Abstract
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) produced by epithelial and inflammatory cells are key mediators of the chronic airway inflammation of asthma. Low L-arginine levels can result in the uncoupling of nitric oxide synthase (NOS) leading to production of both ROS and RNS. Asymmetric dimethylarginine (ADMA) is a competitive endogenous inhibitor of all NOS isoforms and has been demonstrated to inhibit NO formation and increase oxidative stress in vascular endothelial and smooth muscle cells. The effect of ADMA on inducible NOS (iNOS) activity in epithelial cells has not been explored. In this study, we investigated whether addition of exogenous ADMA alters the generation of NO and superoxide anion ( ), leading to peroxynitrite (ONOO−) formation in a mouse epithelial cell line. In stimulated LA-4 cells, ADMA dose-dependently inhibited nitrite accumulation after 24 h of treatment. In addition, ADMA concentrations as low as 10 μM induced rapid increases in
), leading to peroxynitrite (ONOO−) formation in a mouse epithelial cell line. In stimulated LA-4 cells, ADMA dose-dependently inhibited nitrite accumulation after 24 h of treatment. In addition, ADMA concentrations as low as 10 μM induced rapid increases in  production as measured by dihydroethidium oxidation. Furthermore, using dihydrorhodamine to monitor ONOO− formation, ADMA caused a dose-dependent increase in ONOO− after treatment for 24 h. Similar effects of ADMA were seen using purified iNOS protein in a cell-free system. Together, these data indicate that elevated ADMA may contribute to the production of ROS and RNS in airway inflammation.
production as measured by dihydroethidium oxidation. Furthermore, using dihydrorhodamine to monitor ONOO− formation, ADMA caused a dose-dependent increase in ONOO− after treatment for 24 h. Similar effects of ADMA were seen using purified iNOS protein in a cell-free system. Together, these data indicate that elevated ADMA may contribute to the production of ROS and RNS in airway inflammation.
Keywords: ADMA, epithelial cells, LA-4 cells, NOS, oxidative stress
CLINICAL RELEVANCE
This study demonstrates that elevated levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) may contribute to the production of reactive oxygen species and reactive nitrogen species in airway inflammation. These findings suggest that ADMA may play a role in airway diseases including asthma.
Endogenous nitric oxide (NO) plays a key beneficial role in the physiologic regulation of airway functions including the control of vascular and bronchial tone and neuroendocrine regulation of airway mediator release (1, 2). However, overproduction of NO has also been shown to contribute to tissue injury in inflammatory and ischemic conditions (3). One mechanism by which NO can contribute to tissue injury is by its diffusion-controlled reaction with superoxide (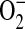 ) to produce peroxynitrite (ONOO−), a potent oxidant thought to be a key mediator of NO-mediated tissue injury in atherosclerosis, congestive heart failure, glutamate excitotoxicity, and other disease states involving inflammatory oxidative stress (4). NO is formed from the semi-essential amino acid L-arginine by the action of three isoforms of the enzyme nitric oxide synthase (NOS) (5). The constitutive isoforms (cNOS) are expressed mainly in nonadrenergic noncholinergic nerves (neuronal NOS [nNOS]), endothelial cells (endothelial NOS [eNOS]), and airway epithelium (nNOS and eNOS) (5–8), and are involved in the physiologic regulation of the airway by the local production of small amounts of NO. The third isoform is induced after exposure to proinflammatory cytokines (inducible NOS [iNOS]) and is expressed in epithelial cells and inflammatory cells of the airway including macrophages (9). In addition to producing NO, NOS can catalyze
) to produce peroxynitrite (ONOO−), a potent oxidant thought to be a key mediator of NO-mediated tissue injury in atherosclerosis, congestive heart failure, glutamate excitotoxicity, and other disease states involving inflammatory oxidative stress (4). NO is formed from the semi-essential amino acid L-arginine by the action of three isoforms of the enzyme nitric oxide synthase (NOS) (5). The constitutive isoforms (cNOS) are expressed mainly in nonadrenergic noncholinergic nerves (neuronal NOS [nNOS]), endothelial cells (endothelial NOS [eNOS]), and airway epithelium (nNOS and eNOS) (5–8), and are involved in the physiologic regulation of the airway by the local production of small amounts of NO. The third isoform is induced after exposure to proinflammatory cytokines (inducible NOS [iNOS]) and is expressed in epithelial cells and inflammatory cells of the airway including macrophages (9). In addition to producing NO, NOS can catalyze 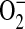 formation in the presence of low L-arginine levels (10, 11). In this case, the oxidation of L-arginine to NO is not complete and the activity of this enzyme can be “uncoupled” (12–14). Furthermore, in L-arginine depleted cells, NOS can generate both
formation in the presence of low L-arginine levels (10, 11). In this case, the oxidation of L-arginine to NO is not complete and the activity of this enzyme can be “uncoupled” (12–14). Furthermore, in L-arginine depleted cells, NOS can generate both 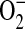 and NO leading to ONOO− formation (15, 16). Both
and NO leading to ONOO− formation (15, 16). Both 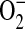 and ONOO− are mediators of cell signaling in airway and pulmonary epithelial cells. Overproduction of ONOO− can cause oxidative damage to proteins, lipids, DNA, and carbohydrates (17) and ONOO-inducible cell death has been demonstrated in multiple cell types including type II epithelial cells (18).
and ONOO− are mediators of cell signaling in airway and pulmonary epithelial cells. Overproduction of ONOO− can cause oxidative damage to proteins, lipids, DNA, and carbohydrates (17) and ONOO-inducible cell death has been demonstrated in multiple cell types including type II epithelial cells (18).
Asymmetric dimethylarginine (ADMA) is an L-arginine analog that serves as an endogenous inhibitor of both the constitutive forms and the inducible form of NOS (19–21). ADMA is involved in the pathophysiology of human vascular disease and has been demonstrated to be not only a marker of endothelial dysfunction (22), but also a novel cardiovascular risk factor (23). Enhanced ADMA levels in both animal models and in patients with pulmonary hypertension have been reported to contribute to vascular abnormalities in pulmonary hypertension (24, 25). Furthermore, a recent report by Bulau and coworkers demonstrated that the lung is not only a major source of NO, but also of ADMA (26). This as well as numerous studies demonstrating an importance of L-arginine metabolism and NO production in inflammatory airway diseases (27, 28), suggest that ADMA may play an important role in regulation of NOS activity and NO production in the lung.
ADMA has been reported to competitively inhibit NO elaboration by displacing L-arginine from NOS, and studies in cultured human endothelial cells have shown that elevated ADMA results in the production of 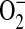 (29). This has led to the hypothesis that ADMA may uncouple NOS, resulting in a switch of the enzymatic activity from NO to
(29). This has led to the hypothesis that ADMA may uncouple NOS, resulting in a switch of the enzymatic activity from NO to 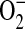 . Although significant strides have been made in the elucidation of the role of ADMA as an inhibitor of the constitutive NOS isoforms, the role of ADMA in iNOS inhibition has been poorly studied. In an in vitro model of Pseudomonas aeruginosa infection-induced iNOS expression of respiratory mucosa, Dowling and colleagues demonstrated that addition of ADMA inhibited nitrite formation (19). More recently, Ueda and colleagues demonstrated that addition of exogenous ADMA to cultured rat smooth muscle cells significantly decreased IL-1β–induced NO production in these cells (21). These findings suggest that ADMA inhibits NO formation via iNOS in these systems. Furthermore, although substantial experimental and clinical evidence suggests that even small modifications of ADMA levels significantly change vascular NO production, vascular tone, and systemic vascular resistance (30), the role of ADMA in the regulation of NO production in the respiratory system has not yet been well explored. In the present study, we hypothesized that ADMA plays a role in regulating cytokine-induced NO production and reactive oxygen species (ROS)/reactive nitrogen species (RNS) formation in lung epithelial cells. To test this hypothesis, the effects of elevated ADMA on iNOS function in a cytokine-stimulated murine lung epithelial cell line and purified iNOS protein were studied.
. Although significant strides have been made in the elucidation of the role of ADMA as an inhibitor of the constitutive NOS isoforms, the role of ADMA in iNOS inhibition has been poorly studied. In an in vitro model of Pseudomonas aeruginosa infection-induced iNOS expression of respiratory mucosa, Dowling and colleagues demonstrated that addition of ADMA inhibited nitrite formation (19). More recently, Ueda and colleagues demonstrated that addition of exogenous ADMA to cultured rat smooth muscle cells significantly decreased IL-1β–induced NO production in these cells (21). These findings suggest that ADMA inhibits NO formation via iNOS in these systems. Furthermore, although substantial experimental and clinical evidence suggests that even small modifications of ADMA levels significantly change vascular NO production, vascular tone, and systemic vascular resistance (30), the role of ADMA in the regulation of NO production in the respiratory system has not yet been well explored. In the present study, we hypothesized that ADMA plays a role in regulating cytokine-induced NO production and reactive oxygen species (ROS)/reactive nitrogen species (RNS) formation in lung epithelial cells. To test this hypothesis, the effects of elevated ADMA on iNOS function in a cytokine-stimulated murine lung epithelial cell line and purified iNOS protein were studied.
MATERIALS AND METHODS
Cell Culture
LA-4 cells were maintained in Ham's modified F-12 medium supplemented with 15% fetal bovine serum (FBS; Hyclone, Logan, UT) antibiotics/antimycotic (MediaTech, Herndon, VA) at 37°C in a humidified atmosphere with 5% CO2–95% air. Cells were seeded at ∼ 50% confluence, and used when 95–100% confluent.
Before experimental treatment, cells were washed and then incubated in the presence or absence (unstimulated) of stimulation (10 μg/ml LPS, 10 ng/ml TNF-α, and 20 ng/ml IFN-γ) ± ADMA or 1400W. LPS, ADMA, and 1400W were obtained from Sigma (St. Louis, MO), TNF-α from BD Biosciences (San Diego, CA), and IFN-γ from Chemicon (Danvers, MA).
Measurement of Nitrite Concentrations
Nitrite concentrations were measured using the Griess reaction (31). Sixty microliters of Griess reagent (1% sulfanilamide and 0.1% naphthly-ethylenediamide in 5% phosphoric acid) purchased from Ricca Chemical Co. (Pocomoke, MD) was added to 60 μl of sample medium. After a 10-min incubation at room temperature, optical density was measured at 550 nm using microplate reader (Molecular Devices, Sunnyvale, CA). Culture medium was used as a blank for cell culture experiments and reaction medium was used as a blank for purified protein experiments. Standard curves were made with NaNO2 (Sigma) dissolved in the culture medium or PBS. There was a linear standard curve for NaNO2 in culture medium for concentrations between 0.06 and 250 μM (R2 = 0.9960, y = 0.02270 [range, −0.004398 to 0.04979], slope = 0.0042 [range, 0.003934 to 0.004465], limit of detection ∼ 0.25 μM). For NaNO2 in PBS, there was a linear standard curve between 0.06 and 62.5 μM (R2 = 0.9998, y = −0.0007918 [range, −0.001798 to 0.0002148], slope = 0.00556 [range, 0.00551 to 0.005608], limit of detection ∼ 0.25 μM). The slight difference in slope values between the NaNO2 standard curves in culture medium and PBS suggests that there may be some differential matrix interference for this assay. Therefore, it would be difficult to make any direct comparisons between nitrite levels determined in culture media and PBS.
Western Blot Analysis
Western blot analysis was performed as previously described (32) with some modifications. Protein extracts (25–50μg), prepared from cell cultures were separated on NuPAGE 4–12% Bis-Tris gels (Invitrogen Life Technologies, Carlsbad, CA) and electrophoretically transferred to Immun-Blot PVDF membrane (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween. After blocking, the membranes were incubated overnight at 4°C with a 1:500 dilution of anti-iNOS (BD Transduction Laboratories, San Jose, CA) or anti-β-actin (Cell Signaling Technology, Danvers, MA) antibodies. The membranes were then washed with TBS containing 0.1% Tween and then incubated for 1 h at room temperature with a 1:1,000 dilution of the rabbit anti-IgG–horseradish peroxidase conjugate (Pierce Laboratories, Rockford, IL). Bands were visualized using chemiluminescnece (SuperSignal West Femto Substrate Kit; Pierce Laboratories) on a Versa Doc Imaging System (Bio-Rad). Bad intensity was quantified using Bio-Rad Quantity One software.
In all electrophoresis experiments, a protein standard ladder (MagicMark XP Western Standards; Invitrogen) was used for estimation of protein molecular weight.
Detection of Superoxide Formation
Dihydroethidium (DHE) is a cell-permeable compound that can undergo a two-electron oxidation to form the DNA-binding fluorophore ethidium/oxyethidium (33). The reaction is relatively specific for 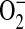 , with minimal oxidation induced by H2O2 or hypochlorous acid (34). However, given that rates of
, with minimal oxidation induced by H2O2 or hypochlorous acid (34). However, given that rates of 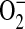 production using DHE may be underestimated due to its capacity to enhance rates of
production using DHE may be underestimated due to its capacity to enhance rates of 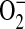 dismutation to H2O2 (34), DHE oxidation is most useful as a qualitative indicator of
dismutation to H2O2 (34), DHE oxidation is most useful as a qualitative indicator of 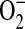 . In our experiments, intracellular
. In our experiments, intracellular 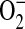 generation was estimated using DHE. After a 24-h incubation of confluent LA-4 cells in 96-well microtiter plates with or without stimuli, ADMA (10–250 μM) or 100 μM 1400W was added the presence of 25 μM DHE (Invitrogen). For
generation was estimated using DHE. After a 24-h incubation of confluent LA-4 cells in 96-well microtiter plates with or without stimuli, ADMA (10–250 μM) or 100 μM 1400W was added the presence of 25 μM DHE (Invitrogen). For 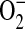 detection in the purified iNOS experiments, the reactions were initiated in the presence of 25 μM DHE and allowed to continue for up to 2 h. After incubation in both the cell culture and purified iNOS experiments, eithidium/oxythidium formation was measured by fluorimetric analysis in a microplate reader (Molecular Devices) at excitation/emission wavelengths of 518 and 605 nm, respectively. Fluorescence due to auto-oxidation of DHE in either culture or reaction medium was subtracted from the original measurements.
detection in the purified iNOS experiments, the reactions were initiated in the presence of 25 μM DHE and allowed to continue for up to 2 h. After incubation in both the cell culture and purified iNOS experiments, eithidium/oxythidium formation was measured by fluorimetric analysis in a microplate reader (Molecular Devices) at excitation/emission wavelengths of 518 and 605 nm, respectively. Fluorescence due to auto-oxidation of DHE in either culture or reaction medium was subtracted from the original measurements.
Determination of Peroxynitrite
Peroxynitrite efficiently oxidizes the nonfluorescent molecule dihydrorhodamine 123 (DHR) to the fluorescent product rhodamine. This method is commonly employed for detection of ONOO−; however, there are several potentially confounding aspects of this assay that should be considered when interpreting results from this assay. In biological systems, DHR may undergo a single electron transfer to ONOO− to form a DHR radical, which can then dismutate to form rhodamine and DHR. Furthermore, the DHR radical may reversibly reduce O2 to form 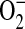 and rhodamine. Compared with the dismutation of DHR radicals, however, this reaction is very slow and therefore its contribution to the total fluorescent product in the absence of SOD is likely minimal (35). In addition, although
and rhodamine. Compared with the dismutation of DHR radicals, however, this reaction is very slow and therefore its contribution to the total fluorescent product in the absence of SOD is likely minimal (35). In addition, although 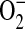 , H2O2, and NO do not appear to react with DHR (35), several cell-derived oxidants including OH,
, H2O2, and NO do not appear to react with DHR (35), several cell-derived oxidants including OH, 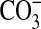 , and NO2 can react with this probe (36), potentially contributing to the oxidation of DHR. Finally, some controversy remains as to whether DHR reacts directly with ONOO− (35), or whether the oxidation of fluorescent probes by peroxynitrite occurs via free radical intermediates formed during the spontaneous decomposition of peroxynitrite (37). These potential confounding factors notwithstanding, the ability of DHR to react efficiently with ONOO− even at very low concentrations makes it useful for detecting ONOO− in vitro. In our studies, ONOO− formation was assayed by following incubation with or without stimuli and inhibitors in the presence of 25 μM DHR (Sigma) in 96-well microtiter plates in culture medium as previously published (38). For ONOO− detection in the purified iNOS experiments, the reactions were initiated in the presence of 25 μM DHR and allowed to continue for up to 2 h. After incubation, DHR conversion to rhodamine was measured by fluorimetric analysis in a microplate reader (Molecular Devices) at excitation/emission wavelengths of 485 and 538 nm, respectively. Fluorescence due to autooxidation of DHR was subtracted from the original measurements.
, and NO2 can react with this probe (36), potentially contributing to the oxidation of DHR. Finally, some controversy remains as to whether DHR reacts directly with ONOO− (35), or whether the oxidation of fluorescent probes by peroxynitrite occurs via free radical intermediates formed during the spontaneous decomposition of peroxynitrite (37). These potential confounding factors notwithstanding, the ability of DHR to react efficiently with ONOO− even at very low concentrations makes it useful for detecting ONOO− in vitro. In our studies, ONOO− formation was assayed by following incubation with or without stimuli and inhibitors in the presence of 25 μM DHR (Sigma) in 96-well microtiter plates in culture medium as previously published (38). For ONOO− detection in the purified iNOS experiments, the reactions were initiated in the presence of 25 μM DHR and allowed to continue for up to 2 h. After incubation, DHR conversion to rhodamine was measured by fluorimetric analysis in a microplate reader (Molecular Devices) at excitation/emission wavelengths of 485 and 538 nm, respectively. Fluorescence due to autooxidation of DHR was subtracted from the original measurements.
Statistical Analysis
The mean ± SEM was calculated for all samples and P values calculated using an unpaired t test or a one-way ANOVA followed by Dunnett's multiple comparison to a single control group. All data are expressed as mean ± SEM. P < 0.05 was accepted as statistically significant.
RESULTS
Stimulation of LA-4 Cells with LPS, IFN-γ, and TNF-α Results in a Significant Increase in iNOS-Mediated Nitrite Accumulation and iNOS Protein Expression
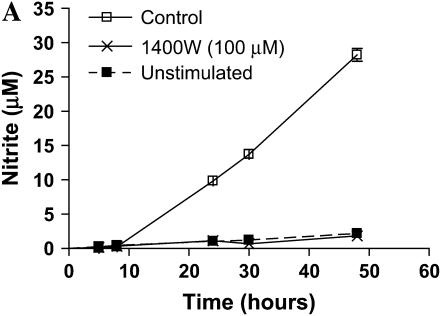
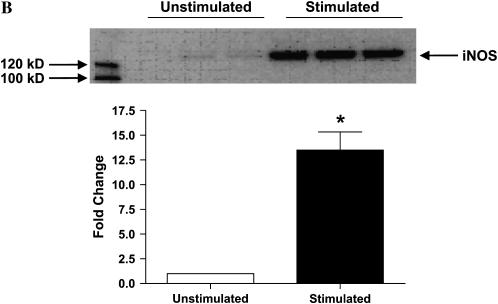
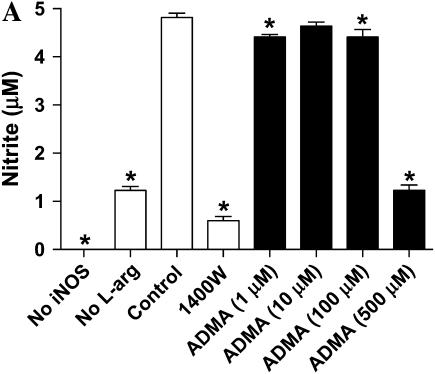
We wished to assess the effects on ADMA on iNOS function in murine epithelial cells. Given that iNOS protein levels are very low in unstimulated LA-4 cells (39), we first determined whether iNOS protein and nitric oxide production could be significantly up-regulated in the LA-4 cell line after stimulation with LPS, IFN-γ, and TNF-α. These molecules were chosen due to their synergism in inducing genes involved in the inflammatory response (40) and because these are factors to which respiratory epithelial cells can be exposed. Cells were exposed to LPS (10 μg/ml), IFN-γ (20 ng/ml), and TNF-α (10 ng/ml) in the presence or absence of the iNOS specific inhibitor 1400W (100 μM) for up to 48 h. Nitrite accumulation increased ∼ 10-fold after 24 h of stimulation and over 25-fold after 48 h (Figure 1A). The iNOS-specific inhibitor 1400W blocked essentially all nitrite accumulation indicating that iNOS was responsible for most of the nitrite formed in this model. In addition, iNOS protein levels increased over 13-fold over protein levels in unstimulated cells (Figure 1B). These data demonstrate that the LA-4 cell line is a good model to assess the effects of ADMA on iNOS function.
Figure 1.
Nitrite accumulation and iNOS protein expression in stimulated LA-4 cells. LA-4 cells were exposed to LPS (10 μg/ml), IFN-γ (20 ng/ml), and TNF-α (10 ng/ml) in the presence or absence of the iNOS-specific inhibitor 1400W (100 μM) for up to 48 h. (A) Time course of nitrite production after stimulation. Cell supernatant samples were assayed for nitrite content at the specified time points (n = 3). (B) Protein extracts (25 μg), prepared from LA-4 cell lysates after 24 h stimulation with LPS (10 μg/ml), IFN-γ (20 ng/ml), and TNF-α were separated on a 4–12% denaturing polyacrylamide gel, electrophoretically transferred to PVDF membrane, and analyzed using a specific antiserum raised against iNOS and β-actin proteins. B is a representative blot of lysates from unstimulated (n = 3) and stimulated (n = 3) cells. The graph below indicates the fold change in densitometric values of iNOS protein (normalized to β-actin levels) in cells stimulated for 24 h over unstimulated controls (n = 5). *P < 0.05 versus control.
ADMA Is a Weak Inhibitor of Nitrite Accumulation in Stimulated LA-4 Cells
ADMA has been shown to inhibit NO production in endothelial cells (20) and smooth muscle cells (21). To our knowledge, the effects of ADMA on NOS function in epithelial cells have not yet been explored. To determine whether exogenously applied ADMA inhibited NO synthesis in murine epithelial cells, we added increasing concentrations of ADMA (10–500 μM) to the culture media along with stimulation. It is important to note that in vivo, the L-arginine/ADMA ratio is in the range of 50:1 to 100:1, given a range of L-arginine levels between 50 and 100 μmol/liter, and ADMA concentrations between 0.3 and 0.7 μmol/liter. The L-arginine concentration in the culture media for all experiments reported here was ∼ 2.4 mM; therefore, the addition of 10–100 μM ADMA in these experiments resulted in an L-arg/ADMA ratio that is within physiologic levels.
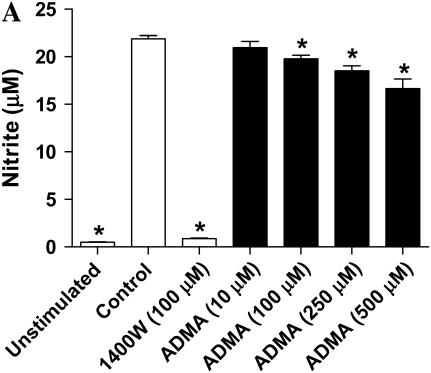
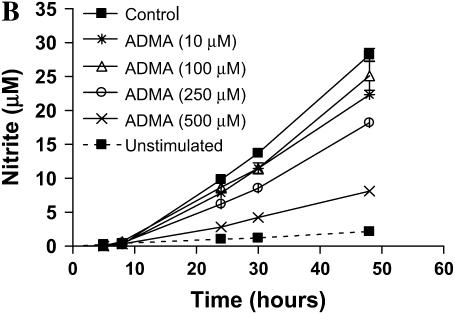
ADMA weakly inhibited nitrite accumulation in a dose-dependent manner after 24 h of stimulation and treatment (Figure 2A). Significant inhibition (P < 0.05) was seen at doses starting at 100 μM; however, even at the highest dose of 500 μM ADMA, nitrite accumulation was only decreased ∼ 25% of control. In contrast, addition of 1400W inhibited nitrite accumulation to nearly baseline (unstimulated cells) level. Even after 48 h of stimulation and treatment, the highest concentration of ADMA (500 μM) inhibited nitrite accumulation by < 75% (Figure 2B).
Figure 2.
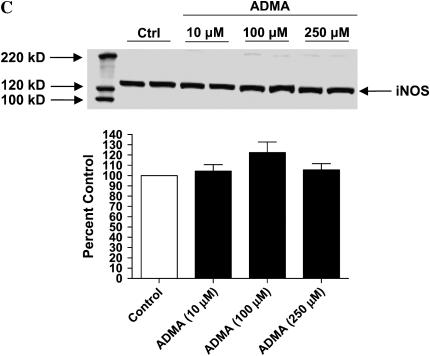
Effects of ADMA on nitrite production and iNOS expression in stimulated LA-4 cells. Cultured LA-4 cells were stimulated for 24 h (A) or up to 48 h (B) in the presence of 1400W (100 μM) or increasing ADMA concentrations (10–500 μM) and nitrite concentrations determined using the Griess reaction (n = 3). *P < 0.05 versus control. (C) Protein extracts (25 μg), prepared from LA-4 cell lysates after 24 h stimulation and treatment were separated on a 4–12% denaturing polyacrylamide gel, electrophoretically transferred to PVDF membrane, and analyzed using a specific antiserum raised against iNOS and β-actin proteins. C is a representative blot of lysates from stimulated untreated cells (n = 2) and stimulated cells treated with ADMA (10–250 μM; n = 2). The graph below indicates the percent change in densitometric values of iNOS protein (normalized to β-actin levels) in stimulated cells treated with ADMA (10–250 μM) versus untreated stimulated controls (n = 8).
We are unaware of any reports documenting effects of elevated ADMA on NOS protein levels. However, to determine whether addition of ADMA has an effect on iNOS protein levels, we performed Western blots of lysates from stimulated LA-4 cells that were either untreated or treated with ADMA (10–250 μM; Figure 2C). Although there appeared to be a slight increase in iNOS protein levels after treatment with 100 μM compared with untreated controls, this difference was not statistically significant.
ADMA Increases Superoxide Formation in LA-4 Cells
In primary endothelial cell cultures, ADMA not only decreases NO formation, but also increases 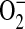 production (29). To address whether ADMA was capable of increasing ROS formation in stimulated epithelial cells, we assessed DHE oxidation after addition of increasing concentrations of ADMA (10–250 μM) or 1400W (100 μM) after overnight stimulation with LPS (10 μg/ml), IFN-γ (20 ng/ml), and TNF-α (10 ng/ml). The oxidation of DHE is relatively specific for
production (29). To address whether ADMA was capable of increasing ROS formation in stimulated epithelial cells, we assessed DHE oxidation after addition of increasing concentrations of ADMA (10–250 μM) or 1400W (100 μM) after overnight stimulation with LPS (10 μg/ml), IFN-γ (20 ng/ml), and TNF-α (10 ng/ml). The oxidation of DHE is relatively specific for 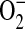 with minimal oxidation induced by H2O2 or ONOO− and can therefore be used as a marker for
with minimal oxidation induced by H2O2 or ONOO− and can therefore be used as a marker for 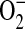 production in intact cells (41). Addition of all concentrations of ADMA resulted in rapid increases in
production in intact cells (41). Addition of all concentrations of ADMA resulted in rapid increases in 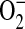 generation (Figure 3A). In contrast, treatment with 1400W did not increase
generation (Figure 3A). In contrast, treatment with 1400W did not increase 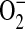 generation over control levels.
generation over control levels.
Figure 3.
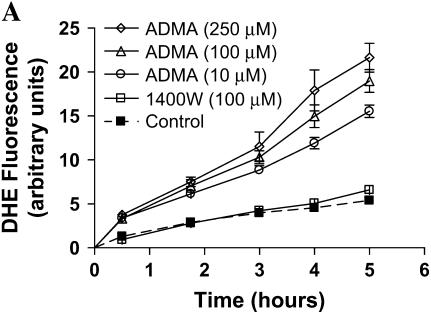
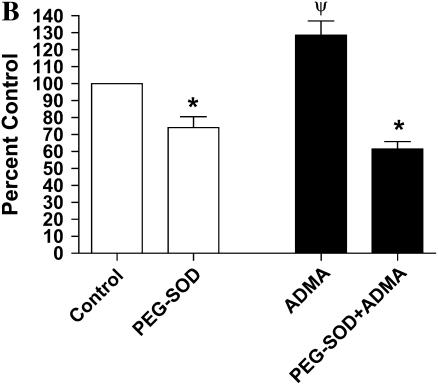
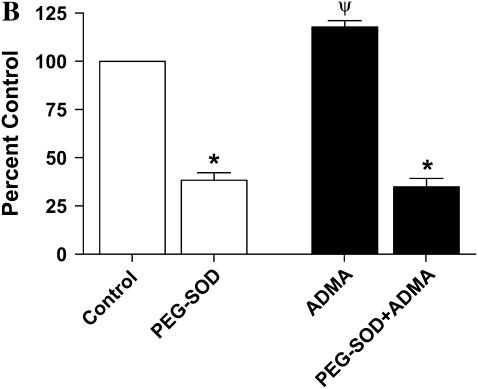
Effects of ADMA on DHE fluorescence in stimulated LA-4 cells. Cultured LA-4 cells were stimulated for 24 h before addition of treatments. (A) ADMA (10–250 μM) or 1400W (100 μM) was added in the presence of 25 μM DHE and fluorescence measured at various time points for 5 h (n = 4). (B) ADMA (100 μM) was added ± PEG-SOD (44 U/ml) and DHE fluorescence determined after 60 min (n = 4). Data are represented as percent change from control (stimulated with no treatment). *P < 0.05 versus no PEG-SOD. ΨP < 0.05 versus control.
It has been shown that concentrations higher than 1 μM DHE can result in  -independent fluorescence from nonspecific ethidium cation binding to nuclear DNA, resulting in an enhancement of fluorescence (42). Furthermore, DHE can be oxidized by routes independent of
-independent fluorescence from nonspecific ethidium cation binding to nuclear DNA, resulting in an enhancement of fluorescence (42). Furthermore, DHE can be oxidized by routes independent of 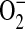 such as ferricytochrome c oxidation in conditions in which mitochondria are the primary source of superoxide production or cytochrome c release into the cytosol during apoptosis (43). To confirm that the increases in DHE oxidation after treatment with ADMA were due to
such as ferricytochrome c oxidation in conditions in which mitochondria are the primary source of superoxide production or cytochrome c release into the cytosol during apoptosis (43). To confirm that the increases in DHE oxidation after treatment with ADMA were due to 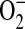 generation, we assessed DHE oxidation of 100 μM ADMA in the presence and absence of 44 U/ml PEG-SOD (Figure 3B). After a 60-min treatment of stimulated cells, the ADMA-induced increase in DHE oxidation was completely negated by addition of PEG-SOD, indicating that the increased DHE oxidation seen after treatment with ADMA was due to increased
generation, we assessed DHE oxidation of 100 μM ADMA in the presence and absence of 44 U/ml PEG-SOD (Figure 3B). After a 60-min treatment of stimulated cells, the ADMA-induced increase in DHE oxidation was completely negated by addition of PEG-SOD, indicating that the increased DHE oxidation seen after treatment with ADMA was due to increased 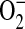 generation.
generation.
Decreased NO and Elevated Superoxide Is Associated with Increased Peroxynitrite Formation in ADMA-Treated LA-4 Cells
Superoxide rapidly and spontaneously reacts with NO to produce the potent oxidant ONOO−, an important mediator of inflammation-induced tissue injury and dysfunction (4). In macrophages, L-arginine depletion results in iNOS-mediated 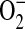 and ONOO− (16). Furthermore, Miles and colleagues have shown that relative rates of NO and
and ONOO− (16). Furthermore, Miles and colleagues have shown that relative rates of NO and 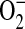 production may be critical in determining the amount and ultimately the reactivity of ONOO− formed in vivo (44). Given that ADMA can decrease NO formation and increase
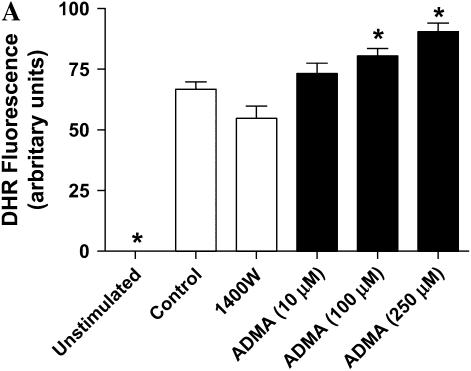
production may be critical in determining the amount and ultimately the reactivity of ONOO− formed in vivo (44). Given that ADMA can decrease NO formation and increase 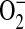 generation, we wished to determine whether ADMA treatment would also result in increased ONOO− formation. Peroxynitrite generation was detected by the oxidation of the nonfluorescent molecule dihydrorhodamine 123 (DHR) to fluorescent rhodamine. Upon stimulation and treatment (24 h) with 100 and 250 μM ADMA, a dose-dependent increase in ONOO− formation compared with stimulated control cells was detectable (Figure 4A). In contrast, no significant increase in ONOO− formation was detected after treatment with 1400W or 10 μM ADMA. DHR oxidation can only be attributed to ONOO− if it is shown to be dependent upon NO and
generation, we wished to determine whether ADMA treatment would also result in increased ONOO− formation. Peroxynitrite generation was detected by the oxidation of the nonfluorescent molecule dihydrorhodamine 123 (DHR) to fluorescent rhodamine. Upon stimulation and treatment (24 h) with 100 and 250 μM ADMA, a dose-dependent increase in ONOO− formation compared with stimulated control cells was detectable (Figure 4A). In contrast, no significant increase in ONOO− formation was detected after treatment with 1400W or 10 μM ADMA. DHR oxidation can only be attributed to ONOO− if it is shown to be dependent upon NO and 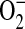 production. Therefore, to confirm that the ONOO− production was dependent upon
production. Therefore, to confirm that the ONOO− production was dependent upon 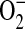 , we also conducted these experiments in the presence of PEG-SOD (44 U/ml), which completely abolished the increased DHR oxidation after treatment with ADMA (Figure 4B). Cellular oxidants other than ONOO−, such as OH− and
, we also conducted these experiments in the presence of PEG-SOD (44 U/ml), which completely abolished the increased DHR oxidation after treatment with ADMA (Figure 4B). Cellular oxidants other than ONOO−, such as OH− and 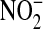 , can react with DHR (36). Therefore, we cannot discount that at least some of increased DHR oxidation may have been due to oxidant radical formation (e.g., OH radical) mediated by
, can react with DHR (36). Therefore, we cannot discount that at least some of increased DHR oxidation may have been due to oxidant radical formation (e.g., OH radical) mediated by 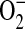 . However, the peroxynitrite anion rapidly decomposes at physiological pH to form free OH− and
. However, the peroxynitrite anion rapidly decomposes at physiological pH to form free OH− and 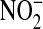 (37), suggesting that there was likely minimal contribution of DHR oxidation from radicals formed independently of ONOO−.
(37), suggesting that there was likely minimal contribution of DHR oxidation from radicals formed independently of ONOO−.
Figure 4.
DHR oxidation in stimulated LA-4 cells treated with ADMA. Cultured LA-4 cells were stimulated for 24 h in the presence of NOS inhibitors and 25 μM DHR and DHR oxidation measured fluorometrically after 24 h. (A) Samples treated with ADMA (10–250 μM) or 1400W (100 μM) (n = 4). (B) Samples treated with ADMA (100 μM) ± PEG-SOD (44 U/ml) (n = 4). Data are represented as percent change from control (stimulated with no treatment). *P < 0.05 versus no PEG-SOD. ΨP < 0.05 versus control.
ADMA Is Capable of Uncoupling Purified iNOS, Resulting in Increased Peroxynitrite Formation
Xia and colleagues have demonstrated that purified iNOS is capable of generating 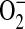 in addition to NO and iNOS-catalyzed
in addition to NO and iNOS-catalyzed 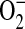 can be significantly decreased by addition of L-arginine (45). To determine whether ADMA could directly affect the function of iNOS, we determined nitrite release as well as
can be significantly decreased by addition of L-arginine (45). To determine whether ADMA could directly affect the function of iNOS, we determined nitrite release as well as 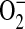 and ONOO− generation by purified iNOS in the presence of increasing concentrations of ADMA. In the presence of 1 mM L-arginine, addition of 10 μM ADMA did not significantly decrease the nitrite accumulation after 2 h (Figure 5A). However, addition of 1 μM and 100 μM ADMA modestly inhibited nitrite formation, while addition of 500 μM ADMA and 1400W (100 μM) inhibited nitrite to levels seen without the addition of L-arginine. To determine whether ADMA could also induce
and ONOO− generation by purified iNOS in the presence of increasing concentrations of ADMA. In the presence of 1 mM L-arginine, addition of 10 μM ADMA did not significantly decrease the nitrite accumulation after 2 h (Figure 5A). However, addition of 1 μM and 100 μM ADMA modestly inhibited nitrite formation, while addition of 500 μM ADMA and 1400W (100 μM) inhibited nitrite to levels seen without the addition of L-arginine. To determine whether ADMA could also induce  production from purified iNOS, DHE oxidation was monitored (Figure 5B). Although the presence of 1400W and 1 μM ADMA did not result in significant increases in
production from purified iNOS, DHE oxidation was monitored (Figure 5B). Although the presence of 1400W and 1 μM ADMA did not result in significant increases in 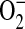 , ADMA at concentrations of 10–500 μM increased DHE oxidation to ∼ 125–130% of control levels. Using DHR fluorescence for detection of ONOO− formation, we found that addition of 10–100 μM ADMA significantly increased ONOO− formation after incubation for 2 h (Figure 5C). Addition of higher concentrations of ADMA resulted in ONOO− levels similar to that seen with 1400W.
, ADMA at concentrations of 10–500 μM increased DHE oxidation to ∼ 125–130% of control levels. Using DHR fluorescence for detection of ONOO− formation, we found that addition of 10–100 μM ADMA significantly increased ONOO− formation after incubation for 2 h (Figure 5C). Addition of higher concentrations of ADMA resulted in ONOO− levels similar to that seen with 1400W.
Figure 5.
Effects of ADMA on nitrite production, DHE fluorescence, and DHR oxidation using purified iNOS. The purified protein reaction system consisted of 1 mM L-arginine, 4 μM tetrahydrobiopterin, 170 μM DTT, 0.5 mM CaCl2, and 10 μg purified iNOS in 50 mM HEPES, pH 7.4. Various concentrations of ADMA (1–500 μM) or 1400W (100 μM) were included in the indicated samples. Samples lacking iNOS or L-arginine were also assessed as controls. The reaction was initiated with the addition of 0.1 mM NADPH and measurements taken after incubation at room temperature for 2 h. (A) Nitrite concentrations of the reactant mixture were assessed using the Griess reaction (n = 4). (B) To assess 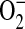 production by purified iNOS, reactions were conducted in the presence of 25 μM DHE and fluorescence monitored specrophotometrically (n = 4). (C) To assess ONOO− production by purified iNOS, reactions were conducted in the presence of 25 μM DHR and fluorescence monitored specrophotometrically (n = 4). *P < 0.05 versus control.
production by purified iNOS, reactions were conducted in the presence of 25 μM DHE and fluorescence monitored specrophotometrically (n = 4). (C) To assess ONOO− production by purified iNOS, reactions were conducted in the presence of 25 μM DHR and fluorescence monitored specrophotometrically (n = 4). *P < 0.05 versus control.
DISCUSSION
Increasing evidence exists for the involvement of 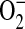 and ONOO− in the pathobiology of inflammatory airway disease. ONOO− is formed by the diffusion-limited reaction of the free radicals NO and
and ONOO− in the pathobiology of inflammatory airway disease. ONOO− is formed by the diffusion-limited reaction of the free radicals NO and 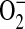 and is a highly reactive compound with various harmful effects on cells (46). It has been demonstrated in a mouse model of allergic inflammation that the development of airway hyperresponsiveness (AHR) is dependent on the release of both
and is a highly reactive compound with various harmful effects on cells (46). It has been demonstrated in a mouse model of allergic inflammation that the development of airway hyperresponsiveness (AHR) is dependent on the release of both 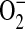 and NO and that the formation of ONOO− is crucial in the development of allergen-induced AHR (47). Moreover, exogenously applied ONOO− induces epithelial damage and AHR in guinea pigs (48), and endogenous ONOO− has pro-contractile actions (49). Studies indicate that in addition to synthesizing NO, iNOS can generate
and NO and that the formation of ONOO− is crucial in the development of allergen-induced AHR (47). Moreover, exogenously applied ONOO− induces epithelial damage and AHR in guinea pigs (48), and endogenous ONOO− has pro-contractile actions (49). Studies indicate that in addition to synthesizing NO, iNOS can generate 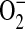 at low concentrations of L-arginine, leading to ONOO−-mediated cellular injury (16, 45). ADMA, a naturally occurring analog of L-arginine, competitively inhibits NO production by NOS. Furthermore, ADMA may uncouple NOS and lead to the generation of
at low concentrations of L-arginine, leading to ONOO−-mediated cellular injury (16, 45). ADMA, a naturally occurring analog of L-arginine, competitively inhibits NO production by NOS. Furthermore, ADMA may uncouple NOS and lead to the generation of 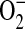 (29). Our findings confirm that ADMA is capable of uncoupling purified iNOS, resulting in both decreased NO production as well as increased
(29). Our findings confirm that ADMA is capable of uncoupling purified iNOS, resulting in both decreased NO production as well as increased 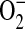 formation. In murine macrophages, depletion of cytosolic L-arginine triggers
formation. In murine macrophages, depletion of cytosolic L-arginine triggers 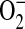 and ONOO− generation from iNOS (16). In the murine airway epithelial cell line LA-4, we have demonstrated that elevated ADMA similarly triggers
and ONOO− generation from iNOS (16). In the murine airway epithelial cell line LA-4, we have demonstrated that elevated ADMA similarly triggers 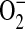 and ONOO− generation from NOS.
and ONOO− generation from NOS.
In our studies, we used LA-4 cells stimulated with LPS, IFN-γ, and TNF-α. This stimulation resulted in a > 13-fold increase in iNOS protein levels. Therefore, we expect that ADMA's action on iNOS accounts for the majority of increased 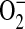 and ONOO− generation. However, we cannot rule out contribution from the two other isoforms of NOS. Indeed, although it is accepted that NO plays a role in asthma, the relative contribution of each of the NOS isoforms to the pathology of this disease remains unclear, as do the effects of altered NO production. NO has been reported to exhibit both beneficial and deleterious effects in asthma.
and ONOO− generation. However, we cannot rule out contribution from the two other isoforms of NOS. Indeed, although it is accepted that NO plays a role in asthma, the relative contribution of each of the NOS isoforms to the pathology of this disease remains unclear, as do the effects of altered NO production. NO has been reported to exhibit both beneficial and deleterious effects in asthma.
In animal models (50) and patients with asthma (51), iNOS protein levels are up-regulated in the airway epithelium, and high concentrations of iNOS-derived NO are thought to be important in the pathophysiology of allergic airway disease. Consequently, the concentration of this molecule in exhaled air is abnormal in activated states of different inflammatory airway diseases. In addition, as discussed above, the production of NO under oxidative stress conditions generates RNS, such as ONOO−, which are strong oxidizing agents that can modulate the development of chronic inflammatory airway diseases. Conversely, it has been well-established that a deficiency of epithelial constitutive NOS-derived NO contributes to allergen-induced AHR in both animal models (52, 53) and patients with asthma (54). In addition to reduced bronchodilation, NO deficiency may induce AHR by promoting airway inflammation (55, 56).
In our study, we found that although ADMA did inhibit NO production in a concentration-dependent manner, it did so weakly compared with the specific iNOS inhibitor 1400W. We cannot discount that at the concentrations utilized in this study, 1400W could have non-specific effects on other NOS isoforms in addition to iNOS. Furthermore, unlike 1400W, which had no significant effect on 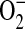 and ONOO− generation, ADMA significantly increased both reactive species. In our model, it is possible that ADMA preferentially inhibited basal NO production by the constitutive NOS isoforms while having a minimal effect on NO production by iNOS.
and ONOO− generation, ADMA significantly increased both reactive species. In our model, it is possible that ADMA preferentially inhibited basal NO production by the constitutive NOS isoforms while having a minimal effect on NO production by iNOS.
The weak inhibition of nitrite production by ADMA was somewhat unexpected given that in smooth muscle cells treated with 1 μM ADMA, nitrite production was reduced to almost baseline levels (21). However, Dowling and coworkers reported that ADMA at concentrations up to 400 μM did not significantly inhibit nitrite formation in an in vitro model of P. aeruginosa infection-induced iNOS expression of respiratory mucosa (19). It is possible that LA-4 cells are somewhat impermeable to uptake of ADMA which could account for the weak inhibition of nitrite production seen in our experiments. However, ADMA has been shown to be effectively transported into cells through the inducible L-arginine transporter CAT-2B (57), suggesting that transport of both L-arginine and ADMA is mediated through the same transporters. Another possible mechanism for the weak inhibition of NO production by ADMA in our system is that the ADMA-metabolizing enzyme dimethylarginine dimethylaminohydrolase (DDAH) activity may be increased after treatment with ADMA. Using Western blotting, we have observed an ∼ 50% increase in DDAH II protein levels in stimulated cells treated with ADMA compared with untreated cells (data not shown), suggesting that increased DDAH activity may reduce intracellular ADMA levels.
The IC50 of ADMA is dependent on the prevailing L-arginine concentration (58). Clinical and experimental evidence suggests elevation of ADMA can cause a relative L-arginine deficiency, even in the presence of normal L-arginine levels. We believe that by addition of exogenous ADMA, we have altered the L-arginine/ADMA balance within the cell. Supplementation with L-arginine has been shown to restore vascular function and to improve the clinical symptoms of various diseases associated with vascular dysfunction (59). L-arginine supplementation has also been explored in inflammatory airway disease. In patients with asthma, oral administration of L-arginine is associated with an increased concentration of NO in exhaled air and an increase in the concentration of L-arginine and nitrate in plasma (60). In vivo, reduced availability of L-arginine in airways of allergen-challenged guinea pigs plays a role in the NO deficiency and subsequent AHR after the early asthmatic reaction (61). To determine whether ADMA exerts the same effects in vivo, it will be important to explore the effects of elevated ADMA in animal models.
The ADMA-induced 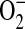 generation in LA-4 cells was scavenged by addition of PEG-SOD, suggesting that the increase in DHE oxidation was due to increased
generation in LA-4 cells was scavenged by addition of PEG-SOD, suggesting that the increase in DHE oxidation was due to increased 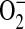 generation. This does not, however, address whether the increased
generation. This does not, however, address whether the increased 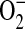 generation was due to increased
generation was due to increased 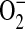 radical flux to the outside of the cell or increased cytosolic production of
radical flux to the outside of the cell or increased cytosolic production of 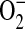 . Using the pegylated form of SOD, we expect that the diminished
. Using the pegylated form of SOD, we expect that the diminished 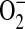 generation was through the action of PEG-SOD on cytosolic
generation was through the action of PEG-SOD on cytosolic 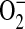 radical flux. However, for this to be true, the PEG-SOD must be internalized by the cells during our experiments. Previous studies have demonstrated that cell uptake of PEG-enzymes likely occurs by both membrane binding and endocytosis (62). Furthermore, these investigators demonstrated that the continual exposure of endothelial cells to PEG-SOD leads to significant augmentation of cellular antioxidant enzymes. Walther and colleagues have also shown that culture of alveolar type II epithelial cells with PEG-SOD resulted in intracellular localization of the enzyme, with maximal uptake during the first 4 h (63). Therefore, we would expect that in experiments in which PEG-SOD was added in the presence or absence of ADMA, at least a portion of the PEG-SOD would be internalized, resulting in the inhibition of the oxidation of cytosolic DHE by
radical flux. However, for this to be true, the PEG-SOD must be internalized by the cells during our experiments. Previous studies have demonstrated that cell uptake of PEG-enzymes likely occurs by both membrane binding and endocytosis (62). Furthermore, these investigators demonstrated that the continual exposure of endothelial cells to PEG-SOD leads to significant augmentation of cellular antioxidant enzymes. Walther and colleagues have also shown that culture of alveolar type II epithelial cells with PEG-SOD resulted in intracellular localization of the enzyme, with maximal uptake during the first 4 h (63). Therefore, we would expect that in experiments in which PEG-SOD was added in the presence or absence of ADMA, at least a portion of the PEG-SOD would be internalized, resulting in the inhibition of the oxidation of cytosolic DHE by 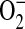 .
.
It has been previously demonstrated in macrophages that iNOS is capable of generating functionally important levels of 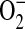 under conditions of L-arginine depletion. Furthermore, L-arginine depletion results production of both
under conditions of L-arginine depletion. Furthermore, L-arginine depletion results production of both 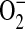 and NO by iNOS, leading to ONOO− formation, suggesting that NOS itself can be a significant source of cellular ONOO− production (16). In a study examining the mechanisms by which excess NO or
and NO by iNOS, leading to ONOO− formation, suggesting that NOS itself can be a significant source of cellular ONOO− production (16). In a study examining the mechanisms by which excess NO or 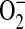 production inhibits peroxynitrite-mediated oxidation reactions, Jourd'heuil and coworkers determined that the efficiency of DHR oxidation by ONOO− was increased from ∼ 40% at equimolar fluxes of NO and
production inhibits peroxynitrite-mediated oxidation reactions, Jourd'heuil and coworkers determined that the efficiency of DHR oxidation by ONOO− was increased from ∼ 40% at equimolar fluxes of NO and 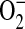 to almost 100% upon generation of a 2-fold excess of NO over
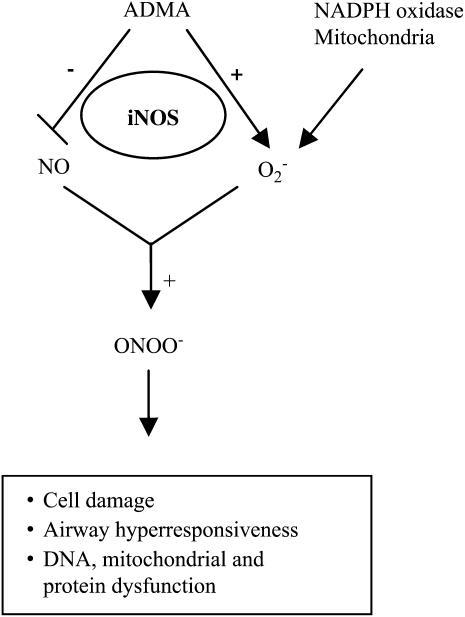
to almost 100% upon generation of a 2-fold excess of NO over 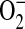 and then decreased at higher NO fluxes (37). On the basis of these data and the findings reported here, we have developed a model describing a role for ADMA in the formation of ONOO− by epithelial cells (Figure 6). In this model, elevated ADMA competitively inhibits NOS binding to its substrate L-arginine, resulting in decreased NO production and increased
and then decreased at higher NO fluxes (37). On the basis of these data and the findings reported here, we have developed a model describing a role for ADMA in the formation of ONOO− by epithelial cells (Figure 6). In this model, elevated ADMA competitively inhibits NOS binding to its substrate L-arginine, resulting in decreased NO production and increased 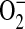 formation. Although the data presented here suggest that increased
formation. Although the data presented here suggest that increased 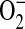 formation in LA-4 cells was predominantly due to iNOS uncoupling, other sources of
formation in LA-4 cells was predominantly due to iNOS uncoupling, other sources of 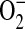 , including mitochondria and NADPH oxidases, may also contribute to elevated
, including mitochondria and NADPH oxidases, may also contribute to elevated 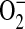 formation in this model. Therefore, we propose that in concert with other cellular sources of
formation in this model. Therefore, we propose that in concert with other cellular sources of 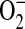 , including mitochondria and NADPH oxidases, increased
, including mitochondria and NADPH oxidases, increased 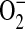 production by ADMA-induced iNOS uncoupling can lead to conditions favoring increased intracellular ONOO− production. This increase in ONOO− may contribute to ONOO−-mediated oxidation of cellular proteins leading to cell damage and dysfunction.
production by ADMA-induced iNOS uncoupling can lead to conditions favoring increased intracellular ONOO− production. This increase in ONOO− may contribute to ONOO−-mediated oxidation of cellular proteins leading to cell damage and dysfunction.
Figure 6.
Model for the role of ADMA in the formation of ONOO− by epithelial cells. In this model, elevated ADMA is responsible for decreased NO production and increased 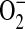 production, resulting in conditions favoring the formation of ONOO−. Additional cellular
production, resulting in conditions favoring the formation of ONOO−. Additional cellular 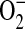 sources such as NADPH oxidases and mitochondria, can also contribute to the
sources such as NADPH oxidases and mitochondria, can also contribute to the 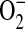 production in the cell.
production in the cell.
Although ADMA has been established as a CV risk factor and its accumulation has been reported in a wide range of CV and other disorders (22, 64–66), there are currently no published reports of the effects of ADMA accumulation in inflammatory airway diseases such as asthma. Experimental and clinical evidence indicates that even small modifications of ADMA concentrations significantly change vascular NO production, vascular tone, and systemic vascular resistance (30). Our findings suggest that alterations in circulating ADMA may also contribute to the pathobiology of airway disease by exacerbating the production of ROS and RNS by airway epithelial cells. The fundamental mechanisms driving the altered NO bioactivity and ONOO− formation under pathologic conditions still need to be fully clarified, as regulating the production of these molecules may serve as a novel target in the prevention and treatment of chronic inflammatory airway diseases.
This work was supported by Grant Number P20RR017670 (A.H.) from the National Center for Research Resources (NCRR), and Grant Number 1F32HL086154 (S.M.W.) from the National Heart, Lung, and Blood Institute (NHLBI), both components of the National Institutes of Health (NIH). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NCRR, NHLBI, or NIH.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0302SM on December 7, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 1994;149:538–551. [DOI] [PubMed] [Google Scholar]
- 2.Zapol WM, Rimar S, Gillis N, Marletta M, Bosken CH. Nitric oxide and the lung. Am J Respir Crit Care Med 1994;149:1375–1380. [DOI] [PubMed] [Google Scholar]
- 3.Mayer B, Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem Sci 1997;22:477–481. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 1996;271:C1424–C1437. [DOI] [PubMed] [Google Scholar]
- 5.Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol 1993;9:371–377. [DOI] [PubMed] [Google Scholar]
- 6.Asano K, Chee CB, Gaston B, Lilly CM, Gerard C, Drazen JM, Stamler JS. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci USA 1994;91:10089–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer A, Mundel P, Mayer B, Preissler U, Philippin B, Kummer W. Nitric oxide synthase in guinea pig lower airway innervation. Neurosci Lett 1993;149:157–160. [DOI] [PubMed] [Google Scholar]
- 8.Tucker JF, Brave SR, Charalambous L, Hobbs AJ, Gibson A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol 1990;100:663–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins DN, Peroni DJ, Basclain KA, Garlepp MJ, Thompson PJ. Expression and activity of nitric oxide synthases in human airway epithelium. Am J Respir Cell Mol Biol 1997;16:629–639. [DOI] [PubMed] [Google Scholar]
- 10.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem 1992;267:24173–24176. [PubMed] [Google Scholar]
- 11.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 1998;273:25804–25808. [DOI] [PubMed] [Google Scholar]
- 12.Klatt P, Schmidt K, Uray G, Mayer B. Multiple catalytic functions of brain nitric oxide synthase. Biochemical characterization, cofactor-requirement, and the role of N omega-hydroxy-L-arginine as an intermediate. J Biol Chem 1993;268:14781–14787. [PubMed] [Google Scholar]
- 13.Pritchard KA Jr, Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res 1995;77:510–518. [DOI] [PubMed] [Google Scholar]
- 14.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 1998;95:9220–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA 1996;93:6770–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci USA 1997;94:6954–6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducrocq C, Blanchard B, Pignatelli B, Ohshima H. Peroxynitrite: an endogenous oxidizing and nitrating agent. Cell Mol Life Sci 1999;55:1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persinger RL, Poynter ME, Ckless K, Janssen-Heininger YM. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol Cell Biochem 2002;234–235:71–80. [PubMed] [Google Scholar]
- 19.Dowling RB, Newton R, Robichaud A, Cole PJ, Barnes PJ, Wilson R. Effect of inhibition of nitric oxide synthase on Pseudomonas aeruginosa infection of respiratory mucosa in vitro. Am J Respir Cell Mol Biol 1998;19:950–958. [DOI] [PubMed] [Google Scholar]
- 20.Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol 1992;20:S60–S62. [DOI] [PubMed] [Google Scholar]
- 21.Ueda S, Kato S, Matsuoka H, Kimoto M, Okuda S, Morimatsu M, Imaizumi T. Regulation of cytokine-induced nitric oxide synthesis by asymmetric dimethylarginine: role of dimethylarginine dimethylaminohydrolase. Circ Res 2003;92:226–233. [DOI] [PubMed] [Google Scholar]
- 22.Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 1998;98:1842–1847. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, Imaizumi T. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation 1999;99:1141–1146. [DOI] [PubMed] [Google Scholar]
- 24.Pullamsetti S, Kiss L, Ghofrani HA, Voswinckel R, Haredza P, Klepetko W, Aigner C, Fink L, Muyal JP, Weissmann N, et al. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. FASEB J 2005;19:1175–1177. [DOI] [PubMed] [Google Scholar]
- 25.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 2004;18:1746–1748. [DOI] [PubMed] [Google Scholar]
- 26.Bulau P, Zakrzewicz D, Kitowska K, Leiper J, Gunther A, Grimminger F, Eickelberg O. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am J Physiol Lung Cell Mol Physiol 2007;292:L18–L24. [DOI] [PubMed] [Google Scholar]
- 27.Meurs H, Maarsingh H, Zaagsma J. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends Pharmacol Sci 2003;24:450–455. [DOI] [PubMed] [Google Scholar]
- 28.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev 2004;84:731–765. [DOI] [PubMed] [Google Scholar]
- 29.Boger RH, Bode-Boger SM, Tsao PS, Lin PS, Chan JR, Cooke JP. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J Am Coll Cardiol 2000;36:2287–2295. [DOI] [PubMed] [Google Scholar]
- 30.Boger RH. Asymmetric dimethylarginine (ADMA) modulates endothelial function–therapeutic implications. Vasc Med 2003;8:149–151. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt HHH, Kelm M. Determination of nitrite and nitrate by the Griess reaction. In: Feelisch M, Stamler JJ, editors. Methods in nitric oxide research. London: Wiley; 1996. pp. 491–497.
- 32.Krajewski S, Zapata JM, Reed JC. Detection of multiple antigens on western blots. Anal Biochem 1996;236:221–228. [DOI] [PubMed] [Google Scholar]
- 33.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 2004;287:C895–C902. [DOI] [PubMed] [Google Scholar]
- 34.Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med 1998;25:826–831. [DOI] [PubMed] [Google Scholar]
- 35.Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 1994;16:149–156. [DOI] [PubMed] [Google Scholar]
- 36.Wrona M, Patel K, Wardman P. Reactivity of 2',7'-dichlorodihydrofluorescein and dihydrorhodamine 123 and their oxidized forms toward carbonate, nitrogen dioxide, and hydroxyl radicals. Free Radic Biol Med 2005;38:262–270. [DOI] [PubMed] [Google Scholar]
- 37.Jourd'heuil D, Jourd'heuil FL, Kutchukian PS, Musah RA, Wink DA, Grisham MB. Reaction of superoxide and nitric oxide with peroxynitrite: implications for peroxynitrite-mediated oxidation reactions in vivo. J Biol Chem 2001;276:28799–28805. [DOI] [PubMed] [Google Scholar]
- 38.Muijsers RB, van Den Worm E, Folkerts G, Beukelman CJ, Koster AS, Postma DS, Nijkamp FP. Apocynin inhibits peroxynitrite formation by murine macrophages. Br J Pharmacol 2000;130:932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins RA, Barnes PJ, Springall DR, Warren JB, Kwon OJ, Buttery LD, Wilson AJ, Geller DA, Polak JM. Expression of inducible nitric oxide in human lung epithelial cells. Biochem Biophys Res Commun 1994;203:209–218. [DOI] [PubMed] [Google Scholar]
- 40.Paludan SR. Synergistic action of pro-inflammatory agents: cellular and molecular aspects. J Leukoc Biol 2000;67:18–25. [DOI] [PubMed] [Google Scholar]
- 41.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ Res 2001;89:224–236. [DOI] [PubMed] [Google Scholar]
- 42.Budd SL, Castilho RF, Nicholls DG. Mitochondrial membrane potential and hydroethidine-monitored superoxide generation in cultured cerebellar granule cells. FEBS Lett 1997;415:21–24. [DOI] [PubMed] [Google Scholar]
- 43.Green DR, Reed JC. Mitochondria and apoptosis. Science 1998;281:1309–1312. [DOI] [PubMed] [Google Scholar]
- 44.Miles AM, Bohle DS, Glassbrenner PA, Hansert B, Wink DA, Grisham MB. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem 1996;271:40–47. [DOI] [PubMed] [Google Scholar]
- 45.Xia Y, Roman LJ, Masters BS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem 1998;273:22635–22639. [DOI] [PubMed] [Google Scholar]
- 46.Muijsers RB, Folkerts G, Henricks PA, Sadeghi-Hashjin G, Nijkamp FP. Peroxynitrite: a two-faced metabolite of nitric oxide. Life Sci 1997;60:1833–1845. [DOI] [PubMed] [Google Scholar]
- 47.Muijsers RB, van Ark I, Folkerts G, Koster AS, van Oosterhout AJ, Postma DS, Nijkamp FP. Apocynin and 1400 W prevents airway hyperresponsiveness during allergic reactions in mice. Br J Pharmacol 2001;134:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadeghi-Hashjin G, Folkerts G, Henricks PA, Muijsers RB, Nijkamp FP. Peroxynitrite in airway diseases. Clin Exp Allergy 1998;28:1464–1473. [DOI] [PubMed] [Google Scholar]
- 49.de Boer J, Meurs H, Flendrig L, Koopal M, Zaagsma J. Role of nitric oxide and superoxide in allergen-induced airway hyperreactivity after the late asthmatic reaction in guinea-pigs. Br J Pharmacol 2001;133:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu SF, Haddad EB, Adcock I, Salmon M, Koto H, Gilbey T, Barnes PJ, Chung KF. Inducible nitric oxide synthase after sensitization and allergen challenge of Brown Norway rat lung. Br J Pharmacol 1997;121:1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo FH, Comhair SA, Zheng S, Dweik RA, Eissa NT, Thomassen MJ, Calhoun W, Erzurum SC. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol 2000;164:5970–5980. [DOI] [PubMed] [Google Scholar]
- 52.De Boer J, Pouw FM, Zaagsma J, Meurs H. Effects of endogenous superoxide anion and nitric oxide on cholinergic constriction of normal and hyperreactive guinea pig airways. Am J Respir Crit Care Med 1998;158:1784–1789. [DOI] [PubMed] [Google Scholar]
- 53.Ten Broeke R, De Crom R, Van Haperen R, Verweij V, Leusink-Muis TA, Van Ark I, De Clerck F, Nijkamp FP, Folkerts G. Overexpression of endothelial nitric oxide synthase suppresses features of allergic asthma in mice. Respir Res 2006;7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricciardolo FL, Timmers MC, Geppetti P, van Schadewijk A, Brahim JJ, Sont JK, de Gouw HW, Hiemstra PS, van Krieken JH, Sterk PJ. Allergen-induced impairment of bronchoprotective nitric oxide synthesis in asthma. J Allergy Clin Immunol 2001;108:198–204. [DOI] [PubMed] [Google Scholar]
- 55.Colasanti M, Suzuki H. The dual personality of NO. Trends Pharmacol Sci 2000;21:249–252. [DOI] [PubMed] [Google Scholar]
- 56.Thomassen MJ, Buhrow LT, Connors MJ, Kaneko FT, Erzurum SC, Kavuru MS. Nitric oxide inhibits inflammatory cytokine production by human alveolar macrophages. Am J Respir Cell Mol Biol 1997;17:279–283. [DOI] [PubMed] [Google Scholar]
- 57.Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide 1997;1:65–73. [DOI] [PubMed] [Google Scholar]
- 58.MacAllister RJ, Whitley GS, Vallance P. Effects of guanidino and uremic compounds on nitric oxide pathways. Kidney Int 1994;45:737–742. [DOI] [PubMed] [Google Scholar]
- 59.Boger RH, Ron ES. L-Arginine improves vascular function by overcoming deleterious effects of ADMA, a novel cardiovascular risk factor. Altern Med Rev 2005;10:14–23. [PubMed] [Google Scholar]
- 60.Sapienza MA, Kharitonov SA, Horvath I, Chung KF, Barnes PJ. Effect of inhaled L-arginine on exhaled nitric oxide in normal and asthmatic subjects. Thorax 1998;53:172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boer J, Duyvendak M, Schuurman FE, Pouw FM, Zaagsma J, Meurs H. Role of L-arginine in the deficiency of nitric oxide and airway hyperreactivity after the allergen-induced early asthmatic reaction in guinea-pigs. Br J Pharmacol 1999;128:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beckman JS, Minor RL Jr, White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem 1988;263:6884–6892. [PubMed] [Google Scholar]
- 63.Walther FJ, Wade AB, Warburton D, Forman HJ. Augmentation of superoxide dismutase and catalase activity in alveolar type II cells. Am J Respir Cell Mol Biol 1991;4:364–368. [DOI] [PubMed] [Google Scholar]
- 64.Boger RH, Bode-Boger SM, Thiele W, Creutzig A, Alexander K, Frolich JC. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol 1998;32:1336–1344. [DOI] [PubMed] [Google Scholar]
- 65.Gorenflo M, Zheng C, Werle E, Fiehn W, Ulmer HE. Plasma levels of asymmetrical dimethyl-L-arginine in patients with congenital heart disease and pulmonary hypertension. J Cardiovasc Pharmacol 2001;37:489–492. [DOI] [PubMed] [Google Scholar]
- 66.Usui M, Matsuoka H, Miyazaki H, Ueda S, Okuda S, Imaizumi T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci 1998;62:2425–2430. [DOI] [PubMed] [Google Scholar]