Abstract
Streptococcus pneumoniae colonizes at the nasopharynx of humans and is able to disseminate and cause various infections. The hallmark of pneumococcal disease is rapid bacterial replication in different tissue sites leading to intense inflammation. The genetic basis of pneumococcal adaptation to different host niches remains sketchy. In this study, we investigated the regulatory effect of RR06, a response regulator protein, on gene expression of S. pneumoniae. Microarray and Northern blot analyses showed that RR06 is specifically required for transcription of spr1996 and cbpA. While the function of Spr1996 is unknown, CbpA has been well characterized as a surface-exposed protective antigen and a virulence factor of S. pneumoniae. A recombinant form of RR06 was able to bind to a 19-bp conserved sequence shared by the spr1996 and cbpA promoter regions. Furthermore, inactivation of rr06 resulted in loss of CbpA expression as detected by antibody staining and bacterial adhesion. CbpA expression was restored in trans by the intact rr06 gene. However, a mutant, RR06(D51A), with a point mutation in the aspartate residue at position 51 (a predicted major phosphorylation site) of RR06, completely abolished the CbpA expression, suggesting that RR06 phosphorylation is required for transcriptional activation of spr1996 and cbpA. Finally, inactivation of rr06 in additional pneumococcal strains also led to the loss of CbpA expression. These data implicate that RR06 activates the expression of spr1996 and cbpA in many other pneumococcal strains.
Streptococcus pneumoniae naturally colonizes at the nasopharynx of humans and behaves as a commensal in immuno-competent adults. In young children, the elderly, and immunocompromised individuals, the bacterium is able to disseminate to remote tissue sites (lung, middle ear, bloodstream, and brain). Pneumococcal replication in the local environments induces intense tissue inflammation, leading to bacterial pneumonia, acute otitis media, bacteremia, and other complications (38). These observations indicate that S. pneumoniae is highly capable of adapting to different host niches during infection, as well demonstrated in many other bacterial pathogens (36). Recent transcriptional studies have identified niche/body site-specific expression patterns for many pneumococcal genes (29, 43). The differentially expressed genes include those involved in capsule biosynthesis and the production of pneumolysin and surface-exposed proteins. The mechanisms of the gene regulation are not well understood except for the well-characterized competence system (44).
Two-component signal transduction systems (TCSs) are common regulatory mechanisms for bacterial responses to various environmental cues (54). Many TCSs are important in bacterial interactions with hosts (12). A typical TCS consists of a membrane-associated histidine kinase and a cytosolic cognate response regulator. Upon receipt of an extracellular stimulus, the histidine kinase is activated by autophosphorylating a conserved histidine residue (54). The phosphorylated histidine kinase further donates the phosphate group to a conserved aspartate residue in its cognate response regulator which, in turn, undergoes a conformational change and regulates gene expression mostly as a transcriptional regulator. There are 13 complete TCSs and 1 orphan response regulator in the complete genome sequences of strains R6 (17) and TIGR4 (56). Eight of the TCSs were reported to contribute to respiratory infection of S. pneumoniae (57), although Lange et al. (25) showed that insertion inactivation in the 13 RR tested did not have significant impact on systemic pneumococcal infection in mice.
Among the TCSs in S. pneumoniae, CiaR/H, the first pneumococcal TCS identified, is involved in competence and antibiotic susceptibility (13). Recent studies also revealed the down-regulation of the major virulence factor HtrA in ciaRH mutants (20, 32, 51). VicRK, also known as YycFG or MicAB, is involved in maintaining integrity of the cell wall (40), fatty acid biosynthesis (37), and expression of pneumococcal surface protein A (PspA) (41). ComD/E was demonstrated to control pneumococcal competence (45) and contribute to virulence (3, 15, 26). BlpR/S regulates the production of bacteriocin-like proteins (9). PnpR/S appears to control the expression of pneumococcal surface antigen A (PsaA) and contributes to pneumococcal virulence and resistance to oxidative stress (33). RitR, the orphan response regulator, represses the piu iron uptake system (59). Standish et al. (53) recently showed that RR06/HK06 regulates the expression of choline binding protein A (CbpA). CbpA is required for pneumococcal colonization (2, 24, 47), lung infection (2, 15, 24), and sepsis (18). In vitro studies have also revealed specific interactions of CbpA with human poly-immunoglobulin (Ig) receptor (pIgR), secretory IgA, secretory component, complement factor H, and C3 (7). Some of these biochemical interactions are consistent with the observations that CbpA is necessary for pneumococcal adhesion to and invasion of airway epithelial cells (11, 47, 63). CbpA is among a few pneumococcal proteins that can stimulate antibody production in humans (34, 35) and is able to confer protective immunity against lethal challenge of virulent pneumococci in animal models (2, 4, 42, 47). However, it is unclear if RR06/HK06 regulates additional pneumococcal genes and virulence factors.
In this study, we have analyzed the impact of RR06 on gene expression in S. pneumoniae. The transcriptional profile of laboratory strain R6 was compared with an isogenic rr06-deficient mutant by microarray. Together with the data from further transcription and protein analyses, RR06 was shown to activate the transcription of its downstream neighbor genes spr1996 and cbpA by binding to a conserved sequence motif in the 5′ untranslated regions of genes spr1996 and cbpA.
MATERIALS AND METHODS
Bacterial strains and growth media.
The bacterial strains and plasmids used in this study are listed in Table 1. G54 is a serotype 19F isolate from human sputum (46) and was kindly provided by Francesco Iannelli. ST556 is a serotype 19F isolate from a patient with otitis media (22) and was kindly provided by Michael R. Jacobs. Pneumococci were routinely grown in Todd-Hewitt broth containing 0.5% yeast extract (THY) or on tryptic soy agar (TSA) plates containing 3% (vol/vol) sheep blood. When necessary, kanamycin (200 μg/ml), spectinomycin (600 μg/ml), chloramphenicol (2 μg/ml), or streptomycin (150 μg/ml) was included in the broth and agar media for selection purposes. Escherichia coli strains DH5α and TB1 were used for routine cloning and protein expression, respectively. E. coli cultures were grown in Luria-Bertani (LB) broth or on LB agar plates. Antibiotic selection in E. coli was performed with the following final concentrations: 100 μg/ml for ampicillin, 100 μg/ml for spectinomycin, 25 μg/ml for chloramphenicol. All ingredients for bacterial culture media and other chemicals used in this work were obtained from Sigma (St. Louis, MO) unless otherwise stated. E. coli strains were grown at 37°C with aeration, and pneumococci were incubated at 37°C with 5% CO2.
TABLE 1.
Bacterial strains used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| D39 | S. pneumoniae isolate, serotype 2, encapsulated; rr06+ cbpA+; Cms | 1 |
| R6 | D39 derivative, unencapsulated; rr06+ cbpA+; Cms Kans Sps Erms | 58 |
| G54 | S. pneumoniae serotype19F, an isolate from human sputum; rr06+ cbpA+; Cms | 46 |
| ST398 | R6 derivative; hk06::pID701t; R6 transformed with pST393; Cmr | This study |
| ST489 | R6 derivative; rr06::pID701t; R6 transformed with pST382; Cmr. | This study |
| ST556 | S. pneumoniae serotype 19F, an isolate from a patient with otitis media; rr06+ cbpA+; Cms | 22 |
| ST568 | R6 derivative; ΔcbpA::Janus cassette; Kanr | This study |
| ST796 | D39 derivative; rr06::pID701t; R6 transformed with pST382; Cmr | This study |
| ST797 | D39 derivative; hk06::pID701t; R6 transformed with pST393; Cmr | This study |
| ST815 | R6 derivative; R6 transformed with pDL278; Spr | This study |
| ST820 | R6 derivative; Δrr06::ermB; Ermr | This study |
| ST822 | R6 derivative; Δspr1996::ermB; Ermr | This study |
| ST918 | ST556 derivative; rr06::pID701t; ST556 transformed with pST382; Cmr | This study |
| ST920 | ST556 derivative; hk06::pID701t; ST556 transformed with pST393; Cmr | This study |
| ST922 | G54 derivative; rr06::pID701t; G54 transformed with pST382; Cmr | This study |
| ST924 | G54 derivative; hk06::pID701t; G54 transformed with pST393; Cmr | This study |
| ST969 | R6 derivative; ST820 transformed with pDL278; Ermr Spr | This study |
| ST970 | R6 derivative; ST820 transformed with pST804; Ermr Spr | This study |
| ST986 | R6 derivative; ST820 transformed with pST982; Ermr Spr | This study |
| Plasmids | ||
| pID701t | Suicide plasmid; Cmr | 26 |
| pJDC9 | Suicide plasmid; Ermr | 5 |
| pMalc2E | Maltose binding protein fusion vector; Apr | NEB |
| pDL278 | E. coli-streptococcal shuttle plasmid; Spr | 28 |
| pST382 | 5′ coding sequence of R6 rr06 cloned in XbaI site of pID701t; Cmr | This study |
| pST393 | 5′ coding sequence of R6 hk06 cloned in SmaI site of pID701t; Cmr | This study |
| pST804 | pDL278::RR06; rr06 coding sequence with whole intergenic region cloned in BamHI/EcoRI site of pDL278; Spr | This study |
| pST825 | pMalE::RR06; coding sequence of R6 rr06 cloned in BamHI/HindIII site of pMALc2E; Apr | This study |
| pST982 | pMalE::RR06; coding sequence of R6 rr06 cloned in BamHI/EcoRI site of pDL278; Spr | This study |
Ap, ampicillin; Cm, chloramphenicol; Erm, erythromycin; Kan, kanamycin; Sp, spectinomycin; r, resistant; s, sensitive.
Construction of S. pneumoniae mutants and plasmids for the complementation study.
The rr06 and hk06 insertion mutants of S. pneumoniae were generated as described elsewhere (62). Briefly, the 5′ coding regions of rr06 and hk06 were amplified by PCR from chromosomal DNA of strain R6 using primers Pr245/Pr246 and Pr227/Pr228, respectively. All of the primers were synthesized by Invitrogen (Carlsbad, CA) and are listed in Table 2. PCR amplifications were performed using a high-fidelity DyNAzyme EXT DNA polymerase (MJ Research, Waltham, MA) under the conditions described previously (61). The PCR products were then cloned into the XbaI (for rr06) or SmaI (for hk06) site of plasmid pID701t (26). The plasmid constructs for insertional inactivation were confirmed by DNA sequencing with vector-based primers. The correct plasmids were transformed into S. pneumoniae by natural transformation as described previously (62). The transformants were selected for resistance to chloramphenicol (2 μg/ml) on the TSA plates.
TABLE 2.
DNA sequences and locations of primers used in this study
| Primer | Sequence | Locationa |
|---|---|---|
| Pr074 | 5′-ACAGAGAACGAGGGAAGTACC-3′ | 4156-4176 |
| Pr227 | 5′-GAGGGGGAAAAGTTTAATGAGAGC-3′ | 6161-6184 |
| Pr228 | 5′-GTTACCTGTTTACCATCTGCTGTAC-3′ | 5937-5961 |
| Pr231 | 5′-TTAGCAATGGCGGGAAAGAA-3′ | 4792-4811 |
| Pr245 | 5′-CTAGTCTAGACGAGGAAATGATTAGAGAAGGAA-3′ | 6912-6934 |
| Pr246 | 5′-CTAGTCTAGATGACAGGAATATCGTGCTTTTG-3′ | 6735-6756 |
| Pr254 | 5′-ATTGGCGCGCCGATATAATGGGAGATAAGACG-3′ | 42-62 |
| Pr255 | 5′-ATTGGCGCGCCGATATAATGGGAGATAAGACG-3′ | 989-1011 |
| Pr257 | 5′-CCTATGGCTGGCGTGACCAACTC-3′ | 12492-12514 |
| Pr311 | 5′-ATTGGCGCGCCACCGTTTGATTTTTAATGGATAATG-3′ | 773-796 |
| Pr312 | 5′-ATTGGCCGGCCCCTTTCCTTATGCTTTTGGAC-3′ | 2080-2100 |
| Pr313 | 5′-ATTGGCGCGCCGTTTATTTCCTTCTATATTTTTTC-3′ | 9241-9264 |
| Pr314 | 5′-ACCGGCCGGCCAACCTAATATAACTAGTTAATACTG-3′ | 11370-11394 |
| Pr722 | 5′-TGACAGATAGCAAGGGACGCAAGG-3′ | 8081-8104 |
| Pr723 | 5′-ATTGGCGCGCCCATCTCTCTCCCTTTCTACTA-3′ | 6944-6975 |
| Pr724 | 5′-ACCGGCCGGCCCTGTGAAAAATGTTGGGTATA-3′ | 6318-6349 |
| Pr725 | 5′-TGACAGATAGCAAGGGACGCAAGG-3′ | 4552-4575 |
| Pr726 | 5′-TTGGCGATATCAGGATGTAACAGT-3′ | 6563-6586 |
| Pr727 | 5′-ATTGGCGCGCCCATATTAGTATCCTTTCTTTTAT-3′ | 4880-4913 |
| Pr728 | 5′-ACCGGCCGGCCATACAAAATTCGATTTATATACAG-3′ | 4342-4376 |
| Pr729 | 5′-TTAGGTAGTACCATGAACCATTGT-3′ | 2742-2765 |
| Pr739 | 5′-CGATGGATCCATTGGTGTCAAAGCAGGCCAGTTA-3′ | 7617-7650 |
| Pr740 | 5′-CGATGAATTCTAGGATTTTTTATCATAAGCTAAT-3′ | 6282-6315 |
| Pr750 | 5′-CCGCGAATTCTTATCTCTTTATGCTTCCATT-3′ | 4356-4386 |
| Pr758 | 5′-ACGATCTGTCTTGATGTTTTCTAA-3′ | 3598-3621 |
| Pr669 | 5′-CCGCGCATGCCAGTTAAAATTTGATATTGCA-3′ | 5933-5949 |
| Pr670 | 5′-CCAACGAATTCGCTATATGAATGACTAGACCAAC-3′ | 7308-7330 |
| Pr781 | 5′-CGCGGATCCATGAACATTTTAGTTGCAGATGAC-3′ | 6934-6966 |
| Pr789 | 5′-CCGCCGAAGCTTCTAGAATTGCAAAACTTCTTA-3′ | 6238-6270 |
| Pr801 | 5′-TCATATCTTGTTTAGGACAGT-3′ | 4550-4570 |
| Pr802 | 5′-CTTTTTGATGCAAACATGTTT-3′ | 4271-4291 |
| Pr803 | 5′-AATACATGTGCGCCTCTAAGT-3′ | 5150-5170 |
| Pr804 | 5′-ACTAAAATAATCGCTAATACC-3′ | 4871-4891 |
| Pr835 | 5′-GCAGGCCAGTTAAAATTTGATATT-3′ | 7605-7628 |
| Pr836 | 5′-TTCCTCGTCATCTGCAACTAAAAT-3′ | 6928-6951 |
| Pr855 | 5′-TCGATTTATATACGGTTCATATTGAA-3′ | 4331-4356 |
| Pr856 | 5′-TTCAATATGAACCGTATATAAATCGA-3′ | 4331-4356 |
| Pr857 | 5′-CATAAAGAGATAAATACAAAATTCGA-3′ | 4353-4378 |
| Pr858 | 5′-TCGAATTTTGTATTTATCTCTTTATG-3′ | 4353-4378 |
| Pr859 | 5′-TGAAGTAATATAGTAAGGTTAAAGAA-3′ | 4309-4334 |
| Pr860 | 5′-TTCTTTAACCTTACTATATTACTTCA-3′ | 4309-4334 |
| Pr865 | 5′-TAGTTTTTTATTAAAGTTCATATAGGG-3′ | 4943-4969 |
| Pr866 | 5′-CCCTATATGAACTTTAATAAAAAACTA-3′ | 4943-4969 |
| Pr867 | 5′-TTAAGATTAGCTTGTAGACAGATTAGT-3′ | 4966-4992 |
| Pr868 | 5′-ACTAATCTGTCTACAAGCTAATCTTAA-3′ | 4966-4992 |
| Pr869 | 5′-AGGGTTAACATAAGTGTGTTATTCTTT-3′ | 4920-4946 |
| Pr870 | 5′-AAAGAATAACACACTTATGTTAACCCT-3′ | 4920-4946 |
| Pr990 | 5′-CAAGATCTCCCTATCCATCTCATGGTATTGGCATTAATG-3′ | 6799-6837 |
| Pr991 | 5′-CAATACCATGAGATGGATAGGGAGATCTTG-3′ | 6808-6837 |
The rr06 and spr1996 deletion mutants in strain R6 were prepared essentially as described previously (31). The upstream (1,146-bp) and downstream (1,798-bp) flanking regions of rr06 in R6 were separately amplified from the R6 genomic DNA by PCR using primer pairs Pr722/Pr723 and Pr724/Pr725, respectively. An erythromycin (ermB) cassette was amplified from plasmid pJDC9 (5) using primers Pr254 and Pr255. The PCR products of the ermB cassette and the cbpA flanking sequences were digested by appropriate restriction enzymes as determined by the nested restriction sites (AscI and FseI) at the 5′ end of the primers, purified from agarose gels using the DNA gel purification kit (QIAGEN, Valencia, CA), and ligated using the Quick ligation kit from New England Biolabs (NEB; Beverly, MA). The ligation mixtures were transformed into S. pneumoniae to select erythromycin-resistant colonies on blood agar plates as described elsewhere (27). The resultant mutant strains were verified by PCR amplification or direct DNA sequencing of the mutation sites using genomic DNA preparations of the mutant strains. The spr1996 deletion mutant in strain R6 was constructed in a similar manner. The spr1996 flanking regions were PCR amplified using primer pairs Pr726/Pr727 and Pr728/Pr729. The cbpA deletion strain ST568 was constructed in R6 by replacing the entire cbpA-coding region with the Janus cassette as described previously (31). The upstream and downstream cbpA-flanking regions in strain R6 were separately PCR amplified from genomic DNA preparations using primer pairs Pr252/Pr313 and Pr314/257, respectively. The PCR fragments were ligated to the AscI/FseI-digested Janus cassette, which confers kanamycin resistance (200 μg/ml) in S. pneumoniae (55). The Janus cassette was PCR amplified with primers Pr311 and Pr312 from the cbpA-null mutant ST588 of S. pneumoniae strain D39 (31).
To prepare the rr06 complementation construct, the sequence consisting of the rr06 coding sequence and its entire 5′ untranslated region was PCR amplified with primers Pr739 and Pr740, digested with BamHI and EcoRI, and cloned into the E. coli-streptococcal shuttle plasmid pDL278 (28). The resulting plasmid, pST804, was verified by DNA sequencing and transformed into the rr06-null mutant ST820 by natural transformation as described previously (62). The mutant RR06(D51A) allele was generated by a PCR-based strategy (31) in which the aspartate residue at position 51 of RR06 was replaced with an alanine. The GAT (D51) codon of the rr06 gene was changed to GCA (A51) by amplifying the up- and downstream segments surrounding the 51st codon using primer pairs Pr739/Pr991 and Pr990/Pr740, respectively. Two PCR fragments were fused by overlap extension PCR using primers Pr739 and Pr740 (16) and cloned in the BamHI/EcoRI-digested pDL278. The resultant plasmid, pST982, was verified by DNA sequencing and transformed into ST820 as described above. In parallel, pDL278 was also introduced into various S. pneumoniae strains as negative controls.
RNA isolation and microarray analysis.
Total RNA extracts were isolated from the cell cultures of the wild-type R6 and rr06 mutant ST489 grown to an optical density at 620 nm (OD620) of 0.5 in the THY broth with the RNeasy mini kit (QIAGEN) according to the manufacturer's instructions. RNA concentrations were determined by measuring the OD values at 260 and 280 nm, checked for RNA integrity by gel electrophoresis based on rRNA bands, and stored at −80°C until later use. To minimize the impact of growth conditions and RNA preparation on the abundance of specific mRNAs, three RNA samples were prepared for each of the S. pneumoniae strains at separate times as biological replicates and analyzed in parallel in the subsequent DNA microarray experiments.
Microarray analysis was performed with the S. pneumoniae R6 GeneChip (Affymetrix, Santa Clara, CA), which contained oligonucleotide probes for the 2,043 protein-coding genes and 73 noncoding RNA genes of S. pneumoniae strain R6 (17) and additional control oligonucleotides. Probe preparation, DNA hybridization, and data collection were carried out according to the manufacturer's instructions at the Bionomics Research and Technology Center at The Rutgers University (Piscataway, NJ). The hybridization probes consisted of differentially labeled cDNA derived from total RNA isolated from R6 and the isogenic rr06 mutant ST489. Data analysis was performed using the Affymetrix Microarray Suite software 5.0 with Affymetrix default parameters. The change was calculated by dividing the median of the normalized red channel intensity by the median of the normalized green channel intensity. An average twofold difference in expression level between R6 and ST489 in the three hybridization experiments was used as a cutoff factor. In addition, the statistical program EBarrays was applied to determine if the two gene expression profiles were statistically significant as described elsewhere (23).
DNA and protein sequence analysis.
DNA and protein sequence analyses were performed using the DNASTAR Lasergene v6.1 program for Macintosh computers (Madison, WI). The nucleotide sequence of the rr06-cbpA locus of strain ST556 was determined by automated DNA sequencing with the primers based on the corresponding DNA sequences of the strains R6 (accession number AE008564) (17) and TIGR4 (accession number AE007507) (56).
Northern blot analysis.
Total RNA was extracted from S. pneumoniae cultures and quantified as described above. Aliquots of RNA (5 μg) were denatured at 65°C for 15 min and separated by electrophoresis through a denaturing formaldehyde-agarose (1.2%) gel according to standard procedures (48). The RNA was then transferred onto positively charged nylon membranes (GE Healthcare Life Sciences, Piscataway, NJ) and baked at 80°C for 2 h. The membranes were hybridized with the cbpA or spr1996 probe. The cbpA and spr1996 probe templates were first amplified from the genomic DNA of strain R6 using primer pairs Pr074/Pr758 and Pr231/Pr750, respectively. Radioactive probes were then prepared with [α-32P]dCTP (Perkin-Elmer, Wellesley, MA) using the DECA prime II random priming DNA labeling kit (Ambion, Austin, TX).
Expression and purification of MalE-RR06.
The full-length rr06 coding sequence was amplified using primers Pr781and Pr789 and cloned into the BamHI-HindIII-digested plasmid pMALc2E (NEB). The resulting plasmid, pST825, was used to overexpress the MalE-RR06 fusion protein in E. coli strain TB1 as recommended by the supplier. Briefly, the E. coli strain was grown in LB at 37°C with aeration. A final concentration of 0.3 mM isopropyl-β-d-thiogalactopyranoside was added to induce the expression of the fusion protein when the OD600 reached 0.5. The culture was incubated for an additional 3 h and centrifuged at 8,000 × g for 15 min. Cell pellets were resuspended with 50 ml of ice-cold column buffer (20 mM Tris-HCl, pH 7.4, 200 mM NaCl, and 1 mM EDTA) in the presence of 1 mM phenylmethylsulfonyl fluoride. The cell suspension was passed three times through a French press (20,000 lb/in2) and centrifuged at 10,000 × g for 30 min to remove the insoluble debris. The supernatants were diluted 1:5 with the ice-cold column buffer in the presence of 1 mM phenylmethylsulfonyl fluoride and loaded onto a 5-ml amylose column (NEB), which had previously been equilibrated with 40 ml of the column buffer. The column was washed with 120 ml of the ice-cold column buffer. The MalE-RR06 protein was eluted with 10 ml of the column buffer containing 10 mM maltose and collected in fractions of 1.0 ml each. Protein concentrations of the fractions were determined by using the Bio-Rad protein assay reagent (Hercules, CA). The three fractions with the highest protein concentrations were pooled and dialyzed. For storage, glycerol was added to a final concentration of 50%. The fusion protein was purified to homogeneity as verified by Coomassie blue staining of the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels.
EMSA.
The ability of the MalE-RR06 protein to bind the rr06, spr1996, and cbpA promoter regions was tested using an electrophoretic mobility shift assay (EMSA). The DNA fragments spanning the 5′ untranslated regions of the rr06, spr1996, and cbpA genes in strain R6 were amplified with primer pairs Pr835/Pr836, Pr801/Pr802, and Pr803/Pr804, respectively. The double-stranded DNA segments spanning the 5′ untranslated region of cbpA were prepared by annealing complementary pairs of oligonucleotides Pr857/Pr858 for Oligo-1, Pr855/Pr856 for Oligo-2, and Pr859/Pr860 for Oligo-3 for 10 min at 90°C in TEN buffer (1 mM Tris-HCl, pH 8.0, 1 mM EDTA, and 100 mM NaCl). The double-stranded DNA oligonucleotides for the spr1996 promoter region were prepared in the same manner by annealing complementary pairs of oligonucleotides Pr867/Pr868 for Oligo-4, Pr865/Pr866 for Oligo-5, and Pr869/Pr870 for Oligo-6. These DNA segments were end labeled with [γ-32P]ATP and T4 polynucleotide kinase (Invitrogen) and used for binding reactions with purified MalE-RR06. For protein-DNA binding, radiolabeled DNA probes (5 ng) were incubated with various concentrations of the MalE-RR06 fusion protein at room temperature for 30 min in 20 μl of binding buffer [20 mM HEPES, pH 8.0, 5 mM MgCl2, 50 mM potassium glutamate, 0.01 mM EDTA, 1 mM NaH2PO4, 20 mM NaCl, 1 mM dithiothreitol, 30 μg/ml bovine serum albumin, and 30 mg/ml poly(dI-dC)]. Where indicated, an excessive amount (100 ng) of specific DNA fragments (cold probes) was included in the reaction mixtures for competitive binding. Samples were subjected to electrophoresis in 5% Tris-borate-EDTA nondenaturing Ready gels (Bio-Rad) at room temperature in 0.5× Tris-borate-EDTA buffer with 1.2% glycerol. The gels were dried with a gel dryer and exposed to X-ray films at −70°C for 3 h.
Western blot and immunofluorescence microscopy.
Western blot analysis with CbpA was carried out as previously described (31). In brief, pneumococcal strains were grown in THY broth to an OD620 of 0.5. Cells were collected by centrifugation and resuspended in lysis buffer (0.1% SDS, 0.1% deoxycholate, and 0.15 M sodium citrate). Protein concentrations were determined using the Bio-Rad protein assay reagent. Samples containing approximately 5 μg of total bacterial proteins were mixed with the standard SDS-PAGE loading buffer (48), boiled for 5 min, and then separated in 10 to 20% Tris-HCl SDS-PAGE gels (Bio-Rad). After semidry electrophoretic transfer of proteins onto the polyvinylidene difluoride membranes (Millipore, Bedford, MA), CbpA was detected with a rabbit antiserum for the full-length CbpA of strain TIGR4 as described previously (31). Following a blocking step in 5% milk (wt/vol), the membranes were reacted with the CbpA antiserum (1:5,000 dilution) and peroxidase-conjugated goat anti-rabbit IgG antibody (1:5,000 dilution). Reactive protein bands were visualized with the enhanced chemiluminescence Western blot kit (Pierce, Rockford, IL). To detect the CbpA variants in strains ST556 and G54 with complement factor H, the blots of bacterial lysates were treated with purified human factor H (Sigma) at a final concentration of 0.4 μg/ml overnight at 4°C. Additional detection and visualization steps were carried out as described elsewhere (31).
Fluorescence staining of live pneumococci with the CbpA antibody was carried out as described previously (31). A final dilution of 1:100 was used for the CbpA antiserum. Bacteria were inspected by standard phase-contrast and fluorescence microscopy using an Olympus BX51 upright fluorescence microscope.
Bacterial adhesion assay.
Pneumococcal adherence was determined in a 24-well plate format as described previously (63). Human airway cell lines Detroit 562 (nasopharyngeal cells) and A549 (type II lung epithelial cells) were obtained from the American Type Culture Collection (Manassas, VA) and maintained as described previously (63). Pneumococci (2 × 107 CFU/well) were incubated with confluent cell monolayers of Detroit 562 or A549 cells in 24-well plates for 30 min. After washing three times with phosphate-buffered saline, epithelial cells were detached with trypsin-EDTA, lysed with ice-cold Triton X-100 (0.05%), diluted in phosphate-buffered saline, and plated onto the TSA blood plates for CFU counts. All assays were performed in four to six replicate wells and repeated at least three independent times. The results of representative experiments are presented as means plus standard deviations.
Nucleotide sequence accession number.
The nucleotide sequence of the rr06-cbpA locus of strain ST556 is contained in GenBank under accession number DQ851866.
RESULTS
Microarray analysis of the rr06 mutant.
A previous study reported that the rr06/hk06 locus is involved in regulating the expression of cbpA (53). We determined if this TCS regulated expression of other pneumococcal genes and virulence factors by transcriptional analysis of an rr06 mutant in laboratory strain R6 by microarray analysis. The complete genome sequence of R6 has identified 2,043 protein-coding genes and 73 noncoding RNA genes (17). The rr06/hk06 locus is highly conserved in all the strains with available sequence information at this locus, including strains R6 (type 2), TIGR4 (type 4), G54 (type 19), and ST556 (type 19). The 3′ coding sequence of rr06 has a 4-bp overlap with the 5′ end of hk06, suggesting these two genes form an operon. The rr06 mutant ST489 was constructed by inserting plasmid pID701t in the 5′ coding sequence of rr06 (see Materials and Methods). Because of the apparent operon structure in the rr06/hk06 locus, this insertion was expected to interrupt the production of both the RR06 and HK06 proteins. Consistent with previous reports (25, 57), ST489 did not show any obvious growth defect in THY (data not shown).
To compare the transcriptional profiles between ST489 and the parent strain R6 by microarray, total RNA extracts were prepared with three independent batches of bacterial cultures for each of ST489 and R6. The RNA preparations were used to prepare the cDNA probes. The differentially labeled cDNA probes of ST489 and R6 were hybridized with the R6 GeneChips representing all of the 2,043 protein-coding regions and other noncoding sequences (intergenic regions and noncoding RNA genes). In this study a gene was considered differentially expressed if (i) hybridization signals between ST489 and R6 differed by a factor of 2 on average and (ii) the calculated probability of differential expression was ≥0.5. A total of 41 genes or intergenic regions had a change of ≥2-fold in three separate biological replicates. To our surprise, only the changes for rr06, hk06, spr1996, and cbpA were consistent in all three separate reactions with statistical significance (Table 3). The rest of the genes were identified in only one of three hybridizations. As a result, the mean fold changes were all below 2 and statistically insignificant.
TABLE 3.
Transcription profiles of pneumococcal genes in the rr06 mutant ST489
| Gene namea | Function | Fold changeb
|
|||
|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Mean | ||
| rr06 | Response regulator | −127.9 | −156.77 | −156.99 | −147.22 |
| hk06 | Histidine kinase | −69.31 | −99.44 | −79.56 | −82.77 |
| spr1996 | Hypothetical protein | −11.03 | −6.09 | −6.43 | −7.85 |
| cbpA | Surface-exposed protein | −10.81 | −6.65 | −7.29 | −8.25 |
The gene names are based on the annotation of the R6 genome (17).
Negative values indicate a reduction in transcription.
The transcription levels of rr06, hk06, spr1996, and cbpA were each decreased more than sixfold in ST489 compared with the parent strain R6. A diminished mRNA level of rr06 in strain ST489 confirmed the rr06 mutation, whereas the loss of hk06 transcription was due to a predicted polar effect of the upstream insertion in rr06. The rr06 mutant had similarly reduced levels of expression of spr1996 (7.85-fold) and cbpA (8.25-fold). Since ST489 had diminished expression of both rr06 and hk06, the microarray result suggested that the rr06/hk06 TCS is required to activate the transcription of spr1996 and cbpA. It should be noted that the mutation in rr06 completely abolished transcription of rr06 and hk06, but there were still low levels of transcription in spr1996 and cbpA in ST489, suggesting that basal levels of transcription occur in spr1996 and cbpA even in the total absence of the rr06/hk06 TCS. Surprisingly, the mutation in rr06 did not result in significantly increased expression of any other pneumococcal genes, suggesting that the rr06/hk06 TCS only activates the expression of spr1996 and cbpA under these in vitro culture conditions.
The biological function(s) of the protein encoded by spr1996 remains unknown. Sequence analysis has suggested that Spr1996 is an integral membrane protein with a putative isoprenylcysteine carboxyl methyltransferase activity (data not shown). Spr1996 has a 21.1% amino acid identity with Saccharomyces cerevisiae Ste14p, an isoprenylcysteine carboxyl methyltransferase and an integral membrane protein. The sequence and genetic localization of spr1996 is highly conserved among the examined pneumococcal strains. Homologues of spr1996 are also present in the genomes of many other bacteria, including Streptococcus agalactiae, Streptococcus suis, Streptococcus pyogenes, and staphylococci.
To determine whether Spr1996 affects the expression of CbpA, spr1996 was replaced with an erythromycin cassette in the R6 background by allelic replacement. The resulting spr1996 mutant ST822 did not show an obvious growth defect, indicating that Spr1996 is not essential for S. pneumoniae growth in vitro (data not shown). Western blotting revealed comparable levels of CbpA production between ST822 and the wild-type strain (data not shown), which argues against a direct role of Spr1996 in CbpA expression. We further tested the adherence and invasion of the spr1996-null mutant in respiratory epithelial cell cultures as shown below in Fig. 6. No obvious difference was observed in the levels of epithelial adherence and invasion between the spr1996-null mutant ST822 and the wild-type strain (data not shown). Extensive characterization of Spr1996 was not further pursued due to the scope of this study.
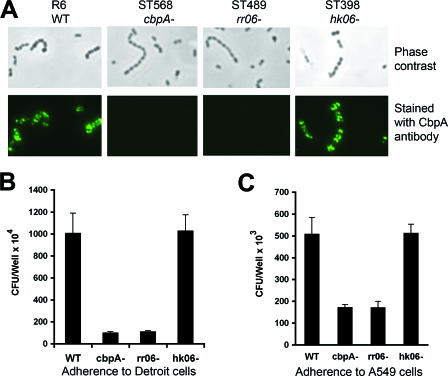
FIG. 6.
RR06 is required for pneumococcal adhesion. A. Expression of CbpA at the cell surface of S. pneumoniae was detected by immunofluorescence microscopy. Intact organisms of strains R6 and its isogenic mutants in cbpA, rr06, and hk06 were visualized under phase-contrast (top panel) or fluorescence (bottom panel) microscopy after being stained with the CbpA antiserum. B. Adherence of R6 and its isogenic mutants to Detroit 562 cells. The results of representative experiments are presented as means ± standard deviations of four duplicate wells. C. Same experiment as in panel B, except for the use of A549 cells.
Cotranscription of cbpA with spr1996.
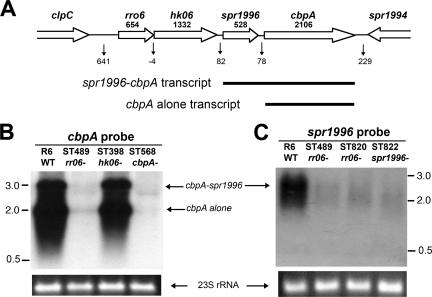
Based on the microarray data, we wondered how the rr06 controls the expression of spr1996 and cbpA. As illustrated in Fig. 1A, sequence analysis did not identify an apparent transcriptional terminator in the 78-nucleotide intergenic region between spr1996 and cbpA. This raised the possibility that spr1996 and cbpA share a single promoter. This idea was tested by Northern blot analysis of various isogenic mutants in the strain R6 background with 32P-labeled probes for coding sequences of spr1996 and cbpA. As represented in Fig. 1B, the cbpA probe revealed two transcripts in strain R6 with sizes of approximately 2.2 and 2.7 kilonucleotides. Both bands were absent in the cbpA-null mutant strain ST568, indicating these were cbpA-specific transcripts. The larger transcript had an equivalent size of the combined cbpA (2,106 nucleotides) and spr1996 (528 nucleotides), suggestive of cotranscription between spr1996 and cbpA. This large transcript was also detected with the spr1996 probe, but the same probe did not detect the 2.2-kilonucleotide transcript (Fig. 1C). We thus conclude that the 2.2- and 2.7-kilonucleotide bands represented a transcript of cbpA alone and a bicistronic message of spr1996 and cbpA, respectively. In agreement with the previous report (53), the Northern blot results also indicated that the rr06/hk06 locus is not cotranscribed with spr1996 and cbpA, because the insertion mutation in hk06 did not affect the size or amount of the spr1996-cbpA transcript (Fig. 1B).
FIG. 1.
RR06 is required for transcription of spr1996 and cbpA. A. Schematic illustration of the rr06-cbpA locus in the chromosome of strain R6 (accession no. AE008564). Open arrows represent the gene directions. The name and length (in bp) of each gene are indicated at the top. The numbers of nucleotides in the intergenic regions are marked below the vertical arrows. Two cbpA transcripts as detected in panel B are illustrated with the thick lines. B. Northern hybridization of strain R6 and its isogenic mutants using the cbpA probe. Total RNA extractions were separated by agarose electrophoresis, blotted, and hybridized with the radiolabeled cbpA probe spanning the 1,065-bp coding sequence at the 5′ region cbpA. The sizes of the RNA molecular mass markers are indicated in kilonucleotides. An ethidium bromide-stained 23S rRNA band in an agarose gel is included to indicate equal loading of the total bacterial RNA. C. Same experiment as in panel B, except for the use of the spr1996 probe, which represents a 446-bp coding sequence of spr1996.
Neither the 2.2- nor 2.7-kilonucleotide band could be detected in the rr06 mutant ST489 with both the cbpA and spr1996 probes (Fig. 1B). The result thus confirmed the microarray data (Table 3) indicating that rr06 is required for expression of both spr1996 and cbpA. Interestingly, inactivation of hk06 itself (strain ST398) did not lead to obvious alteration in the transcription of spr1996 or cbpA, because the mutant and wild-type strains had similar levels of the cbpA-spr1996 cotranscript (large band) and the cbpA transcript (small band) (Fig. 1B). This result suggests that HK06 is not required for the transcription of spr1996 and cbpA under these conditions. The same data have also ruled out the possibility that the polar mutation on hk06 in the rr06 mutant ST489 contributed to the transcription profile in the microarray experiments (listed in Table 3). A predicted transcript for spr1996 alone (∼600 nucleotides in size) was not detected with the spr1996 probe even after extended exposure of the X-ray films (Fig. 1C and data not shown). This observation led to our conclusion that spr1996 is only cotranscribed with cbpA. Taken together, the Northern blot data strongly suggest that spr1996 and cbpA form an operon, and their expression requires RR06 but not HK06.
Binding of recombinant RR06 to the promoter regions of spr1996 and cbpA.
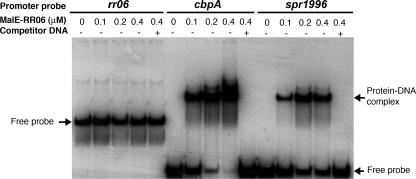
A previous study demonstrated that RR06 can bind to the promoter region of cbpA (53). Our data described above suggested that RR06 may interact with the promoter regions of both spr1996 and cbpA. We tested this possibility by EMSA using a MalE-RR06 fusion protein. The upstream sequences of the rr06, spr1996, and cbpA coding regions were PCR amplified and allowed to interact with the MalE-RR06 fusion protein. It should be noted that a longer PCR product was used for rr06 because of a long intergenic region (641 bp) between the coding sequences of clpC and rr06. As shown in Fig. 2 (middle and right panels), the recombinant RR06 resulted in a dose-dependent mobility shift of the radiolabeled promoter probes for both cbpA and spr1996. Further, the mobility shift was competed off with the unlabeled PCR products, thus supporting the specificity of the protein-DNA binding interactions. In contrast, the recombinant RR06 protein did not show a detectable mobility shift with its own promoter probe even at the highest protein concentration used in the experiments (0.4 μM), which was suggestive of a lack of autoregulation in the rr06 locus as reported previously (53). A faint nonspecific band was visible for the rr06 probe lanes and was also present in the no-protein control lane (Fig. 2, left panel). This observation further supports the specificity of the protein-DNA binding interactions observed with the promoter regions of spr1996 and cbpA.
FIG. 2.
Purified RR06 binds to the 5′ untranslated regions of spr1996 and cbpA. The PCR fragments spanning the 5′ untranslated regions of rr06 (700 bp), spr1996 (300 bp), and cbpA (300 bp) were incubated with purified MalE-RR06 fusion protein for EMSA as described in Materials and Methods. The free probes and protein-DNA complexes are indicated with arrows.
Localization of the RR06 binding motif in promoter regions of spr1996 and cbpA.
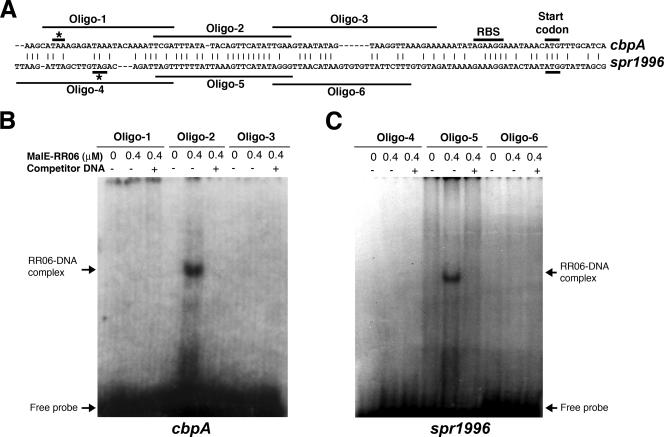
Sequence alignment analysis with the 5′ untranslated regions of spr1996 and cbpA revealed a 19-bp conserved motif with 84% DNA sequence identity, although the rest of the two sequences have little homology, as shown in Fig. 3A. We hypothesized that RR06 binds to the spr1996 and cbpA promoters by specific interaction with this conserved motif. To test this possibility, a series of complementary oligonucleotides were synthesized which spanned the entire 5′ untranslated regions of spr1996 and cbpA. The appropriately annealed double-stranded oligonucleotides were labeled as probes to perform an EMSA. The recombinant RR06 showed detectable binding to the conserved motifs from the 5′ untranslated regions of spr1996 (Fig. 3C, Oligo-5) and cbpA (Fig. 3B, Oligo-2). In contrast, the sequences flanking the conserved motifs from both spr1996 (Fig. 3C, Oligo-4 and Oligo-6) and cbpA (Fig. 3B, Oligo-1 and Oligo-3) failed to show a mobility shift after incubation with the recombinant RR06. The binding specificity was further confirmed by successful titration of the binding signals with unlabeled oligonucleotides representing the conserved motif for spr1996 (Fig. 3C, Oligo-5) and cbpA (Fig. 3B, Oligo-2). Since the sequences flanking the 19-bp conserved motif in spr1996 and cbpA did not show detectable binding to the MalE-RR06 fusion protein, we conclude that RR06 specifically binds to this sequence motif in the 5′ untranslated regions of spr1996 and cbpA. The 5′ untranslated regions of spr1996 and cbpA contain conserved ribosomal binding sites in front of the translational start codon (Fig. 3A). An extensive search of the R6 genome did not identify the RR06 binding sequence elsewhere, supporting the microarray data indicating that RR06 specifically activates the expression of spr1996 and cbpA.
FIG. 3.
The RR06 binding capability is localized to a conserved motif in the 5′ untranslated regions of cbpA and spr1996. A. Sequence comparison between the 5′ untranslated regions of cbpA and spr1996. The appropriate nucleotide sequences from accession no. AE008564 were aligned by the MegAlign program of DNASTAR Lasergene v6.1. Identical sequences are marked with vertical lines. Gaps (indicated by dashes) were introduced to facilitate alignment. The stop codons for spr1996 (top sequence) and hk06 (bottom sequence) are indicated with asterisks. The positions of the six oligonucleotides (Oligo-1 to Oligo-6) used for mapping the RR06 binding regions in panel B are marked with lines. B. RR06 binding of various segments of the 5′ untranslated regions of cbpA. Three pairs of complementary oligonucleotides covering the 5′ untranslated region of cbpA (Oligo-1, Oligo-2, and Oligo-3) were individually synthesized and allowed to anneal as described in Materials and Methods. The resulting DNA fragments were used to perform an EMSA with purified MalE-RR06 protein as described for Fig. 2. C. Same experiment as in panel B, except for the use of three pairs of oligonucleotides corresponding to the 5′ untranslated region of spr1996.
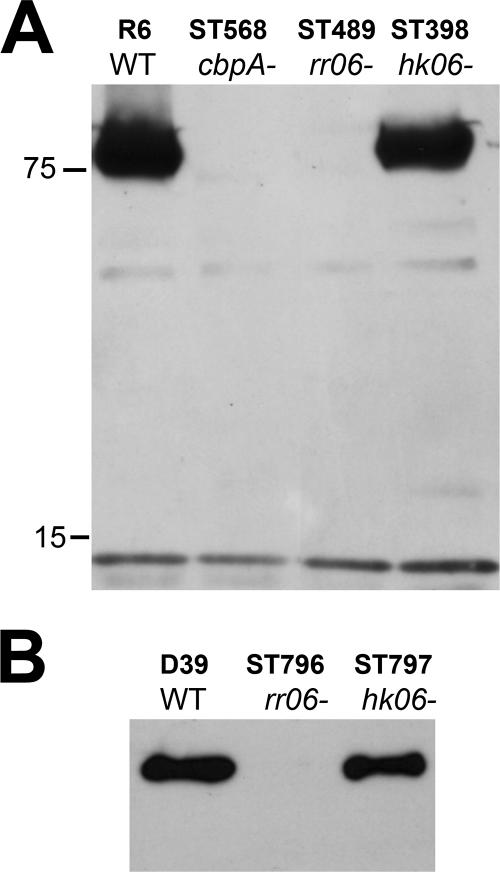
Requirement of RR06 but not HK06 for expression of the CbpA protein.
We further validated the regulatory effect of RR06 on the cbpA locus at the protein level. While CbpA was detected in strain R6 by Western blotting, the protein was undetectable in the rr06 mutant ST489 (Fig. 4A). As a negative control, CbpA was absent in the cbpA-null strain ST568. The result thus confirmed the data from the microarray (Table 3) and Northern blot analyses (Fig. 1). Similar to the Northern blot data (Fig. 1), strain R6 and its isogenic hk06 mutant ST398 showed a comparable level of CbpA production (Fig. 4A), again suggesting that HK06 is not necessary for CbpA expression. We extended this analysis to strain D39, an encapsulated progenitor of strain R6 by inactivation of rr06 and hk06. The rr06 mutant ST796 of strain D39 had undetectable CbpA, whereas the isogenic hk06 mutant ST797 showed comparable levels of CbpA with the wild-type strain (Fig. 4B). Thus, the rr06 and hk06 mutants in the D39 background completely mirrored those of strain R6 in terms of the production of the CbpA protein.
FIG. 4.
RR06 is required for expression of the CbpA protein. A. Whole-cell lysates of strain R6 and isogenic mutants in cbpA, rr06, and hk06 (approximately 5 μg total protein/lane) were subjected to SDS-PAGE. The blot was reacted in a Western blot assay with a CbpA rabbit antiserum. The sizes of the protein mass markers are marked in kDa. B. Western blot of strain D39 and its isogenic mutants in rr06 and hk06, as described for panel A.
We further verified that an rr06-null mutant (strain ST820) in the R6 background also had undetectable CbpA as the insertional mutant ST489 (Fig. 5B), thus ruling out the possibility that the diminished CbpA expression of the rr06 mutant ST489 (Fig. 4A) was due to a polar effect of the insertional mutation in rr06 on HK06 expression and function. To verify that RR06 directly activates the expression of CbpA, the rr06 gene including its entire 5′ untranslated region was placed in an Escherichia coli-S. pneumoniae shuttle plasmid. The complementation construct pST804 was transformed into the rr06-null strain ST820. In contrast to vector controls, the Western blot analysis showed that the rr06 construct pST804 was able to restore CbpA expression in trans in the rr06-null mutant ST820 (Fig. 5B). Together, these data indicate RR06 directly activates the expression of CbpA. Based on the transcription analysis data (Table 3 and Fig. 1), the production of the Spr1996 protein is likely to be regulated in a similar manner.
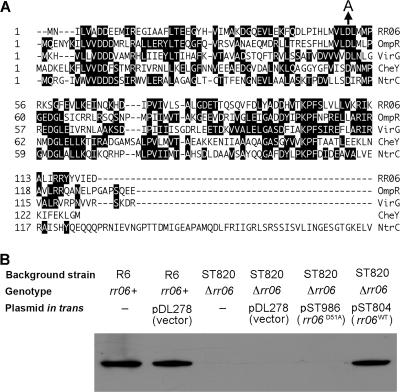
FIG. 5.
Functional impact of RR06 phosphorylation. A. Partial sequence alignment of RR06, OmpR (accession no. AAN82619), VirG (accession no. NP_059810), CheY (accession no. AAA23577), and NtrC (accession no. CAA59425) by the Clustal W method in the MegAlign program of DNASTAR Lasergene v6.1. Identical amino acids are shaded. Gaps were introduced for optimal alignment as indicated by dashed lines. Amino acid positions of each sequence are marked on the left side according to the relative distance from the first amino acid. Only the amino-terminal region of NtrC is shown. The D-to-A mutation at Asp-51 of RR06 is marked with an arrow. B. Restoration of CbpA expression in the rr06-null strain ST820 by genetic complementation with the wild-type rr06 or mutant rr06(D51A) allele. ST820 was transformed with either pDL278 (vector) or the plasmids harboring the wild-type (rr06WT, pST804) or mutant (rr06D51A, pST982) rr06. The resultant strains were used to detect CbpA by Western blotting as described for Fig. 4A. The wild-type strain R6 was also transformed with the empty vector as a negative control.
The regulatory activities of many two-component response regulators are controlled by phosphorylation at a highly conserved aspartate residue in the middle regions of the proteins. Sequence analysis indicated the aspartate residue at position 51 (Asp-51) of RR06 as the major phosphorylation site (Fig. 5A) because the corresponding aspartate residues have been demonstrated as a major phosphorylation site in numerous well-characterized RR06 homologues, including OmpR (8), VirG (21), CheY (50), and NtrC (49). To assess the role of phosphorylation in RR06 activity, an rr06 allele with a D-to-A point mutation at Asp-51 was constructed by site-directed mutagenesis. In contrast to pST804 (carrying the intact rr06), pST982 harboring the RR06(D51A) allele did not result in detectable CbpA when it was transformed in the rr06-null strain ST820 (Fig. 5B). This result was highly reproducible, with additional independent ST820 clones transformed with pST982 (data not shown). We concluded that RR06 phosphorylation at Asp-51 is essential for the activation of CbpA expression by this regulator.
Effects of RR06 on pneumococcal adhesion to respiratory epithelial cells.
CbpA has been shown to be involved in pneumococcal adhesion to human respiratory epithelial cells by interacting with multiple host receptors (14). Based on the above observation that RR06 is required for CbpA expression (Fig. 4), we reasoned that RR06 might also modulate pneumococcal adhesion. We initially assessed CbpA expression on the bacterial surface of different mutant strains by immunofluorescence microscopy. As shown in Fig. 6A, CbpA was readily detectable in the wild-type strain R6 and the isogenic hk06 mutant. In contrast, inactivation of rr06 resulted in the loss of CbpA expression. This result demonstrated that RR06 but not HK06 is required for CbpA expression at the cell surface of S. pneumoniae.
We further tested the adhesion of isogenic mutant strains in two human respiratory epithelial cell lines, Detroit 562 (nasopharyngeal cells) and A549 (type II lung cells). Our previous study showed that CbpA dramatically enhances pneumococcal adhesion and invasion in Detroit 562 cell cultures by binding to human pIgR (63). To a lesser extent, CbpA also promotes pneumococcal adhesion to A549 cells, possibly by interacting with host sialic acid, lacto-N-neotetraose, and the C3 protein (47, 52). As expected, the cbpA mutant showed an approximately 10- and 5-fold reduction in the adhesion levels to Detroit 562 (Fig. 6B) and A549 (Fig. 6C) monolayers, respectively. Consistent with the CbpA expression profile, the rr06 mutant showed similar levels of decreases in adhesion to both cell lines. In contrast, the hk06 mutant did not show apparent reduction in epithelial adhesion. It should be noted that RR06 may have substantially lower levels of impact on CbpA-mediated adhesion with encapsulated S. pneumoniae strains due to the inhibitory effect of the polysaccharide capsule on pneumococcal adhesion as described previously (53). We only used adhesion of the unencapsulated strain R6 as a marker to verify the impact of RR06 on CbpA expression.
Activation of CbpA expression by RR06 in multiple pneumococcal strains.
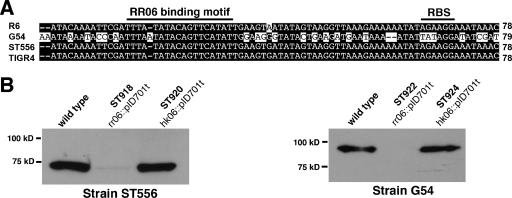
All S. pneumoniae strains tested thus far contain the cbpA locus (4, 19), but there are high levels of sequence polymorphisms among various CbpA alleles. This is in sharp contrast with the fact that the regions flanking the cbpA locus are highly conserved (19). The complete DNA sequences of rr06, hk06, spr1996, and cbpA are available for strains R6 (17), G54 (10), ST556 (accession number DQ851866), and TIGR4 (56). It is unclear if expression of various cbpA allelic variants is regulated in the same manner. There are only a few amino acid sequence differences among the RR06 variants of strains R6, ST556, G54, and TIGR4 (data not shown). Additional sequence alignment also revealed that the 5′ untranslated sequences of cbpA are identical for all the strains except for G54 (Fig. 7A). Even in strain G54, the 19-bp RR06 binding motif of strain R6 is also preserved (Fig. 7A). This is in sharp contrast to the sequence variations in the cbpA coding sequences. CbpA of strain R6 (701 amino acids [aa]) has 85.4%, 32.4%, and 83.7% sequence identities with the CbpA variants of strains ST556 (630 aa), G54 (769 aa), and TIGR4 (693 aa).
FIG. 7.
RR06 is required for CbpA expression in additional isolates of S. pneumoniae. A. Alignment of the 5′ untranslated sequences of cbpA variants. The nucleotide sequences between the stop codon of spr1996 and the start codon of cbpA from strains R6 (accession no. AE008564), G54 (accession no. AL449923), ST556 (accession no. DQ851866), and TIGR4 (accession no. AE007507) were aligned as described for Fig. 3A. The total number of nucleotides in the cbpA intergenic region for each allele is indicated at the end of each sequence. B. Expression of CbpA in the wild-type ST556 and isogenic rr06 and hk06 mutants. CbpA was detected by Western blotting using purified human complement factor H. C. Same experiment as in panel B, except for the use of cellular lysate from strain G54.
To examine the regulatory effect of RR06 on cbpA expression in these strains, the rr06 and hk06 genes were inactivated in strains ST556 and G54 by plasmid insertion. Similar to a previous report (53), our attempt to disrupt rr06 in strain TIGR4 was unsuccessful, likely due to the essentiality of RR06 in this strain. Because of sequence diversity in the CbpA variants, the antiserum against the R6 CbpA protein was weakly reactive to many other CbpA allelic variants, including CbpA of strain G54 (data not shown). Based on the strong interaction of CbpA and human complement factor H (31), factor H was used to detect the CbpA variants of ST556 and G54. As shown in Fig. 7B and C, the wild-type ST556 and G54 strains expressed CbpA variants with expected sizes. Similar to the finding in strains R6 and D39 (Fig. 4), CbpA expression was abolished in the rr06 mutants (ST918 and ST922) of both strain backgrounds (Fig. 7B and C). In contrast, the disruption of hk06 (ST920 and ST924) did not lead to obvious alteration in CbpA expression in strains ST556 and G54. These data thus showed that RR06 but not HK06 is required for CbpA expression in multiple strains of S. pneumoniae.
DISCUSSION
S. pneumoniae is highly capable of adapting to multiple host niches as manifested by its ability to survive and replicate at the mucosal surfaces of the airway, in the bloodstream, and in the brain. In this work, our analyses at both the mRNA and protein levels have demonstrated that RR06, one of the 14 potential DNA binding response regulators, is specifically required for the expression of the immediate downstream genes spr1996 and cbpA under standard culture conditions. The narrow specificity of RR06 in gene regulation is in sharp contrast to the rather broad spectra of the other pneumococcal TCSs that have been characterized thus far. A previous microarray analysis of a type 3 S. pneumoniae strain 0100993 showed that the CiaRH TCS up- and down-regulates 24 and 22 genes, respectively (51). The orphan response regulator RitR was reported to modulate the expression of 54 genes in strain R800, an R6 derivative (59). Similarly, many pneumococcal genes are affected by the mutations in additional TCSs, including comDE (6, 45), vicRK (37, 41), rr04 (33), ciaRH (32, 51), and blpRH (9). Our data cannot exclude the possibility that RR06 regulates the expression of additional pneumococcal genes under other environmental conditions, such as different tissue sites in humans.
The regulatory activity of RR06 appears to be controlled by phosphorylation. Asp-51 of RR06 is a major phosphorylation site, because the RR06(D51A) allele was defective as a transcriptional activator. The Asp-51 equivalents are the major phosphorylation sites in other well-characterized proteins that are members of the phosphate acceptor family, such as OmpR (8), VirG (21), CheY (50), and NtrC (49). Among these, RR06 has the highest sequence similarity to OmpR. OmpR in E. coli modulates the expression of the outer membrane porin proteins OmpF and OmpC in response to changes in the surrounding osmolarity (8). Alanine substitutions in the three aspartate positions 11, 12, and 55 of OmpR, equivalents of positions 7, 8, and 51 of RR06, have identified Asp-55 as a major phosphorylation site, but the OmpR(D55A) allele retains a low level of its regulatory activity (8). Our data have suggested that Asp-51 of RR06 is the only phosphorylation site, because RR06(D51A) lost its activity in activating CbpA expression. In this regard, RR06 behaves like other well-studied bacterial response regulators, such as VirG, CheY, and NtrC. The RR06 Asp-51 equivalents in VirG (21), CheY (50), and NtrC (49) are the only phosphorylation sites to control the activities of these proteins. Further genetic and biochemical analyses are warranted to elucidate the precise mechanism by which RR06 controls the expression of pneumococcal genes under various conditions.
The impact of HK06 on the expression of spr1996 and cbpA is less clear. A previous study showed a fivefold increase in the mRNA level of cbpA with an hk06 mutant of strain D39, suggesting HK06 somehow represses cbpA expression (53). However, additional functional data from epithelial adhesion and mouse infection experiments of the same study implicated an opposite role for HK06 (53). Our data indicated that HK06 is not a major contributor to RR06 phosphorylation under these conditions, because the disruptive mutations in hk06 had no apparent effect on the expression of spr1996 and cbpA. Our Northern blot analysis initially showed that hk06 is not required for the transcription of spr1996 and cbpA. Furthermore, the hk06 mutant did not show obvious alteration in the production of the CbpA protein as determined by Western blotting using the CbpA antibody. In the more sensitive and quantitative adhesion assay, the hk06 mutant and the wild-type strain had similar levels of adherence in two separate airway epithelial culture systems. Together, our data suggest that RR06 receives a phosphorylation signal from other noncognate histidine kinase(s) or some other phosphate donor if the activity of RR06 is controlled by phosphorylation status of the protein.
CbpA protein was consistently undetectable in multiple rr06 mutants by Western blotting, despite a detectable transcription of cbpA in the rr06 disruptive mutant in microarray analysis. This discrepancy may reflect the limited sensitivity of our Western blotting-based detection method compared with more sensitive mRNA detection techniques. In this regard, we also noticed an obvious difference between our data and a previous study (53). In contrast to an undetectable level of the CbpA protein in multiple rr06 mutants of strain D39 in this study, Standish et al. showed a relatively higher level of residual CbpA in an rr06 deletion mutant of strain D39 (53). The same study also observed a higher background level of cbpA mRNA in the absence of RR06 (53). Culture conditions might be a contributing factor, as exemplified by the difference in the amount of yeast extract used in the THY broth (0.5% versus 1%) in the two studies. Consistent with this notion, residual CbpA in the rr06-deficient mutant was undetectable by Western blotting when the pneumococci were cultured in serum broth (53).
Cross-talk among different TCSs has been well documented in many bacterial species (54, 60). In this regard, RR06 is reminiscent of another response regulator, VicR, of S. pneumoniae (also known as RR02). VicR is essential for pneumococcal survival (57) because it is required to activate the expression of the essential PcsB putative murein hydrolase (39, 40). However, VicK, the cognate histidine kinase of VicR, does not share the same tested regulatory pathways with the VicR. Abrogation of VicK has been shown to have little effect on the expression of PcsB (39, 40), the growth rate of S. pneumoniae (39, 40), or the infectivity of the bacterium in the lungs of mice (57). Thus, similar to HK06, VicK is not a major source of phosphorylation for VicR, but the key signal that controls the VicR activity is also unknown. In the chemotaxis system of E. coli, a single histidine kinase, CheA, competitively phosphorylates two response regulators, CheB and CheY, in response to changes in glucose concentration (30). It is thus possible that one or more kinases relay the signal(s) to RR06.
We have demonstrated that RR06 activates the transcription of spr1996 and cbpA in strain R6, but this regulatory relationship is likely to operate in many additional strains. The coding sequence of the cbpA locus is highly variable, likely due to selection pressure from the host immune response against this surface-exposed protein (4, 19). However, the sequence and gene organization of the cbpA-flanking regions, including rr06, hk06, and spr1996, are virtually identical in all tested strains (19). Although the regulatory role of RR06 has not been determined in humans, the natural host of S. pneumoniae, some indirect evidence suggests that RR06 is a key in vivo regulator of S. pneumoniae. The RR06/HK06 system is one of the eight TCS loci in which deletion mutations have resulted in a significant decrease in S. pneumoniae survival in the lungs of mice (57). Differential expression of CbpA has also been well documented. CbpA appears to be present in higher amounts in the transparent colony variants of S. pneumoniae compared with opaque colony variants (47). LeMessurier et al. (29) recently reported that pneumococci recovered from the nasopharynx and lungs of mice express higher levels of cbpA mRNA than those recovered from the bloodstream. These observations are consistent with other reports that CbpA is required for nasopharyngeal colonization and lung infection in animal models (4, 24, 47). The contribution of CbpA to pneumococcal infection in the bloodstream is not completely clear. Some of the previous studies showed no or a minor effect of CbpA mutations on virulence of S. pneumoniae in bacteremia animal models (4, 47), whereas others reported that CbpA-deficient mutants significantly lost virulence in mice after intravenous inoculation (18). It is thus reasonable to postulate that RR06 activates the transcription of spr1996 and cbpA in the colonization/commensal stage of S. pneumoniae at the mucosal surface of the nasopharynx, while its activity as a transcriptional activator may be repressed once the bacterium enters the bloodstream, resulting in lower levels of spr1996 and cbpA expression.
Acknowledgments
We gratefully acknowledge Francesco Iannelli (University of Siena, Siena, Italy) and Michael R. Jacobs (Case Western Reserve University) for generously providing S. pneumoniae strains and Saudi Haataja (University of Turku, Turku, Finland) for kindly providing the pID701t plasmids. We thank our colleagues Thomas D. Friedrich, Odeniel Sertil, Ling Lu, and Yueyun Ma for their technical assistance.
This work was supported in part by research grants from the American Lung Association (RG-178-N) and the National Institutes of Health (AI054753 and DC006917).
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, P., A. Brooks-Walter, A. Virolainen-Julkunen, S. K. Hollingshead, and D. E. Briles. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartilson, M., A. Marra, J. Christine, J. S. Asundi, W. P. Schneider, and A. E. Hromockyj. 2001. Differential fluorescence induction reveals Streptococcus pneumoniae loci regulated by competence stimulatory peptide. Mol. Microbiol. 39:126-135. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Walter, A., D. E. Briles, and S. K. Hollingshead. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J. D., and D. A. Morrison. 1988. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene 64:155-164. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, Q., E. A. Campbell, A. M. Naughton, S. Johnson, and H. R. Masure. 1997. The com locus controls genetic transformation in Streptococcus pneumoniae. Mol. Microbiol. 23:683-692. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, Q., D. Finkel, and M. K. Hostetter. 2000. Novel purification scheme and functions for a C3-binding protein from Streptococcus pneumoniae. Biochemistry 39:5450-5457. [DOI] [PubMed] [Google Scholar]
- 8.Delgado, J., S. Forst, S. Harlocker, and M. Inouye. 1993. Identification of a phosphorylation site and functional analysis of conserved aspartic acid residues of OmpR, a transcriptional activator for ompF and ompC in Escherichia coli. Mol. Microbiol. 10:1037-1047. [DOI] [PubMed] [Google Scholar]
- 9.de Saizieu, A., C. Gardes, N. Flint, C. Wagner, M. Kamber, T. J. Mitchell, W. Keck, K. E. Amrein, and R. Lange. 2000. Microarray-based identification of a novel Streptococcus pneumoniae regulon controlled by an autoinduced peptide. J. Bacteriol. 182:4696-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dopazo, J., A. Mendoza, J. Herrero, F. Caldara, Y. Humbert, L. Friedli, M. Guerrier, E. Grand-Schenk, C. Gandin, M. de Francesco, A. Polissi, G. Buell, G. Feger, E. Garcia, M. Peitsch, and J. F. Garcia-Bustos. 2001. Annotated draft genomic sequence from a Streptococcus pneumoniae type 19F clinical isolate. Microb. Drug Resist. 7:99-125. [DOI] [PubMed] [Google Scholar]
- 11.Elm, C., R. Braathen, S. Bergmann, R. Frank, J. P. Vaerman, C. S. Kaetzel, G. S. Chhatwal, F. E. Johansen, and S. Hammerschmidt. 2004. Ectodomains 3 and 4 of human polymeric immunoglobulin receptor (hpIgR) mediate invasion of Streptococcus pneumoniae into the epithelium. J. Biol. Chem. 279:6296-6304. [DOI] [PubMed] [Google Scholar]
- 12.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guenzi, E., A. M. Gasc, M. A. Sicard, and R. Hakenbeck. 1994. A two-component signal-transducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol. Microbiol. 12:505-515. [DOI] [PubMed] [Google Scholar]
- 14.Hammerschmidt, S. 2006. Adherence molecules of pathogenic pneumococci. Curr. Opin. Microbiol. 9:12-20. [DOI] [PubMed] [Google Scholar]
- 15.Hava, D., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389-1406. [PMC free article] [PubMed] [Google Scholar]
- 16.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 17.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iannelli, F., D. Chiavolini, S. Ricci, M. R. Oggioni, and G. Pozzi. 2004. Pneumococcal surface protein C contributes to sepsis caused by Streptococcus pneumoniae in mice. Infect. Immun. 72:3077-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannelli, F., M. R. Oggioni, and G. Pozzi. 2002. Allelic variation in the highly polymorphic locus pspC of Streptococcus pneumoniae. Gene 284:63-71. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim, Y. M., A. R. Kerr, J. McCluskey, and T. J. Mitchell. 2004. Control of virulence by the two-component system CiaR/H is mediated via HtrA, a major virulence factor of Streptococcus pneumoniae. J. Bacteriol. 186:5258-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin, S. G., R. K. Prusti, T. Roitsch, R. G. Ankenbauer, and E. W. Nester. 1990. Phosphorylation of the VirG protein of Agrobacterium tumefaciens by the autophosphorylated VirA protein: essential role in biological activity of VirG. J. Bacteriol. 172:4945-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joloba, M. L., A. Windau, S. Bajaksouzian, P. C. Appelbaum, W. P. Hausdorff, and M. R. Jacobs. 2001. Pneumococcal conjugate vaccine serotypes of Streptococcus pneumoniae isolates and the antimicrobial susceptibility of such isolates in children with otitis media. Clin. Infect. Dis. 33:1489-1494. [DOI] [PubMed] [Google Scholar]
- 23.Kendziorski, C. M., M. A. Newton, H. Lan, and M. N. Gould. 2003. On parametric empirical Bayes methods for comparing multiple groups using replicated gene expression profiles. Stat. Med. 22:3899-3914. [DOI] [PubMed] [Google Scholar]
- 24.Kerr, A. R., G. K. Paterson, J. McCluskey, F. Iannelli, M. R. Oggioni, G. Pozzi, and T. J. Mitchell. 2006. The contribution of PspC to pneumococcal virulence varies between strains and is accomplished by both complement evasion and complement-independent mechanisms. Infect. Immun. 74:5319-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange, R., C. Wagner, A. de Saizieu, N. Flint, J. Molnos, M. Stieger, P. Caspers, M. Kamber, W. Keck, and K. E. Amrein. 1999. Domain organization and molecular characterization of 13 two-component systems identified by genome sequencing of Streptococcus pneumoniae. Gene 237:223-234. [DOI] [PubMed] [Google Scholar]
- 26.Lau, G. W., S. Haataja, M. Lonetto, S. E. Kensit, A. Marra, A. P. Bryant, D. McDevitt, D. A. Morrison, and D. W. Holden. 2001. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40:555-571. [DOI] [PubMed] [Google Scholar]
- 27.Lau, P. C., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193-205. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc, D. J., L. N. Lee, and A. Abu-Al-Jaibat. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28:130-145. [DOI] [PubMed] [Google Scholar]
- 29.LeMessurier, K. S., A. D. Ogunniyi, and J. C. Paton. 2006. Differential expression of key pneumococcal virulence genes in vivo. Microbiology 152:305-311. [DOI] [PubMed] [Google Scholar]
- 30.Li, J., R. V. Swanson, M. I. Simon, and R. M. Weis. 1995. The response regulators CheB and CheY exhibit competitive binding to the kinase CheA. Biochemistry 34:14626-14636. [DOI] [PubMed] [Google Scholar]
- 31.Lu, L., Y. Ma, and J. R. Zhang. 2006. Streptococcus pneumoniae recruits complement factor H through the amino terminus of CbpA. J. Biol. Chem. 281:15464-15474. [DOI] [PubMed] [Google Scholar]
- 32.Mascher, T., D. Zahner, M. Merai, N. Balmelle, A. B. de Saizieu, and R. Hakenbeck. 2003. The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J. Bacteriol. 185:60-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCluskey, J., J. Hinds, S. Husain, A. Witney, and T. J. Mitchell. 2004. A two-component system that controls the expression of pneumococcal surface antigen A (PsaA) and regulates virulence and resistance to oxidative stress in Streptococcus pneumoniae. Mol. Microbiol. 51:1661-1675. [DOI] [PubMed] [Google Scholar]
- 34.McCool, T. L., T. R. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCool, T. L., T. R. Cate, E. I. Tuomanen, P. Adrian, T. J. Mitchell, and J. N. Weiser. 2003. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect. Immun. 71:5724-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohedano, M. L., K. Overweg, A. de la Fuente, M. Reuter, S. Altabe, F. Mulholland, D. de Mendoza, P. Lopez, and J. M. Wells. 2005. Evidence that the essential response regulator YycF in Streptococcus pneumoniae modulates expression of fatty acid biosynthesis genes and alters membrane composition. J. Bacteriol. 187:2357-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musher, D. M. 2000. Streptococcus pneumoniae, p. 2128-2147. In G. L. Mandell, J. E. Bennett, and R. D. Dolin (ed.), Principles and practice of infectious diseases, vol. 2. Churchill Livingstone, New York, N.Y. [Google Scholar]
- 39.Ng, W. L., K. M. Kazmierczak, and M. E. Winkler. 2004. Defective cell wall synthesis in Streptococcus pneumoniae R6 depleted for the essential PcsB putative murein hydrolase or the VicR (YycF) response regulator. Mol. Microbiol. 53:1161-1175. [DOI] [PubMed] [Google Scholar]
- 40.Ng, W. L., G. T. Robertson, K. M. Kazmierczak, J. Zhao, R. Gilmour, and M. E. Winkler. 2003. Constitutive expression of PcsB suppresses the requirement for the essential VicR (YycF) response regulator in Streptococcus pneumoniae R6. Mol. Microbiol. 50:1647-1663. [DOI] [PubMed] [Google Scholar]
- 41.Ng, W. L., H. C. Tsui, and M. E. Winkler. 2005. Regulation of the pspA virulence factor and essential pcsB murein biosynthetic genes by the phosphorylated VicR (YycF) response regulator in Streptococcus pneumoniae. J. Bacteriol. 187:7444-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogunniyi, A. D., M. C. Woodrow, J. T. Poolman, and J. C. Paton. 2001. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 69:5997-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orihuela, C. J., J. N. Radin, J. E. Sublett, G. Gao, D. Kaushal, and E. I. Tuomanen. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72:5582-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paterson, G. K., C. E. Blue, and T. J. Mitchell. 2006. Role of two-component systems in the virulence of Streptococcus pneumoniae. J. Med. Microbiol. 55:355-363. [DOI] [PubMed] [Google Scholar]
- 45.Pestova, E. V., L. S. Havarstein, and D. A. Morrison. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21:853-862. [DOI] [PubMed] [Google Scholar]
- 46.Pozzi, G., L. Masala, F. Iannelli, R. Manganelli, L. S. Havarstein, L. Piccoli, D. Simon, and D. A. Morrison. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 178:6087-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Sanders, D. A., B. L. Gillece-Castro, A. L. Burlingame, and D. E. Koshland, Jr. 1992. Phosphorylation site of NtrC, a protein phosphatase whose covalent intermediate activates transcription. J. Bacteriol. 174:5117-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders, D. A., B. L. Gillece-Castro, A. M. Stock, A. L. Burlingame, and D. E. Koshland, Jr. 1989. Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J. Biol. Chem. 264:21770-21778. [PubMed] [Google Scholar]
- 51.Sebert, M. E., L. M. Palmer, M. Rosenberg, and J. N. Weiser. 2002. Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH two-component system and contributes to nasopharyngeal colonization. Infect. Immun. 70:4059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, B. L., and M. K. Hostetter. 2000. C3 as substrate for adhesion of Streptococcus pneumoniae. J. Infect. Dis. 182:497-508. [DOI] [PubMed] [Google Scholar]
- 53.Standish, A. J., U. H. Stroeher, and J. C. Paton. 2005. The two-component signal transduction system RR06/HK06 regulates expression of cbpA in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 102:7701-7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 55.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 57.Throup, J. P., K. K. Koretke, A. P. Bryant, K. A. Ingraham, A. F. Chalker, Y. Ge, A. Marra, N. G. Wallis, J. R. Brown, D. J. Holmes, M. Rosenberg, and M. K. Burnham. 2000. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol. Microbiol. 35:566-576. [DOI] [PubMed] [Google Scholar]
- 58.Tiraby, J. G., and M. S. Fox. 1973. Marker discrimination in transformation and mutation of pneumococcus. Proc. Natl. Acad. Sci. USA 70:3541-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulijasz, A. T., D. R. Andes, J. D. Glasner, and B. Weisblum. 2004. Regulation of iron transport in Streptococcus pneumoniae by RitR, an orphan response regulator. J. Bacteriol. 186:8123-8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto, K., K. Hirao, T. Oshima, H. Aiba, R. Utsumi, and A. Ishihama. 2005. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. J. Biol. Chem. 280:1448-1456. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, J.-R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, J.-R., I. Idanpaan-Heikkila, W. Fischer, and E. I. Tuomanen. 1999. Pneumococcal licD2 gene is involved in phosphorylcholine metabolism. Mol. Microbiol. 31:1477-1488. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, J.-R., K. E. Mostov, M. E. Lamm, M. Nanno, S. Shimida, M. Ohwaki, and E. Tuomanen. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827-837. [DOI] [PubMed] [Google Scholar]