Abstract
Discussions of the initiation of pulmonary arterial hypertension (PAH) in man and in experimental models have centered around intimal and medial proliferation in medium-sized pulmonary arteries. The histologic events are thought to include disordered proliferation of enlarged, vacuolated endothelial cells, neo-muscularization of the affected blood vessels, and vascular pruning. The discovery of the association of familial and sporadic PAH with mutations in BMPR2 has generated intense interest in cytokine receptor trafficking and function in the endothelial cell and how this might be disrupted to yield an enlarged proliferative cell phenotype. Nevertheless, considerations of the subcellular machinery of membrane trafficking in the endothelial cell and consequences of the disruption of this outward and inward membrane trafficking are largely absent from discussions of the pathobiology of PAH. Long-standing electron microscopy data in the PAH field has demonstrated marked disruptions of intracellular membrane trafficking in human and experimental PAH. Further, a role of the membrane-trafficking regulator Nef in simian HIV-induced PAH in macaques and in HIV-induced PAH in man is now evident. Additionally, monocrotaline and hypoxia are known to disrupt the function of Golgi tethers, SNAREs, SNAPs, and N-ethylmaleimide–sensitive factor (“the Golgi blockade hypothesis”). These results, along with recent reports demonstrating the trapping of PAH-associated human BMPR2 mutants in the Golgi, highlight the implications of disrupted intracellular membrane trafficking in the pathobiology of PAH. The purpose of this review is to present a brief overview of the molecular basis of intracellular trafficking and relate these considerations to the pathobiology of PAH.
Keywords: pulmonary hypertension, endothelium, Golgi organelle, vesicular trafficking
CLINICAL RELEVANCE
This review highlights an altogether novel way of thinking about the pathobiology of pulmonary arterial hypertension. We posit that the underlying pathogenesis, irrespective of etiology, is related to a disruption of intracellular trafficking.
ELECTRON MICROSCOPY OF CELLULAR ELEMENTS IN ARTERIAL LESIONS IN PULMONARY HYPERTENSION
The hallmark plexiform or onionskin lesions in the intra-alveolar pulmonary arteries in idiopathic pulmonary arterial hypertension (PAH) consist of disordered collections of enlarged (“megalocytotic”) endothelial cells, fibroblasts, and smooth muscle cell elements which lead to reduced arterial lumen, cycles of thrombosis and recanalization, and eventually to progressive right ventricular hypertrophy and cardiac failure (1–3). Electron microscopic (EM) data collected over the last three to four decades reveals that these “plump,” “enlarged,” and “vacuolated” cellular elements contain increased endoplasmic reticulum (ER), Golgi stacks, vacuolation, and Weibel-Palade bodies (these are exocytic vesicles) (4–6). Additional data emphasize the observation of dramatic “tubuloreticular” structures on electron microscopy of pulmonary arterial lesions in patients with HIV-1–induced PAH (7), again indicative of disrupted intracellular trafficking. In an insightful study, Marecki and coworkers report that simian HIV carrying a functional Nef protein, but not one without Nef, could induce PAH in macaques, and that there was enhanced accumulation of Nef in endothelial cells in plexiform lesions in lungs of patients with HIV-induced PAH (8). Remarkably, Nef is known to be a regulator of intracellular trafficking from the trans-Golgi to plasma membrane as well as of clathrin-mediated endocytosis (9–11). Thus, the Nef data (8–11) taken together point to the causal involvement of disrupted membrane trafficking in the pathobiology of PAH.
Turning to the monocrotaline (MCT)- and hypoxia-induced models of experimental PAH, marked alterations in intracellular membrane structures in the cytoplasm of endothelial, smooth muscle, and type II alveolar epithelial cells evident by EM have been reported beginning as early as 40 years ago (12–19). Merkow and Kleinerman reported that rats administered MCT developed pulmonary arterial lesions in which the “medial smooth muscle cells contained increased quantity of mitochondria, rough endoplasmic reticulum, and especially prominent Golgi complexes” (12). These seminal observations, including their applicability to the pulmonary arterial endothelial cell (PAEC), have been extensively confirmed both in vivo and in PAEC in culture (13–20). Moreover, these observations also apply to hypoxia-induced PAH. Increased vacuolation, increased Golgi stacks, and increased rough ER profiles in the intima (endothelium) and media (smooth muscle) were reported by Jaenke and Alexander in hypoxia-induced pulmonary hypertension (PH) in bovine species (17). Meyrick and Reid reported the existence of “hypertrophied smooth muscle cells [with] a significant increase in relative areal proportions of Golgi apparatus and rough sarcoplasmic reticulum” in the tunica media of the hilar pulmonary artery of rats with hypoxia-induced PAH (16). The same authors, while describing subcellular changes in the intima of hilar arteries of rats with hypoxic PH, state that “[the] thickened endothelial cells [have] a more extensive Golgi apparatus than normal and many have swollen cisternae of rough endoplasmic reticulum …” (15). Increases in Golgi stacks in pericytes in hypoxic PAH in rats have also been described (15). Particularly noteworthy are reports of increased accumulations of Weibel-Palade bodies (which are exocytic vesicles) in endothelial cells in rats exposed to neonatal hypoxia (18). This increase points to inhibited anterograde vesicular trafficking. A similar increase in Weibel-Palade bodies has been described in endothelial cells in lesions of human primary PAH (4).
Taken together, this brief overview of the EM data points to disruption of cytoplasmic membrane trafficking within the cellular elements in the arterial lesions in PAH. Thus, at this point we need to take a brief detour and discuss the specific molecules that mediate outward (anterograde) and inward (retrograde) vesicular membrane trafficking through the cytoplasm.
MOLECULAR MACHINERY OF VESICULAR MEMBRANE TRAFFICKING
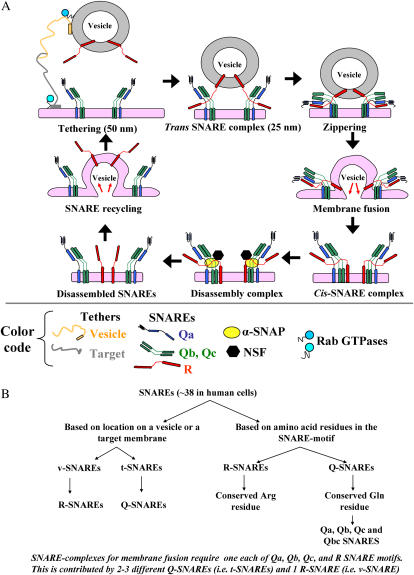
Subcellular membrane trafficking involves the fusion between the membrane of a specific cargo vesicle and that of a specific organellar target. It is mediated by “tether” proteins that bring the respective membranes close to each other (21) (Figure 1). These tether proteins are organelle-specific membrane-associated proteins and at least in part determine the specificity of trafficking. As one example, the Golgi organelle contains at least 12 tethers of the coiled-coil nature and an additional 5 multi-subunit tethering complexes (21). Tethers are not directly involved in the physical step of membrane fusion (21–24).
Figure 1.
Tethers, SNAREs, SNAPs, and NSF in vesicular trafficking. (A) The SNARE cycle in membrane fusion. Initial interaction between a vesicle and its target membrane is mediated by cognate tethers. Membrane fusion is then mediated by the formation of a quaternary-α-helical trans-SNARE complex consisting of one v- (or R-) SNARE on the vesicle and two or three t- (or Q-) SNAREs on the target membrane. After membrane fusion, the cis-SNARE complex is disassembled by the ATPase NSF which is recruited to the cis-SNARE complex from the cytosol by α-SNAP. (B) Classification of SNAREs based on location (on a vesicle [v-] or a target [t-] membrane) or functional amino-acid residue (arginine [R-] or glutamine [Q-]) in their respective SNARE-motifs.
The close approximation of vesicle and target membranes through tether interaction is followed by the formation of four-chain quaternary trans-complexes between specific SNARE proteins on the vesicle and target membranes (SNARE stands for “SNAP receptor protein”; where SNAP stands for “soluble NSF attachment protein” and NSF stands for “N-ethylmaleimide sensitive factor”), the subsequent physical fusion of the respective membranes, conversion of the quaternary complex to cis-complex, and the disassembly of this cis-complex by the recruitment of an ATPase called N-ethylmaleimide sensitive factor (NSF) (22–24) (Figures 1A and 1B). The recruitment of NSF to the cis-SNARE complex requires a soluble SNAP protein (usually α-SNAP) (22–24) (Figure 1A).
There are at least 38 distinct known human SNARE proteins (Figure 1B; Ref. 22). These provide specificity to the fusion of specific subcellular membranes (22–24). Each cell type expresses a different combinatorial basket of SNAREs on distinct subcellular membranes. The v-SNAREs are present on a vesicle membrane, while the t-SNAREs are on the target membrane. More recently these have been renamed R-SNAREs (usually corresponding to v-SNAREs present on vesicles) and Q-SNAREs (usually corresponding to t-SNAREs) based on the central functional amino acid residue in their respective “SNARE” motifs (22–24) (Figure 1B). Confusingly, some proteins nominally called SNAP23, SNAP25, and so on (here the SNAP acronym stands for “synaptosome-associated membrane protein” of designated molecular mass) are Q-SNAREs unrelated to the soluble cytosolic α−, β−, or γ-SNAP proteins (here the SNAP acronym stands for “soluble NSF attachment protein”) (24). The formation of a quaternary-chain SNARE complex consisting of one α-helix from a v-SNARE protein and three α-helices from two or three different t-SNARE proteins (Figure 1A) triggers physical lipid bilayer fusion (22–24). Additional families of proteins called the Sec/Munc or SM proteins regulate the interaction between different SNAREs (23).
NSF, the ATPase required for the disassembly of all cis-SNARE complexes (Figure 1A), is a redox-sensitive protein with nine cysteine residues (25). NSF can be covalently modified by NO-mediated S-nitrosylation, which inhibits its ability to disassemble SNARE complexes without affecting its ATPase function (25). Moreover, mutations in specific Cys residues block the ability of NSF to either associate with the SNARE proteins (mutations on Cys residues 11, 21, 334, 568, and 582) or disassemble SNARE complexes (mutations on Cys residues 91 and 264) (25). Thus, the biologic function of NSF is highly dependent on its cysteine residues.
THE GOLGI BLOCKADE HYPOTHESIS
Afzelius and Schoental (26) suggested that the effects on the Golgi organelle of the pyrrolizidine alkaloid retrorsine (a plant alkaloid related to the PAH-inducing alkaloid MCT) may represent the initiating event leading to the disruption of cytoplasmic membrane trafficking and a block in entry into mitosis resulting in enlarged vacuolated megalocytotic cells. A similar enlargement of Golgi stacks has been reported in affected PAEC and PASMC in pulmonary arterial lesions induced by injection of MCT (12–14). (In the rat, MCT is converted to its bioactive pyrrolic derivative [MCTP] in the liver. MCTP has a half-life of ∼ 3 s in aqueous media and primarily affects only the pulmonary arterial bed. Exposure to MCTP initiates a cascade of changes in affected PAEC culminating in the development of PAH 10–14 d later. In cell culture experiments in endothelial and epithelial cells, the active pyrrolic derivative MCTP is used directly [27, 28]).
MCTP-treated PAEC in culture showed enlarged ER and Golgi stacks by EM within 48–96 h (20). We showed the loss of the cell surface raft/caveolar protein caveolin-1 (cav-1) in PAEC in rat lungs within 48 h after MCT (PAH develops in this model only by 10–14 d) (29; Figure 2). At the single-cell level, the particular cell which showed loss of cav-1 also showed increased nuclear PCNA staining and increased activated PY-STAT3 (29; Figure 2). This loss of cav-1 in PAEC in PAH and its inverse relationship with PY-STAT3 activation have been confirmed by Jasmin and coworkers (30) and extended to a hypoxia-based rat model (31) and also to plexiform lesions in human PAH (32).
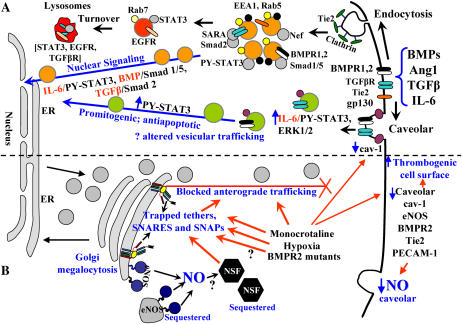
Figure 2.
Dysfunctional intracellular trafficking in the pathobiology of PAH. (A) Productive transcriptional signaling from the plasma membrane to the nucleus along the BMP/Smad 1/5, TGFβ/Smad2, and IL-6/PY-STAT-3 signaling pathways is membrane-associated. IL-6/STAT3 and ERK1/2 signaling is inversely related to loss of caveolar/raft cav-1. (B) Golgi blockade mechanisms in PAH. MCTP and hypoxia lead to a trapping of vesicle tethers, SNAREs, and SNAPs in the Golgi of affected pulmonary arterial endothelial cells. This leads to a block in anterograde trafficking of vasorelevant cargo proteins such as cav-1 and eNOS and reduced caveolar NO production. The intracellularly sequestered eNOS produces NO, which may potentially S-nitrosylate cysteine-rich proteins like NSF, further inhibiting trafficking. Golgi-trapped dominant-negative BMPR2 mutants may also potentially block trafficking of cargo proteins to the plasma membrane.
Inasmuch as cav-1 traffics to the cell surface plasma membrane to/through the Golgi, the above studies were extended to an investigation of membrane trafficking (27, 28, 33–35). MCTP and hypoxia trapped cav-1 in the Golgi in PAEC in culture (27, 34, 35). This trapping of cav-1 in the Golgi of PAEC (“Golgi blockade”) helped focus attention on disruption of intracellular trafficking, and it was discovered that MCTP, hypoxia, and senescence all disrupted the molecular machinery of vesicular trafficking at the level of Golgi tethers, SNAREs, and SNAPs (34, 35) (Figure 2B). Consequences of this disruption included the loss of eNOS from plasma membrane caveolae and its aberrant sequestration in intracytoplasmic compartment(s), and loss of caveolar NO production as assayed in live-cell imaging studies (34) (Figure 2B).
TRAFFICKING OF PAH-ASSOCIATED BMPR2 MUTANTS TO/THROUGH THE GOLGI
Heterogenous mutations in the extracellular ligand binding, kinase, and the cytoplasmic tail domains of the BMPR2 receptor gene have been associated with the development of 25% of cases of sporadic PAH and ∼ 50% of cases of familial PAH (36–40). In the latter instances, mutant BMPR2 alleles have an autosomal dominant phenotype but with low penetrance (10–20%) (38, 40). Thus there is today a considerable, though unsuccessful, effort underway to identify the so-called “second” or “modifier” genes (40, 41). Remarkably, it has been demonstrated that cysteine substitution mutations in the extracellular ligand binding domain (like C60Y) block the trafficking of the mutant BMPR2 receptor protein to the plasma membrane with increased retention and sequestration in intracytoplasmic compartments, including the Golgi (38, 39). Furthermore, cysteine substitution mutants in the kinase domain (like C347Y) are also largely sequestered in intracytoplasmic, perinuclear compartments (38, 39). While the noncysteine mutants in the kinase and cytoplasmic tail domains have been reported to traffic correctly to the cell surface, inspection of the published data reveals that, in fact, there is significant juxtanuclear and cytoplasmic punctate sequestration of these BMPR2 mutants compared with wild-type (wt) BMPR2 (38). The juxtanuclear sequestration of these mutant BMPR2 proteins is clearly indicative, on its face, of dysfunction of intracellular trafficking. Moreover, it has been shown that Golgi-trapped mutant BMPR2 species can bind to functional wt BMPR type 1 receptor exerting a dominant-negative functional effect (38). Moreover, while some of the noncysteine mutants in the kinase and tail domains of BMPR2 may reach the plasma membrane and activate Smad signaling (38), their partial intracytoplasmic retention may be sufficient to initiate dominant-negative effects on trafficking.
In this discussion it is pertinent to emphasize that recent data in the cell biology literature show that trans-cytoplasmic transit of productive inward signaling from the plasma membrane to the cell interior and the nucleus by signaling pathways involving the Smad family and STAT3 transcription factors is associated with endocytic/caveolar vesicular trafficking which, by definition, must thus involve NSF, vesicle tethers, SNAREs, and SNAPs (Figure 2A) (42–44). For instance, it has been shown that transcriptionally productive TGF-β signaling involving Smad transcription factors takes place along the early endocytic pathway (42) and that BMP/Smad signaling is dependent on the integrity of clathrin-mediated endocytic pathways (43)(Figure 2A). Similarly, we have shown that IL-6/STAT3 signaling is dependent upon the endocytic and caveolar trafficking pathways with an inverse relationship between cav-1 and hyperactivation of promitogenic and antiapoptotic IL-6/STAT3 and ERK1/2 signaling (44) (Figure 2A). Nevertheless, recent discussions with respect to BMP/Smad in the context of the pathobiology of PAH completely lack considerations of membrane-associated trafficking in carrying a productive signal from the plasma membrane to the cell interior (see Figure 1 in Ref. 45).
DYSFUNCTION OF TETHERS, SNARES, SNAPS, AND NSF IN ENDOTHELIAL CELLS AFTER HYPOXIA, MONOCROTALINE, AND SENESCENCE
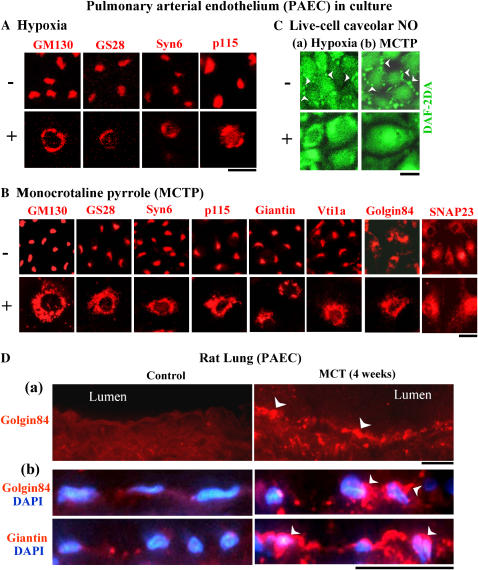
Mechanistically, the loss of cav-1 from cell-surface rafts in PAEC after hypoxia or MCTP was accounted for by the trapping of cav-1 in the Golgi (27, 34, 35). At the same time, there was a marked enlargement of the Golgi organelle into a circumnuclear structure as assayed by immunoflourescent detection of the Golgi tether GM130 (Figures 3A and 3B) (28, 34, 35). A block of anterograde trafficking through the Golgi after MCTP was also confirmed using an assay for secretion of horseradish peroxidase (HRP) (35). In trying to understand the underlying subcellular mechanisms for the anterograde trafficking block through the Golgi, cell fractionation and immunofluorescence techniques revealed the marked trapping not only of cav-1 and eNOS in the Golgi, but also of BMPR2 and of diverse Golgi tethers, SNAREs, and SNAPs (GM130, p115, giantin, golgin 84, clathrin heavy chain; syntaxin-4, -6, Vti1a, Vti1b, GS15, GS27, GS28; SNAP23 and α-SNAP) in the enlarged/circumnuclear Golgi organelle in megalocytotic endothelial cells (34, 35) (Figures 3A and 3B). That diverse tethers, SNAREs, and SNAPs were trapped in the Golgi suggested interference with the disassembly step in membrane trafficking. Indeed, NSF, the ATPase required for disassembly of all cis–SNARE complexes, was found to be largely sequestered in an intracellular location separate from the Golgi (35). This would explain the accumulation of diverse tethers, SNAREs, and SNAPs in the Golgi due to inhibition of SNARE complex disassembly after MCTP. Immunofluorescence studies of lung tissue from MCT-treated rats confirmed enlargement of the perinuclear Golgi in lung arterial endothelial and parenchymal cells as early as 4 d after MCT administration, namely at a time that preceded the appearance of PAH (which usually develops by 10–14 d) (Figure 3D) (35). Thus, after MCT (or MCTP in cell culture) or hypoxia there is dysfunction of Golgi tethers, SNAREs, SNAPs, and NSF.
Figure 3.
Dysfunctional Golgi tethers and SNAREs after hypoxia and monocrotaline. (A and B) Bovine PAEC in culture exposed to hypoxia (1.5% vol/vol) or MCTP (∼ 50 μM equivalent) for 4 d were immunostained for different Golgi tethers (GM130, p115, giantin, golgin 84) and SNAREs (GS28, syntaxin 6, Vti1a, SNAP23). Scale bars = 25 μM in A and 50 μM in B. Adapted from Refs. 34 and 35 with permission. (C) Live-cell caveolar imaging of NO using DAF-2DA in PAEC after 4 d of exposure to hypoxia or MCTP as in A and B. Arrowheads point to caveolar NO. Scale bar = 25 μM. Adapted from Ref. 34 with permission. (D) Enhancement of Golgi tethers (arrowheads) in PAEC in rat lung 4 wk after MCT administration. Scale bars = 20 μM in a and 20 μM in b. Adapted from Ref. 35 with permission.
CONSEQUENCES OF A TRAFFICKING BLOCK: REDUCED CAVEOLAR NITRIC OXIDE WITH INTRACELLULAR SEQUESTRATION OF eNOS
It is now accepted that there are reduced NO levels in the pulmonary arterial walls in human and experimental PAH, even though the levels of eNOS protein have been variably reported as unchanged, decreased, or even increased (34, 46, 47). It is also now clear that the extracellular NO derives from cell-surface caveolar eNOS and that intracellularly active eNOS produces NO in subcellular compartments from where the NO does not reach the extracellular space (48, 49).
Murata and colleagues had previously shown that eNOS was co-trapped in the Golgi with cav-1 in endothelial cells exposed to hypoxia (50). Moreover, we observed that eNOS was increasingly sequestered in the Golgi and in a non-Golgi cytoplasmic vesicular compartment partially overlapping with the endoplasmic reticulum in endothelial cells exposed to MCTP, hypoxia, or following senescence (34). Under these conditions, there was a loss of cell surface caveolar eNOS with colocalization between some of the cell-centric eNOS with Golgi tethers and SNAREs (34). Live-cell imaging studies of NO using DAF-2DA as a membrane-permeant subcellular reporter revealed a loss of caveolar NO with marked increase in cell-centric accumulations of NO (34). The aberrant sequestration of eNOS in an intracellular compartment away from cell-surface caveolae would account for the reduced NO in the pulmonary arterial vasculature despite sustained or even increased protein levels of eNOS (Figure 2B).
The sequestration of eNOS in a cell-centric compartment in endothelial cells after monocrotaline, hypoxia, or senescence within which the enzyme continues to generate NO (but which does not reach the exterior of the cell) might well initiate a self-amplifying loop leading to increased S-nitrosylation of NSF and a further inhibition of trafficking. Indeed, it has been recently shown that the SNARE complex disassembly reaction mediated by NSF is specifically inhibited by NO (25). In fact, Iwakiri and colleagues reported very recently that Golgi-targeted mutants of eNOS produced NO locally within this subcellular compartment and thus modified intracellular NSF by nitrosylation (49). The consequence was a block in trafficking through the Golgi of cargo proteins such as the vesicular stomatitis virus G protein (49). Thus, from the point of view of this essay, we suggest that the aberrantly sequestered eNOS after MCTP, hypoxia, or senescence would generate intracellular NO, which would further inhibit NSF and membrane trafficking through the Golgi in a self-reinforcing inhibitory loop (Figure 2B).
DOMINANT-NEGATIVE EFFECTS OF PAH-CAUSING BMPR2 MUTANTS ON INTRACELLULAR MEMBRANE TRAFFICKING
We posit here the question: to what extent do BMPR2 mutants associated with PAH cause dysfunction of tethers, SNAREs, and SNAPs? To put this question into perspective we note that it is known from the breast cancer literature that the trapping of mutant cav-1 species in the Golgi (such of the P132L mutant) results in a dominant-negative block of anterograde trafficking of wt cav-1 (51). Furthermore, it is known—in the autosomal dominant polycystic kidney disease (ADPDK) literature—that disease-causing mutations in the cell-surface protein polycystin-1 result in trapping of the mutant protein in the Golgi, leading to a dominant-negative block of trafficking of other wild-type cellular proteins such as E-cadherin and cav-1 (52, 53). As with BMPR2 mutation-associated PAH (which is also an autosomal dominant but with a rather low penetrance) a discussion regarding “second-hits” and late penetrance (the kidney disease can appear as late as the eight or ninth decade) is taking place in the ADPDK field (54, 55). It is striking that as with mutant polycystin-1, mutant BMPR2 species are known to be trapped in intracytoplasmic compartments like the ER and Golgi (38, 39).
Haploinsufficiency of BMPR2 in transgenic mice (BMPR2+/− mice) has a limited ability to cause pulmonary hypertension without additional environmental stress (56–58). Further, it is known that transgenic mice with a dominant-negative BMPR2 mutation targeted specifically to smooth muscle cells develop PAH, and that this is further exacerbated with mild hypoxia (59). This BMPR2 mutation is due to a premature termination at 18 amino acids into the kinase domain and has been shown to have a dominant-negative effect on a Smad-responsive luciferase reporter construct (59).
We thus ask whether trapping of mutant BMPR2 proteins in the ER/Golgi leads to a dominant-negative effect on the trafficking of other proteins through that organelle (Figure 2)? Furthermore, we ask whether this trafficking block is evident only in the context of a specific genotypic basket of tethers, SNAREs, and SNAPs? This question points to a different way of approaching the low-penetrance or “modifier gene” issue in familial PAH. These questions remain unanswered at the present time.
CONCLUSIONS
The major objective of this review was to bring aspects of intracellular membrane trafficking and its vocabulary into discussions of the pathobiology of PAH. The prior electron microscopy data indicative of disruption of dysfunction of intracytoplasmic membrane trafficking are clear. We have attempted to relate these data to newer aspects of the molecular machinery involved in vesicular trafficking and membrane-associated inward transcriptional signaling. Existing puzzles relating to the mechanisms by which mutations in BMPR2 cause an autosomal dominant disease with low penetrance might be amenable to resolution in light of the additional compendium of regulatory molecules involved in membrane trafficking—NSF and the tethers, SNAREs, and SNAPs. Rapid discovery screens to find small molecules that restore intracellular trafficking through the Golgi block might yield novel therapeutic solutions for the management of PAH in humans.
This study was supported by Research Grant HL-077301 (to P.B.S) from the National Institutes of Health.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0066TR on March 15, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir KE, Eickelberg O, Voelkel NF, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:13S–24S. [DOI] [PubMed] [Google Scholar]
- 2.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest 1988;114:225–230. [DOI] [PubMed] [Google Scholar]
- 3.Cool CD, Groshong SD, Oakey J, Voelkel NF. Pulmonary hypertension cellular and molecular mechanisms. Chest 2005;128:565S–571S. [DOI] [PubMed] [Google Scholar]
- 4.Heath D, Smith P, Gosney J, Mulcahy D, Fox K, Yacoub M, Harris P. The pathology of early and late stages of primary pulmonary hypertension. Br Heart J 1987;58:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith P, Heath D. Electron microscopy of the plexiform lesion. Thorax 1979;34:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith P, Heath D, Yacoub M, Madden B, Caslin A, Gosney J. The ultrastructure of plexogenic pulmonary arteriopathy. J Pathol 1990;160:111–121. [DOI] [PubMed] [Google Scholar]
- 7.Mette SA, Palevsky HI, Pietra GG, Williams TM, Bruder E, Prestipino AJ, Patrick AM, Wirth JA. Primary pulmonary hypertension in association with human immunodeficiency virus infection. A possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis 1992;145:1196–1200. [DOI] [PubMed] [Google Scholar]
- 8.Marecki JC, Cool CD, Parr JE, Beckley VE, Luciw PA, Tarantal AF, Carville A, Shannon RP, Cota-Gomez A, Tuder RM, et al. HIV Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med 2006;174:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burtney A, Rappoport JZ, Bouchet J, Basmaciogullari S, Guatelli J, Simon SM, Benichou S, Benmerah A. Dynamic interaction of HIV-1 Nef with the clathrin-mediated endocytic pathway at the plasma membrane. Traffic 2007;8:61–76. [DOI] [PubMed] [Google Scholar]
- 10.Roeth JF, Williams M, Kasper MR, Filzen TM, Collins KL. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J Cell Biol 2004;167:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madrid R, Janvier K, Hitchin D, Day J, Coleman S, Noviello C, Bouchet J, Benmerah A, Guatelli J, Benichou S. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J Biol Chem 2005;280:5032–5044. [DOI] [PubMed] [Google Scholar]
- 12.Merkow L, Kleinerman J. An electron microscopic study of pulmonary vasculitis induced by monocrotaline. Lab Invest 1966;15:547–564. [PubMed] [Google Scholar]
- 13.Todorovich-Hunter L, Johnson DJ, Ranger P, Keeley FW, Rabinovitch M. Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline. A biochemical and ultrastructural study. Lab Invest 1988;58:184–195. [PubMed] [Google Scholar]
- 14.Rosenberg HC, Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol Heart Circ Physiol 1988;255:H484–H491. [DOI] [PubMed] [Google Scholar]
- 15.Meyrick B, Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. Lab Invest 1978;38:188–200. [PubMed] [Google Scholar]
- 16.Meyrick B, Reid L. Hypoxia-induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am J Pathol 1980;100:151–178. [PMC free article] [PubMed] [Google Scholar]
- 17.Jaenke RS, Alexander AF. Fine structural alterations of bovine peripheral pulmonary arteries in hypoxia-induced hypertension. Am J Pathol 1973;73:377–398. [PMC free article] [PubMed] [Google Scholar]
- 18.King AP, Smith P, Heath D. Ultrastructure of rat pulmonary arterioles after neonatal exposure to hypoxia and subsequent relief and treatment with monocrotaline. J Pathol 1995;177:71–81. [DOI] [PubMed] [Google Scholar]
- 19.Wilson DW, Segall HJ. Changes in type II cell populations in monocrotaline pneumotoxicity. Am J Pathol 1990;136:1293–1299. [PMC free article] [PubMed] [Google Scholar]
- 20.Reindel JF, Roth RA. The effects of monocrotaline pyrrole on cultured bovine pulmonary artery endothelial and smooth muscle cells. Am J Pathol 1991;138:707–719. [PMC free article] [PubMed] [Google Scholar]
- 21.Sztul E, Lupashin V. Role of tethering factors in secretory membrane traffic. Am J Physiol Cell Physiol 2006;290:C11–C26. [DOI] [PubMed] [Google Scholar]
- 22.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol 2006;6:919–929. [DOI] [PubMed] [Google Scholar]
- 23.Jahn R, Scheller RH. SNAREs – engines for membrane fusion. Nat Rev Mol Cell Biol 2006;7:631–643. [DOI] [PubMed] [Google Scholar]
- 24.Bonifacino JS, Glick BS. The mechanism of vesicle budding and fusion. Cell 2004;116:153–166. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita K, Morrell CN, Cambein B, Yang S-X, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethlymaleimide Sensitive Factor. Cell 2003;115:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afzelius BA, Schoental R. The ultrastructure of the enlarged hepatocytes induced in rats with a single oral dose of retrorsine, a pyrrolozidine (Senecio) alkaloid. J Ultrastruct Res 1967;20:328–345. [DOI] [PubMed] [Google Scholar]
- 27.Shah M, Patel K, Sehgal PB. Monocrotaline induced endothelial cell megalocytosis involves a Golgi blockade mechanism. Am J Physiol Cell Physiol 2005;288:C850–C862. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay S, Sehgal PB. Discordant regulatory changes in monocrotaline-induced megalocytosis of lung arterial endothelial and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L1216–L1226. [DOI] [PubMed] [Google Scholar]
- 29.Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1α/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation 2004;110:1499–1506. [DOI] [PubMed] [Google Scholar]
- 30.Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation 2006;114:912–920. [DOI] [PubMed] [Google Scholar]
- 31.Taraseviciene-Stewart L, Scerbavicius R, Choe H-H, Cool C, Wood K, Tuder RM, Burns N, Kasper M, Voelkel NF. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2006;291:L668–L676. [DOI] [PubMed] [Google Scholar]
- 32.Achcar ROD, Demura Y, Rai PR, Taraseviciene-Stewart L, Kasper M, Voelkel NF, Cool CD. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest 2006;129:696–705. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay S, Shah M, Patel K, Sehgal PB. Monocrotaline pyrrole-induced megalocytosis of lung and breast epithelial cells: disruption of plasma membrane and Golgi dynamics and an enhanced unfolded protein response. Toxicol Appl Pharmacol 2006;211:209–220. [DOI] [PubMed] [Google Scholar]
- 34.Mukhopadhyay S, Xu F, Sehgal PB. Aberrant cytoplasmic sequestration of eNOS in endothelial cells after monocrotaline, hypoxia and senescence: subcellular eNOS localization and live-cell caveolar and cytoplasmic NO imaging studies. Am J Physiol: Heart and Circ Physiol 2006;292:H1373–H1389. [DOI] [PubMed] [Google Scholar]
- 35.Sehgal PB, Mukhopadhyay S, Xu F, Patel K, Shah M. Dysfunction of Golgi tethers, SNAREs and SNAPs in monocrotaline-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2007; 10.1152/ajplung.00463.2006 (In press) [DOI] [PubMed]
- 36.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fisher SG, Barst RJ, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000;67:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane KB, Machado RD, Pauciulo MW, Thompson JR, Phillips JA III, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2 encoding a TGF-β receptor, cause familial pulmonary hypertension. Nat Genet 2000;26:81–84. [DOI] [PubMed] [Google Scholar]
- 38.Nishihara A, Watabe T, Imamura T, Miyazono K. Functional heterogeneity of bone morphogenetic protein receptor-II mutants found in patients with primary pulmonary hypertension. Mol Biol Cell 2002;13:3055–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudrakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet 2002;11:1517–1525. [DOI] [PubMed] [Google Scholar]
- 40.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet 2003;361:1533–1544. [DOI] [PubMed] [Google Scholar]
- 41.Machado RD, James V, Southwood M, Harrison RE, Atkinson C, Stewart S, Morrell NW, Trembath RC, Aldred MA. Investigation of second genetic hits at the BMPR2 locus as a modulator of disease progression in familial pulmonary arterial hypertension. Circulation 2005;111:607–613. [DOI] [PubMed] [Google Scholar]
- 42.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signaling and turnover. Nat Cell Biol 2003;5:410–421. [DOI] [PubMed] [Google Scholar]
- 43.Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergerman JH, Hassel S, Henis YI, Knaus P. Different routes of bone morphogenetic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol 2006;26:7791–7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah M, Patel K, Mukhopadhyay S, Xu F, Guo G, Sehgal PB. Membrane associated STAT3 and PY-STAT3 in the cytoplasm. J Biol Chem 2006;281:7302–7308. [DOI] [PubMed] [Google Scholar]
- 45.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc 2006;3:680–686. [DOI] [PubMed] [Google Scholar]
- 46.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in lungs of patients with pulmonary hypertension. N Engl J Med 1995;333:214–221. [DOI] [PubMed] [Google Scholar]
- 47.Tyler RC, Muramatsu M, Abman SH, Stelzer TJ, Rodman DM, Bloch KD, McMurtry IF. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol 1999;276:L297–L303. [DOI] [PubMed] [Google Scholar]
- 48.Govers R, Oess S. To NO or not to NO: 'where?' is the question. Histol Histopathol 2005;19:585–605. [DOI] [PubMed] [Google Scholar]
- 49.Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, Grozmann RJ, Shah VH, Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci USA 2006;103:19777–19782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 2002;277:44085–44092. [DOI] [PubMed] [Google Scholar]
- 51.Lee H, Park DS, Razani B, Russell RG, Pestell RG, Lisanti MP. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1 (−/−) null mice show mammary epithelial cell hyperplasia. Am J Pathol 2002;161:1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charron AJ, Bacallo R, Wandinger-Ness A. ADPKD: a human disease altering Golgi function and basolateral exocytosis in renal epithelia. Traffic 2000;1:675–686. [DOI] [PubMed] [Google Scholar]
- 53.Charron AJ, Nakamura S, Bacallo R, Wandinger-Ness A. Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J Cell Biol 2000;149:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koptides M, Mean R, Demetriou K, Pierides A, Deltas CC. Genetic evidence for a trans-heterozygous model for cystogenesis in autosomal dominant polycystic kidney disease. Hum Mol Genet 2000;9:447–452. [DOI] [PubMed] [Google Scholar]
- 55.Persu A, Duyme M, Pirson Y, Lens XM, Messiaen T, Breuning MH, Chauveau D, Levy M, Grunfeld J-P, Devuyst O. Comparison between siblings and twins supports a role for modifier genes in ADPKD. Kidney Int 2004;66:2132–2136. [DOI] [PubMed] [Google Scholar]
- 56.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bolch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 2004;287:L1241–L1247. [DOI] [PubMed] [Google Scholar]
- 57.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudrakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res 2006;98:818–827. [DOI] [PubMed] [Google Scholar]
- 58.Song Y, Jones JE, Beppu H, Keaney JF, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 2005;112:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 2004;94:1109–1114. [DOI] [PubMed] [Google Scholar]