were also detected in several other cell types (12–14), including epithelial cells. The alveolar epithelium has a large surface area, and thus is uniquely situated to actively modulate the intra-alveolar environment. We previously reported that A549 cells and primary isolates of human alveolar type II cells release soluble TM into the medium (15). Others have shown that airway epithelial cells in culture can express protein C, EPCR, and TM, and that protein C was activated by these cells in the presence of thrombin. These protein C pathway components were down-regulated by proinflammatory cytokines (16). The findings in these studies suggest that the alveolar epithelium could play a role in modulating intra-alveolar coagulation and inflammation through the protein C pathway similar to the well-described role of the endothelium in modulating intravascular coagulation.
We have previously reported that soluble TM levels are much higher in the alveolar compartment than in simultaneous plasma samples in patients with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), suggesting a local source in the lung (15). To test whether soluble EPCR is also high in the alveolar compartment, we measured levels of EPCR in simultaneous pulmonary edema fluid and plasma samples from patients with ALI/ARDS and found higher levels in the edema fluid, suggesting an intra-alveolar source. We then tested whether alveolar epithelial cells in culture can express TM and EPCR, and therefore activate protein C. A549 cells, human small airway epithelial cells, and primary isolates of human alveolar epithelial cells were able to activate protein C and express TM and EPCR. Finally, we hypothesized that stimulation of alveolar epithelial cells with proinflammatory cytokines would lead to a shift from an anticoagulant to a procoagulant phenotype in alveolar epithelial cells as measured by a reduction in the ability of these cells to activate protein C coupled with shedding of TM and EPCR from the cell surface. Exposure to cytomix (TNF-α, IL-1β, and IFN-γ) led to delayed shedding of TM and EPCR, effects that were inhibited by metalloproteinase inhibitors. These findings indicate that protein C is activated on the alveolar epithelium and that the loss of the ability to activate protein C in response to inflammatory stimuli is a result of protease-mediated shedding of TM and EPCR from the alveolar epithelium, a process that may be of major value for innate immunity in the prevention of lung infections. Portions of this work have been previously reported in abstract form (17, 18).
MATERIALS AND METHODS
Patients
Patients were selected from the University of California Moffitt-Long and San Francisco General Hospital intensive care unit patients who had pulmonary edema fluid and plasma obtained between 1981 and 1998. Inclusion criteria included ALI/ARDS (19) or hydrostatic pulmonary edema (15) and aspiration of pulmonary edema fluid within 1 h of endotracheal intubation. Eligibility for inclusion in the study was based solely on availability of an adequate stored volume of plasma and edema fluid for measurement of EPCR levels. To minimize selection bias, clinical data were not made available to the investigator who chose the samples for measurement of the EPCR levels, other than the etiology of acute pulmonary edema. Patient characteristics are shown in Table 1. The Committee for Human Research at University of California, San Francisco approved this study. To collect edema fluid, a tracheal suction catheter was advanced through the endotracheal tube into a wedged position, and gentle suction was applied. A simultaneous plasma sample was obtained. Plasma samples were also obtained from 10 healthy volunteers. Edema fluid and plasma were centrifuged (3,000 × g, 10 min) and stored at −70°C.
TABLE 1.
CLINICAL CHARACTERISTICS OF 18 PATIENTS WITH HYDROSTATIC PULMONARY EDEMA AND 33 PATIENTS WITH ACUTE LUNG INJURY AND THE ACUTE RESPIRATORY DISTRESS SYNDROME
| Patient Characteristic | Hydrostatic Edema (n = 18) | Acute Lung Injury (n = 33) |
|---|---|---|
| Age, yr | 59 ± 18 | 40 ± 13* |
| Male | 61% | 55% |
| White | 44% | 71% |
| Tobacco smoker | 15% | 25% |
| Lung Injury Score | 2.5 ± 0.7 | 2.9 ± 0.6 |
| SAPSII | 43 ± 16 | 46 ± 15 |
| Days of unassisted ventilation | 19 ± 10 | 9 ± 11* |
| Hospital mortality | 18% | 49%* |
Definition of abbreviation: SAPSII, Simplified Acute Physiology Score II.
Data are shown as mean ± SD or % of patients.
P < 0.05 versus hydrostatic edema group.
Isolation of Human Alveolar Epithelial Cells
Alveolar epithelial type II cells were isolated as previously described (15, 20, 21). Cell viability was assessed by trypan blue exclusion. Purity was > 90% (15, 20, 21). Freshly isolated type II cells were suspended in cell preservation fluid and maintained in liquid nitrogen.
Cell Culture and Treatment
Human alveolar epithelial-like cells (A549 cells; ATCC, Manassas, VA) were grown in MEM (Cellgro, Herndon, VA) with 10% FBS (Cellgro) and penicillin-streptomycin (Sigma, St. Louis, MO). Primary type II cells were grown in DMEM (Cellgro) with 10% FBS. Small airway epithelial cells (SAEC; Cambrex, Walkersville, MD) were grown in small airway cell basal medium (SABM; Cambrex) with growth supplements (SAGM bulletKit). Human vein umbilical endothelial cells (HUVECs; ATCC) were grown in F-12K (ATCC) with 10% FBS, 30 μg/ml endothelial cell growth supplement, and 90 μg/ml heparin (Sigma). For preparation of conditioned medium (CM) and cell lysates, cells were plated in 24-well plates at 5 × 104cells/well for A549 cells/HUVECs/SAECs and 1 × 106 living cells/well (collagen I–coated plate; BD Bioscience, San Jose, CA) for type II cells. All experiments were performed on Day 5 at confluence. The medium was replaced with serum-free medium containing cytomix (TNF-α, IL-1β, and IFN-γ; R&D Systems, Minneapolis, MN) with or without tumor necrosis factor protease inhibitor (TAPI)-0 (Peptides International, Louisville, KY), GM6001 (Calbiochem, San Diego, CA) or tissue inhibitor of metalloproteinase (TIMP)-1, 2, 3 (R&D Systems). After treatment, CM was collected. Cell monolayers were solubilized in lysis buffer (1% Triton X-100, 0.25 M sucrose, 20 mM HEPES, pH 7.5, 5 mM benzamidine-HCl, 0.02% NaN3, 2mM EDTA, 1mM phenylmethanesulfonyl fluoride, and 1 μg/ml leupeptin) for 1 h (4°C) then centrifuged at 10,000 rpm for 3 min. CM and cell lysates were stored at −20°C. Cell viability was measured by CellTiter AQueous Cell Proliferation kit (Promega, Madison, WI) and did not change significantly during the culture and treatment period (data not shown).
Protein C Activation
A549, HUVEC, SAECs or human type II cells were grown to confluence in 96-well microplates. Cells were washed with serum free medium then incubated with 0.1 U/ml α-thrombin and 12 ug/ml protein C for 2h at 37°C. The reaction was stopped with 40ul of hirudin (50 U/ml) (13). Supernatants were transferred to a new microplate and APC was assayed against an APC standard curve after incubation with the chromogenic substrate S2238.
Enzyme Immunoassay
TM and soluble EPCR antigen were measured in triplicate by ELISA (American Diagnostica, Stamford, CT, and Diagnostica Stago, Parsippany, NJ).
Total RNA Isolation and Real-Time PCR
After appropriate stimulus, cells from three identical wells were trypsinized and pooled. mRNA was extracted using a kit (Qiagen) and stored at −70°C. Real-time PCR was done in the Bio-Rad IQ cycle real-time PCR machine (Bio-Rad, Hercules, CA) using primers in Table 2. Before the PCR reaction, cDNA was prepared using Superscript RT (Invitrogen, Carlsbad, CA). All quantitative PCR reactions were run with a no template control for each primer set and a no cDNA control from each experimental condition. TM, EPCR, and β-actin primers were obtained from IDT (Coralville, IA) (22). Semiquantitation was established using the ΔΔCt method. Briefly, the Ct (confidence threshold) for each well was calculated by the Bio-Rad iCycler. For each experimental condition the Ct for β-actin was subtracted from the Ct for TM or EPCR to generate the ΔCt. The difference between the ΔCt of the control experiment and the ΔCt of treated cells was calculated to give the ΔΔCt. Fold increase in mRNA was calculated by 2ΔΔCt as a quantitative estimate.
TABLE 2.
PRIMERS USED FOR RT-PCR
| Gene | Oligonucleotide Sequences | Base Pair |
|---|---|---|
| EPCR | 5′-AACCGCACTCGGTATGAACT-3′ | 279 |
| 5′-TGGCTTCACAGTGAGCTGAA-3′ | ||
| TM | 5′-AGGGGCTGGCACTGGTACTCGCAGT-3′ | 218 |
| 5′-CATGTGCGAAGACCGGCTCCGGCTG-3′ | ||
| β-actin | 5′-GCGGGAAATCGTGCGTGACAT-3′ | 272 |
| 5′-TGGCGTACAGGTCTTTGCGGATG-3′ |
Definition of abbreviations: EPCR, endothelial protein C receptor; TM, thrombomodulin.
Statistical Analysis
All findings are representative of at least two separate experiments that gave qualitatively and quantitatively similar results. Data are expressed as median (interquartile range) or mean ± SD as appropriate. Normally distributed data were analyzed using Student's t test or ANOVA with post hoc Tukey's test. Nonparametric data were analyzed using Mann Whitney U test or Spearman's correlation coefficient as appropriate. A P value of ⩽ 0.05 was considered significant.
RESULTS
Levels of Soluble EPCR in Edema Fluid and Plasma from Human Subjects with ALI/ARDS
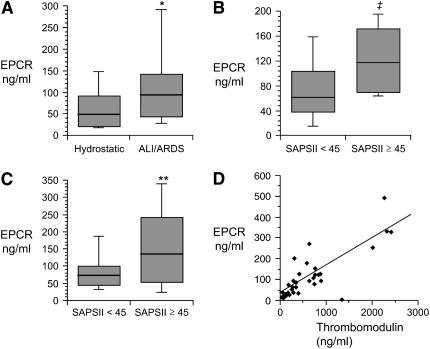
Levels of sEPCR in the undiluted pulmonary edema fluid were 2-fold higher in patients with ALI/ARDS than in a control group of patients with hydrostatic pulmonary edema (median 94.2 ng/ml [interquartile range {IQR}, 43.5–148.7] versus median 48.5 [IQR, 22.2–94.8], P = 0.028) (Figure 1A). When simultaneous edema fluid and plasma samples were compared, levels in the pulmonary edema fluid were higher than the plasma in patients with ALI/ARDS (edema fluid to plasma ratio of 1.46 ± 1.28 in ALI/ARDS versus 0.59 ± 0.37 in hydrostatic edema, P = 0.007), suggesting an intra-alveolar source in the ALI/ARDS group. Among the patients with ALI/ARDS, levels of both lung edema fluid and plasma EPCR were associated with severity of illness as measured by the Simplified Acute Physiology Score II (SAPSII) score. For patients with SAPSII ⩾ 45 (n = 18), plasma and edema fluid EPCR levels were 118 (IQR, 66–175) ng/ml and 135 ng/ml (IQR, 43–261), respectively (Figures 1B and 1C). By comparison, for patients with SAPSII < 45 (n = 15), plasma and edema fluid levels were 61 ng/ml (IQR, 36–108) (P = 0.013) and 72 ng/ml (IQR, 42–108) (P = 0.058), respectively (Figures 1B and 1C). We also compared levels of soluble thrombomodulin and soluble EPCR in the pulmonary edema fluid from patients with ALI/ARDS. Some of the thrombomodulin levels have been previously published (15). There was a strong correlation between soluble thrombomodulin and EPCR levels (r = 0.73, P < 0.001) (Figure 1D), suggesting that they may be released into the alveolar compartment in the acutely injured lung by a common mechanism.
Figure 1.
Levels of EPCR and thrombomodulin in the pulmonary edema fluid and plasma from patients with ALI/ARDS. (A) Boxplot summary of levels of soluble EPCR in the pulmonary edema fluid from 18 patients with hydrostatic pulmonary edema compared to 33 patients with ALI/ARDS. *P = 0.028 by Mann Whitney U test. (B) Boxplot summary of levels of soluble EPCR in the plasma from two groups of patients with ALI/ARDS, those with Simplified Acute Physiology Score II (SAPSII) of < 45 (n = 18) and those with SAPSII ⩾ 45 (n = 15) (‡P = 0.058 by Mann Whitney U test). (C) Boxplot summary of levels of soluble EPCR in the pulmonary edema fluid from two groups of patients with ALI/ARDS, those with SAPSII of < 45 (n = 18) and those with SAPSII ⩾ 45 (n = 15) (**P = 0.013 by Mann Whitney U test). For all boxplots, horizontal line represents median, box encompasses 25th to 75th percentile, and error bars encompass 10th to 90th percentile. (D) Correlation between levels of soluble EPCR and soluble thrombomodulin in the pulmonary edema fluid in 33 patients with ALI/ARDS. Spearman rank correlation coefficient r = 0.73, P < 0.001.
Thrombomodulin and EPCR in Alveolar Epithelial Cells: Effect of Proinflammatory Cytokines
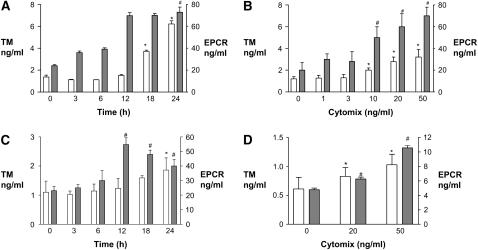
TM and EPCR proteins were detected in the cell lysates and conditioned medium from unstimulated A549 cells (Figures 2A and 2B), human small airway epithelial cells (Figure 2C), and primary isolates of human alveolar epithelial type II cells (Figure 2D). A549 cells were then exposed to 20 ng/ml cytomix (TNF-α, IL-1β, IFN-γ) for 3–24 h or to varying doses from 1–50 ng/ml for 18 h. Exposure to cytomix led to a time- and dose-dependent release of thrombomodulin and EPCR into the conditioned medium by A549 cells (Figures 2A and 2B). After 24 h exposure to 20 ng/ml cytomix, TM protein levels had risen approximately 4-fold compared with 0 h. However, when the sum of cell lysate and conditioned medium levels was considered, there was no difference in total TM protein levels per well with or without cytomix exposure (data not shown), suggesting that the change in levels in the conditioned medium did not reflect new protein synthesis. Similarly, after 24 h exposure to 20 ng/ml cytomix, EPCR protein levels had risen approximately 3-fold compared with 0 h. When cell lysates and conditioned medium levels of EPCR for each well were combined, there was no difference among total EPCR antigen levels per well with or without cytomix exposure (data not shown), again suggesting that the change in levels in the conditioned medium did not reflect new protein synthesis. A similar time- and dose-dependent release of TM and EPCR into the conditioned medium was observed in human SAECs and primary human alveolar epithelial cells (Figures 2C and 2D).
Figure 2.
Exposure to proinflammatory cytokines stimulated the release of soluble TM and EPCR from human distal lung epithelial cells. (A) Confluent monolayers of A549 cells were exposed to 20ng/ml cytomix (TNF-α, IL-1β, and IFN-γ) for several time points (0–24 h). Levels of soluble TM (open bars) and EPCR (shaded bars) in the conditioned medium are shown. *P < 0.05 versus TM at 0 h, #P < 0.05 versus EPCR at 0 h. (B) Confluent monolayers of A549 cells were exposed to varying concentrations (0–50 ng/ml) of cytomix for 18 h. Levels of soluble TM (open bars) and EPCR (shaded bars) in the conditioned medium are shown. *P < 0.001 versus TM level with 0 ng/ml cytomix, #P < 0.001 vs. EPCR level with 0 ng/ml cytomix. (C) Human small airway epithelial cells were exposed to 20 ng/ml cytomix for varying times (0–24 h) and levels of soluble TM (open bars) and EPCR (shaded bars) in the conditioned medium are shown. *P < 0.01 versus TM at 0 h, #P < 0.05 versus EPCR at 0 h. (D) Primary human alveolar epithelial cells were exposed to varying concentrations of cytomix for 6 h, and levels of soluble TM (open bars) and EPCR (shaded bars) in the conditioned medium are shown. *P < 0.05 versus TM at 0 ng/ml cytomix, #P < 0.01 versus EPCR at 0 ng/ml cytomix. For all panels, data are shown as means ± SD.
Activation of Protein C by Alveolar Epithelial Cells
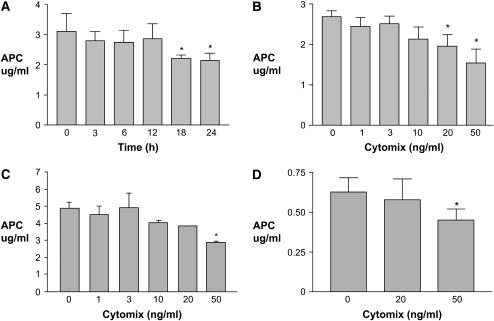
To determine whether the TM and EPCR that were detected in alveolar epithelial cells were functionally active, we determined whether alveolar epithelial cells could activate protein C in the presence of thrombin. A549 cells (Figures 3A and 3B), human SAECs (Figure 3C) and primary human type II cells (Figure 3D) all generated APC in the presence of thrombin and protein C. To compare to a cell type known to activate protein C, human umbilical vein endothelial cells were used in the same system. There was no significant difference between the quantity of activated protein C generated by similar numbers of A549 cells and HUVECs (data not shown). Generation of APC by A549 cells declined significantly with increasing length of exposure to cytomix (Figure 3A). The fall in protein C activation was also dose dependent (Figure 3B). There was also a significant dose-dependent decrease in protein C activation in human SAECs and primary human type II epithelial cells after exposure to cytomix (Figures 3C and 3D).
Figure 3.
Exposure to proinflammatory cytokines impaired the activation of protein C by human distal lung epithelial cells. Activation of protein C in the presence of thrombin was measured using a chromogenic substrate. (A and B) Activation of protein C by A549 cells was decreased by exposure to cytomix (TNF-α, IL-1β, and IFN-γ) in a time- and dose-dependent manner. (C) Activation of protein C by human small airway epithelial cells was decreased by exposure to cytomix for 18 h in a dose-dependent manner. (D) Activation of protein C by primary human alveolar epithelial cells was decreased after exposure to cytomix for 6 h. Data are means ± SD; *P < 0.05 compared with time 0 or 0 ng/ml cytomix by ANOVA with post hoc Tukey's test.
Expression of TM and EPCR by Alveolar Epithelial Cells
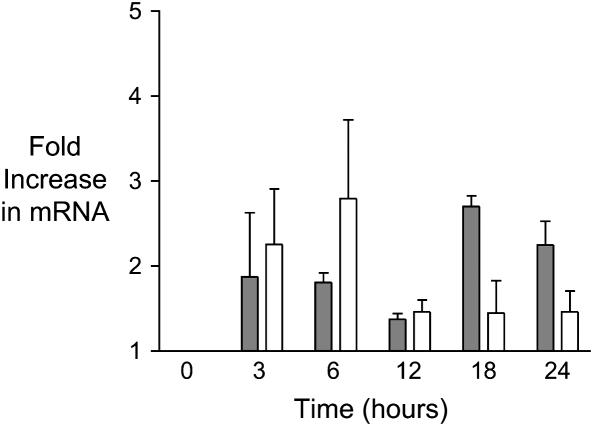
Both the alveolar epithelial cell line (A549) (Figure 4) and primary isolates of human type II cells (not shown) expressed TM and EPCR mRNA. Real-time PCR demonstrated no significant changes in levels of TM or EPCR mRNA expression after cytomix treatment for up to 24 h in A549 cells.
Figure 4.
TM and EPCR mRNA expression in A549 cells in response to cytomix treatment. TM (open bars) and EPCR (shaded bars) mRNA was measured by real-time PCR, and fold-change in mRNA was calculated using the 2ΔΔCt method with normalization to β-actin. Exposure to 20 ng cytomix for increasing lengths of time produced modest but nonsignificant increases in TM and EPCR mRNA. Data are shown as mean ± SD. P = NS by ANOVA with post hoc Tukey's test.
Mechanism of TM and EPCR Antigen Release in Response to Proinflammatory Cytokines
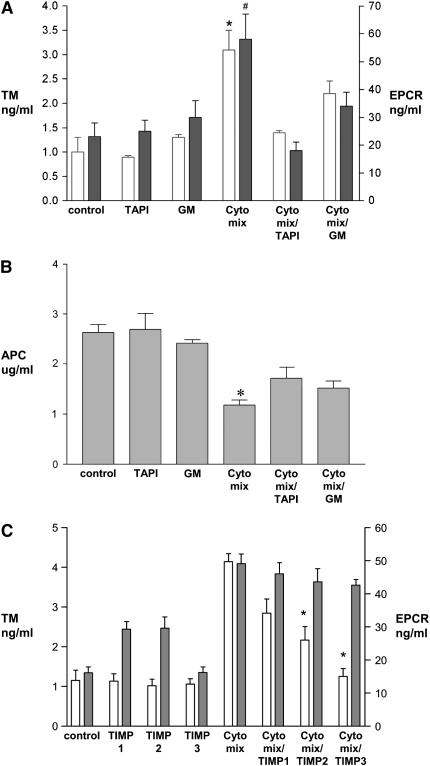
Previous studies in endothelial cells suggest that EPCR shedding is a metalloproteolytic process and that TM shedding is modulated by neutrophils and their release products (22, 23). To investigate the mechanism of TM and EPCR shedding from alveolar epithelial cells in response to cytomix, we tested the influence of metalloproteinase inhibitors on this process. TAPI and GM6001 are hydroxamic-based inhibitors of metalloproteinases. In A549 cells, both TAPI and GM6001 significantly inhibited the shedding of TM and EPCR that was induced by cytomix (Figure 5A). Since cell surface TM and EPCR are essential to PC activation, we hypothesized that the inhibition of TM and EPCR shedding might restore the activation of protein C on the epithelial cell surface. To test this hypothesis, we measured activation of protein C in the presence of TAPI and GM6001 after cytomix exposure. Activation of protein C was partially restored by TAPI and GM 6001 (Figure 5B). To further define the possible metalloproteinase that mediates EPCR shedding, we measured EPCR shedding after cytomix stimulation for 18 h in the presence or absence of the metalloproteinase inhibitors TIMP-1, -2, or -3 (5 μg/ml). The modest and nonspecific inhibitory pattern for shedding of both TM and EPCR observed with the TIMPs (Figure 5C) along with the prolonged time course of cytomix-mediated release was not consistent with rapid up-regulation of a sheddase such as ADAM-17, but rather suggests that a matrix metalloproteinase may be involved.
Figure 5.
Effect of metalloproteinase inhibitors on release of EPCR and TM and activation of protein C in A549 cells stimulated by cytomix. (A) Confluent monolayers of A549 cells were exposed to 20 ng/ml cytomix for 18 h with or without the metalloproteinase inhibitors TAPI (50 μg/ml) or GM6001 (10 μM). Conditioned medium was collected for assay of TM (open bars) and EPCR (shaded bars) by ELISA. Data are means ± SD; *P < .05 versus TM under all other conditions except cytomix + GM6001; #P < 0.05 versus EPCR under all other conditions. (B) Confluent monolayers of A549 cells were exposed to 20 ng/ml cytomix for 18h. Activation of protein C in the presence of thrombin was measured using a chromogenic substrate. The decrease in protein C activation induced by cytomix exposure was partially restored by TAPI and GM6001. Data are means ± SD; *P < 0.05 versus all other conditions except cytomix + GM6001. (C) Confluent monolayers of A549 cells were exposed to 20 ng/ml cytomix for 18 h with or without TIMP-1, -2, or -3 (5 μg/ml). Conditioned medium was collected for assay of TM (open bars) and EPCR (shaded bars) by ELISA. Release of TM was significantly inhibited by TIMP-2 and -3 but not TIMP-1. Release of EPCR was not inhibited by the TIMPs. Data are means ± SD; *P < 0.05 compared with TM level with cytomix alone.
DISCUSSION
The cellular and molecular mechanisms that govern intra-alveolar fibrin deposition in ALI/ARDS are unknown. The major findings of our study can be summarized as follows. First, undiluted pulmonary edema fluid from patients with ALI/ARDS contains soluble EPCR in levels higher than plasma, suggesting a local source in the lung. Second, alveolar epithelial cells express active TM and EPCR, and can activate protein C on the cell surface. Third, TM and EPCR shedding is induced and protein C activation is inhibited by exposure to cytomix, a mixture of proinflammatory cytokines. Finally, the shedding of TM and EPCR does not appear to be mediated by increased protein synthesis, but is likely mediated by a matrix metalloproteinase. These findings provide new evidence that the alveolar epithelium can modulate intra-alveolar coagulation through the protein C pathway, and this pathway is likely to be an important determinant of alveolar fibrin deposition.
The endothelial cell surface has previously been thought to be the primary site of protein C activation in vivo (23). Inflammatory stress such as occurs in sepsis leads to a reduction in endothelial activation of protein C and low circulating levels of activated protein C, factors that may contribute to microvascular thrombosis and organ failure in sepsis (24, 25). In cultured endothelial cells the cell surface activity of both TM and EPCR can be down-regulated by inflammatory cytokines such as IL-1β and TNF-α, thus decreasing the protein C activation potential (26). This down-regulation of TM and EPCR activity appears to be mediated predominantly by cleavage of these proteins from the cell surface (27, 28). The findings of the current study indicate that alveolar epithelial cells are remarkably similar to endothelial cells in their capacity to activate protein C. Protein C activation occurs on the alveolar epithelial cell surface of A549 cells, human small airway epithelial cells, and primary isolates of human alveolar epithelial type II cells, and the degree of protein C activation is down-regulated by proinflammatory cytokines.
This study provides new information about the mechanisms of shedding of TM and EPCR from the cell surface, an area that is incompletely understood, even in endothelial cells. Prior studies in cultured endothelial cells indicate that EPCR release is a metalloproteolytic process, sensitive to both coagulation factors and inflammatory mediators, but the specific metalloproteinase has not been identified (21). Thrombin or the protease-activated receptors (PAR)1 agonist peptide can enhance EPCR shedding from human umbilical vein endothelial cells via activation of a metalloproteinase, but again the identity of the metalloproteinase is not clear (29). By contrast, shedding of TM is thought to be modulated by neutrophil elastase (30). We identified the mechanisms by which TM and EPCR are shed from alveolar epithelial cells into the conditioned medium independent of neutrophils. Cytomix-induced shedding of TM and EPCR peaked at 12–24 h, suggesting that a rapid induction of a sheddase is not involved. The shedding of both TM and EPCR was blocked by the hydroxamic-based metalloproteinase inhibitors TAPI and GM6001; both inhibitors also partially restored the activation of protein C. This finding suggests that shedding of TM and EPCR was mediated by a metalloproteinase. To further identify the metalloproteinase responsible for TM and EPCR shedding, we studied the effect of TIMP-1, -2, and -3 on EPCR shedding. The nonspecific inhibition pattern for EPCR was not suggestive of a specific metalloproteinase. Inhibition of TM shedding by TIMP 2 and TIMP 3 but not TIMP 1 suggests that MT1-MMP, MT2-MMP, MT3-MMP, MT5-MMP, or MMP-19 is the relevant protease (31).
What are the potential consequences of down-regulation of protein C activation in the setting of acute inflammation in the lung? Recent evidence suggests that APC not only inhibits coagulation but also has anti-inflammatory properties that include suppression of production of cytokines such as TNF-α, IL-1, and IL-6, and inhibition of leukocyte attachment to the endothelium (32, 33). APC can inhibit plasminogen activator inhibitor-1, thereby promoting fibrinolysis (34). APC can also modulate several genes in the endothelial apoptosis pathway to block p53-mediated apoptosis (35). Thus, in addition to being procoagulant, decreases in intra-alveolar APC may be proinflammatory, antifibrinolytic, and pro-apoptotic, contributing to the activation of both coagulation and inflammation in the airspaces. Although intra-alveolar levels of APC have not been measured in patients with acute lung injury, APC levels were decreased in the intra-alveolar space of patients with interstitial lung diseases, a finding that was associated with increased intra-alveolar activation of the coagulation system and with enhanced deposition of collagen in the lung (36, 37). If a similar deficiency in APC occurs in human acute lung injury, as is suggested by our initial data, then intravenous recombinant APC (rhAPC) might be used to correct this deficiency. In a recent study, administration of rhAPC to healthy humans challenged with endobronchial LPS instillation produced sustained elevations in BAL levels of activated protein C (38). However, it is also possible that a decreased capacity to activate intra-alveolar protein C could be beneficial, serving to localize and wall off injury and inflammation.
There are some potential limitations of this study. First, many of the studies were done in A549 cells, a cell line that is ultrastructurally similar to the alveolar epithelial type II cell. Although A549 cells are widely used to model the alveolar epithelium, they are derived from a human adenocarcinoma and thus could respond differently than primary type II cells. For this reason we repeated the key experiments in both primary human small airway epithelial cells and primary isolates of human alveolar epithelial type II cells to confirm the findings (Figures 2 and 3). A second possible limitation is that the findings may be pertinent only to cultured alveolar epithelial cells. Although this is a possibility, the finding of high levels of both TM and EPCR in the alveolar space in samples of human pulmonary edema fluid confirms that the intra-alveolar release of these proteins occurs in vivo in ALI/ARDS. The higher levels of TM compared to EPCR in the human pulmonary edema fluid samples may reflect release of TM by additional mechanisms that are not modeled in alveolar epithelial cell culture, such as the previously described shedding of TM by neutrophil elastase (30). A third limitation is that although the findings indicate that both TM and EPCR shedding in response to cytomix is mediated by metalloproteinases, we have not identified the specific metalloproteinases induced by exposure to cytomix. Nevertheless, the information regarding the role of metalloproteolytic cleavage of TM and EPCR from alveolar epithelial cells is new and of likely biological significance.
In conclusion, alveolar epithelial cells express the major components of the protein C pathway, including both TM and EPCR, and can activate protein C on the cell surface. Exposure of alveolar epithelial cells to proinflammatory cytokines decreased alveolar epithelial activation of protein C through delayed shedding of TM and EPCR from lung epithelial cells. We conclude that the alveolar epithelium plays an important role in the modulation of intra-alveolar coagulation and inflammation through regulation of the protein C pathway. Down-regulation of activation of protein C by the alveolar epithelial protein C activation may contribute to the pathogenesis of ALI/ARDS and could be a therapeutic target in this disease. Alternatively, the down-regulation of APC may be a well-conserved biological process that allows the host to confine and localize lung injury from infection, such as bacterial pneumonia, with the purpose of preventing systemic dissemination. This interpretation would add further evidence for a pivotal role of the alveolar epithelium in the response to acute lung injury (39–41).
This study was supported by NIH HL70521, HL81332 (Principal Investigator L.W.), P50HL74005, HL51854 (Principal Investigator M.A.M.), and a Biomedical Research Grant from the American Lung Association.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0425OC on November 10, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Fukuda N, Frank J, Kallet R, Daniel B, Sakuma T. Alveolar epithelial barrier: role in lung fluid balance in clinical lung injury. Clin Chest Med 2000;21:477–490. [DOI] [PubMed] [Google Scholar]
- 3.Spragg RG. Surfactant replacement therapy. Clin Chest Med 2000;21:531–541. [DOI] [PubMed] [Google Scholar]
- 4.Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol 2000;22:401–404. [DOI] [PubMed] [Google Scholar]
- 5.Idell S, Peters J, James KK, Fair DS, Coalson JJ. Local abnormalities of coagulation and fibrinolytic pathways that promote alveolar fibrin deposition in the lungs of baboons with diffuse alveolar damage. J Clin Invest 1989;84:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welty-Wolf KE, Carraway MS, Ortel TL, Piantadosi CA. Coagulation and inflammation in acute lung injury. Thromb Haemost 2002;88:17–25. [PubMed] [Google Scholar]
- 7.Esmon CT, Stenflo J, Suttie JW. A new vitamin K-dependent protein: a phospholipid-binding zymogen of a serine esterase. J Biol Chem 1976;251:3052–3056. [PubMed] [Google Scholar]
- 8.Esmon CT, Gu JM, Xu J, Qu D, Stearns-Kurosawa DJ, Kurosawa S. Regulation and functions of the protein C anticoagulant pathway. Haematologica 1999;84:363–368. [PubMed] [Google Scholar]
- 9.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex Proc Natl Acad Sci USA 1996;93:10212–10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esmon CT, Owen WG. The discovery of thrombomodulin. J Thromb Haemost 2004;2:209–213. [DOI] [PubMed] [Google Scholar]
- 11.Fukudome K, Esmon CT. Molecular cloning and expression of murine and bovine endothelial cell protein C/activated protein C receptor (EPCR): the structural and functional conservation in human, bovine, and murine EPCR. J Biol Chem 1995;270:5571–5577. [DOI] [PubMed] [Google Scholar]
- 12.Lager DJ, Callaghan EJ, Worth SF, Raife TJ, Lentz SR. Cellular localization of thrombomodulin in human epithelium and squamous malignancies. Am J Pathol 1995;146:933–943. [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuyama T, Izumi Y, Shibatate K, Yotsumoto Y, Obama H, Uemura M, Maruyama I, Sueda T. Expression and activity of thrombomodulin in human gingival epithelium: in vivo and in vitro studies. J Periodontal Res 2000;35:146–157. [DOI] [PubMed] [Google Scholar]
- 14.Grey ST, Csizmadia V, Hancock WW. Differential effect of tumor necrosis factor-alpha on thrombomodulin gene expression by human monocytoid (THP-1) cell versus endothelial cells. Int J Hematol 1998;67:53–62. [DOI] [PubMed] [Google Scholar]
- 15.Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003;285:L514–L521. [DOI] [PubMed] [Google Scholar]
- 16.Hataji O, Taguchi O, Gabazza EC, Yuda H, Fujimoto H, Suzuki K, Adachi Y. Activation of protein C pathway in the airways. Lung 2002;180:47–59. [DOI] [PubMed] [Google Scholar]
- 17.Ware LB, Matthay MA. Activation of Protein C by human alveolar epithelial cells is reduced by a pro-inflammatory stimulus. FASEB J 2004;337:60A. [Google Scholar]
- 18.Wang L, Prudhomme J, Wickersham N, Fang X, Matthay MA, Ware LB. Alveolar epithelial cells express thrombomodulin and endothelial Protein C receptor and can activate protein C [abstract]. Proc Am Thorac Soc 2005;2:A916. [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed] [Google Scholar]
- 20.Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, et al. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 2003;278:43939–43950. [DOI] [PubMed] [Google Scholar]
- 21.Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM and Pittet JF. Interleukin-1beta decreases expression of the epithelial sodium channel alphaENaC in alveolar epithelial cells via a p38 MAP kinase-dependent signaling pathway. J Biol Chem 2005;280:18579–18589. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;24:402–408. [DOI] [PubMed] [Google Scholar]
- 23.Fukudome K, Ye X, Tsuneyoshi N, Tokunaga O, Sugawara K, Mizokami H, Kimoto M. Activation mechanism of anticoagulant protein C in large blood vessels involving the endothelial cell protein C receptor. J Exp Med 1998;187:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liaw PC, Esmon CT, Kahnamoui K, Schmidt S, Kahnamoui S, Ferrell G, Beaudin S, Julian JA, Weitz JI, Crowther M, et al. Patients with severe sepsis vary markedly in their ability to generate activated protein C. Blood 2004;104:3958–3964. [DOI] [PubMed] [Google Scholar]
- 25.Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR. Low levels of protein C are associated with poor outcome in severe sepsis. Chest 2001;120:915–922. [DOI] [PubMed] [Google Scholar]
- 26.Laszik ZG, Zhou XJ, Ferrell GL, Silva FG, Esmon CT. Down-regulation of endothelial expression of endothelial cell protein C receptor and thrombomodulin in coronary atherosclerosis. Am J Pathol 2001;159:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iba T, Yagi Y, Kidokoro A, Fukunaga M, Fukunaga T. Increased plasma levels of soluble thrombomodulin in patients with sepsis and organ failure. Surg Today 1995;25:585–590. [DOI] [PubMed] [Google Scholar]
- 28.Krafte-Jacobs B, Brilli R. Increased circulating thrombomodulin in children with septic shock. Crit Care Med 1998;26:933–938. [DOI] [PubMed] [Google Scholar]
- 29.Gu JM, Katsuura Y, Ferrell GL, Grammas P, Esmon CT. Endotoxin and thrombin elevate rodent endothelial cell protein C receptor mRNA levels and increase receptor shedding in vivo. Blood 2000;95:1687–1693. [PubMed] [Google Scholar]
- 30.MacGregor IR, Perrie AM, Donnelly SC, Haslett C. Modulation of human endothelial thrombomodulin by neutrophils and their release products. Am J Respir Crit Care Med 1997;155:47–52. [DOI] [PubMed] [Google Scholar]
- 31.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 2002;115:3719–3727. [DOI] [PubMed] [Google Scholar]
- 32.Esmon CT. The anticoagulant and anti-inflammatory roles of the protein C anticoagulant pathway. J Autoimmun 2000;15:113–116. [DOI] [PubMed] [Google Scholar]
- 33.Nick JA, Coldren CD, Geraci MW, Poch KR, Fouty BW, O'Brien J, Gruber M, Zarini S, Murphy RC, Kuhn K, et al. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood 2004;104:3878–3885. [DOI] [PubMed] [Google Scholar]
- 34.Sakata Y, Loskutoff DJ, Gladson CL, Hekman CM, Griffin JH. Mechanism of protein C-dependent clot lysis: role of plasminogen activator inhibitor. Blood 1986;68:1218–1223. [PubMed] [Google Scholar]
- 35.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med 2003;9:338–342. [DOI] [PubMed] [Google Scholar]
- 36.Yasui H, Gabazza EC, Taguchi O, Risteli J, Risteli L, Wada H, Yuda H, Kobayashi T, Kobayashi H, Suzuki K, et al. Decreased protein C activation is associated with abnormal collagen turnover in the intraalveolar space of patients with interstitial lung disease. Clin Appl Thromb Hemost 2000;6:202–205. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi H, Gabazza EC, Taguchi O, Wada H, Takeya H, Nishioka J, Yasui H, Kobayashi T, Hataji O, Suzuki K, et al. Protein C anticoagulant system in patients with interstitial lung disease. Am J Respir Crit Care Med 1998;157:1850–1854. [DOI] [PubMed] [Google Scholar]
- 38.van der Poll T, Levi M, Nick JA, Abraham E. Activated protein C inhibits local coagulation after intrapulmonary delivery of endotoxin in humans. Am J Respir Crit Care Med 2005;171:1125–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest 1999;104:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376–1383. [DOI] [PubMed] [Google Scholar]
- 41.Wright JR. Pulmonary surfactant: a front line of lung host defense. J Clin Invest 2003;111:1453–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]