Abstract
Cystic fibrosis (CF) is characterized by prolonged and excessive inflammatory responses in the lung and increased activation of NF-κB. Parthenolide is a sesquiterpene lactone derived from the plant feverfew, which has been used in folk medicine for anti-inflammatory activity. Several studies suggest that this compound inhibits the NF-κB pathway, but the exact site is controversial. We hypothesized that parthenolide might ameliorate the excessive inflammatory response in CF models by inhibiting activation of NF-κB. This was tested in vitro, using two pairs of cell lines with defective versus normal CF transmembrane conductance regulator (CFTR) (antisense/sense transfected 16 HBE and IB-3/S9), and in vivo, using CFTR-knockout (KO) mice. All cell lines were pretreated with parthenolide and then stimulated with IL-1β and/or TNF. Parthenolide significantly inhibited IL-8 secretion induced by these cytokines and prevented NF-κB activation, IκBα degradation, and IκB Kinase complex activity. CFTR-KO and wild-type mice were pretreated with parthenolide or vehicle alone then challenged intratracheally with LPS. Bronchoalveolar lavage was performed 3, 6, and 8 h later. Parthenolide pretreatment inhibited PMN influx as well as cytokine and chemokine production. This was also associated with inhibition of IκBα degradation and NF-κB activation. We thus conclude that parthenolide inhibits IκB kinase, resulting in stabilization of cytoplasmic IκBα, which in turn leads to inhibition of NF-κB translocation and attenuation of subsequent inflammatory responses. IκB kinase may be a good target, and parthenolide and/or feverfew might be promising treatments for the excessive inflammation in CF.
Keywords: cystic fibrosis, IκB kinase, lung, NF-κB, parthenolide
CLINICAL RELEVANCE
Natural compounds such as parthenolide, which is a sesquiterpene lactone, have powerful anti-inflammatory properties, and might be used safely to inhibit the inflammatory processes that lead to lung destruction and death in patients with cystic fibrosis.
Most of the morbidity and almost all the mortality in cystic fibrosis (CF) is due to a destructive inflammatory response to chronic infection in the lung, which is characterized by large numbers of polymorphonuclear leukocytes (PMNs) and their products (1, 2). Most studies in humans, knockout (KO) mice, and CF phenotype cell lines suggest that dysregulated production of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and the potent chemoattractant IL-8, are important in pathophysiology of the excessive inflammation in the CF lung (3–7). These cytokines are all dependent on the transcription factor NF-κB for maximal secretion. NF-κB is activated when its inhibitor, IκB, is phosphorylated by IκB Kinase (IKK), then ubiquitinated and degraded (8). The free NF-κB translocates to the nucleus, binds to promoters of NF-κB–dependent genes, and facilitates their transcription. NF-κB may thus be an important target for new anti-inflammatory approaches for treating CF lung disease.
Sesquiterpene lactones (SLs) are active constituents of medicinal plants from the Asteraceae family which have been used for centuries as folk remedies for migraine, inflammation, and arthritis (9). Parthenolide, one of the major SLs found in the medicinal plant, feverfew (Tanacetum parthenium), is responsible for many of its anti-inflammatory effects (9–11). The specific mechanisms by which feverfew and/or parthenolide may inhibit proinflammatory signaling pathways in vivo have not been completely defined. In vitro studies suggest that parthenolide can inhibit IKK (12–14) and can also directly inactivate NF-κB (15), but there is controversy as to whether either or both of these mechanisms are important in vivo (16–18). Both of these actions, independently or together, could contribute to net inhibition of NF-κB–dependent proinflammatory gene expression in the CF lung. Thus, we hypothesized that parthenolide would ameliorate the excessive inflammation in the CF lung and that it might be useful for treatment of CF.
We tested our hypothesis first with in vitro models. We used a human bronchial epithelial cell line (16 HBE) stably transfected with antisense oligonucleotides (AS), which inhibit expression of CFTR (4); and as a control, 16 HBE cells transfected with sense oligonucleotides. We also used the IB3 cell line, from a patient with CF, and the paired cell line S9, in which the CF defect has been corrected by transfection with full-length CFTR. Both CF-phenotype cell lines, AS and IB3, produced significantly more IL-8 in response to cytokine stimulation than the control cell lines. Pretreatment with parthenolide significantly inhibited cytokine-induced IL-8 production in all cell lines. The inhibition of IL-8 production was associated with inhibition of IKK activity, preservation of IκB, and inhibition of NF-κB translocation. We also examined our hypothesis in vivo using CF transmembrane conductance regulator (CFTR)-KO mice and control heterozygote +/− mice. These results also showed that parthenolide inhibited both IκB degradation and NF-κB activation induced by LPS in vivo, and reduced cytokine production and PMN influx into the lung. Parthenolide and/or feverfew may have beneficial therapeutic effects in CF.
MATERIALS AND METHODS
Cell Culture
We used two different sets of cell lines with CF defects and their corresponding controls. The first was the 16 HBE human bronchial epithelial cell line stably transfected with an AS oligonucleotide that inhibits expression of CFTR (4), and a sister cell line transfected with CFTR sense oligonucleotide (S) as its control. These were kindly provided by Dr. Pamela Davis (Case Western Reserve University, Cleveland, OH). The second pair of cell lines were IB3 cells from a patient with CF, and the control cell line, S9 cells, which have been rescued by transfection with full-length functional CFTR (19). These were purchased from ATCC (Manassas, VA). Cells were maintained in a 5% CO2 incubator at 37°C using modified Eagle's medium (Mediatech, Inc., Herndon, VA) for AS and S cell lines and LUC-8 media (Biosource, Camarillo, CA) for IB3 and S9 cell lines. All media contained penicillin/streptomycin and 10% fetal bovine serum.
Experimental Conditions
All cell lines were plated at a density of 1 × 106 cells/well on vitrogen-coated 24-well plates. Twenty-four hours after plating, the cells were switched to serum-free medium for 18 h. IL-8 production was induced by treating AS and S cell lines with TNF and IL-1β (Sigma, St. Louis, MO) used at concentrations of 100 ng/ml each, and IB3 and S9 with TNF at 30 ng/ml (4, 20). The viability of all cell lines was assayed by trypan blue exclusion. Cytotoxicity was determined by incubating the cells with 0.4% trypan blue (Sigma) and assessing the percentage of stained cells within each well.
Cell lines were pretreated with parthenolide (Sigma) for 1 h at the indicated concentration before treatment with TNF/IL-1β or TNF alone (AS/S and IB3/S9, respectively). After preliminary dose–response experiments, parthenolide was subsequently used at 40 μM and 15 μM in AS/S and IB3/S9 cell line pairs, respectively. Parthenolide was dissolved in dimethyl sulfoxide (DMSO), such that the final maximum concentration of DMSO in the media was 0.04%. Equivalent final concentrations of DMSO were added to controls. Cells were pretreated with parthenolide or vehicle alone for 1 h and then stimulated with the mixture of TNF/IL-1β or TNF alone for 15, 30, 180, and 360 min. Unstimulated controls were run in each experiment. At the end of each experiment, media were harvested and assayed for IL-8 with enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN). After removal of media, the epithelial cell monolayers were washed with ice-cold PBS, and cells were harvested by scraping and pelleted at 600 × g for 5 min at 4°C, then lysed with RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 0.5 mM PMSF) containing protease inhibitors cocktail (Sigma). Protein concentrations were determined using the Bradford method (Bio-Rad Laboratories, Hercules, CA) and data for IL-8 production were expressed as pg/mg cellular protein. Cytosolic and nuclear proteins were extracted according to manufacturer's instructions (BioVision Research Products, Mountain View, CA). Extracts were then used for analysis of IκB, protein kinases, and NF-κB.
In Vivo Experimental Model
Mice.
We used 7- to 10-wk old male “gut-corrected” CFTR-KO mice. These are stock CftrtmtUnc-TgN(FABPCFTR)#Jaw mice bearing the S489X mutation in cftr and also a transgene for full-length human CFTR driven by the fatty acid–binding promoter (FABP), which restores CFTR function in the intestinal tract. These mice are much heartier than non–gut-corrected mice, do not require liquid diet, and breed much more productively. We have previously found that these mice have the same excessive inflammatory response to Pseudomonas aeruginosa, when compared with wild-type (WT) mice, as do cftr−/− mice without the added transgene (6). All mice were maintained in sterile micro-isolator cages with sterile water.
Experimental model.
Two groups each of CFTR-KO (n = 59) and heterozygous −/+ (n = 12) mice were pretreated with parthenolide at 3 μg/g body weight or with DMSO (vehicle of parthenolide) in 200 μl saline intraperitoneally 1 h before LPS challenge. Then all mice were anesthetized with avertin and their tracheas were intubated with plastic catheters, through which they were challenged with 25 ng LPS (Escherichia coli serotype 0111:B4 from Sigma) in 50 μl PBS exactly as described previously (5). We have previously found that this dose of LPS induces a robust inflammatory response but does not cause systemic shock or early mortality. In addition, 14 unchallenged controls underwent bronchoalveolar lavage (BAL) for baseline cell count and cytokine concentrations.
Mice were killed 1, 3, or 8 h after intratracheal LPS challenge. Mice were randomized in advance as to the hour of killing. At the designated time, mice were anesthetized with avertin, BAL was performed (21, 22), then mice were exsanguinated, and lungs were perfused with ice-cold saline and removed. Lung homogenates for Western blots for IκBα and nuclear extracts for NF-κB were prepared as described (5, 6, 23).
BAL Fluid Analysis
Bronchoalveolar lavage was performed as previously described (5, 6, 21, 22). Briefly, a 22-gauge bead-tip needle was inserted into the trachea and ligated to prevent leakage, then six 0.5-ml aliquots of ice-cold PBS were instilled into the trachea. Each aliquot was allowed to dwell for 3 s, then gently aspirated and pooled. Total and differential cell counts were performed on 250-μl samples as previously described (21). The remainder was centrifuged at 1,000 × g for 10 min at 4°C, then PMSF and EDTA were added to the supernatants, which were aliquoted and stored at −70°C until analyzed for TNF-α, MIP2, and KC/N51 (KC) using commercial ELISA kits (R&D Systems, Minneapolis, MN).
Electrophoretic Mobility Shift Assay
Aliquots of nuclear extract from cell lines or lung homogenates (5 or 8 μg) were mixed in binding buffer (10 mM Tris-HCl, pH 7.5, 1 mM MgCl2, 50 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, 4% glycerol, 0.5 μg poly [dI-dC]) at room temperature for 10 min, then incubated with 32P-radiolabeled NF-κB consensus oligonucleotide, 5′−AGTTGAGGGGACTTTCCCAGGC-3′ (Promega, Madison, WI) for an additional 20 min. Protein binding of the oligonucleotide was analyzed by 4% nondenatured PAGE and autoradiography.
Immunoblotting for Phospho- and Total IκBα
Aliquots of cytosolic protein or tissue extracts (15 μg protein) were separated by 10% SDS-polyacrylamide gels electrophoresis and transferred onto nitrocellulose membranes. The Western blots were probed using rabbit polyclonal antibodies specific for phosphorylated, total IκBα and IκBβ, and immunoreactive proteins were visualized by enhanced chemiluminescence (5, 6). Blots were also probed with monoclonal anti–β-actin to correct for protein loading.
Immunoprecipitation and IκB Kinase Assay
Cell lysates were prepared using PhosphoSafe (Novagen, Madison, WI) containing protease and phosphatase inhibitors. Monoclonal IκKα antibody (Pharmingen, San Diego, CA) was used to immunoprecipitate IKK complexes from 250 μg of cell extracts as described previously for use in immunoprecipitation kinase assays (24). Kinase activity was assayed in 10 mM Hepes pH 7.6, 5 mM MgCl2, 50 mM NaCl, 20 mM b-glycerophosphate, 5 mM p-nitrophenyl phosphate, 0.1 mM Vanadate, 2 mM DTT, 30 μM “cold” ATP, and protease inhibitors (leupeptin, aprotenin, pepstatin), with 5 μCi γ[32P]ATP at 30°C for 30 min. Kinase activity was determined using GST-IκBα (1–54) WT as substrate, and kinase specificity was established with mutant GST-IκBα (1–54-AA) in which serines 32 and 36 were substituted with alanines (AA). Fold induction of IKK activity was determined after phosphorimaging of the dried SDS-PAGE–fractionated GST-IκB protein-containing gels.
Statistical Analysis
Two-factor ANOVAs were used with mouse groups and times for percent PMNs, total BAL cell counts, and bacteria clearance. For cytokine data, 0 was assigned to values below the limit of detection of the ELISA assays. Data are expressed as mean ± SEM.
RESULTS
CF Cell Lines Over-Produce IL-8 in Response to Proinflammatory Cytokine Stimulation
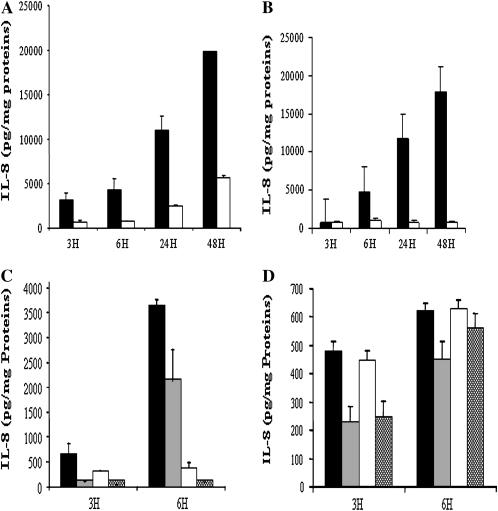
We first measured the kinetics of IL-8 secretion at 3, 6, 24, and 48 h after incubation with TNF/IL-1β (100 ng/ml each) in AS- and S-transfected 16 HBE cells. Stimulated AS cell lines produced very high concentrations of IL-8 (Figure 1A, solid bars), while in similarly stimulated S cells (Figure 1A, open bars) the increase was more modest. Similarly, IB3 cells (Figure 1B, solid bars) also increased IL-8 production much more than S9 cells (Figure 1B, open bars) in response to TNF (30 ng/ml) alone. Moreover, in agreement with Kube and coworkers (4), these results showed that the differences between CF cell lines and their controls widened over time, consistent with continued excessive NF-κB activation of gene transcription. Thus, these CF cell lines present an ideal in vitro model to test the effects and doses of putative inhibitors of NF-κB–dependent proinflammatory gene expression.
Figure 1.
(A) IL-8 production by CFTR AS (solid bars)- and S (open bars)-transfected 16 HBE cells upon stimulation with TNF/IL-1β and (B) by IB3 (solid bars) and S9 (open bars) upon stimulation with TNF. Results are expressed as pg IL-8 secreted/mg cellular protein. AS cells produced significantly more IL-8 compared with S cells (P = 0.003 at 6 h, P = 0.0005 at 24 h, and P = 0.0001 at 48 h). IB3 cells produced significantly more IL-8 as compared with S9 cells (P = 0.01 at 6 h, P = 0.005 at 24 h, and P = 0.001 at 48 h). (C) Parthenolide pretreatment, 40 μM, 1 h before TNF/IL1β in AS/S cells and (D) 15 μM, 1 h before TNF in IB3/S9. Results are from two to three combined experiments. Values represent the mean ± SEM. (C) In AS cells, parthenolide treatment (shaded bars) inhibited IL-8 secretion as compared with nontreated cells (solid bars) (P = 0.02 at 3 h), and in S cells parthenolide treatment (hatched bars) also inhibited IL-8 as compared with nontreated cells (open bars) (P = 0.03 at 6 h). Similarly, in IB3 cells (D) parthenolide treatment (shaded bars) inhibited IL-8 secretion as compared with nontreated cells (solid bars) (P = 0.02 at 3 h), and in S9 cells parthenolide treatment (hatched bars) also inhibited IL-8 as compared with nontreated cells (open bars) (P = 0.04 at 3 h).
Parthenolide Inhibits TNF/IL-1β– or TNF-Induced Production of IL-8
We next determined the effects of parthenolide pretreatment on TNF/IL-1β–mediated production of IL-8 by the AS/S cell pair and TNF-mediated IL-8 production by the IB-3/S9 cell pair. By trypan blue exclusion, we observed that more than 95% of AS/S cells were viable after 48 h of treatment with a range of parthenolide concentrations: from 5–40 μM. However, concentrations of parthenolide exceeding 20 μM resulted in cytotoxic effects on IB3/S9 cells. Thus, in IB3/S9 experiments, parthenolide was used at 15 μM, which had no toxic effect, and cells had a viability exceeding 95% after 24 h incubation. In all cases, control incubations contained the same final concentration of DMSO as in incubations containing parthenolide.
Pretreatment of the AS and S cells with parthenolide at 40 μM caused marked inhibition of the IL-8 production (Figure 1C, shaded and hatched bars). In both AS and S cells, IL-8 production at 3 h after stimulation was inhibited by 75–85%. Even though the IL-8 production by the AS cells was 10 times greater than that of the S controls at 6 h, this was still inhibited by 48% by parthenolide (Figure 1C). Similarly, in IB3 and S9 cells, pretreatment with parthenolide (15 μM) significantly inhibited TNF-mediated IL-8 production by 55% and 44% by 3 h, and 27% and 11% at 6 h, respectively (Figure 1D). Additional application of parthenolide, 1 h after stimulation, had further inhibitory effects, reducing the IL-8 production at 6 h after stimulation to less than 25% of that of untreated cells (data not shown). However, delaying the second application of parthenolide until 5 h after stimulation resulted in a diminished inhibitory effect, consistent with the suggestion that parthenolide acts at an early stage in the signaling that induces IL-8 production, rather than inhibiting synthesis or export of the protein per se. A similar pattern of inhibition was seen in the control S cell line.
Parthenolide Inhibits TNF/IL-1β–Induced Nuclear Translocation of NF-κB in Intact Cells
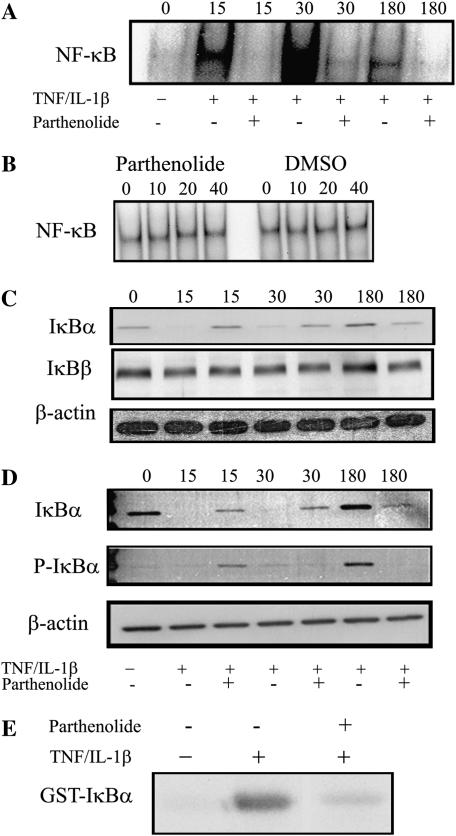
To study the mechanisms of the inhibitory effects of parthenolide on TNF/IL-1β–mediated production of IL-8, we first determined whether parthenolide could suppress TNF/IL-1β–induced NF-κB activation and translocation to the nucleus. To do so, AS cell lines were pretreated with 40 μM of parthenolide for 1 h, then stimulated with TNF/IL-1β for the indicated times. Nuclear extracts were prepared from the cells and DNA-binding activity of NF-κB was measured by EMSA (5, 6, 23). Unstimulated control cells did not have detectable active nuclear NF-κB (Figure 2A, lane 1 = 0 time). However, incubation with TNF/IL-1β markedly increased nuclear translocation of NF-κB, which was easily observed at 15 min (Figure 2A, lane 2) and reached a maximum at 30 min (Figure 2A, lane 4), then decreased by 180 min (Figure 2A, lane 6). Pretreatment with parthenolide decreased nuclear NF-κB at all time points (Figure 2A, lanes 3, 5, and 7). These data suggest that the decreased activation of NF-κB is responsible for the inhibition of IL-8 secretion.
Figure 2.
AS cells were pretreated with 40 μM parthenolide or vehicle for 1 h and stimulated with TNF/IL-1β for the indicated time (shown as numbers at top of A, C, and D). Subsequently, nuclear and cytosolic or total cell protein extracts were prepared and then analyzed for different parameters. Cells not stimulated with TNF/IL-1β are shown as 0 time. (A) AS cells stimulated with TNF/IL-1β demonstrated strong activation of NF-κB at 15 and 30 min, which is inhibited with parthenolide. (B) In vitro effects of parthenolide on NF-κB DNA binding. AS cells were stimulated with TNF/IL-1β for 30 min, then cell nuclear extracts were incubated in vitro with the indicated concentrations of parthenolide or vehicle for 1 h before the EMSA with 32P-labeled NF-κB consensus binding oligonucleutide. Results showed that neither parthenolide nor DMSO altered the ability of the activated NF-κB to bind to the oligonucleotide in vitro. (C) Cytosol extract from same experiment as in A. Stimulation with TNF/IL-1β demonstrated complete degradation of cytosolic IκBα, and parthenolide prevented its degradation (top row); however, IκBβ did not change with TNF/IL-1β nor with parthenolide treatment (middle row). The bottom row shows β-actin to assure equal protein loading. (D) Total IκBα (top row) and p-IkBα (middle row); the lanes are similar to those in C. (E) IKK assay. Extracts from cells pretreated with/or without parthenolide for 1 h before stimulation with TNF/IL-1β for 30 min were subject to immunoprecipitation with antibody against IκKα, then kinase activity was assayed with γ32P-ATP assay using GST-IκBα as substrate. An autoradiogram of an SDS gel from a typical experiment is shown. There was no detectable activity in unstimulated cells (lane 1). TNF/IL-1β strongly activated IKK (lane 2), and parthenolide dramatically inhibits its activation (lane 3).
Effects of Parthenolide on DNA Binding of Active NF-κB in Cell Extracts
Two major mechanisms of action of parthenolide have been suggested: inhibition of activation of IκB, secondarily inhibiting the activation and release of NF-κB from the cytoplasmic IκB complex; and direct binding to NF-κB, preventing its interaction with DNA. To understand which of these might be more important in decreasing production of cytokines from CF cells, we further investigated the level at which parthenolide acts. AS cells were stimulated as above, in the absence of parthenolide, then various concentrations of parthenolide (10, 20, or 40 μM) or DMSO alone were added directly to nuclear extracts and incubated at room temperature for 1 h. 32P-oligonucloeotide probe was then added, and the samples were run out on nondenatured PAGE gels. Results of autoradiograms showed that neither parthenolide nor DMSO altered the ability of the activated NF-κB to bind to the DNA oligonucleotide in vitro (Figure 2B). This strongly suggests that parthenolide acts at a proximal step, preventing the activation of NF-κB, rather than by preventing the nuclear translocation or DNA binding capabilities of activated NF-κB.
Parthenolide Inhibits TNF/IL-1β–Induced Degradation of IκBα
Since degradation of IκB proteins is an essential step for NF-κB activation by diverse stimuli, we examined the effect of parthenolide on the phosphorylation and degradation of IκBα protein induced by TNF and IL-1β. AS cells were pretreated with 40 μM of parthenolide for 1 h, and subsequently stimulated with TNF/IL-1β for the indicated times. The gels shown in Figure 2C are from the same experiment as in Figure 2A, and use cytosol from cells whose nuclei had been separated to use for assays of NF-κB. The results in the top row of Figure 2C show that IκBα was readily detectable in unstimulated AS cells (lane 1, 0 time), but that it was consumed, and undetectable within 15 min after stimulation with TNF/IL-1β in cells not treated with parthenolide (Figure 2C, top row, lanes 2 versus 1). The IκBα had not been replaced by 30 min (lane 4). In contrast, in parthenolide-treated cells, there was marked inhibition of IκBα degradation, with preservation of this protein at 15 and 30 min (Figure 2C, top row, lanes 3 and 5). At 180 min, there is a large increase in the IκBα protein in cells stimulated in the absence of parthenolide (lane 6), but there is much less IκBα in the presence of parthenolide (lane 7). These observations suggest that in the absence of parthenolide, new synthesis of IκBα has been induced by active NF-κB and that this protein is rapidly phosphorylated by persistently active Iκ-Kinase (below). This was not observed in the presence of parthenolide, since there was much less active NF-κB (and presumably much less newly synthesized IκBα) in those cells. Since IL-8 production was not completely inhibited by parthenolide, the latter results are consistent with some activity of IKK causing slight consumption of IκBα over the 3 h.
In contrast to the above results with IκBα, IκBβ protein did not differ in response to TNF/IL-1β stimulation nor to parthenolide treatment (Figure 2C, middle row). The bottom row shows β-actin to demonstrate equal protein loading.
We also studied the phosphorylated intermediate, pIκBα, to assist in determining the level at which parthenolide acts. In Figure 2D total cell protein extracts were prepared from AS cells that were treated the same as those in Figure 2B. In this set of experiments the effects of stimulation in the absence and presence of parthenolide were similar to those shown above in Figure 2C. Only a small amount of phosphorylated IκBα was observed in extracts from non–parthenolide-treated cells at 15 or 30 min (Figure 2D, middle row, lanes 2 and 4), suggesting that under these conditions, most of the phosphorylated protein is rapidly degraded. Some phosphorylated IκB was detectable in the presence of parthenolide at 15 min (Figure 2D, middle row, lane 3). Similarly to what was seen in Figure 2C, at 180 min there is a large increase in the phosphorylated IκBα (Figure 2D, middle row, lane 6), which was not seen in the presence of parthenolide (lane 7). This suggests that the newly synthesized IκBα has been phosphorylated by IKK, which continues to remain active, but that the phosphorylated IκBα has not been degraded. The bottom row shows β-actin to demonstrate equal protein loading.
To determine directly if parthenolide mediates its anti-inflammatory effects through inhibition of IKK, AS cells were pretreated with parthenolide or DMSO alone, then 1 h later stimulated with TNF/IL-1β for 30 min, the time point that corresponds to maximum NF-κB activation, then IKK was immunoprecipitated and its activity was assayed using in vitro Iκ-Kinase assays using (GST)-IκB (residues 1–54) fusion protein and 32P-ATP as substrates (24). The results are shown in Figure 2E. There was little active IKK in unstimulated cells (left lane). TNF/IL-1β stimulation dramatically increased IKK activity (middle lane). In contrast, parthenolide essentially abolished this TNF/IL-1β–stimulated kinase activity (right lane).
Effects of Parthenolide on Response to LPS in CFTR-KO Mice
To test the hypothesis that parthenolide can attenuate the excessive inflammatory response in the lungs of CFTR-KO mice in vivo, we pretreated CFTR-KO mice with 3 μg/gram body weight parthenolide or vehicle (DMSO) intraperitoneally 1 h before inoculating them intratracheally with 25 ng of LPS. We have previously described the excessive response of CFTR-KO mice to LPS in this model (5), and this dose of parthenolide was chosen based on studies of the efficacy of parthenolide in other in vivo systems (25). BAL was performed at various times (1, 3, or 8 h) after the LPS treatment to measure PMN influx and cytokine production, then lungs were removed for analysis of IκB and NF-κB.
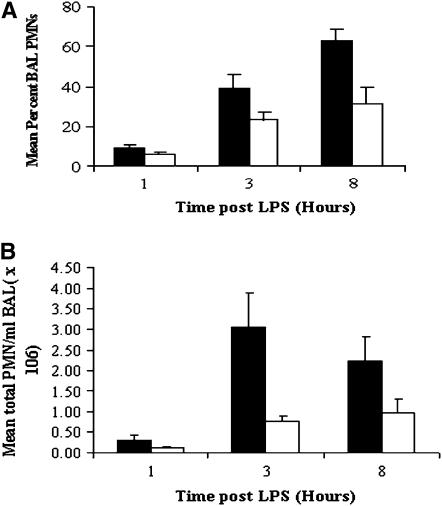
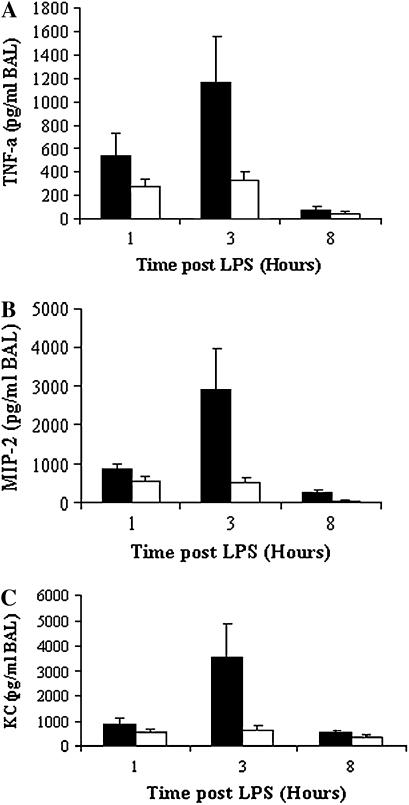
CFTR-KO mice not pretreated with parthenolide had increasing percentages of PMNs over time after intratracheal LPS challenge: (9 ± 1.54% at 1 h, 38.8 ± 7.23% at 3 h, and 63 ± 6.0% at 8 h) (Figure 3A, solid bars). Pretreatment with parthenolide caused marked reductions in the percent PMN in BAL at all time points (Figure 3A, open bars), which were highly significant at 8 h (P = 0.006) as compared with untreated controls. Similar results were seen for the absolute number of PMNs (Figure 3B). In agreement with previous results (5), LPS challenge in CFTR-KO mice induced extremely high levels of the cytokine TNF, and of the chemokines MIP2 and KC, in BAL fluid at all time points, but particularly at 3 h. In parallel with the inhibition of PMN influx by parthenolide, pretreatment with this drug caused marked reductions in the concentration in BAL of TNF (Figure 4A, open bars) and also the major chemoattractants, MIP2 (Figure 4B, open bars) and KC (Figure 4C, open bars) at all time points. The decreased production of NF-κB–dependent chemokines suggest that parthenolide may have similar actions in vivo to those we had detected in the bronchial epithelial cell lines in vitro.
Figure 3.
Effects of in vivo parthenolide treatment on percent and absolute numbers of PMNs in BAL fluid in LPS-challenged CFTR-KO mice. Mice were pretreated with parthenolide (3 μg/g body weight) (open bar) or placebo (solid bar) for 1 h then challenged intratracheally with LPS, and BAL was performed at the indicated times. (A) Mean ± SEM of the percent PMNs in BAL fluid 1, 3, and 8 h after LPS administration (n = 4–11 mice in each group). Parthenolide-treated groups had lower percentage of PMNs in BAL fluid at all time points, which was highly significant at 8 h after LPS (P = 0.006) as compared with placebo group. (B) Total PMNs per milliliter of BAL fluid (mean ± SEM). Parthenolide groups had significantly less total PMN in BAL fluid than the placebo groups at 3 h (P = 0.04) and 8 h (P = 0.03) after LPS.
Figure 4.
BAL fluid proinflammatory cytokines in CFTR-KO mice treated with parthenolide (open bar) or placebo (solid bar). Mice were pretreated with parthenolide 3 μg/g body weight or DMSO alone (placebo) 1 h before intratracheal LPS challenge. BAL was performed at the indicated time. Values represent the mean ± SEM of the concentration (pg/ml) of TNF-α, MIP2, and KC in BAL fluid 1, 3, and 8 h after LPS (n = 6–11 mice in each group). Parthenolide-treated mice had significantly more TNF-α (A) (P = 0.03), MIP2 (B) (P = 0.04), and KC (C) (P = 0.05) at 3 h after LPS as compared with placebo group.
Effects of Parthenolide on Degradation of IκBα and Activation of NF-κB in the CF Lung
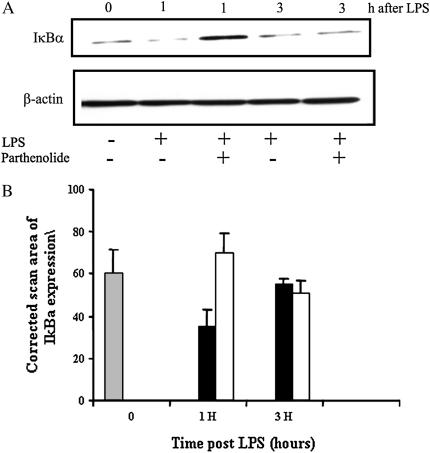
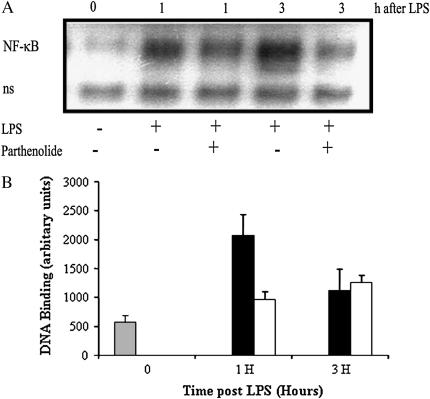
To determine if the intracellular mechanisms by which parthenolide treatment attenuates the excessive inflammatory response in vivo are similar to those by which it inhibits proinflammatory responses by airway epithelial cells in vitro, we evaluated the degradation of IκBα and the subsequent activation of NF-κB in the lung tissue. After BAL to remove infiltrating leukocytes from the airspaces and airways, the lungs were perfused through the right ventricle until they blanched, to remove circulating blood cells. They were then removed and homogenized. IκBα was measured by Western blot in total cell extracts and NF-κB was measured in nuclear extracts by EMSA. As shown in the representative Western blots and summary figure (Figures 5A and 5B), at 1 h after LPS administration, IκBα was markedly reduced in the mice given LPS without parthenolide, while it was increased in the parthenolide-treated mice. There was little difference in the IκBα content of parthenolide-treated versus untreated mice at 3 h. The results for NF-κB by EMSA (Figures 6A and 6B) appear as the inverse of those for IκBα. There is clearly less active NF-κB in parthenolide-treated mice at 1 h than in placebo-treated mice (Figure 6A, lane 3 and graph, middle set of bars). Although the gel in the figure shows more active NF-κB in the sample taken at 3 h in the absence of parthenolide than in its presence, the difference at this time point was not consistently found in the multiple experiments summarized in the graph in Figure 6B.
Figure 5.
Effects of in vivo treatment with parthenolide on IκBα degradation in the CF lung. Mice were pretreated with parthenolide 3 μg/g body weight or placebo 1 h before intratracheal LPS challenge, then underwent BAL and the lungs were perfused and removed at the indicated times. (A) Representative autoradiograph of an immunoblot for IκBα. (B) Summary graph of data for degradation of IκBα determined by image analysis of densitometry from autoradiographs. Data show mean ± SEM of scan area of IκBα expression (n = 3 mice in each time point and group) corrected for scan area of β-actin in the same gel lane. Solid bar, placebo; open bar, parthenolide-treated mice.
Figure 6.
Effect of in vivo treatment with parthenolide on the activation of NF-κB in the CF lung. Mice were pretreated with parthenolide 3 μg/g body weight or placebo 1 h before intratracheal LPS challenge. Mice underwent BAL and the lungs were perfused, removed at the indicated times, and nuclei were prepared and extracted for EMSA. (A) Representative autoradiograph of EMSA for activated NF-κB. 0 = mice not challenged with LPS. Results are shown for 1 and 3 h, with (+) and without (−) parthenolide. (B) Image analysis of activation of NF-κB determined by densitometry from two separate time course experiments (n = 5 mice in each time points and group). Mean ± SEM are shown. Parthenolide-treated mice (open bar) had significantly increased NF-κB activation at 1 h after LPS as compared with placebo group (solid bar) (P = 0.05). (“ns” in lower row is “non-specifically bound”.)
DISCUSSION
Cystic fibrosis is characterized by a chronic, destructive inflammatory process in which massive numbers of PMNs and their products damage the airways (1, 2). Extremely high concentrations of the NF-κB–dependent cytokines TNF-α, IL-1β, IL-6, and IL-8 are believed to play a major role in initiating and perpetuating this inflammatory process, and in mediating its systemic consequences such as cachexia and hypergammaglobulinemia (26, 27). A considerable body of evidence suggests that in CF, the inflammatory response in the lung is dysregulated, and is excessive relative to the burden of infection (28–30). Data from our own and other labs show that CFTR-KO mice have similarly excessive inflammatory responses in the lung, which are also characterized by extremely increased production of NF-κB–dependent cytokines and chemokines, increased PMN influx into the lung, and increased weight loss and mortality in response to LPS or P. aeruginosa challenge (3, 5, 6, 31). The NF-κB family of transcription factors activates the genes for the proinflammatory cytokines, chemokines, and adhesion molecules whose expression are greatly increased in human CF and in mouse models of this disease (3, 5, 6, 27, 29–31). NF-κB normally resides in the cytosol in an inactive complex with an IκB family member such as IκBα, which prevents the NF-κB from entering the nucleus and activating DNA transcription (8). Multiple signaling pathways, from Toll-like receptors of the innate immune systems, as well as from receptors for cytokines such as IL-1 and TNF-α, converge through a cascade of adaptors and “upstream kinases” that result in activation of IKK and phosphorylation of IκB. After phosphorylation of IκBα, it is ubiquitinated and degraded. This frees NF-κB, which rapidly enters the nucleus, binds to the promoters of the proinflammatory cytokines and chemokines, and increases their transcription.
Recently, we showed that after challenge with P. aeruginosa or the prototypic TLR ligand, LPS, CFTR-KO mice have excessive and prolonged activation of NF-κB, which is associated with profound and sustained depletion of IκB (5, 6). Based on this, and on in vitro data, we and others have hypothesized that dysregulation of the IκB→NF-κB pathway plays a major role in the destructive inflammatory process in the CF lung in vivo. Thus, the IκB→NF-κB pathway is likely to be a good target for therapy in CF lung disease. Indeed, previous studies have shown that corticosteroids and nonsteroidal anti-inflammatory agents (which, among other effects, inhibit NF-κB activation) have beneficial activity in CF lung disease (32). Unfortunately, adverse effects limit the widespread use of these agents, and new therapies are needed.
In the present study, we studied the anti-inflammatory effects of parthenolide, a sesquiterpene lactone derived from the widely available medicinal plant, feverfew (Tanacetum parthenium). The medicinal properties of feverfew have been recognized for centuries, and many people use it for prevention and/or relief of migraine as well as for anti-inflammatory effects in arthritis (11, 25). Parthenolide significantly reduced neutrophil influx into the lung and reduced cytokines and chemokines in BAL of CFTR-KO mice challenged intratracheally with LPS. Similarly, parthenolide caused marked inhibition of the excessive IL-8 response in vitro, in cell lines with defective CFTR expression. Both in vitro and in vivo, these anti-inflammatory effects of parthenolide were associated with inhibition of IκBα depletion, which in turn resulted in inhibition of excessive activation of NF-κB. The IKK assays, which showed that parthenolide inhibited activity of this enzyme complex, together with the results in which parthenolide failed to inhibit binding of already activated NF-κB to oligonucleotides, suggests that the major locus of action of parthenolide in these cells is at or proximal to IKK, rather than on the transcription factor itself.
Although much attention has been focused on the ability of parthenolide to inhibit cellular processes that depend on NF-κB activity, there is controversy over the actual site of these actions. Some results suggest that parthenolide directly alkylates NF-κB and prevents it from binding to DNA (33), while other studies indicate that it inhibits I-κK and/or reacts directly with IκBα, preventing its phosphorylation (12–14, 16). Other studies suggest that parthenolide can also alter the function of other kinases that regulate inflammation (34–36). These effects are not necessarily exclusive, and may differ in different model systems. The results of our studies with Western blots of IκBα clearly indicate that parthenolide blocked degradation of IκBα, both in bronchial epithelial cell lines in vitro, and in the lung in vivo. Since in the latter studies, infiltrating inflammatory cells were removed by BAL, and blood was removed by perfusion before the lungs were excised and homogenized, it is likely that these results reflect Iκ-Kinase and NF-κB activity in lung parenchymal cells. Collectively, these data indicate that parthenolide acts proximally to the degradation of IκBα, by inhibiting IKK and/or its activity. These results are concordant with previous studies of Hehner and colleagues, who were the first to demonstrate that parthenolide inhibited degradation of IκBα and subsequent NF-κB activation in Jurkat and HeLA cells (12). In a subsequent in vitro study, they found that parthenolide completely prevented activation of Iκ-Kinase (13). They thus concluded that parthenolide acted on the IKK complex itself rather than by alkylating NF-κB. Mazor and coworkers also showed that parthenolide inhibited IL-8 production as well as IL-8 mRNA expression in the A549 human adenocarcinoma cell line due to inhibition of IκBα degradation and subsequent NF-κB activation (37). In contrast, however, Rungeler and colleagues suggested that the major effects of parthenolide were on NF-κB itself, preventing its interaction with DNA (33). The experiments in which we added parthenolide to the extracts of already activated cells showed no inhibition of the binding of the already activated NF-κB to DNA. This suggests that this mechanism did not play a major role in the experimental systems we studied. Thus, we conclude that parthenolide was acting at or before the level of the IκK complex activity.
Determination of the effects of this compound in vivo is of the greatest relevance. That is why we studied the effect of parthenolide in intact CFTR-KO mice. We have previously shown that CFTR-KO mice had excessive and prolonged inflammation in response to challenge with LPS and P. aeruginosa. This was associated with prolonged depletion of IκB and increased activity of NF-κB (5, 6). In this work we again showed that CFTR-KO mice had an excessive inflammatory response to intratracheal LPS, with increased influx of PMN compared with non-CF controls, and elevated production of TNF and the chemokines MIP2 and KC. Pretreatment with parthenolide caused a marked reduction in the percent PMN in BAL and also in the total cell counts, and the mean absolute numbers of PMN recovered from the airways. This was accompanied by a marked decrease in the BAL concentrations of TNF and the major chemoattractants, KC and MIP2, which was observed at all time points. The reduction in these cytokines is likely responsible for the decreased PMN influx and are indicative of anti-inflammatory activity of parthenolide in vivo. Sheehan and coworkers also showed that parthenolide reduced lung neutrophil infiltration and ameliorated cardiovascular derangement in endotoxic shock in rodents by inhibiting NF-κB (17). However, in that study, LPS was given intravenously and the authors concluded that parthenolide directly interfered with the DNA-binding activity of NF-κB, whereas IκBα degradation was unchanged (17). In a separate study, the same group reported that parthenolide protects against myocardial ischemia and reperfusion injury in rats by selective inhibition of IKK activation and IκBα degradation (18). Our results suggest that the main site of action of parthenolide is inhibiting IκBα degradation and that the effects on NF-κB may be secondary. Studies by Yip and colleagues corroborate our results (38). They reported that parthenolide inhibited degradation of IκB and activation of NF-κB in response to LPS in mouse osteoclast models in vivo and in vitro (38). Discrepancies in the apparent mechanisms of the in vivo effects of parthenolide in different models may be due to the differences in the experimental animals and the dose and timing of parthenolide treatment. Nevertheless, all of these studies agree on the benefits of parthenolide treatment in reducing inflammation by net inhibition of NF-κB.
The IκB→NF-κB pathway may not be the sole target of parthenolide in vivo. Other transcription factors and/or pathways may be involved as well. p38 MAP kinase and Jun N-terminal kinase (JNK) have been implicated in TNF production and/or signaling (34, 39, 40), and ERK may also participate (41). Several reports suggest that parthenolide can inhibit p38 MAPK, JNK, and other kinases and may stabilize some phosphorylated intermediates (34–36, 41). Determining whether those effects are important in CF will be the subject of future work.
Finding safe and effective ways to ameliorate the excessive inflammation in the CF lung continues to be a major research objective. Other drugs whose actions include preventing degradation of IκB and decreasing proinflammatory cytokine production caused reductions in PMN infiltrate, lung inflammation, and weight loss, without increasing the burden of bacteria or causing sepsis in patients with CF. However, adverse effects of steroids and ibuprofen have limited their use (42, 43). IL-10 has similar effects in mouse models (5), but has been withdrawn from human trials. Since we have demonstrated that parthenolide exerts anti-inflammatory effects, with both in vitro and in vivo models of CF, feverfew and/or parthenolide itself may be considered as candidates for therapy in CF disease. Alternatively, parthenolide could be considered a “lead compound” for further drug development.
In summary, our results demonstrate that parthenolide, a sesquiterpene lactone, is a powerful anti-inflammatory agent in cellular and animal models of CF, and support the proposal that natural compounds may be used for development of new therapies to inhibit the inflammatory processes that lead to lung destruction and death in patients with CF. However, further studies are needed to make sure that the use of parthenolide or other anti-inflammatory agents in CF is effective without inhibiting important host defense mechanisms. This is of critical interest in CF.
Acknowledgments
The authors thank Dr. Pamela Davis for gifts of antisense and sense cell lines. They also thank Alma Gente Wilson, Anna van Heeckeren, and other members of the Animal Core of the Case Western Reserve University Cystic Fibrosis Center for providing us with mice.
This work was supported by NIH grants AT 002159 (to M.B.), DK27651 (to Pamela B. Davis), and NIH RO1 CA84406 (to J.D).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0323OC on February 1, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Berger M, Konstan MW. Immunopathogenesis of Cystic Fibrosis Lung Disease. In: Cystic fibrosis in adults. Yankaskas J, Knowles M, editors. Philadelphia, PA: Lippincott-Raven; 1999. pp. 115–143.
- 2.DeRose V. Mechanisms and markers of airway inflammation in cystic fibrosis. Eur J Immunol 2002;19:333–340. [DOI] [PubMed] [Google Scholar]
- 3.Freedman SD, Weinstein D, Blanco PG, Martinez-Clark P, Urman S, Zaman M, Morrow JD, Alvarez JG. Characterization of LPS-induced Lung inflammation in cftr−/− mice and the effectg of docosahexaenoic acid. J Appl Physiol 2002;92:2169–2176. [DOI] [PubMed] [Google Scholar]
- 4.Kube D, Sontich U, Fletcher D, Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol 2001;280:L493–L502. [DOI] [PubMed] [Google Scholar]
- 5.Saadane A, Soltys J, Berger M. Role of IL-10 deficiency in excessive nuclear factor-kappaB activation and lung inflammation in cystic fibrosis transmembrane conductance regulator knockout mice. J Allergy Clin Immunol 2005;115:405–411. [DOI] [PubMed] [Google Scholar]
- 6.Saadane A, Soltys J, Berger M. Acute Pseudomonas challenge in cystic fibrosis mice causes prolonged nuclear factor-kappa B activation, cytokine secretion, and persistent lung inflammation. J Allergy Clin Immunol 2006;117:1163–1169. [DOI] [PubMed] [Google Scholar]
- 7.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl- channel function. Am J Physiol Lung Cell Mol Physiol 2001;281:L71–L78. [DOI] [PubMed] [Google Scholar]
- 8.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 2000;18:621–663. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich M. Ethnopharmacology of mexican asteraceae (compositae). Annu Rev Pharmacol Toxicol 1998;38:539–565. [DOI] [PubMed] [Google Scholar]
- 10.Heptinstall S, Groenewegen WA, Spangenberg P, Losche W. Inhibition of platelet behaviour by feverfew: a mechanism of action involving sulphydryl groups. Folia Haematol Int Mag Klin Morphol Blutforsch 1988;115:447–449. [PubMed] [Google Scholar]
- 11.Jain NK, Kulkarni SK. Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J Ethnopharmacol 1999;68:251–259. [DOI] [PubMed] [Google Scholar]
- 12.Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem 1998;273:1288–1297. [DOI] [PubMed] [Google Scholar]
- 13.Hehner SP, Hofmann TG, Droge W, Schmitz ML. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol 1999;163:5617–5623. [PubMed] [Google Scholar]
- 14.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol 2001;8:759–766. [DOI] [PubMed] [Google Scholar]
- 15.Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett 1997;402:85–90. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Franco O, Suzuki Y, Sanjuan G, Blanco J, Hernandez-Vargas P, Yo Y, Kopp J, Egido J, Gomez-Guerrero C. Nuclear factor-kappa B inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol 2002;161:1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheehan M, Wong HR, Hake PW, Malhotra V, O'Connor M, Zingarelli B. Parthenolide, an inhibitor of the nuclear factor-kappaB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol Pharmacol 2002;61:953–963. [DOI] [PubMed] [Google Scholar]
- 18.Zingarelli B, Hake PW, Denenberg A, Wong HR. Sesquiterpene lactone parthenolide, an inhibitor of IkappaB kinase complex and nuclear factor-kappaB, exerts beneficial effects in myocardial reperfusion injury. Shock 2002;17:127–134. [DOI] [PubMed] [Google Scholar]
- 19.Zeitlin PL, Lu L, Rhim J, Cutting G, Stetten G, Kieffer KA, Craig R, Guggino WB. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12–SV40 infection. Am J Respir Cell Mol Biol 1991;4:313–319. [DOI] [PubMed] [Google Scholar]
- 20.Venkatakrishnan A, Stecenko AA, King G, Blackwell TR, Brigham KL, Christman JW, Blackwell TS. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol 2000;23:396–403. [DOI] [PubMed] [Google Scholar]
- 21.Chmiel JF, Konstan MW, Berger M. Murine models of CF airway infection and inflammation. Methods Mol Med 2002;70:495–515. [DOI] [PubMed] [Google Scholar]
- 22.Chmiel JF, Konstan MW, Saadane A, Krenicky JE, Kirchner LH, Berger M. Prolonged inflammatory response to acute Pseudomonas challenge in interleukin-10 knockout mice. Am J Respir Crit Care Med 2002;165:1176–1181. [DOI] [PubMed] [Google Scholar]
- 23.Lentsch AB, Shanley TP, Sarma V, Ward PA. In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest 1997;100:2443–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiDonato JA. Assaying Ikappa B kinase activity. Methods Enzymol 2000;322:393–400. [DOI] [PubMed] [Google Scholar]
- 25.Reuter U, Chiarugi A, Bolay H, Moskowitz MA. Nuclear factor-kappaB as a molecular target for migraine therapy. Ann Neurol 2002;51:507–516. [DOI] [PubMed] [Google Scholar]
- 26.Beutler B, Cerami A. The biology of cachectin/TNF-a primary mediator of the host response. Annu Rev Immunol 1989;7:625–655. [DOI] [PubMed] [Google Scholar]
- 27.Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol 1999;104:72–78. [DOI] [PubMed] [Google Scholar]
- 28.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 1995;152:2111–2118. [DOI] [PubMed] [Google Scholar]
- 29.Muhlebach MS, Noah TL. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. Am J Respir Crit Care Med 2002;165:911–915. [DOI] [PubMed] [Google Scholar]
- 30.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 1999;160:186–191. [DOI] [PubMed] [Google Scholar]
- 31.van Heeckeren AM, Schluchter MD, Xue W, Davis PB. Response to acute lung infection with mucoid Pseudomonas aeruginosa in cystic fibrosis mice. Am J Respir Crit Care Med 2006;173:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med 1995;332:848–854. [DOI] [PubMed] [Google Scholar]
- 33.Rungeler P, Castro V, Mora G, Goren N, Vichnewski W, Pahl HL, Merfort I, Schmidt TJ. Inhibition of transcription factor NF-kappaB by sesquiterpene lactones: a proposed molecular mechanism of action. Bioorg Med Chem 1999;7:2343–2352. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Kartha S, Iasovovskaia S, Tan A, Bhart RK, Manaligod JM, Page K, Brasier AR, Hershenson MB. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am J Physiol Lung Cell Mol Physiol 2002;283:L390–L399. [DOI] [PubMed] [Google Scholar]
- 35.Hwang D, Fischer NH, Jang BC, Taq H, Kim JK, Lee W. Inhibition of the expression of induceble cyclooxygenase and proinflammatory cytokines by sesquiterpene lactones in macrophages correlates with the inhibition of MAP kinase. Biochem Biophys Res Commun 1996;226:810–818. [DOI] [PubMed] [Google Scholar]
- 36.Uchi H, Arrighi JF, Aubry JP, Furue M, Hauser C. The sesquiterpene lactone parthenolide inhibits LPS- but not TNF-alpha-induced maturation of human monocyte-derived dendritic cells by inhibition of the p38 mitogen-activated protein kinase pathway. J Allergy Clin Immunol 2002;110:269–276. [DOI] [PubMed] [Google Scholar]
- 37.Mazor RL, Menendez IY, Ryan MA, Fiedler MA, Wong HR. Sesquiterpene lactones are potent inhibitors of interleukin 8 gene expression in cultured human respiratory epithelium. Cytokine 2000;12:239–245. [DOI] [PubMed] [Google Scholar]
- 38.Yip KH, Zheng MH, Feng HT, Steer JH, Joyce DA, Xu J. Sesquiterpene lactone parthenolide blocks lipopolysaccharide-induced osteolysis through the suppression of NF-kappaB activity. J Bone Miner Res 2004;19:1905–1916. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol 2002;72:847–855. [PubMed] [Google Scholar]
- 40.Bian ZM, Elner SG, Yoshida A, Kunkel SL, Su J, Elner VM. Activation of p38, ERK1/2 and NIK pathways is required for IL-1beta and TNF-alpha-induced chemokine expression in human retinal pigment epithelial cells. Exp Eye Res 2001;73:111–121. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM. Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J Nutr 2005;135:2096–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai HC, FitzSimmons SC, Allen DB, Kosorok MR, Rosenstein BJ, Campbell PW, Farrell PM. Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N Engl J Med 2000;342:851–859. [DOI] [PubMed] [Google Scholar]
- 43.Oermann CM, Sockrider MM, Konstan MW. The use of anti-inflammatory medications in cystic fibrosis: trends and physician attitudes. Chest 1999;115:1053–1058. [DOI] [PubMed] [Google Scholar]