Abstract
Mutations of Fog2 in mice result in a phenotype that includes pulmonary lobar defects. To determine whether formation of the accessory lobe bronchus is mediated by a Gata family cofactor, we evaluated embryonic lungs from mice carrying missense mutations that cause loss of FOG–GATA protein interaction. Lungs from embryos carrying a missense mutation in Gata6 were structurally normal, while lungs from embryos carrying mutations of either Gata4 or of both Gata4 and Gata6 had a structural phenotype that matched the Fog2 mutant phenotype. Expression analysis showed that Gata4 and Fog2 are expressed in the ventral and medial pulmonary mesenchyme during secondary budding. Although Gata4 has not previously been suspected as playing a role in lung development, we have found that a Fog2–Gata4 interaction is critical for the development of normal pulmonary lobar structure, and this phenotype is not influenced by the additional loss of Gata6 interaction. Fog2 and Gata4 in the early pulmonary mesenchyme participate in patterning the secondary bronchus of the accessory lobe.
Keywords: lung, accessory lobe, Gata, Fog, branching
CLINICAL RELEVANCE
We report that the transcription factor complex, FOG2-GATA4, mediates development of normal pulmonary lobar structure in mice. This establishes Gata4 as a transcription factor critical for early lung patterning.
The mammalian lung develops from budding of the foregut endoderm into a complex lobular structure composed of branching networks of airways and vascular structures. While later stages of pulmonary branching morphogenesis are becoming better understood, the genes that guide the early specification of the lung and the subsequent establishment of stereotyped secondary bronchi are largely unknown. Obstacles to the discovery of these mechanisms include the paucity of early lung-specific markers and the lack of mouse models with specific defects in secondary bronchial patterning. There are mutations that cause abnormal branching morphogenesis and abnormal lobar structure (1, 2); however, these also result in diffuse developmental defects that make it difficult to assess specific effects on secondary bronchial patterning.
We have found that a deficiency of the gene, Fog2 (Friend of Gata 2), also called Zfpm2, causes loss of the accessory lobe and anterior right medial lobe of the mouse lung with relatively good preservation of other structures (3). Because mice homozygous for a hypomorphic allele of Fog2 also have diaphragmatic defects, we used organ culture at a time point before the occurrence of diaphragmatic muscularization to show that this structural defect is primary and is not influenced by diaphragmatic development. Culture of embryonic lungs at the time of secondary bronchial branching demonstrates the development of a bud and a lobe in wild-type mice and the complete absence of an accessory bud and lobe in lungs from Fog2-null mice (3). (Culture during this time is not adequately sensitive to demonstrate the partial right medial lobe defect that is seen in mice that survive to late gestation.) Because the accessory lobe defect is readily evaluated in culture, and has not been variable in Fog2 mutant mice derived from a variety of genetic backgrounds, it is an ideal phenotype to examine in an effort to identify other genes required for normal pulmonary development.
Fog2 is a transcription cofactor for the Gata transcription factor family (4) and interacts with other mediators of development such as COUP-TFII (5) and retinoic acid receptors (6). Our aim in the experiments described here was to determine whether Fog2-dependent lobar development was mediated by Gata transcription factors. Both Gata4 and Gata6 are expressed in the foregut endoderm; however, previous studies have implicated Gata6 and not Gata4 as playing a role in lung development (7–11). Unfortunately, early embryonic lethality precludes the evaluation of in vivo early lung patterning and development in the Gata6 null mutant mouse (12). Gata5 is also expressed in the developing lung, making it a candidate for Fog2-mediated lung development; however, a Gata5-null mutant mouse has no evident pulmonary phenotype (13), suggesting that it is not likely to be a primary moderator of this process.
Alteration of a specific amino acid in the zinc finger domain of Gata transcription factors results in the disruption of Fog-Gata binding while preserving Fog-independent Gata function (14, 15). Mice carrying this mutation, introduced by homologous recombination into the murine Gata4 locus, have cardiac and gonadal defects similar to those seen in Fog2-null mutant mice (16, 17). These mice have increased survival compared with Gata4-null mutant mice, likely as a consequence of the preservation of Fog-independent Gata function. This increase is insufficient to permit evaluation of Gata4 as a mediator of Fog2-dependent diaphragmatic development, although mRNA expression patterns are consistent with this possibility. In this report we describe the generation of the analogous Gata6 mutant line, and our investigation of whether Gata4, Gata6, or both, mediate Fog2-dependent establishment of normal pulmonary lobar structure.
MATERIALS AND METHODS
These experiments were conducted with approval of the Center for Animal Resources and Comparative Medicine (Harvard Medical School, Boston, MA) and the Animal Resources at Children's Hospital (ARCH, Children's Hospital, Boston, MA).
Gata6 Targeted Mouse
A Gata6 genomic clone was provided by Dr. Jeffery Molkentin (Cincinnati Children's Medical Center, Cincinnati, OH). A SmaI-SacI fragment was subcloned into pBluscript KS (+) for site-directed mutagenesis using QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The sequence TGCGGCAACT, part of the N-zinc finger motif currently annotated in Exon 5, was changed to TGCGTGAATT to produce a missense mutation from valine to glycine at position 239 (ENSMUSP00000005989) and to introduce an MunI restriction site. The targeting vector was built on the mutagenized plasmid mentioned above by inserting an HSV-tk cassette into 5′ of the left homology region (the SmaI fragment from original genomic clone) and a loxP-flanked phosphoglycerate kinase (PGK)-neo cassette between the left homology arm and right homology arms (see Figure 1A). The targeting construct was linearized with Asp718 and electroporated into CJ7 ES cells. A correctly targeted clone was identified by Southern blotting (see Figure 1B). To remove the PGK-neo cassette, the positive clone was subjected to transient transfection with GFPcre, followed by fluorescence-activated cell sorting for GFP(+) cells. ES clones with PGK-neo cassette removed were identified and confirmed by both PCR and Southern Blot. After karyotype was determined to be normal, the targeted cells were injected into C57B6 mouse blastocysts to generate chimeras. Genotyping was done thereafter by PCR, followed by digest with MunI.
Figure 1.
Targeting strategy for knocking the missense mutation, V239G into the Gata6 locus. (A) Partial restriction map of the murine wild-type Gata6 locus, the targeting vector, and the targeted locus. The targeting construct contains the HSV-tk and neomycin resistance genes under the control of the mouse PGK promoter. Homologous recombination replaced wild-type Gata6 with genomic DNA mutagenized to change valine to glycine at position 239, to introduce an MunI cut site, and to incorporate a neomycin cassette for selection. The region used as a probe for Southern blot analysis is shown as a black rectangle. The N-zinc finger motif is in exon 5 and the C-zinc finger motif is in exon 6 (B, BglII; X, XhoI; N, NotI; S, SmaI; Sc, SacI). (B) Southern blot analysis of targeted ES cell DNA. DNA was digested with XhoI and BglII, separated on an agarose gel by electrophoresis followed by Southern blotting and hybridization with the 5′ probe indicated. The knock-in allele yields a fragment of 10.4 kb, and the wild-type allele yields a 8.4-kb fragment. (C) PCR genotyping of mouse pups. Tail DNA was used as template for PCR using the primers flanking the mutation. A representative gel is shown with bands corresponding to Gata6 targeted homozygote (Gata6ki/ki) mice (lanes 1 and 2), heterozygote (Gata6ki/+, lane 3), and wild-type mice (lane 4).
Genotyping
Genotyping for Gata6 ki line was performed with standard PCR using these primers: forward, 5′-TGTTGGAGGACCTGTCGGAG-3′; reverse, 5′-CGCTTCTGTGGCTTGATGAG-3′. PCR product was digested with MunI enzyme (Roche) and run on a 2.5% agarose gel (mutant band is 130 bp, wild-type band is 160 bp). Gentoyping for Gata4 ki line was performed with PCR using the following primers: forward, 5′-GGGTGAGCCTGTATGTAATGCCTGCG-3′; reverse, 5′-GATGACACTGCTTCTGTGGGGTCTTGAG-3′. PCR products were digested with Bgl II and run on a 1.2% agarose gel (mutant band is 800 bp, wild-type band is 350 bp).
Lung Culture
Timed pregnancies were set with day of plug detection defined as Day 0.5. Embryos were collected on Days 11.5 and 12.5. Pregnant mothers were killed with carbon dioxide, prepped with alcohol, and the uterus was removed and placed in sterile PBS solution. Lungs were cultured as previously described (3) on polyester membranes with Dulbecco's modified Eagle's medium, nutrient mixture F-12 supplemented with 10% fetal bovine serum, 0.3 mg/ml L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. Lungs were photographed daily with a dissecting microscope (Leica, Wetzlar, Germany) equipped with a transilluminating base and a digital camera.
Gata4 Immunofluoresence
Embryos were collected from timed pregnant A/J × C57BL/6J wild-type mice at Embryonic Day (e)10.5 or e11.5, with day of plug discovery defined as Day 0.5. The embryos were fixed in fresh 4% paraformaldehyde at 4°C for 4 h, and then cryoprotected in 30% sucrose prepared in 1× PBS at 4°C overnight. The embryos were then embedded in OCT and frozen on dry ice. Tissue sections were cut on a Leica cryostat (7 μm) and stored at −80°C.
Tissue sections were air-dried for 30 min, fixed in fresh 4% paraformaldehyde at room temperature for 15 min, and washed 3 × 5 min in 1× PBS. The sections were then blocked for 1.5 h in 5% normal goat serum, M.O.M. blocking reagent (M.O.M Immunodetection Kit, BMK-2202; Vector Laboratories, Burlingame, CA) with 0.3% triton. After washing 3 × 5 min in 1× PBS, the sections were incubated with mouse anti-human Gata4 antibody (sc-25310; Santa Cruz, Santa Cruz, CA) at 1:1,000 dilution in M.O.M. diluent buffer at 4°C overnight. Sections used as negative controls were incubated with M.O.M. buffer only. Slides were then washed 4 × 5 min in 1× PBS before incubation with Cy3-conjugated goat anti-mouse IgG antibody (115–165–003; Jackson Immunologicals, West Grove, PA) diluted in M.O.M. buffer at 1:500 for 1 h at room temperature. The sections were then washed three times in 1× PBS, and coverslips were mounted with Vectashield Mounting Medium with DAPI (H-1200; Vector Laboratories). Slides were examined with a Nikon eclipse 80i microscope (Nikon, Melville, NY) equipped for fluorescent imaging of DAPI and Cy3 and photographed with an Optronics MicroFire digital camera (Optronics, Goleta, CA). DAPI and fluorescent images were merged using PictureFrame imaging application (Optronics).
Fog2 Expression by β-Galactosidase Expression
Fog2-lacZ mice have been previously described (3). These mice carry a lacZ gene knocked into the Fog2 locus to allow β-galactosidase expression as a fusion protein in frame with the first 235 amino acids of the FOG2 protein. Embryos from a C57BL/6J × Fog2-lacZ hemizygote cross were harvested at e10.5 and e11.5. Whole embryos were fixed in fresh 1% formaldehyde, 0.2% glutaraldehyde, 2 mM MgCl2, 5 mM EGTA (pH 8.0), and 0.02% NP-40 in PBS at 4°C for 4 h. Embryos were then washed three times for 30 min each in 1× PBS plus 0.02% NP-40 at room temperature. Whole embryos were stained in 1 mg/ml X-gal (Cat# B4252; Sigma, St. Louis, MO), 5 mM K3Fe (CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, 0.01% deoxycholic acid, and 0.02% NP-40 in PBS in the dark at room temperature overnight. Embryos that did not carry a lacZ allele had no staining and served as negative controls. After staining, embryos were washed in 1× PBS before post fixation in 10% formaldehyde for 12 h. Post-fixed embryos were dehydrated and paraffin embedded. Transverse embryonic sections were cut 7 μm thick. Sections were de-waxed, rehydrated, and counterstained with 0.2% eosin.
RESULTS
Targeted Gata6 Mice Have Normal Pulmonary Lobar Structure
We started with an evaluation of Gata6 mutant mice, as Gata6 is the only Gata factor that has previously been reported to be important for lung development (7–10, 18, 19). Chimeric mice carrying the targeted V238G missense mutation in the Gata6 locus (“Gata6 knock in” mice or Gata6ki/+) and Gata6ki/+ offspring appeared normal. These mice were intercrossed and homozygous Gata6ki/ki mice were healthy and viable with normal fertility. Adult homozygous Gata6ki/ki mice had normal pulmonary lobar structure with no evidence of hypoplasia of the accessory lobe or the right median lobe.
Targeted Gata4 Mice Have Abnormal Lobar Development
Gata4 targeted mice carrying the V238G missense mutation (Gata4ki/ki) were previously described (16) and are embryonic lethal at e11.5–e13.5. Lungs from these embryos were not previously evaluated for abnormal lobar development. For this purpose, four litters were obtained at e11.5 and e12.5 from Gata4ki/+ intercrosses. From these litters, 40 embryos were recovered, of which 38 appeared viable (2 nonviable embryos were Gata4ki/ki mutants). Lung phenotypes were evaluated in all 38 viable embryos, and of these, 8 were Gata4ki/ki mutant. All mutant lungs lacked development of the accessory lobe and the accessory bud and showed mild growth delay, while lungs from wild-type and heterozygous mice were normal. Wild-type and mutant lungs from a single litter dissected at e12.5 and cultured for 48 h are shown in Figure 2. While the growth delay appeared variable, the specific structural defect is present in both mutants. Distal branching is relatively well maintained.
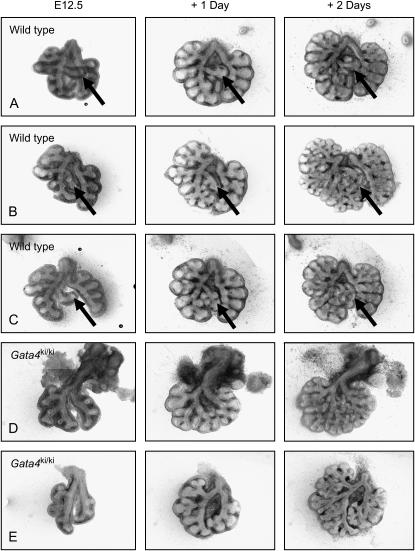
Figure 2.
Lungs removed from Gata4 mutant (Gata4ki/+) intercrossed mice and cultured at e12.5. This litter yielded 10 viable embryos, and all wild-type (A–C) and Gata4ki/ki mutant (D–E) lungs are shown to illustrate the range of normal and abnormal phenotypes. Gata4ki/ki mutants range from having minimal (D) to moderate (E) growth delay at e12.5, but distal branching and mesenchymal mass is relatively well preserved after culture for 2 d. Heterozygote mice (Gata4ki/+) look identical to the wild types. Accessory buds are marked with arrows.
Compound Targeted Gata Mice Have Abnormal Lobar Structure
To evaluate the consequence of loss of both Gata4 and Gata6 Fog interactions, Gata6ki/ki mice were crossed with Gata4ki/+ mice to produce Gata6ki/ki;Gata4ki/+ mice, which have a loss of Gata6-Fog interaction and a partial loss of Gata4-Fog interaction. These mice were healthy and viable with normal fertility. Gata6ki/ki;Gata4ki/+ mice were intercrossed to produce double homozygous mutants. Embryos from four timed pregnant females were evaluated at e11.5. All lungs from the 30 viable embryos obtained were evaluated for the presence of the accessory bud or lobe. These 30 embryos included four compound mutants (Gata6ki/ki; Gata4ki/ki). Lungs from all four of these mutants had a mild delay in developmental branching with complete absence of the accessory lobe and the accessory bud. The pulmonary abnormalities of these doubly mutant mice were not appreciably different than those found in the Gata4ki/ki mutant mice. All littermates (15 Gata6ki/ki and 12 Gata6ki/ki; Gata4ki/+ mice) had normal structural development. Representative examples from these crosses are shown in Figure 3.
Figure 3.
Lungs removed and cultured from e11.5 mice shown on day of harvest and after 1 d in culture. Photos are representative samples from litters containing Gata4 ki mutants (A and B are littermates), and Gata6 ki mutants or Gata6 and Gata4 compound mutants (C and D are littermates). Mice with loss of Fog–Gata4 interaction (Gata4ki/ki, B and D) have loss of the accessory lobe. The additional loss of Fog–Gata6 interaction does not change this phenotype (D). For comparison, lungs from Fog2 null mice at the same developmental stage are shown in E. Accessory buds are marked with arrows.
Gata4 and Fog2 Are Expressed in the Early Pulmonary Mesenchyme
To further assess the role of Gata4 in pulmonary development, embryonic expression of Gata4 protein was evaluated by immunofluorescence during the period of primary and secondary lung branching. At e10.5, primary lung buds have recently formed from the laryngotracheal diverticulum. Expression of Gata4 at a level rostral to the primary bronchial buds is restricted to the mesothelial surface and peripheral ventral mesenchyme (Figure 4A), while at the level of primary branching (Figure 4B) and caudal to the branchpoint (Figure 4C) expression is extensive in the ventral mesoderm and mesothelial tissue. Gata4 is not expressed in lung epithelial cells (Figure 4D). By e11.5, secondary branches have been established. Expression of Gata4 in the lung at e11.5 is mainly limited to the pleural and parietal mesothelial surfaces (Figures 4E and 4F). Expression at these time points is also evident in the heart, septum transversum, gastric epithelium, and pleuroperitoneal folds (PPFs).
Figure 4.
Gata4 (A–F) and Fog2 (G–H) expression are present in the early developing lung. Transverse sections from wild-type embryos at e10.5 (A–D, G) and e11.5 (E, F, H). A–D are sections through the same embryo (A, most rostral). Sections are counterstained with DAPI. At e10.5, Gata4 expression is evident in the mesothelium ventral to the rostral foregut (A), but is strongest in the mesenchyme ventral to the laryngotracheal diverticulum (B) and primary lung buds (C, lung buds marked with white arrows). Magnification of section of ventral pulmonary mesenchyme from panel C shows nuclear localization of Gata4 in the mesenchymal cells with no staining in the epithelium (D). By e11.5, Gata4 expression is strongest in the pleural and parietal mesothelium (E with DAPI counterstain and F without). Fog2 expression is shown by X-gal staining for β-galactosidase activity in mice carrying a lacZ reporter gene knocked into the Fog2 locus. Transverse sections from embryo at e10.5 (G) shows an expression pattern identical to Gata4 with expression limited to the ventral pulmonary mesenchyme and mesothelium. By e11.5, lung, Fog2 expression becomes diffuse (H) in the mid-lung fields and is also heavily expressed in primordial diaphragmatic tissue of the PPFs (marked with black arrows).
Fog2 expression mirrors Gata4 expression in the pulmonary mesenchyme during lobar development (e10.5), while expression patterns diverge after the establishment of secondary bronchi (e11.5). We evaluated β-galactosidase expression in e10.5 and e11.5 embryos of mice carrying a Fog2-lacZ reporter. At the primary bronchial stage (Figure 4G), expression of Fog2, like Gata4, is present in the ventral pulmonary mesenchyme and mesothelium. After secondary bronchi are established (e11.5), expression becomes diffuse in the pulmonary mesenchyme (Figure 4H) in the mid-lung fields adjacent to the pleuroperitoneal folds. Fog2 is also expressed in PPF tissue thought to contribute to the mature posterior diaphragm. There is no pulmonary epithelial expression of Fog2.
DISCUSSION
Early lung formation requires specification of the foregut endodermal tissue to bud, formation and elongation of the primary bronchi, separation of lung bud from the esophagus, and the formation of secondary bronchi (20). Most of the genes and genetic pathways required for the complex process are unknown. We report that Fog2–Gata4 interaction is necessary for the development of normal secondary lung structure. In addition, this function in patterning the ventral and medial lobar structure is correlated with a unique and overlapping expression pattern in the developing pulmonary mesoderm.
As Gata4 was not previously reported to be expressed in the lung, and Gata6 is expressed in the distal lung bud epithelium of all lobes during lobar establishment (7), we anticipated that Fog2–Gata6 interaction would be necessary for lobar development. Unexpectedly, we have found that it is Fog2–Gata4 interaction that mediates early pulmonary patterning. Furthermore, Gata6 does not appear to have even a redundant function, as there was no difference between the lung phenotype of Gata4 mutant mice and doubly homozygous Gata4/Gata6 mutants.
The expression patterns of Gata4 and Fog2 that we found in the early lung support the evidence that they are required for patterning lobar structure. Both Gata4 and Fog2 are expressed in a restricted pattern limited to the ventral and medial regions of the pulmonary mesenchyme and mesothelium during secondary bronchial formation. The lobes that are missing in Fog2 and Gata4 mutant mice are those that are more medial and ventral, suggesting that this pattern of expression reflects the organization of the lung. After secondary lobar structure is established, expression of Fog2 becomes more diffuse in the pulmonary mesenchyme while Gata4 expression quickly becomes restricted to smooth muscle cells surrounding pulmonary arteries.
Recently, pulmonary abnormalities limited mainly to the accessory and right middle lobes were found in some mice heterozygous for a null mutation of Gata4 (21). These mice have normal numbers of bronchi, but have dilated airways, delayed expression of epithelial cell differentiation markers, and ectopic expression of smooth muscle actin. In this model, Gata4 was necessary for normal mesenchymal function, but not differentiation (21). Mice expressing a reduced dose of Gata4 protein preserve lobar structure, just as mice with a reduced dose of FOG2 protein (Fog2null/+) have preserved structure. Fog2 mutant and Gata4 ki/ki mice die by e12.5, precluding an in vivo analysis of late lung development. It is possible that Fog2–Gata4 interactions also mediate later lung development including pulmonary vascular development or function. It is also possible that they have different roles and interact with different binding partners in late lung development. An extensive evaluation of both Fog1, Fog2, and Gata4 expression in later lung development and an evaluation of lung epithelial markers in Gata6ki/ki mice will help to answer these questions.
The establishment of Gata4 as a transcription factor critical for normal pulmonary development may have important implications for understanding abnormal lung development in some human patients with congenital heart disease or congenital diaphragmatic defects, as Gata4 is the third gene (after Fog2 and COUP TFII) identified to be critical for the primary development of the lung, the heart, and the diaphragm in mice (21–23). Humans with diaphragm defects have a high mortality and morbidity secondary to pulmonary hypoplasia, respiratory insufficiency, pulmonary hypertension, and right ventricular heart failure (24), and human structural lung defects may be associated with diaphragmatic defects or cardiac defects (25, 26). Since Gata4 is a human candidate gene for congenital heart disease (27–31)and congenital diaphragmatic hernia (32–34), the pulmonary hypertension, ventricular dysfunction, and lung disease associated with these conditions in some patients may be a consequence of the effect of perturbed Gata4 function in the developing lung. For this reason, further exploration of its role in both development and function of lung and pulmonary vasculature is warranted.
In summary, Fog2 and Gata4 are critical for patterning the developing mouse lung into stereotypic lobes. However, since deficiencies in these genes do not affect lung or body laterality or formation of the other lobes, they are only a subset of the factors necessary for normal early lung patterning. This establishment of Gata4 as a critical transcription factor for lung development helps further our understanding of how field limited gene expression in the early pulmonary mesenchyme contributes to lobar development.
Acknowledgments
The authors acknowledge Dr. Jeffery D. Molkentin (Cincinnati Children's Medical Center/University of Cincinnati) for providing the Gata6 clone and Dr. Sergei Tevosian (Dartmouth Medical School) for use of the Fog2-lacZ mouse line. The authors also acknowledge Alethea Wait and Patricia Follett for their technical assistance. S.H.O. is an investigator of the HHMI.
This study was funded by NIH HL076286 (K.G.A.), Hearst Foundation (K.G.A.), NIH HL032259 (S.H.O.), and NIH HD36404 (D.R.B.).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0211RC on December 1, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Perl AK, Hokuto I, Impagnatiello MA, Christofori G, Whitsett JA. Temporal effects of Sprouty on lung morphogenesis. Dev Biol 2003;258:154–168. [DOI] [PubMed] [Google Scholar]
- 2.Grindley JC, Bellusci S, Perkins D, Hogan BL. Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev Biol 1997;188:337–348. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman KG, Herron BJ, Vargas SO, Huang H, Tevosian SG, Kochilas L, Rao C, Pober BR, Babiuk RP, Epstein JA, et al. Fog2 is required for normal diaphragm and lung development in mice and humans. PLoS Genet 2005;1:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantor AB, Orkin SH. Coregulation of GATA factors by the Friend of GATA (FOG) family of multitype zinc finger proteins. Semin Cell Dev Biol 2005;16:117–128. [DOI] [PubMed] [Google Scholar]
- 5.Huggins GS, Bacani CJ, Boltax J, Aikawa R, Leiden JM. Friend of GATA 2 physically interacts with chicken ovalbumin upstream promoter-TF2 (COUP-TF2) and COUP-TF3 and represses COUP-TF2-dependent activation of the atrial natriuretic factor promoter. J Biol Chem 2001;276:28029–28036. [DOI] [PubMed] [Google Scholar]
- 6.Clabby ML, Robison TA, Quigley HF, Wilson DB, Kelly DP. Retinoid X receptor alpha represses GATA-4-mediated transcription via a retinoid-dependent interaction with the cardiac-enriched repressor FOG-2. J Biol Chem 2003;278:5760–5767. [DOI] [PubMed] [Google Scholar]
- 7.Keijzer R, van Tuyl M, Meijers C, Post M, Tibboel D, Grosveld F, Koutsourakis M. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development 2001;128:503–511. [DOI] [PubMed] [Google Scholar]
- 8.Koutsourakis M, Keijzer R, Visser P, Post M, Tibboel D, Grosveld F. Branching and differentiation defects in pulmonary epithelium with elevated Gata6 expression. Mech Dev 2001;105:105–114. [DOI] [PubMed] [Google Scholar]
- 9.Weidenfeld J, Shu W, Zhang L, Millar SE, Morrisey EE. The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J Biol Chem 2002;277:21061–21070. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development 2002;129:2233–2246. [DOI] [PubMed] [Google Scholar]
- 11.Heikinheimo M, Scandrett JM, Wilson DB. Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Dev Biol 1994;164:361–373. [DOI] [PubMed] [Google Scholar]
- 12.Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev 1998;12:3579–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molkentin JD, Tymitz KM, Richardson JA, Olson EN. Abnormalities of the genitourinary tract in female mice lacking GATA5. Mol Cell Biol 2000;20:5256–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsang AP, Visvader JE, Turner CA, Fujiwara Y, Yu C, Weiss MJ, Crossley M, Orkin SH. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell 1997;90:109–119. [DOI] [PubMed] [Google Scholar]
- 15.Nichols KE, Crispino JD, Poncz M, White JG, Orkin SH, Maris JM, Weiss MJ. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet 2000;24:266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crispino JD, Lodish MB, Thurberg BL, Litovsky SH, Collins T, Molkentin JD, Orkin SH. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes Dev 2001;15:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 2002;129:4627–4634. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Glasser SW, Wan H, Whitsett JA. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem 2002;277:4519–4525. [DOI] [PubMed] [Google Scholar]
- 19.Yin Z, Gonzales L, Kolla V, Rath N, Zhang Y, Lu MM, Kimura S, Ballard PL, Beers MF, Epstein JA, et al. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am J Physiol Lung Cell Mol Physiol 2006;291:L191–L199. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 2006;133:1611–1624. [DOI] [PubMed] [Google Scholar]
- 21.Jay PY, Bielinska M, Erlich JM, Mannisto S, Pu WT, Heikinheimo M, Wilson DB. Impaired mesenchymal cell function in Gata4 mutant mice leads to diaphragmatic hernias and primary lung defects. Dev Biol 2007;301:602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science 2006;313:1922–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura Y, Suzuki T, Kaneko C, Darnel AD, Moriya T, Suzuki S, Handa M, Ebina M, Nukiwa T, Sasano H. Retinoid receptors in the developing human lung. Clin Sci (Lond) 2002;103:613–621. [DOI] [PubMed] [Google Scholar]
- 24.Colvin J, Bower C, Dickinson JE, Sokol J. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics 2005;116:e356–e363. [DOI] [PubMed] [Google Scholar]
- 25.Berrocal T, Madrid C, Novo S, Gutierrez J, Arjonilla A, Gomez-Leon N. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics 2004;24:e17. [DOI] [PubMed] [Google Scholar]
- 26.Nose K, Kamata S, Sawai T, Tazuke Y, Usui N, Kawahara H, Okada A. Airway anomalies in patients with congenital diaphragmatic hernia. J Pediatr Surg 2000;35:1562–1565. [DOI] [PubMed] [Google Scholar]
- 27.Barber JC, Maloney V, Hollox EJ, Stuke-Sontheimer A, du Bois G, Daumiller E, Klein-Vogler U, Dufke A, Armour JA, Liehr T. Duplications and copy number variants of 8p23.1 are cytogenetically indistinguishable but distinct at the molecular level. Eur J Hum Genet 2005;13:1131–1136. [DOI] [PubMed] [Google Scholar]
- 28.Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 2003;424:443–447. [DOI] [PubMed] [Google Scholar]
- 29.Sarkozy A, Conti E, Neri C, D'Agostino R, Digilio MC, Esposito G, Toscano A, Marino B, Pizzuti A, Dallapiccola B. Spectrum of atrial septal defects associated with mutations of NKX2.5 and GATA4 transcription factors. J Med Genet 2005;42:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirayama-Yamada K, Kamisago M, Akimoto K, Aotsuka H, Nakamura Y, Tomita H, Furutani M, Imamura S, Takao A, Nakazawa M, et al. Phenotypes with GATA4 or NKX2.5 mutations in familial atrial septal defect. Am J Med Genet A 2005;135:47–52. [DOI] [PubMed] [Google Scholar]
- 31.Nemer G, Fadlalah F, Usta J, Nemer M, Dbaibo G, Obeid M, Bitar F. A novel mutation in the GATA4 gene in patients with Tetralogy of Fallot. Hum Mutat 2006;27:293–294. [DOI] [PubMed] [Google Scholar]
- 32.Lopez I, Bafalliu JA, Bernabe MC, Garcia F, Costa M, Guillen-Navarro E. Prenatal diagnosis of de novo deletions of 8p23.1 or 15q26.1 in two fetuses with diaphragmatic hernia and congenital heart defects. Prenat Diagn 2006;26:577–580. [DOI] [PubMed] [Google Scholar]
- 33.Slavotinek AM, Moshrefi A, Davis R, Leeth E, Schaeffer GB, Burchard GE, Shaw GM, James B, Ptacek L, Pennacchio LA. Array comparative genomic hybridization in patients with congenital diaphragmatic hernia: mapping of four CDH-critical regions and sequencing of candidate genes at 15q26.1–15q26.2. Eur J Hum Genet 2006;14:999–1008. [DOI] [PubMed] [Google Scholar]
- 34.Slavotinek AM. The genetics of congenital diaphragmatic hernia. Semin Perinatol 2005;29:77–85. [DOI] [PubMed] [Google Scholar]