Abstract
Cultures of differentiating fetal human type II cells have been available for many years. However, studies with differentiated adult human type II cells are limited. We used a published method for type II cell isolation and developed primary culture systems for maintenance of differentiated adult human alveolar epithelial cells for in vitro studies. Human type II cells cultured on Matrigel (basolateral access) or a mixture of Matrigel and rat tail collagen (apical access) in the presence of keratinocyte growth factor, isobutylmethylxanthine, 8-bromo-cyclicAMP, and dexamethasone (KIAD) expressed the differentiated type II cell phenotype as measured by the expression of surfactant protein (SP)-A, SP-B, SP-C, and fatty acid synthase and their morphologic appearance. These cells contain lamellar inclusion bodies and have apical microvilli. In both systems the cells appear well differentiated. In the apical access system, type II cell differentiation markers initially decreased and then recovered over 6 d in culture. Lipid synthesis was also increased by the addition of KIAD. In contrast, type II cells cultured on rat tail collagen (or tissue culture plastic) slowly lose their lamellar inclusions and expression of the surfactant proteins and increase the expression of type I cell markers. The expression of the phenotypes is regulated by the culture conditions and is, in part, reversible in vitro.
Keywords: type I cell, type II cell, surfactant, lipogenesis
CLINICAL RELEVANCE
This report is important to our understanding of alveolar type II cell biology and defines critical differentiation factors required for the surfactant system in adult humans.
The alveolar surface is covered by an epithelium composed of two main cell types (1, 2). Type I cells are large flat cells that cover ∼ 95% of the alveolar surface and through which gas exchange takes place, and their metabolic properties are just now being discovered (2). Type II cells have a distinct morphology with characteristic lamellar bodies, the intracellular storage form of pulmonary surfactant, and apical microvilli. Type II cells make and secrete pulmonary surfactant, and they proliferate to restore the epithelium after damage to the more sensitive type I cells. Type II cells also transport sodium and fluid from the apical to the basolateral surface and play an important role in innate immunity.
Although methods for isolating and culturing adult rat and mouse type II cells and fetal human type II cells have been available for years, there has been limited success maintaining the differentiated functions of adult human alveolar epithelial cells in primary culture. In addition, there are no human cell lines that produce surfactant. H441 cells express surfactant protein (SP)-A and SP-B, whereas A549 cells do not express any of the surfactant proteins nor contain a higher percentage of surfactant phospholipids than fibroblasts (3). A variety of methods for isolating human type II cells have been published and some of their properties have been described (4–8). However, maintenance of surfactant protein expression in adult human type II cells has not been established. Maintenance of differentiation of type II cells and conversion of the type II cell phenotype into a type I–like phenotype is thought to be dependent on the extracellular matrix on which the cells are cultured and on the addition of factors in the medium that promote differentiation. In fetal human type II cells, dexamethasone (Dex), isobutylmethylxanthine (IBMX), and 8-bromo-cyclic AMP (8Br-cAMP) have been shown to be important for surfactant production (9). Markers for human type II cells include SP-A, SP-B, SP-C, and fatty acid synthase (FAS), and markers for human type I cells include receptor for advanced glycation end products (RAGE) and caveolin (10–12). There are some reports of SP-C and RAGE expression in human alveolar epithelial cells in primary culture (5, 10, 13). However, in general expression of SP-C decreases over the first few days of culture (8).
The goals of this study were to determine if we could develop a system for maintaining the differentiated functions of adult human type II cells, and to determine if the phenotypes of adult human type II cells and type I–like cells are reversible in vitro.
MATERIALS AND METHODS
Alveolar Type II Cell Isolation
We modified the human type II cell isolation method published by Fang and coworkers (4). Deidentified human lungs not suitable for transplantation and donated for medical research were obtained through the National Disease Research Interchange (Philadelphia, PA) and the International Institute for the Advancement of Medicine (Edison, NJ). The Committee for the Protection of Human Subjects at National Jewish Medical and Research Center approved this research. Briefly, the middle lobe was perfused, lavaged, and then instilled with elastase (12.9 U/ml; Roche Diagnostics, Indianapolis, IN) for 50 min at 37°C. The lung was minced, and the cells were isolated by filtration and partially purified by centrifugation on a discontinuous density gradient made of Optiprep (Accurate Chemical Scientific Corp., Westbury, NY) with densities of 1.080 and 1.040, and by negative selection with CD14-coated magnetic beads (Dynal Biotech ASA, Oslo, Norway) and binding to IgG-coated Petri dishes (Sigma, St. Louis, MO). The cells were counted and cytocentrifuge cell preparations were made to assess cell purity by staining for cytokeratin (CAM 5.2; DakoCytomation, Carpinteria, CA).
Culture of Human Type II and Type I–Like Cells
The freshly isolated cells were resuspended in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine, 2.5 μg/ml amphotericin B, 100 μg/ml streptomycin, 100 μg/ml penicillin G, and 10 μg/ml gentamicin (GIBCO BRL, Life Technologies Inc., Rockville, MD). For the Matrigel system (basolateral access), 5 × 106 cells were plated on Matrigel (BD Biosciences, Bedford, MA) in a 35-mm well. After adherence for 24–48 h, the medium was switched to 1% charcoal-stripped FBS (CS-FBS), 10 ng/ml keratinocyte growth factor (KGF, K; R&D Systems, Minneapolis, MN), 0.1 mM isobutylmethylxanthine (IBMX, I), and 0.1 mM 8Br-cAMP (A). Dex (D, 10 nM; Sigma) was added 2 d later along with the other additives and every other day thereafter. For the apical access system, 2.5 million cells were plated on 4.2 cm2 millicell inserts (Millipore Corp., Bedford, MA) that had been previously coated with a mixture of 60–65% Matrigel and 40–35% rat-tail collagen in DMEM with 10% FBS (14). After 24–48 h the media was changed to 5% FBS plus KIA additives or 1% CS-FBS plus KIA additives. Dex was added to the KIA additives 2 d later and KIAD was added every other day thereafter. To transdifferentiate type II cells to type I–like cells, type II cells were plated on rat tail collagen–coated dishes at a density of 0.5–1.0 × 105/cm2 in DMEM with 10% FBS (15). After 24–48 h the medium was changed to 5% FBS without additives.
Morphology
Cells were visualized by phase microscopy, electron microscopy, and immunofluorescence as described previously (16). Samples were prepared for electron microscopy with standard techniques (17). For immunofluorescence the cells were fixed in paraformaldehyde or acid alcohol and then filters were embedded in paraffin as described (18). In addition, some filter inserts were fixed in paraformaldehyde or ice-cold methanol and stained directly. The primary antibodies included mouse anti-human SP-A (PE-10), a gift from Yoshio Kuroki, Sapporo, Japan; rabbit anti–SP-B, rabbit anti–proSP-C, and mouse anti–Ep-CAM (Chemicon International, Temecula, CA); mouse anti-vimentin (V9; Santa Cruz Biotechnology, Santa Cruz, CA); goat anti-caveolin, mouse anti-CD45, mouse anti-cytokeratin (CAM5.2), and mouse anti–CD-31 (BD Biosciences, San Jose, CA); mouse anti-CD68 (DakoCytomation); rabbit anti–proSP-C (Fitzgerald Industries International, Concord, MA); goat anti-RAGE (R&D Systems); and rabbit anti-FAS, a gift of Stuart Smith (Oakland Children's Medical Center, Oakland, CA). The secondary antibodies included donkey anti-mouse IgG (Alexa 594); donkey anti-rabbit IgG (Alexa 488), and donkey anti-goat IgG (Alexa 594) from Molecular Probes (Eugene, OR).
Immunoblotting and Real-Time RT-PCR
Expression of protein and mRNA of corresponding genes were measured by Western blotting and real-time RT-PCR according to protocols as described previously (14). Polyacrylamide gradient gels (8–16%; Invitrogen Corporation, Carlsbad, CA) run in tris glycine buffer were used to separate proteins. Proteins were run in the reduced state except for SP-B, which was run unreduced. For Western blotting, protein loading was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or actin. For real-time RT-PCR, the expression levels of genes were expressed as a ratio to the expression of the constitutive probe 36B4, acidic ribosomal phosphoprotein P0 (19). The specific primers and probes used in these experiments are listed in Table 1.
TABLE 1.
SEQUENCE OF PRIMERS AND PROBES USED IN THIS STUDY
| Gene Name | Forward Primer | Probe | Reverse Primer |
|---|---|---|---|
| SP-A | GCCATTCAGGAGGCATGTG | CGGCCGCATTGCTGTCCCA | GCCTCATTTTCCTCTGGATTCC |
| SP-B | TGGGAGCCGATGACCTATG | CAAGAGTGTGAGGACATCGTCCACATCC | GCCTCCTTGGCCATCTTGT |
| SP-C | CGGGCAAGAAGCTGCTTCT | CCACACCGCAGGGACAAACCCT | CCCCAAGCTCCCATTTCTC |
| FAS | GAACTCCTTGGCGGAAGAGA | CACCCGCTCGGCATGGCTATCTT | GGACCCCGTGGAATGTCA |
| 36B4 | CCACGCTGCTGAACATGCT | AACATCTCCCCCTTCTCCTTTGGGCT | TCGAACACCTGCTGGATGAC |
Definition of abbreviations: FAS, fatty acid synthase; SP, surfactant protein.
Lipid Synthesis
Lipid synthesis by type II cells cultured on the apical system was performed as described previously (14). Briefly, on Day 7 of culture (after 6 d with additives), the cells were incubated with 5 μCi/ml [1-14C] acetate (specific activity 25–60 μCi/mmol; MP Biomedicals, Irvine, CA) for 4 h. Lipids were extracted with methanol and chloroform and separated by two-dimensional thin-layer chromatography on silica gel 60-well plates, as described previously (14). The first solvent system was chloroform:methanol:acetic acid (130:50:20, vol/vol/vol) and the second solvent system was chloroform:methanol:formic acid (130:50:20, vol/vol/vol). The plates were exposed to iodine vapor, individual lipids spots were scraped into vials for scintillation counting, and their incorporated radioactivity was determined. Separate inserts were used to measure DNA as described previously (14).
Statistic Analysis
Data from gene expression was processed through a nonparametric, paired t test and lipid data were analyzed by paired t tests.
RESULTS
Cell Isolation
In general we used the right middle lobe so that most of the lobe was surrounded by pleura and leaked minimally during lavage or instillation of elastase. After the negative selection by panning on IgG-coated Petri dishes, the yield was 344 ± 40 × 106 cells (mean ± SE, n = 16), and the cell purity was 82 ± 0.5%. The purity was further increased by adherence to the matrix in culture. The purity of the epithelial monolayers on EHS or the apical access system was > 90% as determined by immunostaining for SP-A, cytokeratin, or Ep-CAM and < 10% for other cells as determined by staining for vimentin, CD-45, CD-31, and CD-68.
Matrigel Basolateral Access System
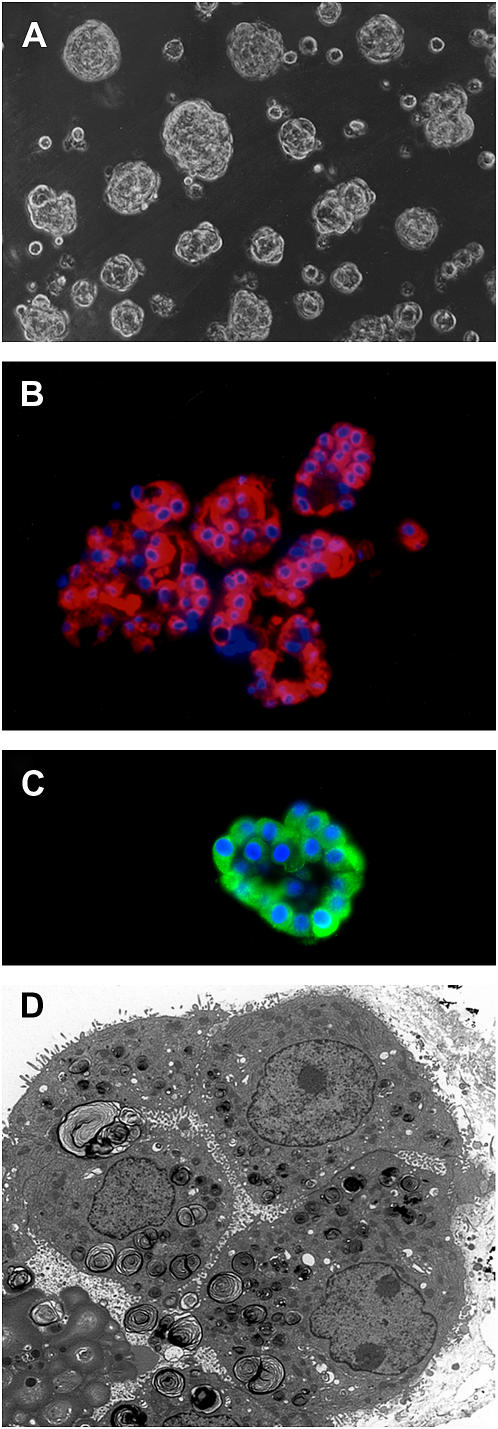
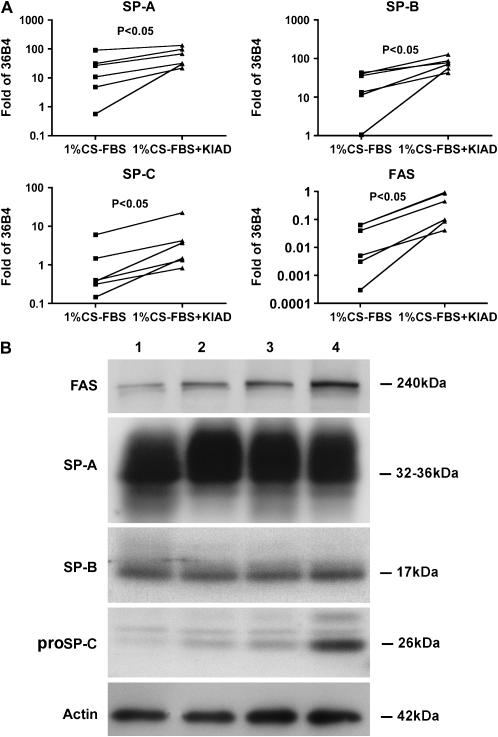
The type II cells formed spherules when they were cultured on Matrigel, as well described for rat type II cells (20) (Figure 1A). By immunostaining nearly all the cells expressed SP-A and proSP-C (Figures 1B and 1C). Some of the SP-A was in the lumen of the spherules. There were only rare cells that stained for CD-68, a macrophage marker (data not shown). An electron micrograph through one of these aggregates is shown in Figure 1D. Most of the apical surface, as indicated by microvilli, faced the internal lumen. Cells cultured on Matrigel had abundant lamellar inclusions, well formed intracellular organelles, and were joined by tight junctions. The mRNA levels for differentiation markers normalized to the expression of a constitutive probe varied from experiment to experiment. However, as shown in Figure 2A, there was a consistent increase in the mRNA levels for SP-A, SP-B, SP-C, and FAS relative to the constituent probe 36B4 upon the addition of KIAD. Because of the variability from experiment to experiment and the relatively high level of expression of SP-A without any addition, detailed evaluation of the individual additives at the mRNA level was not done. Protein expression in the Matrigel system was measured by immunoblotting (Figure 2B). FAS showed the highest level of expression with the combination of KIAD. There was a similar increase in proSP-C with KIAD, but there was little effect of the additives on the level of SP-A and SP-B in these experiments.
Figure 1.
Morphology of adult human type II cells cultured on Matrigel. Human type II cells were cultured on Matrigel in 1% CS-FBS with KIAD. After addition of KIA for a total of 6 d and Dex for 4 d, the cells were processed for phase microscopy (A), immunocytochemistry for SP-A (B), immunocytochemistry for proSP-C (C), and electron microscopy (D). For immunocytochemistry the cells were fixed in acid alcohol (SP-A) or 4% paraformaldehyde (proSP-C), embedded in paraffin, and stained for SP-A with the monoclonal antibody PE-10 or a rabbit polyclonal antibody to proSP-C (18).
Figure 2.
Expression of FAS and surfactant proteins in cells cultured on Matrigel. Type II cells were cultured on Matrigel and harvested 6 d after the addition of the additives as described in Materials and Methods. A shows mRNA values for FAS, SP-A, SP-B, and SP-C normalized to the constitutive probe 36B4 by quantitative real-time PCR for six independent experiments. B shows protein levels by immunoblotting. Lane 1, 1% CS-FBS; lane 2, 1% CS-FBS + K; lane 3, 1% CS-FBS + K + Dex; lane 4, 1% CS-FBS + KIAD. These results are representative of four independent experiments.
Apical Access System
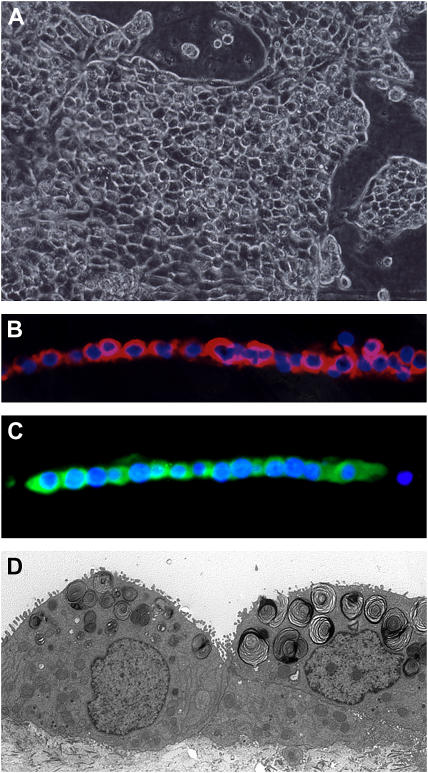
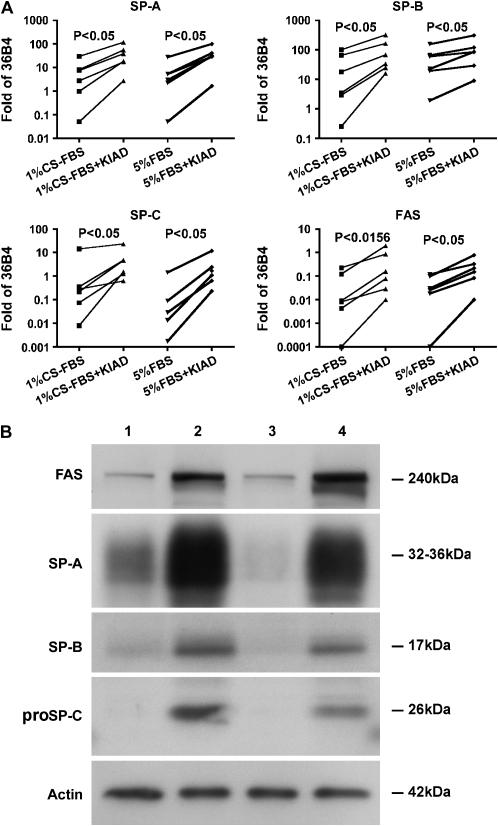
One of the difficulties with the Matrigel system is that the cells form aggregates, and most of the apical surface faces inward toward the lumen (20). Surfactant accumulates in the lumen of these aggregates, and therefore the immunoblotting for SP-A represents both intracellular and extracellular secreted SP-A. To provide access to the apical surface, we developed a system in which most of the cells form a monolayer (14–16, 21). With rat type II cells, KGF causes obvious proliferation, and the cells form a complete monolayer in the presence of rat serum. However, KGF does not have as dramatic proliferative response with human type II cells. To prevent cell spreading, the percentage of Matrigel was increased. The cells form islands or aggregates on this matrix (Figure 3A). However, there were also some spherules seen in individual wells. By immunocytochemistry, most cells expressed SP-A and proSP-C (Figures 3B and 3C). These cells also expressed SP-B, cytokeratin, Ep-CAM, and low levels of SP-D; these cells were negative for vimentin, CD-68, CD-45, and CD-31 (data not shown). There were a few other cells near the aggregates that stained for CD-68, CD-45, vimentin, or CD-31 in these cultures. The amount of contaminating cells varied with the different isolations. By electron microscopy, the alveolar epithelial cells have apical microvilli, and the lamellar bodies appear to be distributed preferentially toward the apical surface (Figure 3D). The cells were cultured with 5% FBS or 1% CS-FBS, and the results were quite similar. As shown in Figure 4A, the mRNA levels for SP-A, SP-B, SP-C, and FAS all increased with the addition of KIAD in the presence of 5% FBS or 1% CS-FBS. At the protein level, the addition of the combination of the KIAD increased expression of FAS, SP-A, SP-B, and proSP-C in the presence of 5% FBS or 1% CS-FBS (Figure 4B). The expression of SP-D was significantly less than that of SP-A, and was excluded from our analyses.
Figure 3.
Human type II cells cultured in the apical access system with KIAD additives. The cells were processed for phase microscopy (A), immunocytochemistry for SP-A (B), immunocytochemistry for proSP-C (C), and electron microscopy (D) as described in Materials and Methods and in Figure 1.
Figure 4.
Expression of FAS and surfactant proteins in the apical access system. Type II cells were cultured on the apical access system and harvested 6 d after the addition of KIAD as described in Materials and Methods. A shows the mRNA for FAS, SP-A, SP-B, and SP-C normalized to the constitutive probe 36B4 by quantitative real-time PCR for six independent experiments. KIAD was added to cultures with either 1% CS-FBS or 5% CS-FBS. B shows protein levels by immunoblotting. Lane 1, 1% FBS; lane 2, 1% CS-FBS + KIAD; lane 3, 5% FBS; lane 4, 5% FBS + KIAD. The results are representative of four independent experiments.
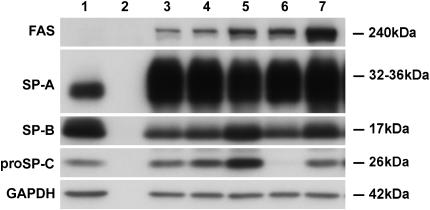
Additional studies were done to evaluate the effects of individual additives and time in culture at the protein level in the apical access system. The combination of all the additives increased the expression of FAS (Figure 5). SP-A was increased by KGF and was highly expressed under all conditions. SP-B and proSP-C were stimulated by Dex. However, proSP-C was inhibited by the combination of IBMX and 8Br-cAMP. As seen in the time course, freshly isolated cells expressed surfactant proteins at the beginning of culture, lost expression over the next few days, and then regained their expression after 4–6 d in the presence of KIAD (Figure 6A). Compared with type II cells, there was no detectable expression of SP-A, SP-B, or proSP-C after 7 d of culture on rat-tail collagen-coated wells (Figure 6A). In these studies, we saw no effect of removing the medium from the apical surface to provide an air–liquid interface at the surface of the monolayer, which is known to be important for ciliated cells of the bronchial epithelium (Figure 6A).
Figure 5.
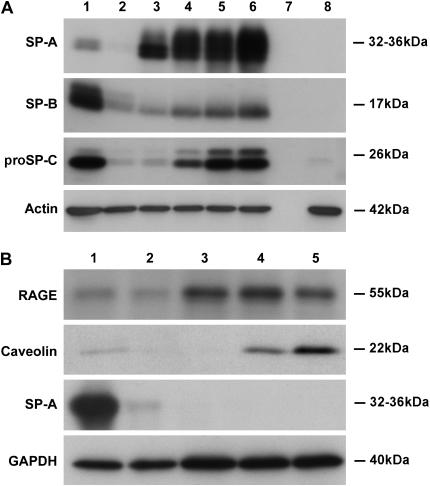
Effect of different additives on protein expression in the apical access system. Cells were cultured in the apical access system and individual additives were added as stated in Materials and Methods. Protein expression was estimated by immunoblotting. Lane 1, freshly isolated cells; lane 2, a no cell control; lane 3, 1% CS-FBS + K; lane 4, 1% CS-FBS + K + ethanol + DMSO; lane 5, 1% CS-FBS + K + Dex; lane 6, 1% CS-FBS + K + A + I; lane 7, 1% CS + KIAD. DMSO is the vehicle for I, and ethanol is the vehicle for D. The results are representative of three independent experiments.
Figure 6.
Time course for protein expression of surfactant proteins or type I cell markers. In A, type II cells were cultured in the apical access system in 5% FBS with the usual KIAD additives. Proteins were detected by immunoblotting. Lane 1, freshly isolated cells; lane 2, Day 1 of culture before the addition of additives; lane 3, Day 3 of culture (2 d with KIA); lane 4, 5 d of culture (4 d of KIA and 2 d of Dex); lane 5, 6 d with KIAD (6 d of KIA and 4 d of Dex, 7 d of culture); lane 6, KIAD cultured with no apical fluid; lane 7, a no cell control; lane 8, culture on a collagen-coated well in 5% FBS for 7 d. This result is representative of three independent experiments. In B, type II cells were plated on collagen-coated wells and cultured with 5% FBS and the proteins identified by immunoblotting. Lane 1, freshly isolated type II cells; lane 2, Day 1 in culture; lane 3, Day 3 of culture; lane 4, Day 5 of culture; lane 5, Day 7 of culture. This result is representative of three independent experiments.
Since type II cells are the major lipogenic cell type in the lung, differentiation of these cells should include increased lipogenesis. KIAD significantly increased FAS mRNA and protein levels (Figures 4A and 4B). We also measured acetate incorporation as an indicator of lipogenesis. As shown in Table 2, there was an increase in the rate of acetate incorporation due to KIAD in the presence of 1% CS-FBS, which was predominantly due to an increase in phospholipids as contrasted to neutral lipids. However, there was not much change in the types of phospholipids synthesized. Individual species of phosphatidylcholine were not determined in these experiments. The rate of acetate incorporation into phosphatidylcholine was increased by KIAD 5.47- ± 0.03-fold in 1% CS-FBS and 3.33- ± 0.73-fold in 5% FBS (n = 4).
TABLE 2.
ACETATE INCORPORATION IN CULTURED HUMAN TYPE II CELLS
| 1% CS-FBS | 1% CS-FBS+KIAD | 5% FBS | 5% FBS+KIAD | |
|---|---|---|---|---|
| μg DNA / well | 4.0 ± 0.1 | 7.2 ± 0.4* | 5.7 ± 0.2 | 7.8 ± 0.2* |
| Acetate incorporation, (cpm × 10−3/μg DNA) | 35.2 ± 9.0 | 170 ± 33.7* | 126.3 ± 37.1 | 298.7 ± 77.3 |
| % total lipids | ||||
| Phospholipids | 67.8 ± 2.1 | 82.8 ± 1.9* | 79.9 ± 4.5 | 85.6 ± 1.6 |
| Neutral lipids | 32.2 ± 2.1 | 17.2 ± 1.9* | 20.1 ± 4.5 | 14.5 ± 1.6 |
| % phospholipids | ||||
| Lyso PC | 1.1 ± 0.2 | 0.5 ± 0.1* | 1.0 ± 0.2 | 0.5 ± 0.1 |
| SM | 8.0 ± 0.7 | 3.7 ± 0.4* | 6.4 ± 1.0 | 2.9 ± 0.4* |
| PC | 75.7 ± 1.6 | 82.6 ± 1.1 | 77.6 ± 1.5 | 82.7 ± 1.1 |
| PS | 2.3 ± 0.4 | 1.8 ± 0.4 | 2.2 ± 0.4 | 1.5 ± 0.2 |
| PI | 5.5 ± 0.6 | 5.6 ± 0.5 | 5.2 ± 0.2 | 6.9 ± 1.0 |
| PE | 6.9 ± 0.4 | 5.5 ± 0.5 | 7.0 ± 0.3 | 4.9 ± 0.5 |
| PG | 0.6 ± 0.1 | 0.3 ± 0.0 | 0.5 ± 0.1 | 0.6 ± 0.1 |
Definition of abbreviations: CS-FBS, charcoal-stripped FBS; FBS, fetal bovine serum; KIAD, keratinocyte growth factor + 3-isobutyl-1-methylxanthine + cyclic AMP + dexamethasone; LysoPC, lysophosphatidylcholine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine; SM, sphingomyelin.
Type II cells were cultured in the apical access system with the specified media for 6 d. Incorporation of (1-14C) acetate was measured over the last 4 h. The data are expressed at cpm/μg DNA or as percent incorporation into total lipids or individual phospholipids. The results represent the mean ± SE for four independent experiments. The phospholipids measured were LysoPC, SM, PC, PS, PI, PE, and PG.
Indicates there is significant difference between with and without KIAD (P < 0.05).
Type I–Like Cells
Type II cells plated on tissue culture plastic or collagen-coated wells characteristically spread and flatten, lose their ability to express type II cell markers, and express some type I cell markers (15, 21, 22). The adult human type II cells cultured on plastic or collagen-coated coverslips or wells behaved similarly. As shown in Figure 6B, the level of SP-A decreases rapidly, whereas the type I cell markers, receptor for advanced glycation end-products (RAGE) and caveolin 1, appear over a few days. These type I–like cells are large and flat, but they still contain some residual lamellar bodies (data not shown).
Reversibility of the Phenotype
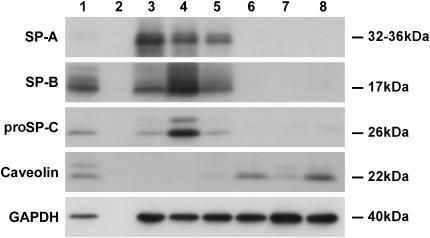
The type II cell phenotype is thought to be dependent on the culture conditions and to be reversible in part (23, 24). Rat type II cells cultured on Matrigel express markers of the type II cell phenotype, whereas type II cells cultured on tissue culture plastic or rat tail collagen lose markers of the type II phenotype and express markers of the type I cell phenotype (15). As shown in Figure 7, this also occurs with adult human type II cells. Type II cells were cultured with 1% CS-FBS plus KIAD on the Matrigel system for 7 d to maintain the type II cell phenotype or they were cultured with 5% FBS on collagen-coated tissue culture plastic to transdifferentiate into type I–like cells. On Day 7 of culture, cells grown on the Matrigel system were digested with dispase and then plated on the tissue culture plastic coated with collagen for another 7 d. Similarly, cells grown on the collagen-coated plastic were digested with trypsin and then plated on Matrigel for another 7 d. The appropriate media were also switched at the time for changing the culture systems. Cells maintained on Matrigel (the type II cell phenotype) or on collagen-coated wells (type I–like cell phenotype) for 14 d served as controls. Cells were harvested for immunoblotting on Day 7 or Day 14. Hence, type II cells initially maintained on Matrigel for 7 d could transdifferentiate into type I–like cells on collagen-coated wells as shown by the loss of SP-A, SP-B, and proSP-C and the acquired expression of caveolin. Similarly, type II cells cultured on collagen to express type I cell features could re-express type II cell characteristics when they were transferred to Matrigel, as shown by the expression of SP-A, SP-B, and proSP-C (Figure 7). These data indicate that the phenotype of these cells is determined by the in vitro conditions and is, in part, reversible.
Figure 7.
Reversibility of the type II and type I phenotype. Type II cells were cultured on Matrigel to promote the type II cell phenotype or collagen-coated wells to promote the type I cell phenotype. Some of the cultures were switched on Day 7 to the opposite culture conditions. The expression levels of proteins were estimated by immunoblot analyses as described in Materials and Methods. GAPDH was used to confirm that equal amounts of protein were loaded. Lane 1, freshly isolated cells; lane 2, an empty well; lane 3, 7 d on Matrigel; lane 4, 14 d on Matrigel; lane 5, 7 d on a collagen-coated well and then switched to Matrigel for 7 d; lane 6, 7 d on a collagen-coated well; lane 7, 14 d on a collagen-coated well; lane 8, 7 d on Matrigel switched to 7 d on collagen. The results are representative of three independent experiments.
DISCUSSION
To study the diverse functions of adult human alveolar type II cells, which include surfactant production, alveolar repair, fluid transport, and innate immunity, it would be useful to have a highly differentiated primary culture system. As stated previously, cell lines of differentiated type II cells are not available. Reported culture systems for adult human type II cells have shown some expression of SP-A or SP-C, but complete characterization of these cultures is not available, and expression of surfactant proteins was not maintained in culture (5, 8, 13). In addition, it would be useful to have epithelial cells that express some type I–like characteristics for comparative studies. However, it is highly likely that these type I–like cells, derived from isolated type II cells, are different from type I cells in vivo or freshly isolated type I cells (11). In this study we were able to show that type II cells could re-express the surfactant proteins after the initial loss in the first few days of culture, that KGF stimulated differentiation as it does with rat type II cells, and that the phenotype was greatly dependent upon the culture micro-environment.
In the present study, we modified other published methods to isolate and culture human type II cells. We used both the apical access and Matrigel systems to maintain differentiation as we have done previously with rat type II cells (14, 25). We developed culture conditions sufficient to induce and maintain differentiation of adult human alveolar type II cells. Cells cultured in both systems displayed distinct characteristics of type II cells in vivo such as cuboidal shape, lamellar inclusions, microvilli, and tight junctions and expression of surfactant proteins (1). However, our studies indicate that individual functions or expression of specific proteins in type II cells will likely vary with specific culture conditions. For example, cAMP and IBMX inhibited the expression of proSP-C in our system. However, human fetal type II cells respond differently. Gonzales and colleagues reported that cAMP + IBMX increased SP-C in fetal type II cells (9). In general, for fetal human type II cells, the combination of Dex, IBMX, and cAMP produced a synergistic effect on type II cell differentiation (9) With rat type II cells cultured on Matrigel, Shannon and colleagues showed that forskolin or IBMX plus forskolin reduced the expression of SP-A but not SP-C in the presence of KGF (26). Hence, the response of human adult alveolar type II cells to different additives in culture is different from fetal human type II cells or adult rat type II cells. Since the basic interest in our laboratory is lipogenesis, the combination of KGF, Dex, 8Br-cAMP, and IBMX appears to produce the maximal expression of FAS. As such, we feel that it is unlikely that one set of additives will maximize all human type II cell functions and that, instead, the functional characteristics of type II cells will greatly depend upon specific culture conditions and microenvironment, which includes the extracellular matrix. Specific culture conditions should probably be tailored to the specific function being investigated.
It is likely that we and other researchers will improve upon this culture system. The current series of experiments evaluated only a limited number of soluble additives or growth factors. It is probable that other additives or growth factors such as transforming growth factor-α or hepatocyte growth factor will be important. In our experiments there was a marked variation in level of mRNA for the differentiation markers SP-A, SP-B, SP-C, and FAS from isolation to isolation. The reasons for these variations are not clear at this time. Unfortunately, there are numerous potential sources for this variation, and these include the age, sex, administered medicines, and smoking history of the donor; the cause of death; the techniques used to harvest the lung; the transport media; the time of transport; the actual isolation procedure; the culture reagents; use of fresh versus frozen cells; and so on. These variables will have to be evaluated in future experiments.
There have been reports that the phenotype of the type II cells is reversible in vitro (23, 24). Our studies support the concept of the plasticity of the phenotype and modulation of the phenotype in vitro by the culture microenvironment. Type II cells cultured on tissue culture plastic or collagen-coated dishes express some of the markers of type I cells. We refer to these cells as type I–like cells. It is becoming clear from the work of Dahlin and coworkers that freshly isolated type I cells are different from these type I–like cells in culture and different still from type I cells maintained in culture (11). These type I–like cells in vitro might be similar to the intermediate or transitional cells described in lung injury that retain some lamellar bodies but are spreading to reform the epithelial surface. To test if these changes were reversible and regulated by the culture microenvironment, we designed the switch experiment. First, we cultured freshly isolated human alveolar type II cells on tissue culture plastic coated with rat tail collagen for 7 d to assume a type I–like cell phenotype, and then cultured these cells on Matrigel for 7 d to determine if they would express type II markers again. These results indicate that the type II and type I phenotypes are reversible in vitro.
These studies and the studies of others indicate that type II cells can be isolated from adult human lung and that their differentiated functions can be maintained in primary culture. However, the actual phenotype of these cells is dependent upon the culture microenvironment that includes the extracellular matrix on which the cells are grown. These cells should be useful to define the regulation of specific type II cell functions, their role in innate immunity, and their interactions with other cells in the alveolar compartment.
Acknowledgments
The authors thank Michael Matthay and Leland Dobbs for their advice on isolating human type II cells, Kevin Brown for his help in obtaining lungs for research, Lynn Cunningham for the histology, Galeen Smith for the electron microscopy, and Teneke Warren for preparing and editing this manuscript.
This research was supported by grants from the National Institutes of Health (HL-29891 and AI059576) and the ExxonMobil Foundation.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0410OC on January 25, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mason RJ. Biology of alveolar type II cells. Respirology 2006;11:S12–S15. [DOI] [PubMed] [Google Scholar]
- 2.Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol 2003;65:669–695. [DOI] [PubMed] [Google Scholar]
- 3.Mason RJ, Williams MC. Phospholipid composition and ultrastructure of A549 cells and other cultured pulmonary epitheial cells of presumed type II cell origin. Biochim Biophys Acta 1980;617:36–50. [DOI] [PubMed] [Google Scholar]
- 4.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L242–L249. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, Lehr CM. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res 2003;311:31–45. [DOI] [PubMed] [Google Scholar]
- 6.Mason RJ, Apostolou S, Power J, Robinson P. Human alveolar type II cells: stimulation of DNA synthesis by insulin and endothelial cell growth supplement. Am J Respir Cell Mol Biol 1990;3:571–577. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H, Kuroki Y, Tanaka H, Saito T, Kurokawa K, Chiba H, Sagawa A, Nagae H, Abe S. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med 2000; 162:258–263. [DOI] [PubMed] [Google Scholar]
- 8.Witherden IR, Vanden Bon EJ, Goldstraw P, Ratcliffe C, Pastorino U, Tetley TD. Primary human alveolar type II epithelial cell chemokine release: effects of cigarette smoke and neutrophil elastase. Am J Respir Cell Mol Biol 2004;30:500–509. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol 2002;283:L940–L951. [DOI] [PubMed] [Google Scholar]
- 10.Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res 2006;323:475–488. [DOI] [PubMed] [Google Scholar]
- 11.Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, Dobbs L. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol 2004;31:309–316. [DOI] [PubMed] [Google Scholar]
- 12.Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, Hata Y. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells 2004;9:165–174. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Mitsui M, Takeuchi K, Uwabe Y, Kobayashi K, Sawasaki Y, Matsuoka T. Preservation of the characteristics of the cultured human type II alveolar epithelial cells. Lung 2004;182:213–226. [DOI] [PubMed] [Google Scholar]
- 14.Mason RJ, Pan T, Edeen KE, Nielsen LD, Zhang F, Longphre M, Eckart MR, Neben S. Keratinocyte growth factor and the transcription factors C/EBP{alpha}, C/EBP{delta}, and SREBP-1c regulate fatty acid synthesis in alveolar type II cells. J Clin Invest 2003;112:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Wang S, Manzer R, McConville G, Mason RJ. Ozone induces oxidative stress in rat alveolar type II and type I-like cells. Free Radic Biol Med 2006;40:1914–1928. [DOI] [PubMed] [Google Scholar]
- 16.Mason RJ, Lewis MC, Edeen KE, McCormick-Shannon K, Nielsen LD, Shannon JM. Maintenance of surfactant protein A and D secretion by rat alveolar type II cells in vitro. Am J Physiol Lung Cell Mol Physiol 2002;282:L249–L258. [DOI] [PubMed] [Google Scholar]
- 17.Williams MC. Conversion of lamellar body membranes into tubular myelin in alveoli of fetal rat lungs. J Cell Biol 1977;72:260–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, McCormick-Shannon K, Voelker DR, Mason RJ. KGF increases SP-A and SP-D mRNA levels and secretion in cultured rat alveolar type II cells. Am J Respir Cell Mol Biol 1998;18:168–178. [DOI] [PubMed] [Google Scholar]
- 19.Krowczynska AM, Coutts M, Makrides S, Brawerman G. The mouse homologue of the human acidic ribosomal phosphoprotein PO: a highly conserved polypeptide that is under translational control. Nucleic Acids Res 1989;17:6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shannon JM, Emrie PA, Fisher JH, Kuroki Y, Jennings SD, Mason RJ. Effect of a reconstituted basement membrane on expression of surfactant apoproteins in cultured adult rat alveolar type II cells. Am J Respir Cell Mol Biol 1990;2:183–192. [DOI] [PubMed] [Google Scholar]
- 21.Manzer R, Wang J, Nishina K, McConville G, Mason RJ. Alveolar epithelial cells secrete chemokines in response to IL-1beta and lipopolysaccharide but not to ozone. Am J Respir Cell Mol Biol 2006;34:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobbs LG, Williams MC, Brandt AE. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta 1985;846:155–166. [DOI] [PubMed] [Google Scholar]
- 23.Olsen CO, Isakson BE, Seedorf GJ, Lubman RL, Boitano S. Extracellular matrix-driven alveolar epithelial cell differentiation in vitro. Exp Lung Res 2005;31:461–482. [DOI] [PubMed] [Google Scholar]
- 24.Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol 1995;12:497–502. [DOI] [PubMed] [Google Scholar]
- 25.Kawada H, Shannon JM, Mason RJ. Improved maintenance of adult rat alveolar type II cell differentiation in vitro: effect of serum-free, hormonally defined medium and a reconstituted basement membrane. Am J Respir Cell Mol Biol 1990;3:33–43. [DOI] [PubMed] [Google Scholar]
- 26.Shannon JM, Pan T, Nielsen LD, Edeen KE, Mason RJ. Lung fibroblasts improve differentiation of rat type II cells in primary culture. Am J Respir Cell Mol Biol 2001;24:235–244. [DOI] [PubMed] [Google Scholar]