Abstract
Transforming growth factor-β1 (TGF-β1) has been implicated as a major negative regulator of lung branching morphogenesis. Since connective tissue growth factor (CTGF) is a downstream mediator of TGF-β1 effects on mesenchymal cells, we hypothesized that TGF-β1 induces CTGF expression in mouse embryonic lung explants and that CTGF mediates TGF-β1 inhibition of branching morphogenesis. We show that addition of TGF-β1 to the serum-free medium of embryonic day (E)12.5 lung explant cultures inhibited branching morphogenesis and induced CTGF mRNA expression in time- and dose-dependent manners. In contrast to basal endogenous CTGF protein, which was exclusively localized in the distal airway epithelium, TGF-β1–induced CTGF protein was localized in both the epithelium and the mesenchyme. Addition of exogenous CTGF to culture medium directly inhibited branching morphogenesis. To identify the signal transduction pathway through which TGF-β1 induces CTGF, we used SB431542, a specific inhibitor for TGF-β type I receptor (TβRI)/ALK-5 to block TGF-β1–induced Smad2/3 phosphorylation. Consequently, SB431542 stimulated normal branching morphogenesis and blocked TGF-β1 inhibition of branching. Furthermore, SB-431542 blocked both endogenous and TGF-β1–induced expression of CTGF mRNA and protein. These results demonstrate for the first time that TGF-β1 induces CTGF expression in mouse embryonic lung explants, that CTGF inhibits branching morphogenesis, and that both endogenous and TGF-β1–induced CTGF expression are mediated by the TβRI/ALK-5–dependent Smad2 signaling pathway.
Keywords: connective tissue growth factor, TGF-β, 1, ALK-5, Smad2, lung
CLINICAL RELEVANCE
This research will enhance our knowledge of the regulation of lung branching morphogenesis. It may also provide better understanding of lung diseases that are caused by abnormal development and repair processes.
Normal lung development is highly coordinated by autocrine/paracrine signaling, and by cell–extracellular matrix (ECM) and cell–cell interactions between the epithelium and mesenchyme (1–3). Peptide growth factors and transcriptional factors play an important role during the processes of lung branching morphogenesis (4). Small changes in the temporal or spatial expression of growth factors or transcriptional factors can lead to significant alterations in the final architecture of the lung and severe defects in the pulmonary function.
Multiple lines of evidence have indicated that TGF-βs are key negative regulators for lung branching morphogenesis. TGF-βs belong to a family of closely related peptides, including TGF-β1, TGF-β2, and TGF-β3. TGF-β1, often referred to simply as TGF-β, is the most extensively studied member of the family (5–7). TGF-βs initiate their cellular action by binding to the two cell membrane receptors termed type I (TβRI) and type II (TβRII) receptors. Activin receptor-like kinase 5 (ALK-5) is the principal TβRI that mediates most cellular responses to TGF-βs (6–8). Upon ligand binding, constitutively active TβRII kinase phosphorylates TβRI/ALK-5 which, in turn, activates the downstream signal transduction cascades. ALK-5–activated Smad2 and Smad3 phosphorylation is the most prominent pathway (7). Once activated, Smad2/3 associate with Smad4 and translocate to the nucleus, where the complex transcriptionally regulates the expression of the target genes.
TGF-βs and their receptors are expressed in the developing lung (9–12). In situ hybridization studies have demonstrated prominent expression of TGF-β1 mRNA in the mesenchyme during embryonic lung development (9, 12). Abrogation of TβRII or Smad2 and Smad3 signaling pathways stimulates branching morphogenesis in cultured mouse embryonic lung explants, suggesting that endogenous TGF-β signaling negatively regulates lung branching (13, 14). Studies using embryonic lung explant cultures have demonstrated that addition of exogenous TGF-βs to the culture medium inhibits branching morphogenesis (15, 16). In a transgenic mouse model, overexpression of TGF-β1 in the distal lung epithelial cells with the surfactant protein C (SP-C) promoter arrests lung development at the late pseudoglandular stage (17, 18). In both embryonic lung explant culture and transgenic mouse models, TGF-β1 increases production of ECM and results in ectopic accumulation of α smooth muscle actin (α-SMA), a marker for myofibroblast differentiation in the distal mesenchyme (12, 17). These molecular alterations are thought to play a role in TGF-β inhibition of branching morphogenesis.
Connective tissue growth factor (CTGF) is a member of the CCN (CTGF/Fisp12, Cyr61/Cef10, Nov, WISP-1, WISP-2, and WISP-3) family of early gene products with a high degree of amino acid sequence homology and 38 conserved cysteine residues (19–21). The CCN family members exhibit multiple activities including stimulation of extracellular matrix (ECM) production, fibroblast proliferation and migration, and myofibroblast differentiation (22–24). Studies have suggested that CTGF plays an important role during embryogenesis. CTGF is expressed in a variety of tissues and organs during development (25–27). Disruption of the CTGF gene in the mouse embryo results in skeletal dysmorphisms and death shortly after birth due to respiratory distress, suggesting lung developmental defect (28). We have studied the role of CTGF in mouse embryonic lung morphogenesis (29). Our results demonstrated that CTGF expresses in the epithelial cells of terminal buds during the pseudoglandular stage and that peak expression of CTGF correlates with the termination of branching and induction of sacculation at embryonic day (E)16.5. The lungs of CTGF-null mice presented with sacculation defect (unpublished data). Using in vitro embryonic lung explant cultures, we showed that addition of recombinant CTGF to the culture medium reduces branching morphogenesis (29). Our data suggest that CTGF may play an important role in the termination of branching morphogenesis.
The biological importance of CTGF as a downstream mediator of TGF-β effects on mesenchymal cells is highlighted by numerous observations. TGF-β1 selectively up-regulates CTGF gene expression in mesenchymal cell types such as fibroblasts via a unique TGF-β response element present in the CTGF promoter (30–32). TGF-β1 stimulation of fibroblast proliferation, collagen synthesis, and myofibroblast differentiation is mediated via a CTGF-dependent pathway (24, 32–34). Although TGF-β1 is one of the major inducers of CTGF expression in cultured fibroblasts, regulation of CTGF expression by the TGF-β1 in the embryonic lungs has not yet been studied.
The objective of this study was to investigate whether TGF-β1 induces CTGF expression in mouse embryonic lung explants and to identify the downstream signaling pathway involved in the endogenous and TGF-β1–induced CTGF expression during branching morphogenesis. We demonstrate that TGF-β1 inhibits branching and induces CTGF expression in mouse E12.5 lung explants. Furthermore, inhibition of TβRI/ALK-5 activity by a specific synthetic inhibitor, SB431542 (35), blocks endogenous and TGF-β1–induced CTGF expression. We propose that ALK-5–dependent Smad2 activation plays a key role in endogenous and TGF-β1–induced CTGF expression in lung development.
MATERIALS AND METHODS
Materials
ICR strain mice were purchased from Harlan (Indianapolis, IN). F12/Dulbecco's modified Eagle's medium (DMEM) tissue culture media, bovine serum albumin (BSA), gentamicin, transferrin, Trizol reagents, and first-strand cDNA synthesis kits were obtained from Invitrogen (Carsbad, CA). Recombinant CTGF was prepared using a baculovirus expression system as previously described (33). Recombinant TGF-β1 was purchased from R&D Systems (Minneapolis, MN). SB431542 was from Tocris Bioscience (Ellisville, MO). PCR kits for semiquantitative RT-PCR were purchased from Applied Biosystems (Foster City, CA). Primers and reagents for quantitative real-time RT-PCR were obtained from Supperarray Bioscience Corp. (Frederick, MD). Cycloheximide and a mouse monoclonal anti–β-actin antibody were from Sigma (St. Louis, MO). A rabbit polyclonal anti-CTGF antibody was from Torrey Pines Biolabs (Houston, TX). Rabbit polyclonal anti-pro–SP-C and anti-phosphospecific Smad2 (p-Smad2) antibodies were from Chemicon (Temecula, CA). Goat polyclonal anti-CTGF and total anti-Smad2/3 antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Biotinylated anti-rabbit IgG, horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-goat IgGs, streptavidin–alkaline phosphatase reagent, and Vector Red substrate were obtained from Vector Laboratories (Burlingame, CA). Enhanced chemiluminescence (ECL) reagents were from Amersham (Piscataway, NJ).
Breeding of Mice, Embryonic Lung Dissection, and Explant Culture
The study protocol was reviewed and approved by the Animal Care and Use Committee of the University of Miami. Eight-week-old female mice were mated to males. Noon on the day of vaginal plug formation was set as E0.5. On E12.5, pregnant females were killed by cervical dislocation after receiving CO2 narcosis. Embryos were obtained by hysterectomy. Lungs were dissected under dissection microscope and placed individually on 8-μm nucleopore membranes floating on 0.5 ml of chemical-defined serum-free medium in each well of a 24-well plate. The medium consisted of 1:1 mixture of Ham's F12 and DMEM with 1 μg/ml of BSA, 10 μg/ml of transferrin, and 50 μg/ml of gentamicin (15). Recombinant CTGF (5–250 ng/ml) or TGF-β1 (1–100 ng/ml) was added to the culture medium of lung explants. Cycloheximide (5 μM) or SB43152 (0.2–20 μM) was added to culture medium 1 h before the addition of TGF-β1. The cultures were maintained at 37°C in a humidified 5% CO2 incubator for 2–48 h.
Quantification of Branching Morphogenesis
Branching morphogenesis was measured as the number of the terminal sacs around the circumference of the lung explants. Terminal sacs were counted under the microscope before administration of inhibitors or growth factors (baseline) and daily after treatment for 48 h. Lung explants in culture on the nucleopore membranes were photographed daily using an Olympus digital camera (Olympus Optical Co., Tokyo, Japan) attached to an inverted microscope for permanent imaging analysis. Five to six lung explants were used for each condition, and individual experiments were repeated at least three times.
RT-PCR
Total RNA was isolated from pooled lung explants by using Trizol reagent. Two micrograms of total RNA was reverse-transcribed in a 20-μl reaction by using a first-strand cDNA synthesis kit according to manufacture's protocol (Invitrogen). PCR was performed using 1 μl of first-strand cDNA and gene-specific primers. Using mouse CTGF forward primer 5′-CTGGACGGCTGCGGCTGCTG-3′ (positions 285–304) and reverse primer 5′-GGTCCTTGGGCTCGTCACAC-3′ (positions 629–648) yielded a CTGF fragment of 363 bp. As an internal control, GAPDH primers were also included in each PCR reaction. GAPDH primers were: forward, 5′-ACCACAGTCCATGCCATCAC-3′ (position 593–613); reverse, 5′-TCCACCACCCTGTTGATGTA-3′ (1090–1110) that produce a GAPDH fragment of 497 bp. The reactions included denaturing for 30 s at 94°C, annealing at 55°C for 30 s, and extension for 30 s at 72°C for 30 cycles followed by further extension for 5 min at 72°C. The 30 cycles were chosen to be in the linear amplification phase. DNA contamination was excluded by performing PCR on each sample without first transcribing mRNA with reverse transcriptase. Negative control was performed by substituting 1 μl of H2O for cDNA in a PCR reaction. The amplified cDNA fragments were then separated on 2% agarose gels and visualized by ethidium bromide staining. The intensity of the cDNA products was determined by Quantity One Imaging Analysis Program (Bio-Rad, Hercules, CA). The relative mRNA levels of CTGF were determined after normalization to GAPDH.
Quantitative Real-Time RT-PCR
The real-time RT-PCR was performed on a Light-cycler (Roche, Indianapolis, IN). Each reaction included diluted first-strand cDNA generated as above, mouse CTGF or mouse GAPDH primers, and RT2 Real-Time PCR SYBR Green master mix according to the manufacturer's instruction (Superarray Bioscience). Real-time RT-PCR conditions were 95°C for 15 min, followed by 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. RNase-free water was used as a negative control. For each target gene, a standard curve was established by performing a series of 2-fold dilutions of the first-strand cDNA. The mRNA expression levels of CTGF or GAPDH were determined from the standard curve. The relative CTGF mRNA levels were normalized to GAPDH.
Western Blot Analysis
Total protein was extracted from pooled lung explants with a lysis buffer from Active Motif according to manufacturer's protocol (Carlsbad, CA). The protein concentrations were measured by BCA protein assay using a commercial kit from Pierce Biotechnology, Inc. (Rockford, IL). Seventy-five micrograms of total protein was fractionated by SDS-PAGE on a 10% Tris-glycine precast gel (Bio-Rad, Richmond, CA) and then transferred to a nitrocellulose membrane (Amersham). The membrane was incubated with a goat anti-CTGF antibody (Santa Cruz), a mouse anti–β-actin antibody (Sigma), a rabbit anti-p-Smad2 antibody (Chemicon) or a goat anti-total Smad2/3 antibody (Santa Cruz) overnight at 4°C and then incubated for 1 h at room temperature with an HRP-conjugated secondary antibody raised against respective primary antibodies. Antibody-bound proteins were detected using ECL chemiluminescence methodology. The intensities of protein bands were quantified by Quantity One Imaging Analysis Program. The relative protein levels of CTGF were normalized to β-actin, and p-Smad2 levels were normalized to total Smad2/3.
Immunohistochemistry
Lung explants were fixed with 4% paraformaldehyde in PBS at 4°C for 16 h, then dehydrated through series of methanol and embedded in paraffin, and 5-μm sections were prepared. Expression of CTGF protein was localized by immunostaining with a rabbit anti-CTGF antibody (Torrey Pines Biolabs). A rabbit anti-pro–SP-C antibody was used to detect the epithelial cells of distal lung buds. Nonimmune rabbit IgG was used as a negative control. Tissue sections were deparaffinized in xylene and rehydrated through graded ethanol into PBS. The sections were incubated with blocking buffer (2% milk plus 10% horse serum) for 1 h at room temperature. The blocking buffer was removed from the slides, and the tissue sections were then incubated with either the primary antibodies or the negative control antibody for 16 h at 4°C. The slides were washed with Tris-buffered saline (TBS), and incubated with biotinylated secondary IgGs for 1 h at room temperature. After washing with TBS, the slides were incubated with a streptavidin-biotin-alkaline phosphatase complex for 45 min at room temperature. The slides were then washed with TBS again, and incubated with Vector Red alkaline phosphatase substrate, mounted and coverslipped.
Data Presentation and Statistical Analysis
Results are expressed as means ± SD. Comparison was performed by one-way ANOVA followed by Student-Newman-Keuls test. P < 0.05 was considered significant.
RESULTS
TGF-β1 Inhibits Branching Morphogenesis in E12.5 Lung Explants
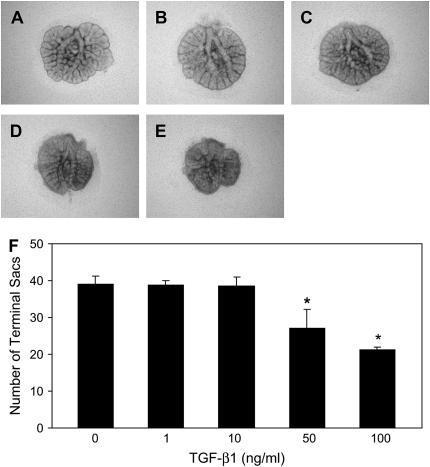
Previous studies have demonstrated that addition of TGF-β1 to the culture medium of mouse E11.5 lung explants inhibits branching morphogenesis (15). In this study, E12.5 lung explants were used to assess the effect of TGF-β1 on branching morphogenesis. TGF-β1 decreased lung size and inhibited terminal branching in a dose- and time-dependent manner. TGF-β1 at concentration 1 or 10 ng/ml did not affect lung size, but did decrease lung size at concentrations of 50 and 100 ng/ml. While the decrease in lung size was apparent at 24 h (data not shown), maximal reductions were seen at 48 h (Figures 1A–1E). TGF-β1 at concentration 1 and 10 ng/ml did not cause branching inhibition at 24 or 48 h. However, at concentrations 50 and 100 ng/ml, TGF-β1 significantly decreased terminal branching by 17% and 27% at 24 h (data not shown), and by 45% and 50% at 48 h, respectively (Figure 1F).
Figure 1.
TGF-β1 reduces branching morphogenesis in mouse E12.5 lung explants. E12.5 lung explants were treated with increasing concentration of TGF-β1 for 48 h as described in Materials and Methods. Photomicrographs were taken under the same magnification at 48 h. (A) Control lung explant; lung explants treated with TGF-β1 1 ng/ml (B), 10 ng/ml (C), 50 ng/ml (D), and 100 ng/ml (E). (F) Graphical representation of the quantification of terminal branching after cultured for 48 h. Each bar represents the mean ± SD from six lung explants. TGF-β1 dose-dependently inhibited branching morphogenesis. *P < 0.001 compared with control.
CTGF mRNA Is Induced in the Presence or Absence of TGF-β1
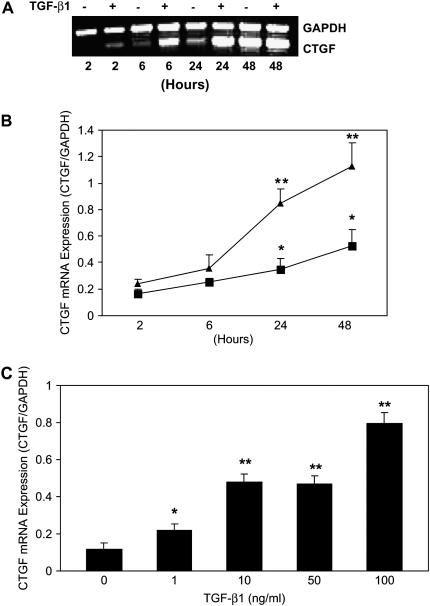
To determine endogenous expression of CTGF mRNA and the effect of TGF-β1 on CTGF mRNA expression, E12.5 lung explants were cultured for 2–48 h in the presence or absence of TGF-β1 (100 ng/ml). Expression of CTGF mRNA was detected by semiquantitative RT-PCR and quantitative real-time RT-PCR. The semiquantitative RT-PCR and quantitative real-time RT-PCR with different sets of primers yielded similar results. As shown in Figures 2A and 2B, CTGF mRNA was detected with increasing levels in control E12.5 lung explants in a time-dependent manner. When compared with 2 h control, endogenous CTGF mRNA was increased 2.1-fold at 24 h and more than 3-fold at 48 h. These results indicate that as lung explants undergo branching morphogenesis in culture, they increase their endogenous CTGF mRNA expression. Treatment with TGF-β1 induced a time-dependent greater CTGF mRNA expression in the E12.5 lung explants. Compared with 2 h control, treatment with 100 ng/ml of TGF-β1 up-regulated CTGF mRNA expression 5.1- to 6.8-fold from 24 to 48 h. To determine the dose–response of TGF-β1 induction of CTGF mRNA expression, E12.5 explants were treated with different concentrations (1–100 ng/ml) of TGF-β1 for 24 h. Expression of CTGF mRNA was increased 1.9- to 6.8-fold by 1–100 ng/ml of TGF-β1 (Figure 2C). The maximal induction was achieved by the highest dose (100 ng/ml) tested. Thus, TGF-β1 induced rapid and sustained CTGF mRNA expression during early embryonic lung development.
Figure 2.
Endogenous and TGF-β1–induced CTGF mRNA expression in E12.5 lung explants. E12.5 lung explants were incubated with medium (control) or with 100 ng/ml TGF-β1 for 2 to 48 h. CTGF and GAPDH mRNA were detected by (A) semiquantitative RT-PCR and (B) quantitative real-time RT-PCR. Relative CTGF mRNA expression levels were determined after normalization to GAPDH. Data are expressed as mean ± SD from three separate experiments. Endogenous CTGF mRNA was induced in control lung explants after cultured for 24–48 h. *P < 0.04 compared with 2 h control. TGF-β1 time-dependently induced CTGF mRNA expression in E12.5 lung explants. **P < 0.001 compared with corresponding controls. Triangles, TGF; squares, control. (C) E12.5 lung explants were treated with 1–100 ng/ml TGF-β1 for 24 h. CTGF mRNA levels were analyzed and quantified as described above. TGF-β1 dose-dependently induced CTGF mRNA expression in E12.5 lung explants. *P < 0.05 compared with control; **P < 0.001 compared with control.
CTGF Protein Is Induced in the Presence or Absence of TGF-β1
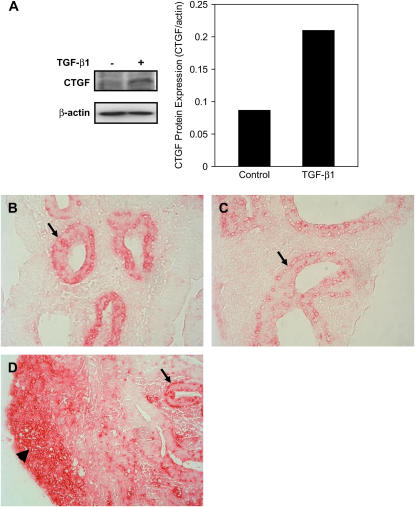
To determine whether CTGF mRNA expression is correlated with CTGF protein expression, total proteins were extracted from control and TGF-β1 (100 ng/ml)–treated lung explants at 48 h. Expression of CTGF protein was analyzed by Western blot. Compared with control where low level of CTGF protein was detected, treatment with TGF-β1 up-regulated CTGF protein nearly 2.5-fold (Figure 3A).
Figure 3.
Endogenous and TGF-β1–induced CTGF protein expression in E12.5 lung explants. E12.5 lung explants were treated with 100 ng/ml TGF-β1 for 48 h. Western blot was performed to analyze CTGF and β-actin proteins. (A) The relative CTGF protein levels were normalized to β-actin. Treatment with TGF-β1 resulted in a 2.5-fold increase in CTGF protein expression. Immunhistochemistry was performed to localize CTGF and pro–SP-C. Red precipitation indicates positive staining. (B) In control lung explant, CTGF was localized in the distal airway epithelium (arrow). (C) In control lung explant, pro–SP-C was also localized in the distal airway epithelium (arrow). (D) In TGF-β1–treated explant, CTGF was strongly detected in both epithelial cells (arrow) and subsets of mesenchymal cells (arrowhead). Images were taken under ×400 magnifications.
Localization of CTGF protein was examined by immunohistochemistry with an anti-CTGF antibody in control and 100 ng/ml TGF-β1–treated lung explants at 48 h. Staining with an anti-pro–SP-C antibody was used to help identify the distal airway epithelial cells. In control lung explants, CTGF was exclusively detected in the distal airway epithelial cells (Figure 3B), which were also positively stained by pro–SP-C antibody (Figure 3C). In TGF-β1–treated lung explants, CTGF was strongly detected in the epithelial cells and mesenchymal cells (Figure 3D). Thus, endogenous CTGF protein is expressed in the distal airway epithelium, but TGF-β1 induces CTGF protein in both epithelial and mesenchymal cells.
Induction of CTGF mRNA Expression Is Independent of New Protein Synthesis
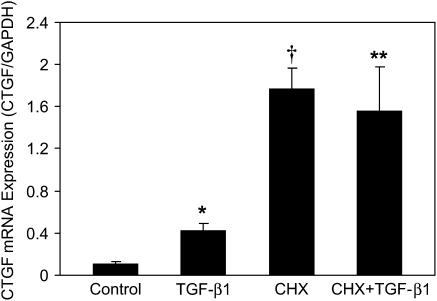
To determine whether the CTGF gene is directly controlled by TGF-β1 or secondarily induced by other TGF-β1–regulated gene products, we examined the effect of a protein synthesis inhibitor, cycloheximide, on TGF-β1 induction of CTGF mRNA expression. E12.5 lung explants were pretreated with cycloheximide (5 μM) for 1 h and then cultured in the presence or absence of 100 ng/ml TGF-β1 for 24 h. Expression of CTGF mRNA was analyzed by quantitative real-time RT-PCR. As shown in Figure 4, compared with control, exposure to cycloheximide alone dramatically up-regulated CTGF mRNA expression. Compared with TGF-β1 alone, incubation with cycloheximide plus TGF-β1 superinduced CTGF mRNA expression. These results demonstrate that induction of CTGF mRNA by TGF-β1 does not require de novo protein synthesis, thereby suggesting that the increase in CTGF mRNA is directly regulated by TGF-β1.
Figure 4.
Effect of cycloheximide on TGF-β1 induction of CTGF mRNA expression. E12.5 lung explants were pretreated with 5 μM cycloheximide or vehicle for 1 h, followed by incubating in the presence or absence of 100 ng/ml TGF-β1 for 24 h. Quantitative real-time RT-PCR was performed to detect CTGF and GAPDH mRNA. The relative CTGF mRNA levels were normalized to GAPDH. Data are expressed as mean ± SD from three separate experiments. TGF-β1 up-regulated CTGF mRNA expression in the presence and absence of cycloheximide. *P = 0.001 compared with control; †P < 0.001 compared with control; **P < 0.001 compared with TGF-β1 treatment. CHX, cycloheximide.
CTGF Inhibits Branching Morphogenesis in E12.5 Lung Explants
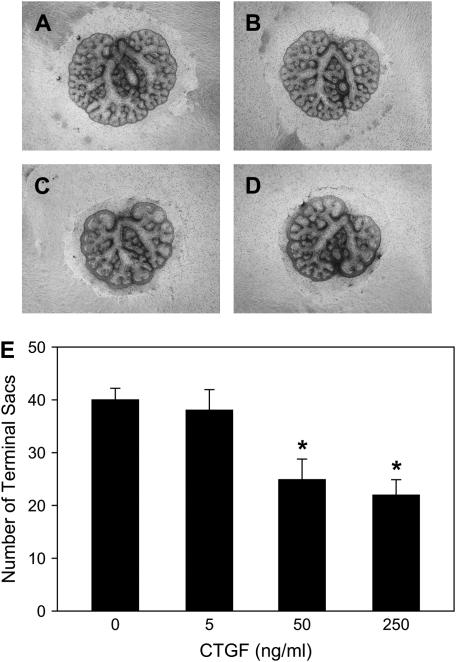
To link the inhibitory effect of TGF-β1 on branching morphogenesis to the observed induction of CTGF by TGF-β1 in lung explants, E12.5 lung explants were incubated directly with recombinatant CTGF for 48 h. As shown in Figure 5, CTGF inhibited branching morphogenesis in dose- and time-dependent manners similar to those observed for TGF-β1. CTGF at concentration 5 ng/ml did not affect lung size or branching formation. However, CTGF at concentrations of 50–250 ng/ml did decrease lung size (Figures 5A–5D) and significantly inhibited branching 37–45% (Figure 5E). These results strongly suggest that CTGF mediates TGF-β1 inhibition of branching morphogenesis.
Figure 5.
CTGF inhibits branching morphogenesis in E12.5 mouse embryonic lung explants. E12.5 lung explants were treated with CTGF (5–250 ng/ml) for 48 h and photomicrographs were taken under the same magnification at 48 h. (A) Control lung explant; lung explants treated with CTGF 5 ng/ml (B), 50 ng/ml (C), and 250 ng/ml (D). (E) Graphical representation of the quantification of terminal branching after culture for 48 h. Each bar represents mean ± SD from six lung explants. CTGF dose-dependently inhibited branching morphogenesis. *P < 0.001 compared with control.
Inhibition of ALK-5 Activity Stimulates Branching Morphogenesis and Blocks TGF-β1 Inhibition of Branching Morphogenesis
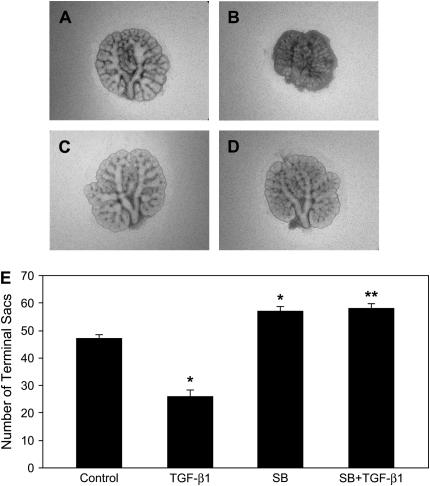
Since TGF-β1 predominantly signals via the TβRI/ALK-5–dependent pathway, we hypothesized that TGF-β1 inhibition of branching morphogenesis and induction of CTGF expression are also mediated by TβRI/ALK-5 activity. To test this hypothesis, we used an inhibitor strategy in the embryonic lung explant culture. SB431542 is a novel imidazole compound with selectivity for the ALK4, ALK5, and ALK7 that mediate signaling for the TGF-β/Activin/Nodal branch of the TGF-β superfamily (35). This inhibitor has been shown to effectively abrogate Smad2 phosphorylation and has no effect on other, more divergent ALK family type I receptors that bind BMPs, or on other kinases including ERK, JNK, and P38 MAPK. To determine the effect of SB431542 on branching morphogenesis, E12.5 lung explants were incubated with different concentration of SB431542 (0.2–20 μM) for 48 h. SB431542 stimulated branching 14–28% with concentrations of 2 and 20 μM (data not shown). To block TGF-β1 activity, E12.5 lung explants were preincubated with 20 μM SB431542 for 1 h and then cultured in the presence of 100 ng/ml TGF-β1 for 48 h. Compared with control (Figure 6A), when SB431542 was added to the culture in the absence of TGF-β1, it significantly increased branching, ∼ 20% (Figure 6C). Compared with TGF-β1 treatment (Figure 6B), pre-incubation with SB431542 completely blocked TGF-β1 inhibition of terminal branching (Figure 6D). In fact, branching was significantly increased 23% in the presence of both SB431542 and TGF-β1 when compared with control explants. These results demonstrate that blocking TβRI/ALK-5 activity stimulates branching, indicating that the ALK-5 activity modulated by endogenous TGF-β signaling plays a negative role in terminal branching formation. Furthermore, inhibition of branching morphogenesis by exogenous TGF-β1 is also mediated by TβRI/ALK-5 activity.
Figure 6.
ALK-5 inhibitor stimulates branching morphogenesis and blocks TGF-β1 inhibition of branching morphogenesis. E12.5 lung explants were pretreated with 20 μM SB431542 for 1 h, followed by incubation with 100 ng/ml TGF-β1 for 48 h. Representative photomicrographs of lung explants are shown: (A) control explant; (B) explant treated with 100 ng/ml TGF-β1; (C) explant incubated with SB431542; (D) explant incubated with both SB431542 and TGF-β1. (E) Graphical representation of the quantification of terminal branching at 48 h. Each bar represents mean ± SD from six explants. SB431542 stimulated branching and blocked TGF-β1 inhibition of branching. *P < 0.001 compared with control; **P < 0.001 compared with TGF-β1 treatment. SB, SB431542.
ALK-5 Activity Is Required for Endogenous and TGF-β1–Induced CTGF mRNA Expression
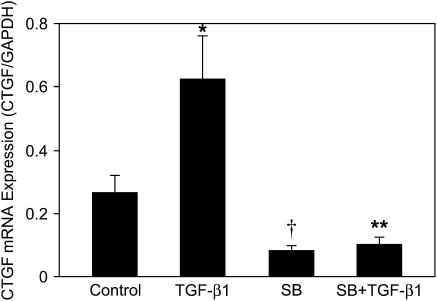
We next examined the effects of SB431542 on endogenous and TGF-β1–induced CTGF mRNA expression in the above-described culture system. Expression of CTGF mRNA was determined by quantitative real-time RT-PCR (Figure 7). Compared with control, SB43152 alone decreased CTGF mRNA expression. Compared with TGF-β1 treatment, pre-incubation with SB431542 completely blocked TGF-β1–induced CTGF mRNA expression. These results suggest that ALK-5 activity is required for endogenous and TGF-β1–induced CTGF mRNA expression.
Figure 7.
ALK-5 inhibitor blocks endogenous and TGF-β1–induced CTGF mRNA expression. E12.5 lung explants were pretreated with 20 μM SB431542 for 1 h, followed by incubation with 100 ng/ml TGF-β1 for 48 h. CTGF and GAPDH mRNA were analyzed by quantitative real-time RT-PCR. Relative CTGF mRNA levels were normalized to GAPDH. Results are mean ± SD from three separate experiments. SB431542 significantly decreased endogenous CTGF mRNA expression and blocked TGF-β1–induced CTGF mRNA expression. *P < 0.001 compared with control; †P < 0.05 compared with control; **P < 0.001 compared with TGF-β1 treatment.
Endogenous and TGF-β1–Induced CTGF Protein Expression Depends on ALK-5 Activity
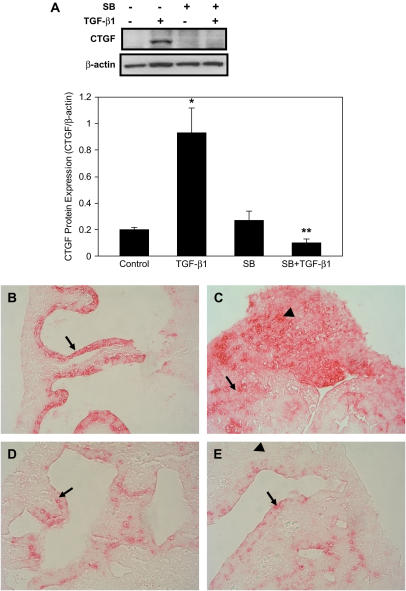
To determine whether the effect of SB431542 on CTGF mRNA expression is correlated with CTGF protein expression, E12.5 lung explants were cultured as described above. Expression of CTGF protein was analyzed by Western blot. Compared with TGF-β1 treatment, pre-incubation with SB431542 completely abolished TGF-β1 up-regulation of CTGF protein expression (Figure 8A). Thus, the effect of SB431542 on CTGF protein expression correlates with its effect on CTGF mRNA expression. Localization of CTGF protein was detected by immunostaining with a CTGF antibody. Compared with control, where CTGF protein was extensively detected in the distal airway epithelium (Figure 8B), incubation with SB431542 markedly decreased CTGF protein expression (Figure 8D). In contrast, in TGF-β1–treated lung explant, CTGF protein was strongly detected in the epithelium and mesenchyme (Figure 8C), while in SB431542 plus TGF-β1–treated lung explant, CTGF protein was weakly detected in the epithelium but not in the mesenchyme (Figure 8E). These data indicate that TβRI/ALK-5 activation is crucial for endogenous as well as TGF-β1–induced CTGF protein expression.
Figure 8.
ALK-5 inhibitor blocks endogenous and TGF-β1–induced CTGF protein expression. E12.5 lung explants were pretreated with SB431542 for 1 h, followed by incubation with 100 ng/ml TGF-β1 for 48 h. (A) CTGF and β-actin proteins were analyzed by Western blot. The relative CTGF protein levels were determined after normalization to β-actin. Data shown are mean ± SD from three separate experiments. SB431542 blocked TGF-β1–induced CTGF protein expression. *P < 0.001 compared with control; **P < 0.001 compared with TGF-β1 treatment. Immunohistochemistry was performed to localize CTGF protein. (B) CTGF was strongly detected in the distal airway epithelium in the control lung explant (arrow). (C) CTGF was strongly detected in the epithelial cells (arrow) and subsets of mesenchymal cell (arrowhead) in TGF-β1–treated explants. (D) Incubation of lung explants with SB431542 reduced CTGF protein expression in the epithelium (arrow). (E) Pre-incubation with SB431542 decreased TGF-β1–induced CTGF protein expression in epithelial cells (arrow) and completely blocked CTGF protein expression in mesenchymal cells (arrowhead). Images were taken under ×400 magnifications.
Inhibition of ALK-5 Activity Abolishes Endogenous and TGF-β1–Induced p-Smad2 Expression
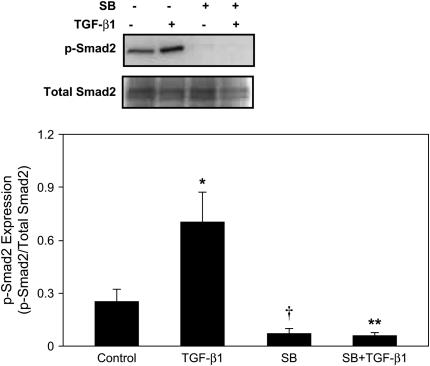
Finally, we evaluated the effect of SB431542 on expression of p-Smad2. E12.5 lung explants were cultured for 48 h as described above. Expression of p-Smad2 was determined by Western blot and normalization to total Smad2/3 (Figure 9). Expression of p-Smad2 was detected in the control lung explants. Exposure to SB431542 alone completely abolished endogenous p-Smad2 expression compared with control. Treatment with TGF-β1 alone up-regulated p-Smad2 expression 2.8-fold when compared with control. However, pre-incubation with SB431542 completely blocked TGF-β1–induced p-Smad2 expression. These findings imply that ALK-5 activity mediates endogenous and TGF-β1–induced Smad2 phosphorylation.
Figure 9.
ALK-5 inhibitor abolishes endogenous and TGF-β1–induced Smad2 phosphorylation. E12.5 lung explants were preincubated with 20 μM SB431542 for 1 h, followed by incubation with 100 ng/ml TGF-β1 for 48 h. Expression of p-Smad2 and total Smad2/3 were analyzed by Western blot. Relative abundance of p-Smad2 was normalized to total Smad2/3. Results are presented as mean ± SD from three separate experiments. Endogenous expression of p-Smad2 was detected in the control lung explants. Treatment with TGF-β1 up-regulated p-Smad2 expression without changing total Smad2/3 expression. Preincubation with SB431542 completely abolished both endogenous and TGF-β1–induced p-Smad2 expression. *P < 0.001 compared with control; †P < 0.05 compared with control; **P < 0.001 compared with TGF-β1 treatment.
DISCUSSION
In this study, we demonstrate for the first time that TGF-β1 induces rapid and sustained CTGF expression in mouse E12.5 lung explants. Inhibition of ALK-5 activity by a specific inhibitor abolished endogenous and TGF-β1 up-regulation of Smad2 phos- porylation, blocked endogenous and TGF-β1–induced CTGF mRNA and protein expression and increased branching morphogenesis. These data strongly suggest that the ALK-5–dependent Smad2 pathway inhibits branching and is crucial for endogenous as well as TGF-β1–induced CTGF expression. Our findings provide new insights into identifying mediators and elucidating the molecular mechanisms underlying the negative effect of TGF-β1 on lung branching morphogenesis.
Many studies have indicated that endogenous and exogenous TGF-β signaling negatively regulates lung branching morphogenesis (12–14). It has been postulated that the inhibitory effect of TGF-β1 on branching morphogenesis is associated with increased production of ECM and accumulation of α-SMA in the distal mesenchyme (12, 13). Thus, in light of its pivotal role as a mediator of TGF-β1 on fibroblast proliferation, ECM production, and myofibroblast differentiation, we hypothesized that CTGF could be a target gene playing a role in TGF-β1 inhibition of branching morphogenesis. And indeed, our results provide evidence that CTGF likely is a downstream mediator of TGF-β1, because exogenous CTGF inhibits branching morphogenesis and expression of CTGF during early mouse embryonic lung development was found to be tightly controlled by a signaling pathway common to both endogenous and exogenous TGF-β.
Embryonic lung explant culture provides a useful experimental model to study branching morhopgenesis and gene regulation. Many growth factors and their receptors such as VEGF, VEGF receptor (F1k-1), FGF10, and BMP-4 (36), as well as transcriptional factors such as N-myc (15) and Smad2/3 (14) that play a critical role in lung morphogenesis are expressed in cultured embryonic lung explants, suggesting that their expression is controlled by autocrine or paracrine factors in the lung explant. We have shown that when mouse E12.5 lung explants are placed in culture under serum-free condition, CTGF mRNA expression was induced in a time-dependent manner. This finding indicates that CTGF is an important factor for lung branching morphogenesis and its expression during branching morphogenesis is controlled by autocrine or paracrine factor(s) in the lung explant. Previous studies have indicated that TGF-β1 is expressed in the mesenchyme, particularly in the mesenchyme underlying distal epithelial branching points of embryonic lungs (9, 11, 12) while Smad2/3 is expressed in the distal airway epithelial cells (14). A functional Smad-binding site has recently been identified in human CTGF promoter and that is essential for TGF-β induction of CTGF gene expression (37, 38). In this study, endogenous CTGF protein was localized in the distal airway epithelium, which correlates with where Smad2/3 are reportedly expressed. We used SB431542, a specific inhibitor of TβRI/ALK-5–mediated Smad2 phosphorylation to antagonize endogenous TGF-β signaling. Consequently, SB431542 drastically decreased endogenous expression of CTGF mRNA as well as CTGF protein in the distal airway epithelium. These data indicate that endogenous TGF-β signaling via ALK-5–Smad2/3 pathway controls CTGF expression during early mouse embryonic lung development. We have also shown that inhibition of endogenous ALK-5 activity increased branching morphogenesis. Thus, our results are in agreement with previous studies showing abrogation of TβRII and TβRI signal transduction pathways stimulates early mouse embryonic lung branching morphogenesis in culture (13, 14), possibly through reversing the negative influence of endogenous TGF-β signaling upon lung branching morphogenesis.
We have demonstrated that exogenous TGF-β1 induced rapid and sustained CTGF expression in E12.5 lung explant culture. To our knowledge, this is the first report of TGF-β1 induction of CTGF expression in embryonic lung explants. The induction of CTGF expression by TGF-β1 was time- and dose-dependent. We found that TGF-β1 induced CTGF mRNA expression peaked at 24–48 h during the 2- to 48-h study period. This is in contrast with published data with fibroblast cell cultures, which showed that the peak of CTGF mRNA induction by TGF-β1 was at 2 to 6 h (39, 40). We have also observed that induction of CTGF mRNA in lung explant culture required 1–100 ng/ml TGF-β1. This also contrasts with the studies in lung fibroblast cell culture, where only 0.05–1 ng/ml of TGF-β1 was needed to induce CTGF mRNA expression (40). Other investigators have reported similar pattern for Smad7 mRNA expression by TGF-β in lung cell culture and lung explant culture (41, 42). Therefore, induction of CTGF expression in lung explant culture requires higher dose of TGF-β1 and longer exposure than in cell culture systems. It is possible that the organ cultures present a greater barriers to growth factor penetration than signal layer cell cultures.
To provide insight into the molecular mechanisms of TGF-β1 up-regulation of CTGF expression, we examined the effects of cycloheximide, a protein synthesis inhibitor, on embryonic lung explant culture. CTGF mRNA expression was superinduced by TGF-β1 in the presence of cycloheximide. This phenomenon has also been observed in studies with cultured fibroblasts (31, 32, 39). While the phenomenon of “superinduction” has not been specifically investigated for CTGF mRNA, it can be explained by three general mechanisms. First, new protein synthesis is not required for TGF-β1 stimulation of CTGF mRNA expression. Second, CHX can inhibit synthesis of RNA-degrading enzymes and prevent ribosome translocation of CTGF mRNA; therefore, CHX can increase CTGF mRNA stability and the steady-state level of CTGF mRNA. Finally, CHX may inhibit the synthesis of transcription factors that repress the CTGF gene promoter, thus allowing increased transcription of CTGF mRNA.
Smad2/3 has been reported to be predominately expressed in the distal airway epithelium in embryonic lung explants (14). However, ALK-5 has been found located in both epithelium and mesenchyme in rat embryonic lungs (43) and in the mesenchymal layers underlying the epithelium in mouse embryonic lungs (44). Moreover, at least one previous study has demonstrated that addition of TGF-β1 to the culture medium affects both epithelial cells and mesenchymal cells of lung explants (12). Similarly, our immunohistology results showed that exogenous TGF-β1 induced CTGF in both epithelial cells and peripheral mesenchymal cells, and our blocking experiments with SB431542 indicated that exogenous TGF-β1 regulates CTGF expression via ALK-5–dependent Smad2 signaling. SB431542 completely blocked TGF-β1–induced CTGF protein expression in the mesenchyme, suggesting that Smad2 may be the sole signaling pathway that mediates TGF-β1–induced CTGF expression in the mesenchyme. However, epithelial expression of CTGF was only partially blocked by SB431542, suggesting that additional signal transduction pathways other than Smad2 may also mediates TGF-β1–induction of CTGF in the epithelial cells of embryonic lung explants. In fact, MAPK signaling pathways involving ERK and JNK have been reported to mediate TGF-β induction of CTGF expression in cell culture models (38, 45). It is unknown whether the MAPK pathways play any role in CTGF regulation during embryonic lung development. Future studies to investigate the role of MAPK pathways in TGF-β induction of CTGF expression during embryonic lung development may provide new insights into other molecular mechanisms regulating CTGF expression and branching morphogenesis.
We conclude that expression of CTGF during early embryonic lung development is tightly controlled by endogenous and exogenous TGF-β via the TβRI/ALK-5–dependent Smad2 singaling pathway. CTGF inhibits branching morphogenesis and down-regulation of CTGF expression by blocking ALK-5–Smad2 pathway stimulates branching morphogenesis. We speculate that CTGF may be a critical downstream responsive gene that mediates TGF-β inhibition of lung branching morphogenesis.
Acknowledgments
The authors thank Ms. Brenda Roberts for her excellent technical support in processing embryonic lung explant sections.
This work was supported by funding from NIH grant K08 HD046582, Project NewBorn University of Miami, and by a grant from the Bank of America Charitable Foundation, Inc.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0320OC on December 29, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn 1998;212:482–494. [DOI] [PubMed] [Google Scholar]
- 2.Demayo F, Minoo P, Plopper CG, Schuger L, Shannon J, Torday JS. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am J Physiol Lung Cell Mol Physiol 2002;283:L510–L517. [DOI] [PubMed] [Google Scholar]
- 3.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol 2004;66:625–645. [DOI] [PubMed] [Google Scholar]
- 4.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 2000;92:55–81. [DOI] [PubMed] [Google Scholar]
- 5.Massague J, Cheifetz S, Boyd FT, Andres JL. TGF-beta receptors and TGF-beta binding proteoglycans: recent progress in identifying their functional properties. Ann N Y Acad Sci 1990;593:59–72. [DOI] [PubMed] [Google Scholar]
- 6.Massague J. TGF-beta signal transduction. Annu Rev Biochem 1998;67:753–791. [DOI] [PubMed] [Google Scholar]
- 7.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev 2000;14:627–644. [PubMed] [Google Scholar]
- 8.Franzen P, ten Dijke P, Ichijo H, Yamashita H, Schulz P, Heldin CH, Miyazono K. Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. Cell 1993;75:681–692. [DOI] [PubMed] [Google Scholar]
- 9.Pelton RW, Dickinson ME, Moses HL, Hogan BL. In situ hybridization analysis of TGF beta 3 RNA expression during mouse development: comparative studies with TGF beta 1 and beta 2. Development 1990;110:609–620. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Young SL. Expression of transforming growth factor-beta type II receptor in rat lung is regulated during development. Am J Physiol 1995;269:L419–L426. [DOI] [PubMed] [Google Scholar]
- 11.Schmid P, Cox D, Bilbe G, Maier R, McMaster GK. Differential expression of TGF beta 1, beta 2 and beta 3 genes during mouse embryogenesis. Development 1991;111:117–130. [DOI] [PubMed] [Google Scholar]
- 12.Bragg AD, Moses HL, Serra R. Signaling to the epithelium is not sufficient to mediate all of the effects of transforming growth factor beta and bone morphogenetic protein 4 on murine embryonic lung development. Mech Dev 2001;109:13–26. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Bu D, Lee M, Slavkin HC, Hall FL, Warburton D. Abrogation of transforming growth factor-beta type II receptor stimulates embryonic mouse lung branching morphogenesis in culture. Dev Biol 1996;180:242–257. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Lee M, Smith S, Warburton D. Abrogation of Smad3 and Smad2 or of Smad4 gene expression positively regulates murine embryonic lung branching morphogenesis in culture. Dev Biol 1998;194:182–195. [DOI] [PubMed] [Google Scholar]
- 15.Serra R, Pelton RW, Moses HL. TGF beta 1 inhibits branching morphogenesis and N-myc expression in lung bud organ cultures. Development 1994;120:2153–2161. [DOI] [PubMed] [Google Scholar]
- 16.Serra R, Moses HL. pRb is necessary for inhibition of N-myc expression by TGF-beta 1 in embryonic lung organ cultures. Development 1995;121:3057–3066. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Dey CR, Wert SE, Whitsett JA. Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-beta 1 chimeric gene. Dev Biol 1996;175:227–238. [DOI] [PubMed] [Google Scholar]
- 18.Zeng X, Gray M, Stahlman MT, Whitsett JA. TGF-beta1 perturbs vascular development and inhibits epithelial differentiation in fetal lung in vivo. Dev Dyn 2001;221:289–301. [DOI] [PubMed] [Google Scholar]
- 19.Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett 1993;327:125–130. [DOI] [PubMed] [Google Scholar]
- 20.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev 1997;8:171–179. [DOI] [PubMed] [Google Scholar]
- 21.Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol 2003;81:355–363. [DOI] [PubMed] [Google Scholar]
- 22.Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol 1999;19:2958–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 2000;32:1805–1819. [DOI] [PubMed] [Google Scholar]
- 24.Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J 2004;18:469–479. [DOI] [PubMed] [Google Scholar]
- 25.Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF. Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res 1997;233:63–77. [DOI] [PubMed] [Google Scholar]
- 26.Surveyor GA, Brigstock DR. Immunohistochemical localization of connective tissue growth factor (CTGF) in the mouse embryo between days 7.5 and 14.5 of gestation. Growth Factors 1999;17:115–124. [DOI] [PubMed] [Google Scholar]
- 27.Friedrichsen S, Heuer H, Christ S, Winckler M, Brauer D, Bauer K, Raivich G. CTGF expression during mouse embryonic development. Cell Tissue Res 2003;312:175–188. [DOI] [PubMed] [Google Scholar]
- 28.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 2003;130:2779–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu S, Duncan M, Peng JH, Grotendorst G, Bancalari E. The role of connective tissue growth factor in lung morphogenesis [abstract]. Proc Am Thorac Soc 2005;2:A463. [Google Scholar]
- 30.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 1993;4:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ 1996;7:469–480. [PubMed] [Google Scholar]
- 32.Kothapalli D, Hayashi N, Grotendorst GR. Inhibition of TGF-beta-stimulated CTGF gene expression and anchorage-independent growth by cAMP identifies a CTGF-dependent restriction point in the cell cycle. FASEB J 1998;12:1151–1161. [DOI] [PubMed] [Google Scholar]
- 33.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol 1996;107:404–411. [DOI] [PubMed] [Google Scholar]
- 34.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J 1999;13:1774–1786. [PubMed] [Google Scholar]
- 35.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 2002;62:65–74. [DOI] [PubMed] [Google Scholar]
- 36.Van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of developing mouse lung. Am J Physiol Lung Cell Mol Physiol 2005;288:L167–L178. [DOI] [PubMed] [Google Scholar]
- 37.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem 2001;276:10594–10601. [DOI] [PubMed] [Google Scholar]
- 38.Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene expression: Requirments for its induction by transforming growth factor-β2 in fibroblasts. J Biol Chem 2003;278:13008–13015. [DOI] [PubMed] [Google Scholar]
- 39.Kucich U, Rosenbloom JC, Herrick DJ, Abrams WR, Hamilton AD, Sebti SM, Rosenbloom J. Signaling events required for transforming growth factor-beta stimulation of connective tissue growth factor expression by cultured human lung fibroblasts. Arch Biochem Biophys 2001;395:103–112. [DOI] [PubMed] [Google Scholar]
- 40.Utsugi M, Dobashi K, Ishizuka T, Masubuchi K, Shimizu Y, Nakazawa T, Mori M. C-Jun-NH2-terminal kinase mediates expression of connective tissue growth factor induced by transforming growth factor-beta1 in human lung fibroblasts. Am J Respir Cell Mol Biol 2003;28:754–761. [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Crowe DL, Castillo C, Wuenschell C, Chai Y, Warburton D. Smad7 is a TGF-beta-inducible attenuator of Smad2/3-mediated inhibition of embryonic lung morphogenesis. Mech Dev 2000;93:71–81. [DOI] [PubMed] [Google Scholar]
- 42.Afrakhte M, Moren A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin CH, Heldin NE, ten Dijke P. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. Biochem Biophys Res Commun 1998;249:505–511. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Tseu I, Wang J, Tanswell K, Post M. Transforming growth factor β2, but not β1 and β3, is critical for early rat lung branching. Dev Dyn 2000;217:343–360. [DOI] [PubMed] [Google Scholar]
- 44.Seki T, Hong KH, Oh SP. Nonoverlapping expression patterns of ALK1 and ALK5 reveal distinct roles of each receptor in vascular development. Lab Invest 2006;86:116–129. [DOI] [PubMed] [Google Scholar]
- 45.Xie S, Sukkar MB, Issa R, Oltmaans U, Nicholson AG, Chung F. Regulation of TGF-β1-induced connective tissue growth factor expression in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2005;288:L68–L76. [DOI] [PubMed] [Google Scholar]