Abstract
Despite its importance as a human pathogen, information on population structure and global epidemiology of Staphylococcus epidermidis is scarce and the relative importance of the mechanisms contributing to clonal diversification is unknown. In this study, we addressed these issues by analyzing a representative collection of S. epidermidis isolates from diverse geographic and clinical origins using multilocus sequence typing (MLST). Additionally, we characterized the mobile element (SCCmec) carrying the genetic determinant of methicillin resistance. The 217 S. epidermidis isolates from our collection were split by MLST into 74 types, suggesting a high level of genetic diversity. Analysis of MLST data using the eBURST algorithm revealed the existence of nine epidemic clonal lineages that were disseminated worldwide. One single clonal lineage (clonal complex 2) comprised 74% of the isolates, whereas the remaining isolates were clustered into 8 minor clonal lineages and 13 singletons. According to our evolutionary model, SCCmec was acquired at least 56 times by S. epidermidis. Although geographic dissemination of S. epidermidis strains and the value of the index of association between the alleles, 0.2898 (P < 0.05), support the clonality of S. epidermidis species, examination of the sequence changes at MLST loci during clonal diversification showed that recombination gives rise to new alleles approximately twice as frequently as point mutations. We suggest that S. epidermidis has a population with an epidemic structure, in which nine clones have emerged upon a recombining background and evolved quickly through frequent transfer of genetic mobile elements, including SCCmec.
Staphylococcus epidermidis is one of the most prevalent causes of nosocomial infections associated with the immunocompromised and patients with indwelling medical devices. An international survey performed in Europe, Asia, and Latin America revealed that approximately 70% of the S. epidermidis strains circulating in the hospital environment are resistant to methicillin and that the overwhelming majority of them are also resistant to other antimicrobial classes (35). Resistance to methicillin in staphylococci is known to be associated with the presence of the mecA gene, which codes for a penicillin-binding protein with low affinity for β-lactam antibiotics (PBP2A) (14, 42). The mecA gene is carried by a genetic mobile element called the staphylococcal chromosomal cassette mec (SCCmec) (20), which integrates specifically in an open reading frame of unknown function, orfX (18). To date, six different SCCmec structures have been identified in methicillin-resistant Staphylococcus aureus (17, 19, 20, 31) and evolutionary models proposed for the emergence of nosocomial methicillin-resistant S. aureus suggest that SCCmec has been acquired at least 20 times by this species (34).
Molecular typing of nosocomial S. epidermidis strains by several typing methods has shown considerable diversity within the S. epidermidis population (2, 5, 10, 11, 25, 27, 29). This was observed not only in studies involving isolates from diverse geographic or clinical origins (27, 29, 45) but also in collections which originated from the same hospital (6) and even in a single intensive care unit (2). Variations in the pulsed-field gel electrophoresis (PFGE) macrorestriction pattern were even detected among isolates recovered from the same infection site of a single patient (10, 43, 48). Paradoxically, the same studies also reported the dissemination of S. epidermidis strains among different patients, wards, hospitals, and even different countries, supporting the hypothesis of the clonal nature of the S. epidermidis population.
PFGE has been the most widely used method for studying methicillin-resistant S. epidermidis (MRSE) nosocomial outbreaks (5) and local transmission (1, 16, 33, 37), allowing the identification of locally disseminated MRSE clones. The molecular characterization of nosocomial MRSE from Iceland and Denmark by PFGE provided the first evidence for geographic dissemination of S. epidermidis strains (27). Nevertheless, PFGE is not the method of choice when the purpose is to identify clones disseminated worldwide, since it analyzes the whole chromosome, parts of which may be evolving relatively rapidly. Multilocus sequence typing (MLST), based on sequencing of conserved housekeeping genes, is proving to be the typing tool most appropriate to the study of the global epidemiology of many pathogenic bacteria due to the unambiguous nature of DNA sequences and the ease with which this and other information can be stored and retrieved via the internet (www.mlst.net).
Three different MLST schemes have been proposed for S. epidermidis (45, 46; S. J. Peacock, personal communication) that differ in several of the genes analyzed. Nevertheless, the application of these MLST schemes to geographically and clinically diverse S. epidermidis collections fails to provide adequate resolution to study S. epidermidis epidemiology. Very recently, an improved MLST scheme based on a comparative study of the previously reported MLST schemes was proposed (41) to be the one adopted and recognized as the MLST scheme for S. epidermidis.
The origin of diversity in S. epidermidis is still unclear; nevertheless, insertion sequences appear to play an important role in genome flexibility, as proven by chromosomal rearrangements promoted by IS256 that affected biofilm production and antibiotic resistance (48, 49). Our own studies revealed that variation in S. epidermidis may also be a consequence of frequent acquisition of mobile elements, such as SCCmec, at the orfX region (26). However, the extent to which homologous recombination contributes to clonal diversification in S. epidermidis is at present unexplored. The clarification of the origins of diversity in S. epidermidis will be of critical importance in the selection of the most appropriate typing strategy for successful epidemiological surveillance.
In the present study, we examined the diversity/clonality paradox by characterizing the population structure of S. epidermidis using MLST. From MLST data, we estimated the relative contributions of recombination and mutation in strain diversification and examined the dynamics of the SCCmec element in S. epidermidis.
MATERIALS AND METHODS
Bacterial isolates.
A total of 863 S. epidermidis nosocomial isolates were collected in 17 collaborating national centers between 1996 and 2001 and stored in the culture collection of the Laboratory of Molecular Genetics of the Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa, Oeiras, Portugal. A representative sample of our large strain collection was selected as follows. (i) Among our collections of isolates from Iceland (80 isolates), Denmark (121 isolates), Cape Verde (45 isolates), Uruguay (10 isolates), Mexico (66 isolates), Greece (33 isolates), and Portugal (23 isolates) previously characterized by PFGE, we selected 1 isolate of each PFGE subtype (Iceland and Denmark) or PFGE type (remaining countries), resulting in 170 isolates. (ii) In our collections from Argentina (36 isolates), Bulgaria (24 isolates), China (17 isolates), Colombia (57 isolates), Spain (57 isolates), Hungary (21 isolates), Italy (160 isolates), Japan (105 isolates), Poland (5 isolates), and Taiwan (3 isolates), between 2 and 9 isolates from an infection origin from each country were selected, resulting in 45 isolates. (iii) All methicillin-susceptible S. epidermidis (MSSE) isolates found in the collection (20) were included. Two S. epidermidis strains for which the entire genome is available, ATCC 12228 (47) and RP62A (12), were also included. The final representative S. epidermidis sample included a total of 217 nosocomial isolates (197 MRSE and 20 MSSE) collected between 1996 and 2001 in 17 different countries from disease (107 isolates) and from carriage (87 isolates). No information on the remaining 23 isolates concerning disease or colonization origin was available.
S. aureus strains COL (32), N315 (23), HU25 (32), and WIS (19) were included as controls for SCCmec types I, II, III, and V, respectively. Strain N315 was used as control for ccrAB2 complex, and strain COL was used as a control for mec complex class B, associated with SCCmec types IV and I.
Criteria used for classification of isolates as originated from infection or colonization.
The following criteria were used to classify the origin of S. epidermidis isolates from Denmark: (i) for blood, a judgment call was formed based on the analysis of additional data regarding other possible infection sites in the same patients (e.g., urine, sputum, other blood cultures, abscess, and medical devices); (ii) for urine, significant growth in a pure culture was regarded as infection; (iii) for respiratory tract, microbial growth in the sputum visible under the microscope was classified as infection; (iv) for a wound, that from abscess was considered to be from infection and that from chronic ulcers was regarded as colonization; and (v) for continuous ambulatory peritoneal dialysis devices, significant growth from tips was classified as infection. The following criteria were used to classify the origin of S. epidermidis isolates from Iceland: (i) for blood, a single blood culture set was regarded as colonization and the presence of bacteria from more than one set was regarded as infection; (ii) for urine, significant growth (>100,000 bacteria/ml) in relatively pure culture was classified as infection; (iii) for respiratory tract, the majority of isolates were considered to be from colonization; (iv) for a wound, open heart surgery wounds or wounds from catheter sites with more than 15 colonies of pure culture were regarded as infection. Isolates from the remaining countries were considered as being from infection or colonization sites, according to local clinicians' evaluation criteria.
DNA preparation.
Genomic DNA for PCR was extracted by cell lysis (a mixture of 0.2 μg/μl of lysostaphin, 1 mM EDTA, and 10 mM Tris [pH 8]) at 37°C for 1 h, followed by heating at 95°C for 15 min.
Analysis of SCCmec structure.
The structures of the ccr and mec complexes were determined by conventional PCRs as described by Hiramatsu and colleagues, and SCCmec types I through V were defined by the combination of the type of ccr complex and the class of mec complex (19, 30) as follows: SCCmec type I (mec complex B, ccrAB1); SCCmec type II (mec complex A, ccrAB2); SCCmec type III (mec complex A, ccrAB3), SCCmec type IV (mec complex B, ccrAB2), and SCCmec type V (mec complex C, ccrC). SCCmec was considered as nontypeable when the ccr complex, the mec complex, or both were nontypeable. The mec complex was considered nontypeable when no PCR amplification occurred for any of the primer pairs used. The ccr complex was considered nontypeable when a positive PCR amplification signal was obtained only for βc/αc primers or when no PCR amplification occurred for any of the primer pairs used.
MLST.
MLST was carried out using the new MLST scheme described by Thomas et al. (41), which is based on the sequencing of internal fragments of seven housekeeping genes. The fragments were amplified by PCR using primers within highly conserved regions. Sequences of both strands of all amplicons were resolved with an ABI Prism 3700 DNA sequencer using BigDye (version 3) fluorescent terminators. Numbers for alleles and sequence types (STs) were assigned according to the S. epidermidis MLST database (http://sepidermidis.mlst.net/).
eBURST algorithm.
The most likely patterns of evolutionary descent in our collection were assessed using the eBURST algorithm (http://eburst.mlst.net) (9). The most restrictive group definition was used, in which STs were included within the same group only if they shared a minimum of six of the seven MLST loci with at least one other ST in the group. The statistical confidence in the assigned primary founders was determined by a bootstrap resampling procedure (1,000 samples). In our study, an ST was considered as a subgroup founder if it had at least three single locus variants (SLVs). Clonal complexes were represented by the abbreviation “CC” followed by the number of the clonal complex founder or by the number of the ST that had the highest number of isolates inside that clonal complex. For example, CC2 is the clonal complex for which ST2 is the founder. Singletons were represented by the abbreviation “S” followed by the corresponding ST number.
Phylogenetic analysis.
The in-frame sequences at the seven loci for each sequence type defined within the collection analyzed were concatenated, in the order of loci used to define the allelic profile. Sequences were aligned by CLUSTALW, and a minimum evolution (ME) tree was then constructed from the concatenated sequences (3,003 bp) by using the Kimura two-parameter model for estimating pairwise genetic distances. An initial tree was obtained by the neighbor-joining method, and the minimum-evolution method was used to search for the tree which minimizes the sums of the branch length estimates, by branch swapping and by closest-neighbor interchange (40). The degree of statistical support for the nodes on the minimum evolution tree was evaluated by examining their percent recovery in 1,000 resample trees by the bootstrap test (38). In addition, evolutionary trees were also constructed for each MLST locus. The sequences of all of the alleles identified within each locus were aligned by CLUSTALW, and trees were built through the application of the neighbor-joining method and the Kimura two-parameter as a model for estimating pairwise distances. The nucleotide diversity for each MLST locus and the respective standard error were calculated, considering the mean diversity in overall population and the Kimura two-parameter model. The ratio between the number of synonymous (dS) and nonsynonymous (dN) substitutions was calculated by the method of Nei and Gojobori with the Jukes-Cantor correction. The dS/dN ratio indicates purifying selection (negative selection) if values are >1, positive selection if values are <1, and balancing selection or neutral evolution if values are close to 1. The alignments, minimum evolution tree, neighbor-joining trees, nucleotide diversity, and dS/dN calculation were performed using the program MEGA version 3.1 (22).
Estimates of recombination rates.
The per-allele and per-site recombination/mutation (r/m) parameter was empirically calculated by counting the number of polymorphisms introduced by mutation or recombination according to previously described methodology (8). According to this methodology, if the variant allele in one isolate differed at a single nucleotide site from the corresponding allele in the descendant SLV, the emergence of the variant allele was considered as having arisen by mutation. If, however, the difference involved a multiple-nucleotide change or single-nucleotide change previously observed within the collection analyzed, the emergence of the variant allele was considered to have resulted from a recombination event.
Tests for recombination.
The index of association standardized (IAS) between the different MLST loci was calculated using LIAN program (version 3.1, Department of Biotechnology and Bioinformatics University of Applied Sciences Weihenstephan; http://adenine.biz.fh-weihenstephan.de/lian_3.1/) (15). If there is linkage equilibrium, the expected value of the index of association (IAS) is zero and recombination events must occur frequently. If the IAS (P < 0.05) value differs significantly from zero, recombination should be rare.
Genotypic diversity.
Genotypic diversity (h) (36) was calculated for MLST data for the entire collection and for specific epidemiologically relevant subgroups of the collection (methicillin resistant/susceptible or from infection/colonization origins). Confidence intervals were calculated according to Grundmann et al. (13).
RESULTS
Nucleotide sequence variation for each MLST locus.
The sequences of internal fragments of the seven housekeeping genes, ranging in size from 412 to 465 bp, were determined for 217 S. epidermidis isolates. Between 8 (mutS) and 16 (yqiL) alleles were found for each locus (see Table 1), and the number of polymorphic nucleotide sites at the seven loci varied between 9 (gtr) and 27 (aroE). No positive correlation was found between the number of polymorphic sites and the number of alleles for each gene. Except for mutS and tpi, which showed lower values of nucleotide diversity (π = 0.007 and 0.008, respectively), the remaining genes showed a comparable nucleotide diversity (π = 0.011 to 0.018).
TABLE 1.
Summary of nucleotide sequence variation for each MLST locus
| MLST locus | Sequence length (bp) | No. of alleles | No. of polymorphic sites | Nucleotide diversity (SE) | dS/dN ratio (P value)a |
|---|---|---|---|---|---|
| arcC | 465 | 14 | 20 | 0.018 (0.004) | 2.499 (0.014) |
| aroE | 420 | 13 | 27 | 0.014 (0.003) | 3.255 (0.001) |
| gtr | 438 | 9 | 18 | 0.014 (0.003) | 3.403 (0.001) |
| mutS | 412 | 8 | 9 | 0.007 (0.002) | 0.232 (0.817) |
| pyrR | 428 | 11 | 18 | 0.016 (0.004) | 3.403 (0.001) |
| tpi | 424 | 11 | 12 | 0.008 (0.002) | 2.929 (0.004) |
| yqiL | 416 | 16 | 16 | 0.011 (0.003) | 2.549 (0.012) |
The hypothesis tested is neutrality (dS = dN). The P value indicates the significance of the difference between dS and dN and must be <0.05 for hypothesis rejection at a 5% level.
Identification of main clonal lineages.
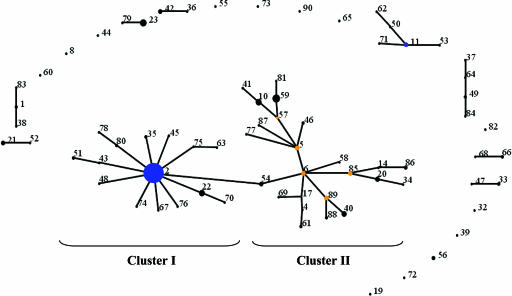
The molecular characterization of the 217 S. epidermidis isolates by MLST identified 74 different STs, indicating a high genotypic diversity (h = 0.89, with a confidence interval [CI] of 0.85 to 0.93). Forty-nine of these STs correspond to single isolates, while 16 STs included between 2 and 4 isolates. The most represented STs were ST2, which comprised 31% of all isolates (67 out of 217 isolates); ST59 (6%); ST23 (5%); ST10 and ST22 (4% each); ST40 (3%); and ST5, ST20, and ST89 (2% each). The 74 STs were divided by eBURST into 1 major clonal complex (CC2), 8 minor clonal complexes (CC1, -11, -21, -23, -33, -42, -49, -66), and 13 singletons (S8, -19, -32, -39, -44, -55, -56, -60, -65, -72, -73, -82, and -90) (Fig. 1 and Tables 2 and 3).The major clonal complex, CC2, comprised 39 different STs and included 159 S. epidermidis isolates (10 MSSE and 149 MRSE). CC2 consisted of two main domains, one containing the predicted ancestor (ST2) of this clonal complex and respective SLV and double-locus variants (DLV)—cluster I—and the other containing the remaining STs related in a multiple-branch-like manner that included several subgroup founders (ST5, -6, -57, -85, and -89)—cluster II (Fig. 1).
FIG. 1.
Application of eBURST algorithm to MLST data for the collection of 217 S. epidermidis isolates. Each ST is represented by a black dot. Blue and yellow dots correspond to group and subgroup founders, respectively. SLVs are linked by lines and clonal complexes, or eBURST groups correspond to the group of connected STs. In order to facilitate data analysis, CC2 was artificially subdivided into two clusters: cluster I, including the founder of the CC (ST2) and its SLVs and DLVs, including ST22, -35, -43, -45, -48, -51, -54, -63, -67, -70, -74, -75, -76, -78, and -80; and cluster II, including all of the other STs from CC2.
TABLE 2.
Summary of MLST and SCCmec results for CC2 strains that account for 74% of the population
| ST | MLST profile (arcC, aroE, gtr, mutS, pyrR, tpi, yqiL) | SCCmeca | Country (no. of isolates) | Originb |
|---|---|---|---|---|
| 2 | 7, 1, 2, 2, 4, 1, 1 | II, IV, III, A/C, NT/3 | Denmark (17), Italy (3), Iceland (22), Argentina (2), Mexico (9), Greece (2), Cape Verde (2), Spain (2), Hungary (2), Colombia (1), Uruguay (3), Japan (1), Bulgaria (1) | I, C |
| 4 | 1, 1, 6, 6, 2, 1, 1 | IV | Denmark (1) | C |
| 5 | 1, 1, 1, 2, 2, 1, 1 | IV, C/2 | Bulgaria (1), Cape Verde (1), Denmark (2), Iceland (1) | I, C |
| 6 | 1, 1, 2, 2, 2, 1, 1 | III | Iceland (1) | I |
| 10 | 1, 1, 1, 1, 3, 1, 1 | II, IV, NT/1 | Denmark (4), Spain (1), Control RP62A (1), Iceland (2) | I, C |
| 14 | 1, 1, 2, 1, 1, 1, 1 | MSSE | Denmark (1) | C |
| 17 | 1, 1, 6, 2, 2, 1, 1 | MSSE | Portugal (1) | C |
| 20 | 1, 1, 2, 2, 1, 1, 3 | IV, A/NT | Iceland (1), Japan (1), Cape Verde (1), Italy (1), Portugal (1) | I, C |
| 22 | 7, 1, 2, 2, 4, 7, 1 | III, IV, V A/C, NT/C | Denmark (3), Iceland (3), Greece (2) | I, C |
| 34 | 1, 1, 2, 2, 1, 13, 3 | IV | Iceland (1) | C |
| 35 | 2, 1, 2, 2, 4, 1, 1 | A/C | Portugal (1) | C |
| 40 | 1, 1, 2, 1, 3, 1, 1 | A/C, IV | Denmark (4), Iceland (2) | I, C |
| 41 | 1, 1, 1, 1, 3, 1, 11 | IV | Cape Verde (1) | C |
| 43 | 7, 1, 2, 2, 1, 1, 1 | III | Iceland (1) | I |
| 45 | 20, 1, 2, 2, 4, 1, 1 | IV | Denmark (1) | C |
| 46 | 1, 1, 1, 2, 2, 1, 7 | IV | Mexico (2) | I |
| 48 | 7, 1, 2, 2, 4, 1, 4 | IV | Denmark (1) | C |
| 51 | 7, 1, 2, 2, 1, 1, 8 | IV | Colombia (1), Italy (1) | I |
| 54 | 1, 1, 2, 2, 4, 1, 1 | IV, C/2, MSSE | Denmark (2), Japan (1), Italy (1) | I, C |
| 57 | 1, 1, 1, 1, 2, 1, 1 | MSSE | Portugal (1) | C |
| 58 | 1, 1, 2, 2, 2, 13, 1 | IV | Bulgaria (1) | I |
| 59 | 2, 1, 1, 1, 2, 1, 1 | V, MSSE | Taiwan (1), Denmark (3), China (3), Mexico (1), Cape Verde (2), Spain (1), Hungary (1), Italy (1) | I, C |
| 61 | 2, 1, 6, 6, 2, 1, 1 | NT/2 | Mexico (2) | I |
| 63 | 22, 1, 2, 2, 4, 13, 1 | NT/NT | Argentina (1) | I |
| 67 | 7, 1, 18, 2, 4, 1, 1 | III | Denmark (1) | I |
| 69 | 1, 18, 6, 2, 2, 1, 1 | NT/2 | Greece (1) | - |
| 70 | 7, 1, 2, 2, 14, 7, 1 | IV | Denmark (1) | C |
| 74 | 7, 1, 2, 12, 4, 1, 1 | III | Spain (1) | I |
| 75 | 7, 1, 2, 2, 4, 13, 1 | III | Poland (1) | I |
| 76 | 7, 1, 2, 14, 4, 1, 1 | A/NT | Greece (1) | I |
| 77 | 23, 1, 1, 2, 2, 1, 1 | V | Bulgaria (1) | I |
| 78 | 21, 1, 2, 2, 4, 13, 1 | II | Argentina (1) | I |
| 80 | 21, 1, 2, 2, 4, 1, 1 | III | Iceland (1), Japan (1) | I, C |
| 81 | 2, 17, 1, 1, 2, 1, 1 | IV | Denmark (1) | C |
| 85 | 1, 1, 2, 2, 1, 1, 1 | IV, MSSE | Denmark (1), Taiwan (1) | I |
| 86 | 1, 2, 2, 1, 1, 1, 1 | IV, MSSE | Uruguay (1), Portugal (1), Cape Verde (1) | I, C |
| 87 | 7, 1, 1, 2, 2, 1, 1 | MSSE | Hungary (1) | - |
| 88 | 1, 1, 2, 1, 1, 1, 7 | IV, MSSE | Denmark (1), Portugal (1) | C |
| 89 | 1, 1, 2, 1, 2, 1, 1 | IV, MSSE | China (1), Denmark (1), Portugal (2), Iceland (1) | I, C |
Based on the ccr and mec complex type only. SCCmec entries with mec complex class followed by ccr complex type are shown when an SCCmec type was not attributable because ccr and/or the mec complex class was nontypeable or when a not-yet-described association between ccr and the mec complex was found.
I, infection origin; C, colonization origin.
TABLE 3.
Summary of MLST and SCCmec typing results for strains belonging to minor CCs and singletons identified in our collection
| CC or singleton (% of strains)a | ST | MLST profile (arcC, aroE, gtr, mutS, pyrR, tpi, yqiL) | SCCmecb | Country (no. of strains) | Originc |
|---|---|---|---|---|---|
| CCs | |||||
| CC23 (5) | 23 | 7, 1, 2, 1, 3, 3, 1 | IV, B/3 | Greece (2), Iceland (1), Mexico (3), Argentina (1), Portugal (1), Hungary (1), Uruguay (1) | I |
| 79 | 21, 1, 2, 1, 3, 3, 1 | IV | Cape Verde (1) | C | |
| CC11 (3) | 11 | 3, 1, 5, 5, 3, 4, 11 | IV | Denmark (1), Greece (1) | C |
| 50 | 3, 1, 5, 5, 3, 4, 4 | IV | Denmark (1) | ||
| 53 | 3, 1, 5, 5, 11, 4, 11 | NT/NT | Cape Verde (1) | C | |
| 62 | 3, 21, 5, 5, 3, 4, 4 | IV | Cape Verde (1) | C | |
| 71 | 3, 1, 5, 5, 3, 1, 11 | II | Mexico (1) | I | |
| CC21 (2) | 21 | 2, 1, 1, 2, 1, 1, 1 | IV, C/2 | Denmark (2), Iceland (2) | I, C |
| 52 | 2, 2, 1, 2, 1, 1, 1 | IV | Iceland (1) | I | |
| CC42 (2) | 36 | 11, 6, 2, 1, 1, 13, 1 | I | Iceland (1) | I |
| 42 | 1, 6, 2, 1, 1, 13, 1 | I | Denmark (2), Iceland (2) | I, C | |
| CC49 (2) | 37 | 18, 1, 5, 5, 11, 4, 20 | IV | Portugal (1) | C |
| 49 | 12, 1, 5, 5, 3, 4, 20 | IV | Denmark (1), Portugal (1) | I, C | |
| 64 | 12, 1, 5, 5, 11, 4, 20 | NT/2 | Poland (1) | I | |
| 84 | 12, 2, 5, 5, 3, 4, 20 | IV | Denmark (1) | C | |
| CC1 (2) | 1 | 1, 2, 2, 2, 1, 1, 10 | IV | Iceland (1), Denmark (1) | I, C |
| 38 | 1, 2, 2, 5, 1, 1, 10 | IV | Iceland (1) | ||
| 83 | 1, 2, 1, 2, 1, 1, 10 | IV | Greece (1) | I | |
| CC66 (1.3) | 66 | 12, 3, 5, 5, 7, 14, 11 | IV | Denmark (1), Italy (1) | I |
| 68 | 12, 3, 5, 5, 7, 1, 11 | IV | Denmark (1) | C | |
| CC33 (1.3) | 33 | 12, 10, 5, 5, 13, 5, 10 | MSSE | Colombia (1), Japan (1) | I |
| 47 | 12, 1, 5, 5, 13, 5, 21 | B/3 | Hungary (1) | ||
| Singletons | |||||
| S8 (0.5) | 8 | 2, 1, 7, 1, 1, 1, 1 | MSSE | Control strain ATCC 12228 (1) | |
| S19 (0.5) | 19 | 8, 7, 12, 4, 12, 2, 2 | MSSE | Bulgaria (1) | C |
| S32 (0.5) | 32 | 1, 1, 7, 1, 3, 5, 14 | MSSE | Italy (1) | I |
| S39 (0.5) | 39 | 22, 1, 5, 5, 10, 13, 12 | MSSE | Greece (1) | |
| S44 (0.5) | 44 | 1, 6, 6, 2, 1, 1, 1 | MSSE | Cape Verde (1) | C |
| S55 (0.5) | 55 | 1, 2, 1, 1, 2, 1, 10 | MSSE | Denmark (1) | |
| S56 (1.3) | 56 | 8, 15, 9, 11, 9, 10 | V, C/2 | Cape Verde (2), Denmark (1) | C |
| S60 (0.5) | 60 | 1, 1, 2, 6, 2, 1, 16 | MSSE | Portugal (1) | C |
| S65 (0.5) | 65 | 1, 19, 17, 4, 9, 10, 2 | IV | Cape Verde (1) | C |
| S72 (0.5) | 72 | 8, 2, 2, 4, 9, 6, 9 | IV | Cape Verde (1) | C |
| S73 (0.5) | 73 | 1, 5, 2, 6, 2, 1, 6 | MSSE | Portugal (1) | C |
| S82 (0.5) | 82 | 17, 20, 5, 5, 3, 4, 4 | IV | Mexico (1) | I |
| S90 (0.5) | 90 | 16, 1, 2, 1, 2, 12, 1 | MSSE | Portugal (1) | C |
The percentage corresponds to the proportion of isolates that have that specific sequence type in the entire collection (217 isolates).
Based on the ccr and mec complex type only. SCCmec entries with mec complex class followed by ccr complex type are shown when a SCCmec type was not attributable because ccr and/or the mec complex class were nontypeable or when a not-yet-described association between ccr and mec complex was found.
I, infection origin; C, colonization origin.
Geographical dissemination of S. epidermidis strains.
Isolates analyzed in this study comprised S. epidermidis isolates from different geographic regions, including 17 countries and four continents. Among the 74 STs identified in our collection, 22 were attributed to strains isolated in more than one country. The most widely disseminated type was ST2, which was identified in strains isolated in as many as 13 different countries (Table 2) across four continents (South America, Europe, Africa, and Asia). A similar dissemination was observed for S. epidermidis strains with ST59, ST23, and ST20, which were identified in eight, seven, and five countries, respectively (Tables 2 and 3). Strains of the remaining STs were also found to be spread among various countries, yet on a narrower scale (less than five countries). Of importance, we observed that strains within each of the two clusters inside CC2 did not originate from a specific geographic location, excluding the possibility of any kind of geographic subspeciation in the formation of these two clusters. On the other hand, we found 52 STs that were assigned to strains isolated in a particular nation, as in the case of ST41, -44, -53, -62, -65, -72, and -79, which were only identified in strains isolated in Cape Verde, and ST17, -35, -37, -57, -60, -73, and -90, which were identified in strains collected in Portugal.
Distribution of disease and carriage isolates.
Eighty-seven S. epidermidis isolates collected from colonization sources and 107 isolates from infection were distributed into 42 different STs each. The calculation of genotypic diversity (h) revealed that isolates from carriage were more diverse (h = 0.93, with a CI of 0.90 to 0.97) than those from disease (h = 0.81, with CI of 0.74 to 0.88). The most frequently found sequence type among carriage isolates was ST2 (21%), followed by ST59 (7%), ST22 (6%), and ST5 (5%), while the remaining STs accounted for less than 5% of isolates each. From infections, the most frequently found ST was also ST2 (41%), followed by ST23 (8%) and ST59 (7%). Each of the remaining STs represented less than 5% of the infection isolates. A total of 16 STs were common to carriage and disease isolates, including all of the STs predominant in the collections of each type of origins (ST2, -5, -22, -23, -40, and -59).
Distribution of MSSE and MRSE isolates.
Isolates analyzed in this study included 20 MSSE isolates that were clustered into 19 different STs and 197 MRSE isolates that were split into 63 STs. Genotypic diversity, in terms of MLST results, was higher among MSSE (h = 0.95, with a CI of 0.93 to 0.96) than among MRSE (h = 0.91, with a CI of 0.87 to 0.95) isolates. Sequence type 2 was the most predominant ST among MRSE (34%), followed by ST59 (6%) and ST23 (5%). Regarding MSSE, half of the isolates corresponded to STs within CC2 (10 isolates), while the remaining isolates belonged to singletons (9 isolates) and to an ST included in CC33 (1 isolate). Six STs (ST33, -54, -59, -85, -86, -88, and -89) were identified in both susceptible and resistant isolates. In addition, we observed that with the exception of MSSE strains with ST54, all of the MSSE isolates belonging to CC2 were localized inside cluster II, whereas cluster I of the same complex comprised MRSE isolates only.
Distribution of SCCmec among main MRSE clonal lineages.
A total of 139 MRSE isolates, including at least one of each ST, were selected for SCCmec characterization. Almost half (41%) of the 139 S. epidermidis isolates selected for characterization harbored SCCmec type IV, whereas 27% carried SCCmec type III. In contrast, SCCmec types V, I, and II were poorly represented in the collection studied (6%, 4%, and 4%, respectively). In addition, we identified 15 isolates carrying SCCmec structures with new associations between ccr complex and mec complex that may correspond to three novel SCCmec structures: mec complex A associated with ccrC (8 isolates), mec complex C associated with ccrAB2 (5 isolates), and mec complex B associated with ccrAB3 (2 isolates). The remaining isolates, corresponding to 7% of the collection analyzed, carried SCCmec types that were nontypeable by the method used.
SCCmec type IV was present in a wide range of distinct genetic backgrounds, namely seven different clonal complexes (CC1, -2, -11, -21, -23, -49, and -66) and three singletons (S65, S72, and S82), which altogether accounted for 39 STs. In contrast, the distribution of the remaining SCCmec types was restricted to only a few different genetic backgrounds: SCCmec type I was present in strains within CC42, SCCmec type II was detected in strains belonging to both CC2 and CC11, SCCmec type III was found to be exclusively associated with strains belonging to CC2, and SCCmec type V was carried by strains within CC2 and S56.
Estimate of SCCmec acquisitions.
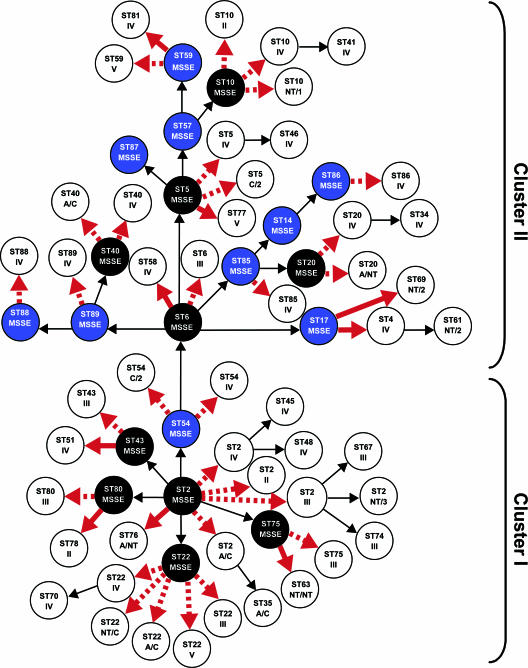
Considering the evolutionary relationships as defined by eBURST as well as SCCmec typing results, we attempted to estimate the number of times SCCmec was acquired by S. epidermidis. In order to achieve this goal, an evolutionary model for SCCmec acquisition was proposed based on the following assumptions: (i) there is a low probability of SCCmec excision occurring, since all strains were isolated in the hospital environment, where antibiotic pressure is high, and (ii) there is a low probability that the exact same mutation occurs twice. Taking these assumptions into consideration for the entire S. epidermidis collection analyzed, we estimated that SCCmec was acquired 56 times by S. epidermidis. Noticeably, SCCmec acquisitions occurred preferentially within CC2 (38 out of 56 acquisitions) (see Table 4 and Fig. 2 for estimates of acquisitions among CC2 strains).
TABLE 4.
Estimates of SCCmec acquisition in the S. epidermidis collection of 217 isolates analyzed
| CC or singleton | SCCmec typea | No. of acquisitions |
|---|---|---|
| CCs | ||
| CC2 | II, III, IV, V, A/C, C/2, A/NT, NT/1, NT/2, NT/3, NT/C, NT/NT | 38 |
| CC23 | IV, B/3 | 2 |
| CC11 | II, IV, NT/NT | 3 |
| CC33 | B/3 | 1 |
| CC21 | IV, C/2 | 2 |
| CC42 | I | 1 |
| CC49 | IV, NT/2 | 2 |
| CC1 | IV | 1 |
| CC66 | IV | 1 |
| Singletons | ||
| S82 | IV | 1 |
| S56 | V, C/2 | 2 |
| S65 | IV | 1 |
| S72 | IV | 1 |
| Total | 56 |
Based on the ccr and mec complex type only. SCCmec entries with mec complex class followed by ccr complex type are shown when a SCCmec type was not attributable because ccr and/or the mec complex class were nontypeable or when a not-yet-described association between ccr and the mec complex was found.
FIG. 2.
Evolutionary model proposed for SCCmec acquisition in S. epidermidis within CC2. Inside each circle, we present the ST (first text line) and respective SCCmec (second line). MRSE strains identified in this study are represented by white circles, while MSSE strains included in this study are represented by blue circles. Black circles correspond to hypothetical MSSE strains. Arrows indicate either SCCmec acquisition or ST variation; the red color indicates the occurrence of SCCmec acquisition; red arrows indicate that an SCCmec acquisition occurred together with an ST variation. Whenever an SCCmec acquisition was observed and the ST was maintained, a dotted red arrow is presented.
Clustering and phylogenetic analysis.
With the purpose of validating the clustering and evolutionary model proposed by eBURST, a ME tree from the concatenated sequences of the seven MLST genes of all 74 STs was constructed.
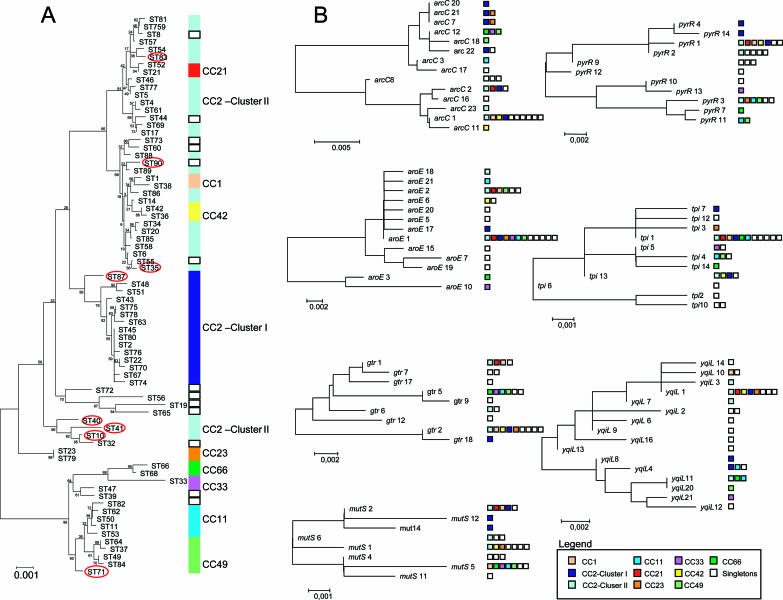
The clustering performed by the eBURST and ME tree agreed globally, but the minimum evolution tree showed a higher resolution of phylogenetic relations, as expected (Fig. 3A). CC2 was clearly split into two distinct groups, as was observed in the eBURST scheme; however, this method further separated the former cluster II into two subclusters. In addition, we noticed that certain STs (ST10, -35, -40, -41, -71, -83, and -87) in the ME tree were detached from the original eBURST CC. Analysis of the type of variation occurring between the detached STs and their direct ancestors revealed that variant loci differed at multiple sites (between 7 and 14 nucleotides), which leads to the separation observed in the ME tree. In addition, we observed that certain singletons were included in the same branch as particular CC, such as S8, S44, S55, S60, S73, and S90, in CC2 cluster II. Similarly, CC1, CC21, and CC42 in the ME tree were clustered together with the CC2 branch. When we scrutinized the allelic profiles of the singletons and of STs belonging to CC1, CC21, and CC42, we confirmed that they were no more than triple- or double-locus variants of STs belonging to CC2. These evolutionary relations were not found using eBURST, perhaps due to deficiencies in strain sampling which failed to recover an SLV able to make the link between those STs in the collection analyzed.
FIG. 3.
(A) Minimum evolution tree constructed from the concatenated sequences of seven MLST loci for the 74 STs identified in this study. Vertical rectangles correspond to eBURST groups or clonal complexes: each eBURST group or clonal complex is represented by a different color (see legend in the figure). Clonal complex 2 was subdivided into two clusters: cluster I in light blue and cluster II in dark blue. The scale corresponds to evolutionary distance, and bootstrap values are shown for each branch. Red circles correspond to STs that due to recombination events are detached from the clusters defined by eBURST. (B) Neighbor-joining trees constructed from the sequences of all the alleles of each the seven genes included in the MLST. Squares correspond to eBURST groups or clonal complexes. The color scheme for squares is the same as that used in panel A. The scale corresponds to evolutionary distance.
With the objective of examining the possible impact of recombination on the structure of the population analyzed, we compared the ME tree from the concatenated sequences with evolutionary trees for each of the MLST genes analyzed (Fig. 3B). For comparison we included in each evolutionary tree the CCs in which that specific gene allele was identified. The gene tree that best fits the concatenated ME tree is the tree for mutS. In this tree, as in the ME tree, two branches can be discerned: one containing CC1, CC2 (clusters I and II), CC21, CC23, and CC42 and the other containing CC11, CC33, CC49, and CC66. Although in the remaining gene trees two independent branches can be also identified, there is no clearly defined distribution of the different CCs between these two branches. Particularly, CC2 cluster II could not be placed with confidence within either branch and CC2 cluster I was consistently assigned to the same branch in only four gene trees constructed (aroE, gtr, mutS, and pyrR). However, an association of CC1, CC21, and CC42 with CC2 cluster II could be observed, as evidenced by the clustering of each of these CC complexes in the same branch or cluster of the gene trees as CC2 cluster II. An identical type of association was also observed for CC11, CC33, CC49, and CC66, which, in different combinations, were observed to be grouped consistently together in each of the gene trees constructed, as was previously observed in the ME tree.
Contribution of mutation and recombination to clonal diversification. (i) r/m parameter.
The comparison of the sequence of each variant allele with the corresponding allele in the descendant SLV showed that 37 out of the 52 single-locus variations occurring in our collection originated from a recombination event, whereas only 15 arose by mutation (Table 5). This resulted in a per-allele r/m parameter of 2.5:1. A value of 10:1 was obtained for the per-site r/m parameter, suggesting that individual nucleotide sites are at least 10 times more likely to change by recombination than by mutation.
TABLE 5.
Variant SLV alleles among all CCs and singletons identified in the study collection and identification of the genetic events involved
| ST of clonal ancestor | ST of SLV | Variant locus | Ancestral allele | SLV allele | No. of nucleotide differences | Other CC(s) containing SLV allele | Amino acid changea | Genetic eventb |
|---|---|---|---|---|---|---|---|---|
| 1 | 83 | gtr | 2 | 1 | 7 | CC1, CC2, CC21, S55 | 2 Syn + E→V, L→H, A→V, C→H, L→S | R |
| 1 | 38 | mut | 2 | 5 | 4 | CC11, CC33, CC49, CC66, S39, S82 | 1 Syn + A→S, I→T, E→K | R |
| 2 | 80 | arc | 7 | 21 | 1 | CC23 | 1 Syn | M |
| 2 | 54 | arc | 7 | 1 | 13 | CC1, CC42, S32, S44, S55, S60, S65, S73 | 10 Syn + K→E, I→V, K→Q | R |
| 2 | 35 | arc | 7 | 2 | 14 | CC21, S8 | 10 Syn + K→E, I→V, K→Q, E→K | R |
| 2 | 67 | gtr | 2 | 18 | 1 | None | D→H | M |
| 2 | 76 | mut | 2 | 14 | 1 | None | 1 Syn | M |
| 2 | 74 | mut | 2 | 12 | 2 | None | 1 Syn + S→C | R |
| 2 | 43 | pyr | 4 | 1 | 2 | CC1, CC21, CC42, S44, S8 | 2 Syn | R |
| 2 | 22 | tpi | 1 | 7 | 1 | None | T→S | M |
| 2 | 75 | tpi | 1 | 13 | 1 | CC42, S39 | 1 Syn | R |
| 2 | 48 | yqiL | 1 | 4 | 6 | CC11 | 6 Syn | R |
| 2 | 45 | arc | 7 | 20 | 2 | None | G→? | R |
| 4 | 61 | arc | 1 | 2 | 1 | CC21, S8 | E→K | R |
| 5 | 77 | arc | 1 | 23 | 1 | None | 1 Syn | M |
| 5 | 87 | arc | 1 | 7 | 13 | CC23 | 10 Syn + K→D, I→V, K→Q | R |
| 5 | 57 | mut | 1 | 2 | 2 | CC1, CC21, S44 | 1 Syn + Y→R | R |
| 5 | 46 | yqiL | 1 | 7 | 1 | None | Y→H | M |
| 6 | 17 | gtr | 2 | 6 | 8 | S44 | 2 Syn + V→A, E→V, A→V, P→T, C→H, L→S | R |
| 6 | 5 | gtr | 2 | 1 | 7 | CC1, CC21, S55 | 2 Syn + E→V, L→H, A→V, C→H, L→S | R |
| 6 | 58 | pyr | 1 | 13 | 1 | CC42, S39 | Y→R | R |
| 6 | 85 | pyr | 2 | 1 | 1 | CC1, CC21, CC42, S8, S44 | 1 Syn | R |
| 6 | 89 | mut | 2 | 1 | 2 | CC2, CC23, CC42, S8, S32, S55, S90 | A→S, I→L | R |
| 10 | 41 | yqiL | 1 | 11 | 7 | CC11, C66 | 1 Syn + Y→H, E→G, Y→C, L→S, F→C, I→T | R |
| 11 | 53 | pyr | 3 | 11 | 2 | CC49 | 2 Syn | R |
| 11 | 71 | tpi | 4 | 1 | 3 | CC1, CC2, CC21, CC66, S8, S44, S55, S60, S73 | 1 Syn, G→S, W→R | R |
| 11 | 50 | yqiL | 11 | 4 | 1 | CC2, S82 | S→L | R |
| 14 | 86 | aro | 1 | 2 | 1 | CC1, CC21, CC49, S55, S72 | I→K | R |
| 17 | 69 | aro | 1 | 18 | 1 | None | 1 Syn | M |
| 17 | 4 | mut | 2 | 6 | 1 | S60, S73 | 1 Syn | R |
| 20 | 34 | tpi | 1 | 13 | 1 | CC42, S39 | 1 Syn | R |
| 21 | 52 | aro | 1 | 2 | 1 | CC1, CC2, CC49, S55, S72 | I→K | R |
| 22 | 70 | pyr | 4 | 14 | 1 | None | I→N | M |
| 23 | 79 | arc | 21 | 7 | 1 | CC2 | 1 Syn | R |
| 33 | 47 | aro | 10 | 1 | 13 | CC2, CC11, CC21, CC23, CC49, S8, S32, S60, S90 | 8 Syn + R→S, Y→N, K→I, I→L, I→V | R |
| 42 | 36 | arc | 1 | 11 | 1 | None | V→L | M |
| 43 | 51 | yqiL | 1 | 8 | 5 | None | 1 Syn + E→G, Y→C, I→T, Y→H | R |
| 49 | 84 | aro | 1 | 2 | 1 | CC1, CC2, CC21, S55, S72 | I→K | R |
| 49 | 64 | pyr | 3 | 11 | 2 | CC11 | 2 Syn | R |
| 50 | 62 | aro | 1 | 21 | 1 | None | V→G | M |
| 54 | 6 | pyr | 4 | 2 | 1 | S55, S60 S73, S90 | 1 Syn | R |
| 57 | 59 | arc | 1 | 2 | 1 | CC21, S8 | E-K | R |
| 57 | 10 | pyr | 2 | 3 | 10 | CC11, CC23, CC49, S32, S82 | 9 Syn + Y→R | R |
| 59 | 81 | aro | 1 | 17 | 1 | None | 1 Syn | M |
| 64 | 37 | arc | 12 | 18 | 1 | None | I→Y | M |
| 66 | 68 | tpi | 14 | 1 | 2 | CC1, CC2, CC11, CC21, S8, S44, S55, S60, S73 | 2 Syn | M |
| 75 | 63 | arc | 7 | 22 | 2 | S39 | N→H, I→M | R |
| 80 | 78 | tpi | 1 | 13 | 1 | CC42, S39 | 1 Syn | R |
| 85 | 14 | mut | 2 | 1 | 2 | CC23, CC42, S8, S32, S55, S90 | 1 Syn + A→S | R |
| 85 | 20 | yqiL | 1 | 3 | 1 | None | U→E | M |
| 89 | 40 | pyr | 2 | 3 | 10 | CC11, CC23, CC49, S32, S82 | 9 Syn + Y→R | R |
| 89 | 88 | yqiL | 1 | 7 | 1 | None | Y→H | M |
| Total | 95 Syn + 71 Non-Syn | 37R+15M |
Syn, synonymous substitutions; Non-syn, nonsynonymous substitutions. Capital letters correspond to amino acids.
R, recombination event; M, mutation event.
(ii) Index of association.
The value of the index of association standardized (IAS) was 0.2898 (P < 0.05) (Table 6), indicating that there is linkage disequilibrium or association between the alleles. Restriction of analysis to phylogenetic significant subgroups, such as STs belonging to CC2 or STs included in clusters I and II of CC2, led to a consistent reduction in the IAS values, indicating that association between the alleles is less significant in some subgroups of the population. Actually, within CC2, no association between the alleles was found, when only the STs from this CC were considered (IAS = −0.0011; P > 0.05). On the other hand, when we restricted the analysis to STs taken as units (74 isolates, representing one of each ST) as opposed to the entire collection (217 isolates), a parallel decrease in the IAS values was observed (to 0.1685; P < 0.05) and the same type of effect was observed for CC2 clusters I and II.
TABLE 6.
IAS calculated for the entire collection, for the STs only, and for selected clusters of the collection
| Group selection | IAS |
P valuea
|
|
|---|---|---|---|
| Parametric | Simulation (100 iterations) | ||
| Entire collection (217 isolates) | 0.2898 | 0.0000 | 0.0100 |
| STs only (74 STs) | 0.1685 | 0.0000 | 0.0100 |
| CC2 | |||
| 160 isolates | 0.1713 | 0.0000 | 0.0100 |
| 39 STs | −0.0011 | 1.0000 | 0.4500 |
| Cluster I | |||
| 91 isolates | 0.0206 | 0.1430 | 0.1500 |
| 15 STs | −0.0935 | 1.0000 | 1.0000 |
| Cluster II | |||
| 69 isolates | 0.0325 | 0.0001 | 0.0100 |
| 24 STs | −0.0179 | 1.0000 | 0.8400 |
The measure of linkage disequilibrium is performed by testing the null hypothesis (H0), VD = Ve, where VD is the variance calculated from the distribution of mismatch values of variance and Ve is the variance expected for linkage equilibrium. P values are derived from the parametric and simulation methods and indicate the significance of linkage disequilibrium. A P value must be <0.05 for hypothesis rejection.
Synonymous and nonsynonymous substitutions.
Within all of the SLVs from our collection, the number of synonymous substitutions (n = 95) exceeded the number of nonsynonymous substitutions (n = 71). With the exception of the mutS locus (dS/dN, <1; P = 0.221), in all loci analyzed the ratio between the number of synonymous changes per synonymous site and nonsynonymous changes per nonsynonymous site had values of >1 (dS/dN > 1, with P = 0.001 to 0.014) (Table 1).
DISCUSSION
The purpose of this study was to reveal S. epidermidis population structure by analysis of a geographically diverse and representative collection of nosocomial S. epidermidis isolates by MLST. Moreover, we aimed to verify the existence of epidemic S. epidermidis clonal lineages and to analyze the dynamics of SCCmec in this species.
The molecular characterization of 217 nosocomial S. epidermidis isolates by the improved MLST scheme identified 74 sequence types and an average of 12 distinguishable alleles per locus, indicating a high level of genetic diversity at slowly evolving loci. The existence of such diversity can be due in part to the fact that we intentionally selected isolates from a wide range of geographic and clinical origins. However, a high level of diversity within S. epidermidis was also observed in other studies, when the analysis was restricted to a single country (27) or to a single clinical origin (10). This genetic diversity may be caused by the need for isolates to adapt to different environments in hospital and community settings, leading to increased frequency of horizontal gene transfer and dissemination of mobile genetic elements.
Despite the variability observed, a large number of STs (22 types) were found in more than one country, on several occasions up to 6 years apart. The recurrence of the same ST is indicative of a clonal population structure. Also, the existence of linkage disequilibrium between MLST alleles (IAS = 0.2898 with P < 0.05) when the entire collection was analyzed is a factor supporting the clonal nature of this species. However our sampling, biased as it is towards disease-causing isolates, does not fairly represent the global natural population, where isolates from infection are not so common. Our data suggest that S. epidermidis cannot be considered as a highly clonal microorganism since we estimate that recombination contributes approximately twice as much to clonal diversification as point mutations do. Moreover, a history of recent recombination was also evidenced by the lack of consistency within the trees for each MLST gene locus and between the tree for each gene locus and the ME tree for concatenated sequences. These apparently contradictory results are similar to those from studies on other bacteria such as Neisseria meningitidis, for which an epidemic population structure has been proposed (39). In this type of population, emergent clones appear superimposed on a background of frequent recombination. We suggest that the S. epidermidis population has a similar type of population structure, in which recombination leads to a higher frequency of nucleotide substitution than point mutation, but it is not sufficiently frequent to prevent the emergence of clones. The hypothesis of an epidemic population structure, in which occasional clones emerge and spread, is supported by the observed decrease in the value of IAS when only the 74 STs were considered, as opposed to the entire collection. These results could in part be explained by the existence in our collection of a single ST (ST2) that accounts for 31% of the isolates, which has weakened the observed effect of recombination on the entire population. However, we cannot rule out the possibility that ST2 is not genuinely that common in the natural population and that its high frequency could result from a sampling artifact. Particularly interesting was the observed consistent decrease in the value of IAS as different groups within the collection were selected, indicating that recombination may be occurring at different rates throughout the population. Actually, equilibrium between the alleles was observed within CC2, indicating that recombination seems to be occurring at a particularly high rate within this CC. The favored occurrence of recombination within a given CC, as opposed to recombination between different CCs, was suggested before for S. aureus (21) and was recently proposed to derive from the fact that strains of the same genetic lineage have specific restriction modification systems (44). As a consequence, DNA from different lineages of S. aureus will be recognized as foreign and the transfer of genetic material between the two lineages will be prevented, whereas the transfer of DNA within strains of the same lineage will be allowed. A similar type of mechanism might be occurring in S. epidermidis. The higher recombination rates observed within CC2 might also be a result of exclusive restriction modification systems between different S. epidermidis lineages. This mechanism would favor the recombination between S. epidermidis strains belonging to CC2 as opposed to recombination between strains of CC2 and strains of other lineages.
The estimates of recombination (r/m of 2.5:1) as well as the type of population structure proposed here are quite different from those proposed by others for S. epidermidis ' close relative, S. aureus, which has an r/m of 1:15 and a highly clonal population structure (7). These differences are illustrative of completely distinct epidemiologies, evolutionary histories, and capacities for DNA transfer between these two Staphylococcus species.
A single genetic lineage (CC2) comprising an unusually large number of STs accounted for the great majority of the population analyzed (74%). The identification of a single predominant clonal lineage with such a large number of STs could be explained in part by the high rates of recombination detected in this study for S. epidermidis. The generation of new SLVs via recombinational replacements from a common gene pool in the long term may have had the effect of homogenizing the population, as a larger number of alleles became shared. Since in S. epidermidis, recombination seems to be allied to a very diverse gene pool, as evidenced by the large number of alleles in the collection, it is expected that a large number of different STs would be produced. The result is the generation of a group of extremely diverse STs with a large number of common alleles that in the eBURST figure will inevitably be clustered together, as is the case of CC2. Although part of this clonal complex (cluster II) seems to be particularly incoherent, probably due to recombination events, as is illustrated by the lack of phylogenetic consistency in the minimum evolution tree, the other part (cluster I), which contains the most represented ST (ST2), appeared to have a strong phylogenetic signal. The clonality of this cluster (cluster I of CC2) was further supported by the fact that all S. epidermidis strains included in this cluster were MRSE strains, whereas the other cluster integrated all of the MSSE strains belonging to CC2.
Besides CC2, another eight minor emergent epidemic clonal lineages were identified which seem to also have the ability to spread widely. Transmission promoted by the high rate of migration of the human population probably contributes to dispersal of S. epidermidis strains between different countries and continents. The success of strains belonging to these eight clonal lineages and specifically of strains of CC2 may be associated with an improved capacity for dissemination, as demonstrated by the identification of this CC in 13 different countries. This could derive from the fact that these strains are either better equipped for colonization or can better evade the host's immune system. In addition, strains of CC2 appear to have an enhanced capacity for transfer of genetic material, as was illustrated by the high estimates of recombination rates when only STs from CC2 were considered and by the large number of SCCmec acquisitions within this clonal complex. The predominance of this lineage in the hospital environment may be connected to its aptitude for frequent recombination that could have brought genomic plasticity to this S. epidermidis lineage and contributed to the acquisition and modulation of antibiotic resistance determinants.
A surprisingly large number of SCCmec acquisitions by S. epidermidis were estimated to have occurred when we applied our evolutionary model to study isolates. Aware that the robustness of such a model is dependent on the representativity of the collection analyzed, the isolate collection used to estimate the number of SCCmec acquisitions by S. epidermidis was carefully selected to include representatives of the entire nosocomial population, namely isolates from colonization and infection from distinct sources and methicillin-susceptible and methicillin-resistant isolates from several countries. However, taking into consideration that S. epidermidis is mainly a commensal microorganism and that the frequency of MRSE in the hospital environment is around 60 to 70% in the majority of countries, the collection analyzed still holds two biases, such as the low number of isolates from nonclinical origin and the underrepresentation of susceptible isolates. Results from the present study demonstrated that susceptible and colonization isolates have a higher genotypic diversity than isolates that are resistant or have an infection origin. On the one hand, the sampling bias introduced may have lead to the underestimation of SCCmec acquisition due to the noninclusion of eventual MRSE strains that were singletons. On the other hand, it may have resulted on an overestimation of the number of SCCmec acquisitions since we may have considered that two unlinked STs had acquired the same SCCmec independently when in fact a link between the two was missing and a single acquisition may have occurred instead. Although the number of SCCmec acquisitions calculated may be biased because of the reasons presented, the magnitude of such a number is impressive and is illustrative of the enhanced capacity of S. epidermidis to acquire this type of mobile element. This observation is in agreement with our previous finding of a high genetic variability near the site of integration of SCCmec in S. epidermidis (26). SCCmec type IV was the SCCmec most frequently acquired by S. epidermidis (23 out of 56 acquisitions), which is in accordance with the enhanced mobility of this type of SCCmec already observed in S. aureus (34). The reason why the estimates for SCCmec acquisitions in S. epidermidis were much higher than those for S. aureus (34) is unclear. We hypothesize that S. epidermidis may be better adapted, due to an earlier contact with SCCmec, or may have the capacity to adapt faster to this piece of foreign DNA than S. aureus. In addition to the large number of SCCmec acquisitions, a high rate of homologous replacement of MLST genes was observed in S. epidermidis, evidencing an overall enhanced capacity for horizontal gene transfer compared to S. aureus. A recent review by Narra and Ochman suggests that generally there is no tight coupling between the degree of reassortment of genes through recombination and the amount of laterally acquired genes within a genome (28). However, one may speculate that mechanisms common to both events, like the ones involved in the blocking of horizontal gene transfer (restriction modification system) or in the maintenance of the acquired gene in the chromosome (recombination), may be adjusted to make S. epidermidis a more permissive species to the acquisition of foreign DNA than S. aureus. Alternatively, the difference in capacities for horizontal gene transfer between S. aureus and S. epidermidis may reflect the different ecologies of the two species. The broader habitat of S. epidermidis may promote a more frequent contact with other strains and species, leading to an increase in the rates of homologous recombination and SCCmec acquisition. Another possibility is that horizontal gene transfer may be favored during biofilm formation, the major virulence factor of S. epidermidis.
At least 12% of the isolates from our collection carry new variants of SCCmec (either nontypeable or new associations of mec complex/ccr complex), which is evidence of a high genetic diversity in SCCmec carried by S. epidermidis, as previously seen (26). This observation contrasts sharply to what is known for S. aureus, in which only five SCCmec types were identified among a wide collection of isolates. So far little is known regarding the origin and evolution of SCCmec: even so, the existence of similar regions among different SCCmec types (3) indicates that SCCmec seems to have undergone several sequential recombinational events, giving rise to mosaic-like structures. We believe that a reservoir of SCCmec variants is being produced in S. epidermidis and subsequently transferred to S. aureus and to other staphylococcal species.
All of the MLST loci, with the exception of mutS, seem to be under purifying selection, indicating that functionality of the housekeeping genes from the scheme used is, as expected, very important for bacterial survival and that there is no or little accumulation of deleterious mutations. The mutS gene, which is involved both in mismatch repair and in prevention of recombination between homologous fragments, seems to have been subjected to positive selection, meaning that natural selection favored particular alleles most probably due to the necessity of adaptation to any pressure in the environment. This observation is particularly striking since mutations in this gene are already described to create mutator phenotypes with high rates of mutation and promiscuous recombination in E. coli (24). Mutations in the S. epidermidis mutS gene during diversification may also lead to the emergence of mutator phenotypes that might function as a source of genetic variation and adaptation in S. epidermidis.
In contrast to the older MLST schemes, which gathered all of the isolates analyzed from distinct collections into only one or two clonal groups (45, 46), the improved MLST scheme applied in this study (41) allowed the identification of eight minor clonal complexes and several singletons in addition to the major clonal complex. These results confirmed that the improved MLST scheme has a better discriminatory ability than the previous ones and should be adopted henceforth as the unique and universal S. epidermidis MLST scheme (4).
MLST together with SCCmec typing data provides a clear picture of S. epidermidis population structure, evolution, and dynamics. Our data indicate that nosocomial S. epidermidis has an epidemic population that evolves quickly by means of recombination and frequent transfer of genetic mobile elements, including SCCmec.
Acknowledgments
Partial support for this study was provided by project POCTI/SAU-ESP/57841/2004 from Fundação para a Ciência e Tecnologia, Lisbon, Portugal, and project no. FCG/61052 from Fundação Calouste Gulbenkian, Lisbon, Portugal, which were both awarded to H. de Lencastre; and a Wellcome Trust project grant awarded to M. C. Enright. Grant SFRH/BD/6476/2001 from the Fundação para a Ciência e Tecnologia, Portugal, provided support for M. Miragaia. M. C. Enright is a Royal Society University Research Fellow.
We would like to thank M. Kuroda for providing strain N315 and T. Ito and W. Grubb for providing strain WIS, included in this study. Also, we are grateful to Ed Feil for helpful discussions.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Aires De Sousa, M., I. Santos Sanches, M. L. Ferro, and H. de Lencastre. 2000. Epidemiological study of staphylococcal colonization and cross-infection in two West African Hospitals. Microb. Drug Resist. 6:133-141. [DOI] [PubMed] [Google Scholar]
- 2.Bogado, I., A. Limansky, E. Sutich, P. Marchiaro, M. Marzi, J. Putero, and A. Viale. 2002. Molecular characterization of methicillin-resistant coagulase-negative staphylococci from a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 23:447-451. [DOI] [PubMed] [Google Scholar]
- 3.Chongtrakool, P., T. Ito, X. X. Ma, Y. Kondo, S. Trakulsomboon, C. Tiensasitorn, M. Jamklang, T. Chavalit, J.-H. Song, and K. Hiramatsu. 2006. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob. Agents Chemother. 50:1001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper, J. E., and E. J. Feil. 2006. The phylogeny of Staphylococcus aureus—which genes make the best intra-species markers? Microbiology 152:1297-1305. [DOI] [PubMed] [Google Scholar]
- 5.de Mattos, E. M., L. A. Teixeira, V. M. Alves, C. A. Rezenda e Resende, M. V. da Silva Coimbra, M. C. da Silva-Carvalho, B. T. Ferreira-Carvalho, and A. M. Figueiredo. 2003. Isolation of methicillin-resistant coagulase-negative staphylococci from patients undergoing continuous ambulatory peritoneal dialysis (CAPD) and comparison of different molecular techniques for discriminating isolates of Staphylococcus epidermidis. Diagn. Microbiol. Infect. Dis. 45:13-22. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez, M. A., J. Linares, A. Pulido, J. L. Perez, and H. de Lencastre. 1996. Molecular tracking of coagulase-negative staphylococcal isolates from catheter-related infections. Microb. Drug Resist. 2:423-429. [DOI] [PubMed] [Google Scholar]
- 7.Feil, E. J., J. E. Cooper, H. Grundmann, D. A. Robinson, M. C. Enright, T. Berendt, S. J. Peacock, J. M. Smith, M. Murphy, B. G. Spratt, C. E. Moore, and N. P. J. Day. 2003. How clonal is Staphylococcus aureus? J. Bacteriol. 185:3307-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feil, E. J., M. C. Enright, and B. G. Spratt. 2000. Estimating the relative contributions of mutation and recombination to clonal diversification: a comparison between Neisseria meningitidis and Streptococcus pneumoniae. Res. Microbiol. 151:465-469. [DOI] [PubMed] [Google Scholar]
- 9.Feil, E. J., B. C. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galdbart, J.-O., A. Morvan, N. Desplaces, and N. El Solh. 1999. Phenotypic and genomic variation among Staphylococcus epidermidis strains infecting joint prostheses. J. Clin. Microbiol. 37:1306-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geary, C., J. Z. Jordens, J. F. Richardson, D. M. Hawcroft, and C. J. Mitchell. 1997. Epidemiological typing of coagulase-negative staphylococci from nosocomial infections. J. Med. Microbiol. 46:195-203. [DOI] [PubMed] [Google Scholar]
- 12.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. DeBoy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartman, B. J., and A. Tomasz. 1984. Low-affinity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J. Bacteriol. 158:513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haubold, B., and R. R. Hudson. 2000. LIAN 3.0: detecting linkage disequilibrium in multilocus data. Linkage analysis. Bioinformatics 16:847-848. [DOI] [PubMed] [Google Scholar]
- 16.Huebner, J., G. B. Pier, J. N. Maslow, E. Muller, H. Shiro, M. Parent, A. Kropec, R. D. Arbeit, and D. A. Goldmann. 1994. Endemic nosocomial transmission of Staphylococcus epidermidis bacteremia isolates in a neonatal intensive care unit over 10 years. J. Infect. Dis. 169:526-531. [DOI] [PubMed] [Google Scholar]
- 17.Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiensasitorn, and K. Hiramatsu. 2001. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito, T., Y. Katayama, and K. Hiramatsu. 1999. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob. Agents Chemother. 43:1449-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, T., X. X. Ma, F. Takeuchi, K. Okuma, H. Yuzawa, and K. Hiramatsu. 2004. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob. Agents Chemother. 48:2637-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katayama, Y., T. Ito, and K. Hiramatsu. 2000. A new class of genetic element, Staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn, G., P. Francioli, and D. S. Blanc. 2006. Evidence for clonal evolution among highly polymorphic genes in methicillin-resistant Staphylococcus aureus. J. Bacteriol. 188:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, H. Yuzawa, I. Kobayashi, L. Cui, A. Oguchi, K. Aoki, Y. Nagai, J. Lian, T. Ito, M. Kanamori, H. Matsumaru, A. Maruyama, H. Murakami, A. Hosoyama, Y. Mizutani-Ui, N. K. Takahashi, T. Sawano, R. Inoue, C. Kaito, K. Sekimizu, H. Hirakawa, S. Kuhara, S. Goto, J. Yabuzaki, M. Kanehisa, A. Yamashita, K. Oshima, K. Furuya, C. Yoshino, T. Shiba, M. Hattori, N. Ogasawara, H. Hayashi, and K. Hiramatsu. 2001. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 24.Li, B., H. C. Tsui, J. E. LeClerc, M. Dey, M. E. Winkler, and T. A. Cebula. 2003. Molecular analysis of mutS expression and mutation in natural isolates of pathogenic Escherichia coli. Microbiology 149:1323-1331. [DOI] [PubMed] [Google Scholar]
- 25.Lina, B., F. Vandenesch, J. Etienne, B. Kreiswirth, and J. Fleurette. 1992. Comparison of coagulase-negative staphylococci by pulsed-field electrophoresis. FEMS Microbiol. Lett. 71:133-138. [DOI] [PubMed] [Google Scholar]
- 26.Miragaia, M., I. Couto, and H. de Lencastre. 2005. Genetic diversity among methicillin-resistant Staphylococcus epidermidis (MRSE). Microb. Drug Resist. 11:83-93. [DOI] [PubMed] [Google Scholar]
- 27.Miragaia, M., I. Couto, S. F. F. Pereira, K. G. Kristinsson, H. Westh, J. O. Jarløv, J. Carriço, J. Almeida, I. Santos-Sanches, and H. de Lencastre. 2002. Molecular characterization of methicillin-resistant Staphylococcus epidermidis clones: evidence of geographic dissemination. J. Clin. Microbiol. 40:430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narra, H. P., and H. Ochman. 2006. Of what use is sex to bacteria? Curr. Biol. 16:R705-R710. [DOI] [PubMed] [Google Scholar]
- 29.Nunes, A. P., L. M. Teixeira, C. C. Bastos, M. G. Silva, R. B. Ferreira, L. S. Fonseca, and K. R. Santos. 2005. Genomic characterization of oxacillin-resistant Staphylococcus epidermidis and Staphylococcus haemolyticus isolated from Brazilian medical centres. J. Hosp. Infect. 59:19-26. [DOI] [PubMed] [Google Scholar]
- 30.Okuma, K., K. Iwakawa, J. D. Turnidge, W. B. Grubb, J. M. Bell, F. G. O'Brien, G. W. Coombs, J. W. Pearman, F. C. Tenover, M. Kapi, C. Tiensasitorn, T. Ito, and K. Hiramatsu. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 40:4289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira, D. C., C. Milheiriço, and H. de Lencastre. 2006. Redefining a structural variant of staphylococcal cassette chromosome mec, SCCmec type VI. Antimicrob. Agents Chemother. 5:3457-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira, D. C., A. Tomasz, and H. de Lencastre. 2001. The evolution of pandemic clones of methicillin-resistant Staphylococcus aureus: identification of two ancestral genetic backgrounds and the associated mec elements. Microb. Drug Resist. 7:349-361. [DOI] [PubMed] [Google Scholar]
- 33.Raimundo, O., H. Heussler, J. B. Bruhn, S. Suntrarachun, N. Kelly, M. A. Deighton, and S. M. Garland. 2002. Molecular epidemiology of coagulase-negative staphylococcal bacteraemia in a newborn intensive care unit. J. Hosp. Infect. 51:33-42. [DOI] [PubMed] [Google Scholar]
- 34.Robinson, D. A., and M. C. Enright. 2003. Evolutionary models of the emergence of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3926-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos Sanches, I., R. Mato, H. de Lencastre, A. Tomasz, S. Nunes, C. R. Alves, M. Miragaia, J. Carriço, I. Couto, I. Bonfim, M. Aires de Sousa, D. Oliveira, A. Gomes, M. Vaz, S. Fernandes, S. C. Verde, A. Ávila, F. Antunes, R. Sá-Leão, J. Almeida, O. Melter, M. Chung, M. C. Brandileone, E. Castañeda, C. Cocuzza, G. Echaniz-Aviles, I. Heitmann, M. Hortal, W. Hryniewicz, F. Jia, K. Kikuchi, M. Konkoly-Thege, K. G. Kristinsson, J. Liñares, A. Rossi, E. Z. Savov, J. Schindler, F. Solorzano-Santos, K. Totsuka, M. Venditti, P. Villari, H. Westh, J.-S. Wu, and R. C. Zanella. 2000. Patterns of multidrug resistance among methicillin-resistant hospital isolates of coagulase-positive and coagulase-negative staphylococci collected in the international multicenter study RESIST in 1997 and 1998. Microb. Drug Resist. 6:199-211. [DOI] [PubMed] [Google Scholar]
- 36.Selander, R. K., D. A. Caugant, H. Ochman, J. M. Musser, M. N. Gilmour, and T. S. Whittam. 1986. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl. Environ Microbiol. 51:873-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva, F. R., E. M. Mattos, M. V. Coimbra, B. T. Ferreira-Carvalho, and A. M. Figueiredo. 2001. Isolation and molecular characterization of methicillin-resistant coagulase-negative staphylococci from nasal flora of healthy humans at three community institutions in Rio de Janeiro City. Epidemiol. Infect. 127:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitnikova, T., A. Rzhetsky, and M. Nei. 1995. Interior-branch and bootstrap tests of phylogenetic trees. Mol. Biol. Evol. 12:319-333. [DOI] [PubMed] [Google Scholar]
- 39.Smith, J. M., E. J. Feil, and N. H. Smith. 2000. Population structure and evolutionary dynamics of pathogenic bacteria. Bioessays 22:1115-1122. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi, K., and M. Nei. 2000. Efficiencies of fast algorithms of phylogenetic inference under the criteria of maximum parsimony, minimum evolution, and maximum likelihood when a large number of sequences are used. Mol. Biol. Evol. 17:1251-1258. [DOI] [PubMed] [Google Scholar]
- 41.Thomas, J. C., M. R. Vargas, M. Miragaia, S. J. Peacock, G. L. Archer, and M. C. Enright. 2007. An improved multilocus sequence typing scheme for Staphylococcus epidermidis. J. Clin. Microbiol. 45:616-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ubukata, K., R. Nonoguchi, M. Matsuhashi, and M. Konno. 1989. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J. Bacteriol. 171:2882-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Eldere, J., W. E. Peetermans, M. Struelens, A. Deplano, and H. Bobbaers. 2000. Polyclonal staphylococcal endocarditis caused by genetic variability. Clin. Infect. Dis. 31:24-30. [DOI] [PubMed] [Google Scholar]
- 44.Waldron, D. E., and J. A. Lindsay. 2006. Sau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J. Bacteriol. 188:5578-5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, X. M., L. Noble, B. N. Kreiswirth, W. Eisner, W. McClements, K. U. Jansen, and A. S. Anderson. 2003. Evaluation of a multilocus sequence typing system for Staphylococcus epidermidis. J. Med. Microbiol. 52:989-998. [DOI] [PubMed] [Google Scholar]
- 46.Wisplinghoff, H., A. E. Rosato, M. C. Enright, M. Noto, W. Craig, and G. L. Archer. 2003. Related clones containing SCCmec type IV predominate among clinically significant Staphylococcus epidermidis isolates. Antimicrob. Agents Chemother. 47:3574-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, Y. Q., S. X. Ren, H. L. Li, Y. X. Wang, G. Fu, J. Yang, Z. Q. Qin, Y. G. Miao, W. Y. Wang, R. S. Chen, Y. Shen, Z. Chen, Z. H. Yuan, G. P. Zhao, D. Qu, A. Danchin, and Y. M. Wen. 2003. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49:1577-1593. [DOI] [PubMed] [Google Scholar]
- 48.Ziebuhr, W., K. Dietrich, M. Trautmann, and M. Wilhelm. 2000. Chromosomal rearrangements affecting biofilm production and antibiotic resistance in a Staphylococcus epidermidis strain causing shunt-associated ventriculitis. Int. J. Med. Microbiol. 290:115-120. [DOI] [PubMed] [Google Scholar]
- 49.Ziebuhr, W., V. Krimmer, S. Rachid, I. Lossner, F. Gotz, and J. Hacker. 1999. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol. Microbiol. 32:345-356. [DOI] [PubMed] [Google Scholar]