Abstract
Exhaled nitric oxide (NO) is altered in numerous diseases including asthma, and is thought broadly to be a noninvasive marker of inflammation. However, the precise source of exhaled NO has yet to be identified, and the interpretation is further hampered by significant inter-subject variation. Using fully differentiated normal human bronchial epithelial (NHBE) cells, we sought to determine (1) the rate of NO release (flux, pl·s−1.cm−2) into the gas; (2) the effect of IL-13, a prominent mediator of allergic inflammation, on NO release; and (3) inter-subject/donor variability in NO release. NHBE cells from three different donors were cultured at an air–liquid interface and stimulated with different concentrations of IL-13 (0, 1, and 10 ng/ml) for 48 h. Gas phase NO concentrations in the headspace over the cells were measured using a chemiluminescence analyzer. The basal NO flux from the three donors (0.05 ± 0.03) is similar in magnitude to that estimated from exhaled NO concentrations, and was significantly increased by IL-13 in a donor-specific fashion. The increase in NO release was strongly correlated with inducible nitric oxide synthase (iNOS) gene and protein expression. There was a trend toward enhanced production of nitrate relative to nitrite as an end product of NO metabolism in IL-13–stimulated cells. NO release from airway epithelial cells can be directly measured. The rate of release in response to IL-13 is strongly dependent on the individual donor, but is primarily due to the expression of iNOS.
Keywords: asthma, cytokines, inflammation, allergy
CLINICAL RELEVANCE
The results from this study provide a direct in vitro link between inducible nitric oxide synthase expression in the human bronchial epithelium and nitric oxide gas phase release. The release is increased by IL-13, a prominent Th2 cytokine present in asthma
The concentration of nitric oxide (NO) in exhaled breath (FeNO) is widely recognized as a marker of inflammation in the lungs (1, 2), and perhaps systemic sites (3, 4), but there is significant variability within clinically similar groups (5). It is elevated 3- to 4-fold in untreated asthma (6, 7), providing the possibility of using exhaled NO as a noninvasive means of asthma diagnosis and management. However, the precise mechanism that leads to NO release into the gas of the lungs remains largely unknown, thus significantly handicapping our ability to interpret alterations in exhaled NO concentration.
The airway epithelium lies adjacent to the exhaled gas, and has been proposed to be a source of NO in the exhaled breath (8–10); however, no study has directly detected NO release into the gas phase from the airway epithelium, and determined the underlying source. Three known isoforms of nitric oxide synthase (NOS) produce NO. NOSI (neuronal) and NOSIII (endothelial) are constitutive forms expressed in numerous cells in the lungs. Mutations in the genes for NOSI and NOSIII have been correlated with changes in the level of NO in the exhaled breath of individuals with asthma (11, 12). NOSII (inducible) is induced by numerous mediators, has been detected in the airway epithelium (13–15), and its expression has also been correlated with exhaled NO (8).
IL-13 is a cytokine produced primarily from Th2 cells as part of the allergic response. It is elevated in bronchoalveolar lavage fluid of individuals with asthma, and thought to play a major role in the disease progression of atopic asthma. It has been shown to modulate TNF-α–, IL-1β–, and IFN-γ–induced inducible NOS (iNOS) gene expression and/or nitrite (an end product of NO metabolism) levels in cultures of undifferentiated human airway epithelial cells and an alveolar epithelial cell (A549) line (16). Similar results have been found in cultures of nonpulmonary cells (17, 18). Another study found that IL-13 had no effect on nitrite production by A549 cells (19). None of these studies have examined the effect of IL-13 in isolation on mucociliary differentiated airway epithelium, nor on NO gas phase release.
We hypothesized that IL-13 would enhance iNOS expression and lead to an increase in NO release to the gas phase. Using mucociliary differentiated human bronchial epithelial cells cultured at an air–liquid interface, we demonstrate for the first time that (1) the airway epithelium releases NO to the gas phase at a rate equivalent to that predicted from whole organ models, (2) IL-13 upregulates gene and protein expression of iNOS in a dose-dependent fashion, (3) the increase in iNOS expression leads directly to an increase in NO release to the gas phase, (4) IL-13 enhances the production of nitrate relative to nitrite as a stable end-product of iNOS-derived NO, and (5) there is significant inter-subject variability in the rate of NO release to gas phase in response to IL-13. This study provides the first direct gas-phase measurement of NO from human bronchial epithelial cells, and a direct link between iNOS expression and NO release. In doing so, the results significantly enhance our understanding of the cellular-based mechanisms that control the level of NO in the exhaled breath.
MATERIALS AND METHODS
Cell Culture
Cryopreserved passage 1 normal human bronchial epithelial (NHBE) cells from three different donors (donor 1: specific lot number not known, donor 2: 4F1430, donor 3: 4F1624) were obtained from Cambrex (Walkersville, MD). Fully mucociliary differentiated monolayers were obtained by seeding passage 3 cells on Costar Transwell inserts as described previously (20). See online supplement for details.
IL-13 Stimulation and Addition of NOS Inhibitors
Culture medium was changed 24 h before the start of the experiment. On the day of the experiment IL-13 was added to fresh culture medium to a final concentration of 0, 1, or 10 ng/ml. For the inhibitor studies an iNOS inhibitor N6-(1-iminoethyl)-L-lysine (L-NIL; 30 μM) or nNos inhibitor L-Nω-nitroarginine-2, 4-L-diaminobutyric amide (L-NA-DBA; 10 μM) was added to the culture medium 24 h later. The total duration of each experiment was 48 h.
Gas Phase NO Measurement
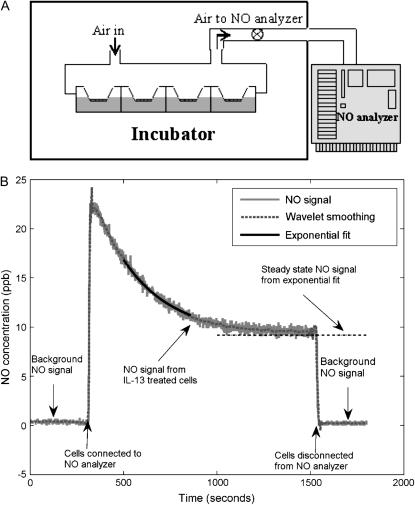
Twelve-well Transwell plates were fitted with modified lids and edges sealed with Parafilm M (Fisher, Waltham, MA) to form a gas-tight seal. Holes were drilled into the top surface of the lids; one of the holes was connected via a flowmeter (RMA-151; Dwyer Instruments, Michigan City, IN) to the inlet of a chemiluminescent nitric oxide analyzer (NOA 280; Ionics Instruments, Boulder, CO) (Figure 1A). The operation of the analyzer pump was used to sample air from either the headspace over the cells or the incubator at a constant flow rate of Q = 40 ml/min and determine the NO concentrations. Four Transwells of a 12-well plate were used to obtain accurate gas phase NO signals. Real-time NO data from the NOA was stored on computer for further analysis.
Figure 1.
Real-time measurement of gas phase NO release by NHBEs (A) Schematic of gas phase NO measurement apparatus. The lids of 12-well Transwell plates were modified to form a gas tight seal. The headspace of the Transwells containing epithelial monolayers was perfused with air at a constant flow rate. The effluent air was fed to an NO analyzer to measure NO concentrations. (B) One representative measurement of the real-time response of NO analyzer shows a low background level in the incubator air. Switching the analyzer intake to effluent air from the headspace over the cells causes a sharp spike in the response that gradually decays to a plateau. Switching the intake back to the incubator air results in a low background NO reading. The raw analyzer response (solid gray line) is smoothed using a wavelet transform (dotted gray line). The steady-state NO concentration at the plateau is determined by fitting an exponential equation to 300 data points of the smoothed curve.
NO Flux Calculation
Ambient NO concentration in the incubator air was measured before and after each cell measurement. The real-time NO response from NHBEs consists of an initial spike followed by an exponential decay to a plateau value, CP (Figure 1B), representing the washout of accumulated NO from the headspace and the steady-state NO release into the gas phase. Steady-state NO concentrations were determined by fitting an exponential form to the smoothed transient response and the NO flux based on the surface area (AS) of the Transwells was calculated as F = QCP/(60AS) (pl·s−1.cm−2). The accuracy of the fitting procedure was verified by comparing to steady-state values achieved by the real-time data in a fraction of measurements.
Cell Extract and Culture Medium Analysis
Total RNA and protein were extracted as previously described (21) and used for PCR and Western analysis (see online supplement for details). Culture medium was analyzed for nitrite 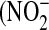 ) and total nitrate
) and total nitrate 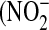 +
+ 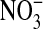 ).
).
Statistics
Four Transwells were used for a single gas phase NO measurement, and culture medium from each Transwell was separately assayed for nitrite and nitrate. Data are presented as mean ± SD with an n of at least 3 for gas phase and 6 for nitrate/nitrite measurements. Statistical significance was tested using a two-tailed, homoscedastic Student's t test in Microsoft Excel; P values < 0.05 were considered significant.
RESULTS
IL-13 Induces NO Release into the Gas Phase and Nitrate/Nitrite Formation
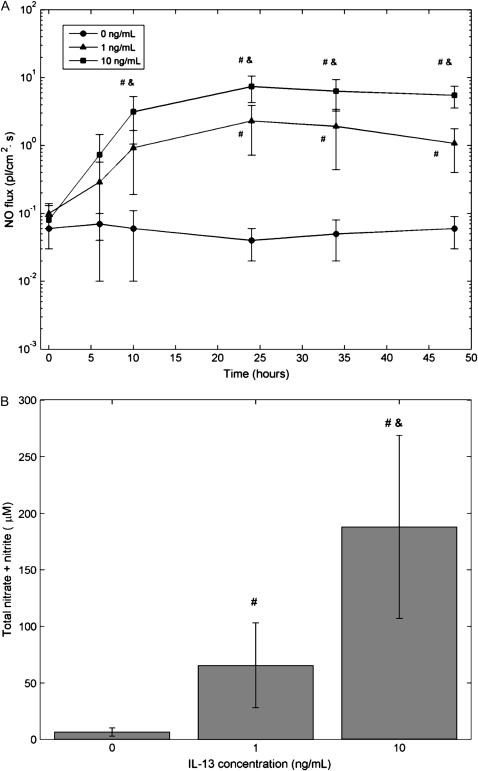
NO concentration in the headspace over IL-13–treated (1, 10 ng/ml) and control (0 ng/ml) cells was measured at various times over the course of 48 h and the NO flux calculated (Figure 2A). Control cells exhibited a low basal level of NO release (0.05 ± 0.03 pl·cm−2.s−1) that remained fairly constant over the entire duration. IL-13 caused an increase in the NO flux that was first significant at 10 h (10 ng/ml) or 24 h (1 ng/ml) after IL-13 addition. The maximum flux observed at 24 h after addition (2.31 ± 1.59 and 7.41 ± 3.12 pl·cm−2.s−1 for 1 and 10 ng/ml, respectively) was 40- to 100-fold greater than the control condition. Total nitrate content in the culture medium at 48 h after addition mirrored the trends of the gas phase flux (Figure 2B).
Figure 2.
IL-13 increases gas phase NO and total nitrate content in culture medium. (A) Different concentrations of IL-13 (0, 1, 10 ng/ml) were introduced in the culture medium of NHBEs at time t = 0. Gas phase NO release was followed over a period of 48 h by determining steady-state NO concentrations at different times and calculating a flux as described in Materials and Methods. Basal (0 ng/ml) NO flux was small and relatively constant. IL-13 caused a dose-dependent increase in NO flux by up to 2 orders of magnitude (n = 4 or 12; #,&: P < 0.05 compared with 0 and 1 ng/ml, respectively). Results for donor 3 cells; other donors showed similar trends with smaller magnitudes. (B) Total nitrate content in the culture medium shows a dose-dependent increase after the cells were exposed to IL-13 for 48 h (n = 13 or 14) (#,&: P < 10−5 compared with 0 and 1 ng/ml, respectively).
IL-13–Induced NO Release Is Mediated by iNOS
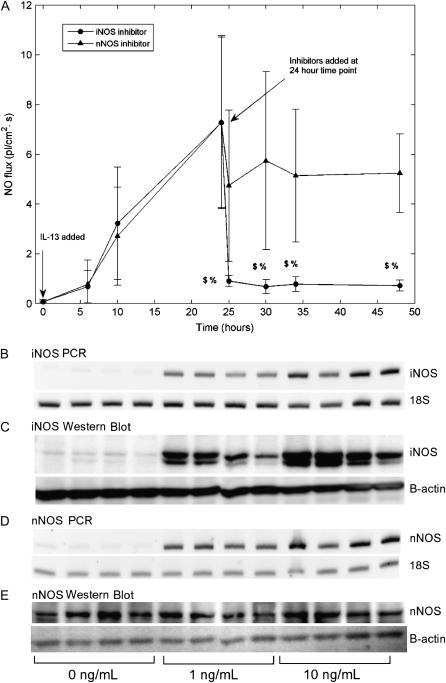
Initial experiments showed that IL-13–induced NO release was inhibited by a nonspecific NOS inhibitor N(G)-mono-methyl-L-arginine (L-NMMA) and the presence of iNOS and nNOS, but not eNOS, mRNA in cell extracts. Therefore later experiments focused on elucidating the roles of the first two isoforms. NO release was first induced by IL-13 addition and iNOS- or nNOS-specific inhibitors added to the culture medium 24 h later. The iNOS inhibitor rapidly and effectively reduced the NO flux by more than 85% 1 h after addition (Figure 3A), while the nNOS inhibitor caused a more modest decrease (∼ 30%). iNOS-inhibited flux was significantly different at all time points from the uninhibited flux at 24 h (P < 0.01) as well as the nNOS-inhibited flux (P < 0.05). However, nNOS-inhibited flux was not significantly different compared with the uninhibited flux at 24 h. PCR and Western blot analysis showed low basal levels of iNOS gene and protein expression that were strongly up-regulated by IL-13 in a dose-dependent fashion (Figures 3B and 3C). nNOS gene expression was upregulated by IL-13 stimulation, but protein expression seemed to be independent of IL-13 dose (Figures 3D and 3E).
Figure 3.
IL-13–mediated increase in NO flux shows a significant decrease with iNOS, but not nNOS, inhibitor and correlates with iNOS mRNA and protein expression. (A) IL-13 (10 ng/ml) was added to the culture medium at time t = 0 (2 groups, n = 4 each) and an increase in NO flux observed for the first 24 h. At t = 24 h, an iNOS or nNOS competitive inhibitor was added to the culture medium of each group. The iNOS inhibitor reduced NO flux by more than 85% within 1 h of addition, while the nNOS inhibitor reduced NO flux by < 30% ($: P < 0.01 compared with flux at 24 h, %: P < 0.05 compared with nNOS inhibitor). IL-13 upregulates iNOS gene (B) and protein expression (C). nNOS gene (D), but not protein (E) expression is upregulated at an IL-13 concentration of 1 ng/ml. RT-PCR products and Western blot show replicates from four experiments using donor 3 cells; other donors showed similar trends.
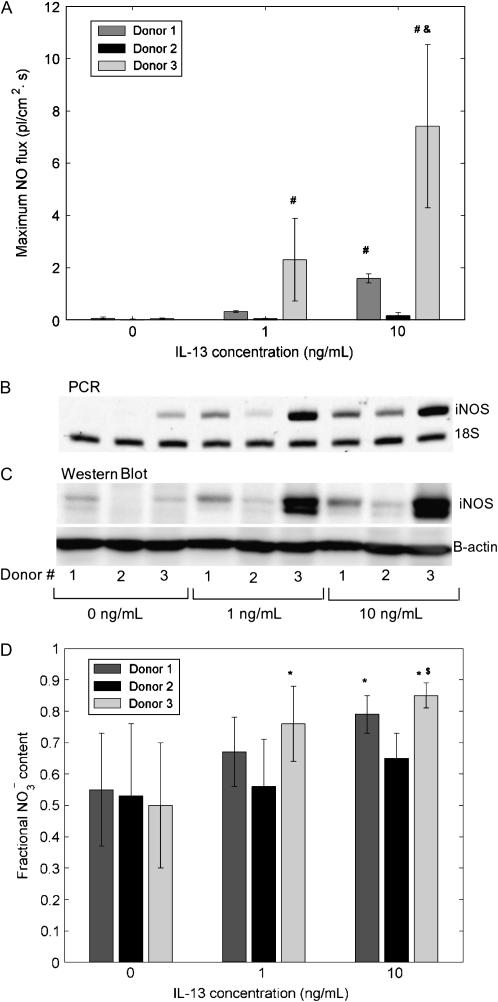
Significant Donor-to-Donor Variation in NO Release and Metabolism Correlates with iNOS Gene and Protein Expression
The issue of donor variability in NO release was addressed by examining the response of cells from three different donors to IL-13 stimulation. Basal NO flux in control cells was similar in all three donors (0.07 ± 0.05, 0.03 ± 0.01, 0.05 ± 0.03 pl/[cm2.s], respectively). However, cells exhibited varying degrees of sensitivity to IL-13 stimulation: at IL-13 concentrations of 1 and 10 ng/ml, peak NO flux (measured 24 h after IL-13 addition) from donors 1 and 3 was significantly higher than that from donor 2 (Figure 4A). Further, donor 3 produced significantly more NO than donor 1 at the 10 ng/ml concentration. These differences in the response to IL-13 were reflected at the iNOS-gene and protein expression level (Figures 4B and 4C): the strongest upregulation was seen in donor 3, and the weakest in donor 2. To assess if IL-13 stimulation affects the ultimate fate of iNOS-derived NO, the relative amounts of nitrite 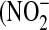 ) and nitrate
) and nitrate 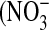 ), which are stable end products of NO metabolism, were determined in the culture medium and expressed as the fraction of
), which are stable end products of NO metabolism, were determined in the culture medium and expressed as the fraction of 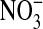 in the total nitrate/nitrite
in the total nitrate/nitrite 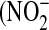 +
+ 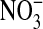 ) content. Under basal conditions approximately equal amounts of
) content. Under basal conditions approximately equal amounts of 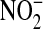 and
and 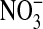 were formed in all three donors. However the relative amounts in IL-13–stimulated cells depend on donor sensitivity to IL-13: the fractional nitrate content increases with IL-13 concentration in donor 3, whereas in donor 1 it is significantly higher only at 10 ng/ml and in donor 2 the differences are not significant (Figure 4D).
were formed in all three donors. However the relative amounts in IL-13–stimulated cells depend on donor sensitivity to IL-13: the fractional nitrate content increases with IL-13 concentration in donor 3, whereas in donor 1 it is significantly higher only at 10 ng/ml and in donor 2 the differences are not significant (Figure 4D).
Figure 4.
Significant donor variation in NO flux and relative nitrate/nitrite content in culture medium correlates with iNOS gene and protein expression. Cells from different donors show significantly different response to IL-13 as measured by the maximum NO flux after exposure (A), iNOS gene (B), and protein (C) expression. Donor 3 cells are most responsive to increasing IL-13 concentrations, while donor 2 cells are least responsive (n = 6 or 12; #: P < 0.001 compared with donor 2, &: P < 0.01 compared with donor 1). Individual amounts of nitrite 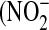 ) and nitrate
) and nitrate 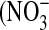 ) in the culture medium were determined after 48 h exposure to IL-13, and the fractional nitrate content [
) in the culture medium were determined after 48 h exposure to IL-13, and the fractional nitrate content [ 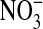 /
/ 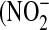 +
+ 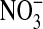 ) ] was calculated. Fractional
) ] was calculated. Fractional 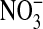 content was close to 0.5 for all donors under basal conditions (0 ng/ml) and increased concomitant with the IL-13–induced upregulation in iNOS mRNA and protein (D) (n = 6, 13, or 14; *: P < 0.02 compared with 0 ng/ml, $: P < 0.0001 compared with 1 ng/ml).
content was close to 0.5 for all donors under basal conditions (0 ng/ml) and increased concomitant with the IL-13–induced upregulation in iNOS mRNA and protein (D) (n = 6, 13, or 14; *: P < 0.02 compared with 0 ng/ml, $: P < 0.0001 compared with 1 ng/ml).
DISCUSSION
Exhaled NO has many potential cellular sources within the respiratory tract (22), but the airway epithelium is thought to be the primary source due to its proximity to the air space and its large surface area. The evidence for this hypothesis is primarily correlative and based on the expression of NOS isoforms in the epithelium (9, 13), detection of NO adducts and metabolites in airway lining fluid (23), and the requirement of an airway as well as alveolar source to account for the dynamics of exhaled breath measurements (24–26). The airway epithelium is also thought to be the source of increased levels of exhaled NO in asthma in response to cytokine-induced inflammation (8). The Th2 cytokine IL-13 has been shown to play a central role in allergic asthma (27, 28) through its action on the airway epithelium (29), but its effect on iNOS expression in the epithelium has not been studied. Our study demonstrates that NO is released into the gas phase from primary cultures of normal human bronchial epithelial cells. In addition, IL-13 increases the rate of NO release and enhances nitrate formation over nitrite, but the response is strongly dependent on the donor.
We used mucociliary differentiated cultures of normal human airway epithelial cells (NHBEs) to perform the experiments. Such cultures have been to shown to be morphologically and functionally similar to in vivo epithelium (20) and offer a useful alternative to technically and ethically difficult whole organ measurements, with the added advantage of being amenable to tight experimental control to isolate the effect of different experimental variables. Our results showed that unstimulated NHBE cultures released NO into the gas phase at a basal level of 0.05 ± 0.03 pl·cm−2.s−1. The steady-state NO production rate from the airways of healthy adults has been estimated at 500–2,000 pl·s−1 (30), which, when normalized with the surface area of the Weibel airway tree (∼ 10,000 cm2, generations 0–17) (31) corresponds to a range of the NO flux of 0.05–0.2 pl·cm−2.s−1. Given the relative simplicity of our cell culture system, this result is remarkably similar to the basal flux from the in vitro model, suggesting that the bronchial epithelium may account for the NO in the exhaled breath. Unstimulated NHBE cultures expressed iNOS and nNOS, but not eNOS, gene and protein (Figures 3B, 3C, 4B, and 4C). These results are consistent with previous studies that have found iNOS and nNOS, but not eNOS, gene and/or protein expression in primary airway epithelial cells (13, 32, 33).
We found that IL-13 stimulation of NHBEs results in a striking increase in the NO flux and the formation of nitrite and nitrate in the culture medium (Figure 2), accompanied by an up-regulation in iNOS and nNOS gene, and iNOS (but not nNOS) protein levels (Figures 3B–3E). Furthermore, the inhibitor to iNOS had a much more significant impact on NO release to gas phase. Although each of the inhibitors exhibits a higher specificity for one of the two isoforms, there is some overlap. For example, the IC50 (concentration which inhibits 50% of enzyme activity) for L-NA-DBA is ∼ 40 times smaller for nNOS (1.13 μM) relative to iNOS (55 μM), whereas the IC50 for L-NIL is 30 times smaller for iNOS (3.3 μM) relative to nNOS (92 μM). If one assumes the inhibition obeys a typical sigmoidal curve (y = 1/(1 + c50/c) (where y is the fractional inhibition, c is the inhibitor concentration, and c50 is the IC50 value), the nNOS inhibitor at a concentration of 10 μM would reduce nNOS activity by 90% and iNOS activity by ∼ 15% and the iNOS inhibitor at a concentration of 30 μM would reduce iNOS activity by 90% and nNOS activity by 25%. In the presence of L-NA-DBA, IL-13–induced NO release from NHBE cells was reduced by ∼ 30%. This could be due to partial inhibition of the iNOS enzyme. Nonetheless, the data strongly implicate iNOS as the major source of IL-13–induced NO release.
Current literature suggests an inhibitory effect of IL-13 on Th1 cytokine- or lipopolysaccharide-induced iNOS expression (16–18). IL-13 was found to have no effect on nitrite production in cultures of A549 cells (19). However, it has been shown that gene expression and iNOS activity in response to IL-13 depends on both cell type (34, 35) and differentiation status (36). Thus IL-13–induced upregulation of iNOS may be specific to mucociliary differentiated NHBEs. This result also suggests that the direct effects of IL-13 on the airway epithelium could be responsible for one of the central observable features in asthma—elevated levels of exhaled NO.
An unexpected result was the finding that IL-13 up-regulated nNOS gene, but not protein, expression (Figures 3D and 3E). While nNOS has been traditionally considered a “constitutive” NOS isoform, its expression can be modulated by a number of factors, including cytokines (37). The difference between nNOS gene and protein expression may be related to post-transcriptional control of protein synthesis or protein turnover rate (38).
Exhaled NO measurements exhibit high levels of variability within groups of similar subjects (5, 12, 39). These variations may be related to environmental factors such as smoking (40) as well as genetic variations (12, 41, 42). In vitro NHBE cultures from different donors show significant variability in the number of goblet cells (43). To assess donor variability in NO production, we tested cells from three different donors. One of the donors (donor 2) was a smoker, but none of the subjects had a history of asthma or other pulmonary diseases. While basal NO flux from unstimulated cells was not significantly different between donors, there were striking differences in NO flux and iNOS gene and protein expression in response to IL-13 stimulation. Although smoking can reduce exhaled NO levels (44), the insensitivity of donor 2 cells to IL-13 may simply reflect natural donor to donor variability in NO metabolism. These results suggest that subject-to-subject variability in macroscopic (exhaled breath) measurements may be recapitulated in vitro, and cell culture studies may be useful in determining the underlying mechanisms of such variations.
NO has multiple, opposing roles in asthma: on the one hand it can protect against bronchoconstriction, either alone or in another form such as S-nitrosoglutathione, while on the other hand it can have proinflammatory and cytotoxic effects (45). These deleterious effects are mediated by reactive nitrogen species (RNS), the most abundant of which is peroxynitrite (ONOO−) formed by the reaction of NO with superoxide 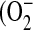 ). The relative amounts of nitrite
). The relative amounts of nitrite 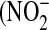 ) and nitrate
) and nitrate 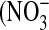 ) in the culture medium provides an initial clue about the dominant intermediates in NO metabolism. While
) in the culture medium provides an initial clue about the dominant intermediates in NO metabolism. While 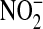 is the major product of NO oxidation in aqueous solutions (46),
is the major product of NO oxidation in aqueous solutions (46), 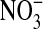 is the predominant product of ONOO− decomposition (47). Our results indicate that approximately equal amounts of
is the predominant product of ONOO− decomposition (47). Our results indicate that approximately equal amounts of 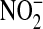 and
and 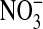 are formed under basal conditions, but IL-13 stimulation enhances
are formed under basal conditions, but IL-13 stimulation enhances 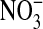 formation in a donor-dependent fashion that mirrors the donor variation in iNOS gene and protein expression (Figure 4D). This could be indicative of enhanced
formation in a donor-dependent fashion that mirrors the donor variation in iNOS gene and protein expression (Figure 4D). This could be indicative of enhanced 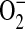 , and hence ONOO−, formation. Increased
, and hence ONOO−, formation. Increased 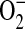 could result from IL-13–induced up-regulation of Duox1 and Duox2 (48), or L-arginine limitation (49).
could result from IL-13–induced up-regulation of Duox1 and Duox2 (48), or L-arginine limitation (49).
In summary, we have performed the first direct measurement of NO release into the gas phase from human bronchial epithelial (NHBE) cells. We found that unstimulated NHBEs produce and release NO at a low basal rate equal in magnitude to that observed in the exhaled breath of healthy subjects. IL-13 stimulation results in a significant increase in NO production due to iNOS induction, and also alters NO metabolism, resulting in an increase in the amount of nitrate relative to nitrite. Cells from different donors exhibit significantly different responses to IL-13 stimulation as measured by NO flux and iNOS gene and protein expression. We conclude that the bronchial epithelium is the likely source of NO in the exhaled breath, and increased levels observed in inflammatory diseases such as asthma are likely due to iNOS upregulation. Furthermore, this experimental system should prove useful in providing additional mechanistic insight into epithelial production and storage of NO.
Supplementary Material
This study was funded by National Institutes of Health grants HL070645 and HL67954.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2006-0419OC on March 8, 2007
Conflict of Interest Statement: V.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.D.M. has received a NIOX instrument as a gift from Aerocrine AB. (AAB), and has patents issued and pending related to exhaled NO for which AAB has entered a licensing agreement with the University of California, Irvine. S.C.G. has received a NIOX instrument as a gift from AAB, and has patents issued and pending related to exhaled NO for which AAB has entered a licensing agreement with the University of California, Irvine.
References
- 1.Barnes P, Kharitonov S. Exhaled nitric oxide: a new lung function test. Thorax 1996;51:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005;352:2163–2173. [DOI] [PubMed] [Google Scholar]
- 3.Moodley YP, Lalloo UG. Exhaled nitric oxide is elevated in patients with progressive systemic sclerosis without interstitial lung disease. Chest 2001;119:1449–1454. [DOI] [PubMed] [Google Scholar]
- 4.Rolla G, Colagrande P, Brussino L, Bucca C, Bertero M, Caligaris-Cappio F. Exhaled nitric oxide and pulmonary response to iloprost in systemic sclerosis with pulmonary hypertension. Lancet 1998;351:1491–1492. [DOI] [PubMed] [Google Scholar]
- 5.Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol 2005;115:1130–1136. [DOI] [PubMed] [Google Scholar]
- 6.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 1993;6:1368–1370. [PubMed] [Google Scholar]
- 7.Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994;343:133–135. [DOI] [PubMed] [Google Scholar]
- 8.Lane C, Knight D, Burgess S, Franklin P, Horak F, Legg J, Moeller A, Stick S. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax 2004;59:757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins RA, Barnes PJ, Springall DR, Warren JB, Kwon OJ, Buttery LDK, Wilson AJ, Geller DA, Polak JM. Expression of inducible nitric-oxide in human lung epithelial-cells. Biochem Biophys Res Commun 1994;203:209–218. [DOI] [PubMed] [Google Scholar]
- 10.Shin H-W, George SC. Microscopic modeling of no and s-nitrosoglutathione kinetics and transport in human airways. J Appl Physiol 2001;90:777–788. [DOI] [PubMed] [Google Scholar]
- 11.van's Gravesande KS, Wechsler ME, Grasemann H, Silverman ES, Le L, Palmer LJ, Drazen JM. Association of a missense mutation in the nos3 gene with exhaled nitric oxide levels. Am J Respir Crit Care Med 2003;168:228–231. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler ME, Grasemann H, Deykin A, Silverman EK, Yandava CN, Israel E, Wand M, Drazen JM. Exhaled nitric oxide in patients with asthma: Association with nos1 genotype. Am J Respir Crit Care Med 2000;162:2043–2047. [DOI] [PubMed] [Google Scholar]
- 13.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA 1995;92:7809–7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS. Nitric oxide synthase in human and rat lung: Immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol 1993;9:371–377. [DOI] [PubMed] [Google Scholar]
- 15.Robbins RA, Springall DR, Warren JB, Kwon OJ, Buttery LD, Wilson AJ, Adcock IM, Riveros-Moreno V, Moncada S, Polak J, et al. Inducible nitric oxide synthase is increased in murine lung epithelial cells by cytokine stimulation. Biochem Biophys Res Commun 1994;198:835–843. [DOI] [PubMed] [Google Scholar]
- 16.Berkman N, Robichaud A, Robbins RA, Roesems G, Haddad EB, Barnes PJ, Chung KF. Inhibition of inducible nitric oxide synthase expression by interleukin-4 and interleukin-13 in human lung epithelial cells. Immunology 1996;89:363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borderie D, Hilliquin P, Hernvann A, Lemarechal H, Kahan A, Menkes CJ, Ekindjian OG. Inhibition of inducible no synthase by th2 cytokines and tgf beta in rheumatoid arthritic synoviocytes: effects on nitrosothiol production. Nitric Oxide 2002;6:271–282. [DOI] [PubMed] [Google Scholar]
- 18.Saura M, Martinez-Dalmau R, Minty A, Perez-Sala D, Lamas S. Interleukin-13 inhibits inducible nitric oxide synthase expression in human mesangial cells. Biochem J 1996;313:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao YJ, Piedra PA, Larsen GL, Colasurdo GN. Induction and regulation of nitric oxide synthase in airway epithelial cells by respiratory syncytial virus. Am J Respir Crit Care Med 2001;163:532–539. [DOI] [PubMed] [Google Scholar]
- 20.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 1996;14:104–112. [DOI] [PubMed] [Google Scholar]
- 21.Kolodziejski PJ, Musial A, Koo JS, Eissa NT. Ubiquitination of inducible nitric oxide synthase is required for its degradation. Proc Natl Acad Sci USA 2002;99:12315–12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen-oxides in the airways. Am J Respir Crit Care Med 1994;149:538–551. [DOI] [PubMed] [Google Scholar]
- 23.Gaston B, Reilly J, Drazen JM, Fackler J, Ramdev P, Arnelle D, Mullins ME, Sugarbaker DJ, Chee C, Singel DJ, et al. Endogenous nitrogen-oxides and bronchodilator s-nitrosothiols in human airways. Proc Natl Acad Sci USA 1993;90:10957–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persson MG, Zetterstrom O, Agrenius V, Ihre E, Gustafsson LE. Single-breath nitric-oxide measurements in asthmatic-patients and smokers. Lancet 1994;343:146–147. [DOI] [PubMed] [Google Scholar]
- 25.Schedin U, Frostell C, Persson MG, Jakobsson J, Andersson G, Gustafsson LE. Contribution from upper and lower airways to exhaled endogenous nitric-oxide in humans. Acta Anaesthesiol Scand 1995;39:327–332. [DOI] [PubMed] [Google Scholar]
- 26.Tsoukias NM, Tannous Z, Wilson AF, George SC. Single-exhalation profiles of no and co2 in humans: Effect of dynamically changing flow rate. J Appl Physiol 1998;85:642–652. [DOI] [PubMed] [Google Scholar]
- 27.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for il-13 independently of il-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wills-Karp M, Luyimbazi J, Xu XY, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 29.Kuperman DA, Huang XZ, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002;8:885–889. [DOI] [PubMed] [Google Scholar]
- 30.George SC, Hogman M, Permutt S, Silkoff PE. Modeling pulmonary nitric oxide exchange. J Appl Physiol 2004;96:831–839. [DOI] [PubMed] [Google Scholar]
- 31.Weibel E. Morphometry of the human lung. Berlin: Springer-Verlag; 1963.
- 32.Asano K, Chee CB, Gaston B, Lilly CM, Gerard C, Drazen JM, Stamler JS. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci USA 1994;91:10089–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norford D, Koo JS, Gray T, Alder K, Nettesheim P. Expression of nitric oxide synthase isoforms in normal human tracheobronchial epithelial cells in vitro: dependence on retinoic acid and the state of differentiation. Exp Lung Res 1998;24:355–366. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Kaminski N, Dolganov G, Grunig G, Koth L, Solomon C, Erle DJ, Sheppard D. Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am J Respir Cell Mol Biol 2001;25:474–485. [DOI] [PubMed] [Google Scholar]
- 35.Paoliello-Paschoalatoa AB, Oliveira SHP, Cunha FQ. Interleukin 4 induces the expression of inducible nitric oxide synthase in eosinophils. Cytokine 2005;30:116–124. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi T, Shively JD, Foley JS, Drazen JM, Tschumperlin DJ. Differentiation-dependent responsiveness of bronchial epithelial cells to il-4/13 stimulation. Am J Physiol Lung Cell Mol Physiol 2004;287:L119–L126. [DOI] [PubMed] [Google Scholar]
- 37.Forstermann U, Boissel JP, Kleinert H. Expressional control of the 'constitutive' isoforms of nitric oxide synthase (nos i and nos iii). FASEB J 1998;12:773–790. [PubMed] [Google Scholar]
- 38.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mrna expression levels on a genomic scale. Genome Biol 2003;4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivieri M, Talamini G, Corradi M, Perbellini L, Mutti A, Tantucci C, Malerba M. Reference values for exhaled nitric oxide (reveno) study. Respir Res 2006;7:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kharitonov SA, Robbins RA, Yates D, Keating V, Barnes PJ. Acute and chronic effects of cigarette-smoking on exhaled nitric-oxide. Am J Respir Crit Care Med 1995;152:609–612. [DOI] [PubMed] [Google Scholar]
- 41.Grasemann H, van's Gravesande KS, Buscher R, Drazen JM, Ratjen F. Effects of sex and of gene variants in constitutive nitric oxide synthases on exhaled nitric oxide. Am J Respir Crit Care Med 2003;167:1113–1116. [DOI] [PubMed] [Google Scholar]
- 42.Gravesande KSV, Wechsler ME, Grasemann H, Silverman ES, Le L, Palmer LJ, Drazen JM. Association of a missense mutation in the nos3 gene with exhaled nitric oxide levels. Am J Respir Crit Care Med 2003;168:228–231. [DOI] [PubMed] [Google Scholar]
- 43.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol 2003;285:L730–L739. [DOI] [PubMed] [Google Scholar]
- 44.Hoyt JC, Robbins RA, Habib M, Springall DR, Buttery LDK, Polak JM, Barnes PJ. Cigarette smoke decreases inducible nitric oxide synthase in lung epithelial cells. Exp Lung Res 2003;29:17–28. [DOI] [PubMed] [Google Scholar]
- 45.Ricciardolo FLM, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev 2004;84:731–765. [DOI] [PubMed] [Google Scholar]
- 46.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from l-arginine. Proc Natl Acad Sci USA 1993;90:8103–8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen B, Deen WM. Analysis of the effects of cell spacing and liquid depth on nitric oxide and its oxidation products in cell cultures. Chem Res Toxicol 2001;14:135–147. [DOI] [PubMed] [Google Scholar]
- 48.Harper RW, Xu CH, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual nadph oxidases/peroxidases, duox1 and duox2, by th1 and th2 cytokines in respiratory tract epithelium. FEBS Lett 2005;579:4911–4917. [DOI] [PubMed] [Google Scholar]
- 49.Meurs H, Maarsingh H, Zaagsma J. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends Pharmacol Sci 2003;24:450–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.