Abstract
A survey of 30 representative strains of human gram-positive intestinal bacteria indicated that Roseburia species were among the most active in metabolizing linoleic acid (cis-9,cis-12-18:2). Different Roseburia spp. formed either vaccenic acid (trans-11-18:1) or a 10-hydroxy-18:1; these compounds are precursors of the health-promoting conjugated linoleic acid cis-9,trans-11-18:2 in human tissues and the intestine, respectively.
Linoleic acid (LA) (cis-9,cis-12-18:2) is metabolized in the human colon via conjugated linoleic acids (CLA) (mainly cis-9,trans-11-18:2) to vaccenic acid (VA) (trans-11-18:1) (both of the latter compounds are considered to be beneficial for health [5, 11, 27, 28, 31, 41]) and then to stearic acid (18:0) (17, 26). A similar pathway occurs in the rumen (12, 20), where this process, commonly known as biohydrogenation, has important implications for the fatty acid composition of meat and milk (22, 38). The microbiology of biohydrogenation in the rumen has received a great deal of attention (12, 32, 40, 42), but similar investigations have not been carried out for the human intestinal microbiota. Therefore, the aims of the present study were to identify human gut bacteria that can perform fatty acid biohydrogenation and to assess their likely importance in the mixed intestinal ecosystem. We found that Roseburia spp. were probably the most important organisms; some Roseburia species metabolized LA by the same pathway found in ruminal bacteria (20, 32), while others formed a hydration product that is a precursor of CLA in the mixed community.
Thirty bacterial strains (2 lactobacilli, 1 lactococcus, 5 propionibacteria, 3 bifidobacteria, and 19 strains of the low-G+C-content Clostridium cluster) isolated from or related to bacteria found in the human large intestine were studied to determine their ability to metabolize LA (Table 1). The bacteria were grown either in the liquid form of anaerobic basal M2 medium (13) or in the same medium supplemented with 50 μg/ml LA. For labeling experiments the medium was prepared using enough deuterium oxide to provide around 50% enrichment of the medium water. The linoleate isomerase activity in bacteria grown on unsupplemented M2 medium was determined by the method described by Wąsowska et al. (43). The methods used for extraction and derivatization of the total fatty acids to fatty acid methyl esters (FAME) and the identification methods were similar to the methods described by Devillard et al. (7). In order to determine the fate of the hydroxy-18:1 fatty acid (HFA) produced by some of the Roseburia isolates, Roseburia intestinalis L1-952 was grown in M2 medium containing 50 μg/ml LA, the culture was centrifuged (10,000 × g, 10 min, 4°C), and the supernatant was used to prepare a modified M2 medium enriched in HFA. This medium was inoculated with freshly voided human feces from two omnivorous volunteers consuming a Western diet, which had been diluted (0.2 g in 1 ml) in sterile anaerobic 0.1 M potassium phosphate buffer (pH 7.0). Duplicate aliquots of each fecal sample were removed after different times for fatty acid determination for up to 72 h.
TABLE 1.
Metabolism of linoleic acid by different bacterial species
| Strain | Group | Source (reference) | Linoleate isomerase activity (nmol CLA mg protein−1 min−1) | Lag time (h) | % LA metabolizeda | Main products formeda |
|---|---|---|---|---|---|---|
| Lactobacillus reuteri DSM 20016T | Firmicutes | Human adult intestine (18) | 0.7 ± 0.1 | 0 | 28 ± 3 | HFA |
| Lactobacillus delbrueckii subsp. bulgaricus DSM 20081T | Firmicutes | Bulgarian yoghurt (39) | 0.6 ± 0.1 | 72 | 14 ± 5 | None |
| Lactococcus lactis subsp. lactis DSM 20729 | Firmicutes | Swiss cheese (35) | 0.0 ± 0.0 | 0 | 44 ± 6 | HFA |
| Propionibacterium freudenreichii DSM 20271T | Actinobacteria | Swiss cheese (16) | 1.2 ± 0.1 | 72 | 11 ± 1 | None |
| Propionibacterium freudenreichii subsp. shermani DMS 20270 | Actinobacteria | Cheese (36) | 14.3 ± 1.1 | 24 | 96 ± 9 | CLA (c9t11, t9t11, t10c12) |
| Propionibacterium freudenreichii subsp. shermani DMS 4902T | Actinobacteria | Unknown (16) | 17.0 ± 1.7 | 72 | 85 ± 7 | CLA (c9t11, t9t11, t10c12) |
| Propioniobacterium jensenii DSM 20274 | Actinobacteria | Silage (36) | 2.4 ± 0.1 | 0 | 12 ± 1 | None |
| Propionibacterium thoenii DSM 20276T | Actinobacteria | Emmental cheese (36) | 3.5 ± 0.2 | 48 | 31 ± 9 | HFA |
| Bifidobacterium adolescentis NCFB 2204 | Actinobacteria | Human adult intestine (6) | 0.7 ± 0.0 | 24 | 68 ± 6 | HFA |
| Bifidobacterium breve NCFB 2258 | Actinobacteria | Human infant intestine (6) | 15.4 ± 0.9 | 0 | 95 ± 8 | CLA (c9t11, t9t11) |
| Bifidobacterium infantis NCFB 2256 | Actinobacteria | Human infant intestine (6) | 3.6 ± 0.2 | 24 | 79 ± 4 | HFA |
| Faecalibacterium prausnitzii L2-6 | Cluster IV | Human feces (3) | 0.5 ± 0.0 | 48 | 32 ± 9 | HFA |
| Eubacterium siraeum DSM 15702T | Cluster IV | Human feces (29) | 2.7 ± 0.0 | 24 | 26 ± 5 | HFA |
| Anaerostipes caccae L1-92T | Cluster XIVa | Human feces (24) | 0.0 ± 0.0 | 0 | 15 ± 4 | None |
| Eubacterium hallii L2-7 | Cluster XIVa | Human feces (24) | 3.5 ± 0.5 | 24 | 10 ± 2 | None |
| Eubacterium ventriosum L2-12 | Cluster XIVa | Human feces (3) | 0.0 ± 0.0 | 0 | 14 ± 5 | None |
| Eubacterium ruminantium L2-50 | Cluster XIVa | Human feces (24) | 0.0 ± 0.0 | 24 | 38 ± 3 | HFA |
| Eubacterium rectale T1-815 | Cluster XIVa | Human feces (24) | 1.3 ± 0.0 | 24 | 11 ± 2 | None |
| Eubacterium rectale A1-86 | Cluster XIVa | Human feces (24) | 3.5 ± 0.2 | 24 | 15 ± 1 | None |
| Eubacterium rectale M104/1 | Cluster XIVa | Human feces (3) | 2.4 ± 0.2 | 24 | 10 ± 2 | None |
| Roseburia inulinivorans A2-194T | Cluster XIVa | Human feces (8) | 23.3 ± 1.6 | 24 | 100 ± 6 | VA |
| Roseburia inulinivorans L1-83 | Cluster XIVa | Human feces (8) | 30.0 ± 2.3 | 24 | 100 ± 4 | VA |
| Roseburia hominis A2-183T | Cluster XIVa | Human feces (8) | 10.6 ± 0.8 | 0 | 100 ± 6 | VA |
| Roseburia hominis A2-181 | Cluster XIVa | Human feces (8) | 11.6 ± 0.6 | 0 | 100 ± 8 | VA |
| Roseburia intestinalis L1-82T | Cluster XIVa | Human feces (8) | 0.4 ± 0.0 | 24 | 84 ± 11 | HFA |
| Roseburia intestinalis L1-952 | Cluster XIVa | Human feces (8) | 0.5 ± 0.1 | 24 | 85 ± 13 | HFA |
| Roseburia faecis M6/1 | Cluster XIVa | Human feces (8) | 0.9 ± 0.1 | 24 | 86 ± 9 | HFA |
| Roseburia faecis M88/1 | Cluster XIVa | Human feces (8) | 0.9 ± 0.0 | 24 | 76 ± 8 | HFA |
| Roseburia faecis M72/1T | Cluster XIVa | Human feces (8) | 1.0 ± 0.0 | 24 | 88 ± 12 | HFA |
| Butyrivibrio fibrisolvens 16.4 | Cluster XIVa | Human feces (24) | 45.3 ± 3.1 | 24 | 100 ± 4 | VA |
When the optical density at 600 nm of a culture reached 0.6, fatty acids were extracted from the culture, the percentages of linoleic acid metabolized were calculated, and the products formed during growth were identified. These analyses were conducted in triplicate.
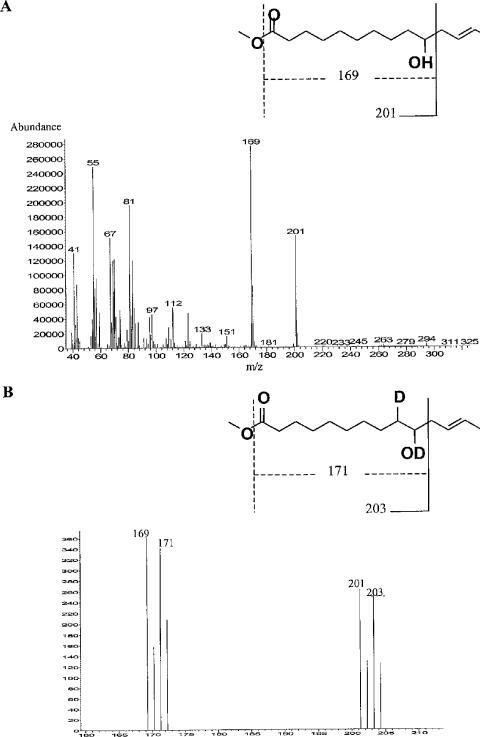
The linoleate isomerase activity was >10 nmol CLA formed (mg protein)−1 min−1 in 8 of the 30 isolates (Table 1), and Butyrivibrio fibrisolvens 16.4 and two strains of Roseburia inulinivorans (A2-194 and L1-83) exhibited the highest activity. When inoculated into medium containing LA, most of the 30 strains showed a lag time before they started to grow (Table 1). The same eight isolates that had high levels of isomerase activity also metabolized LA most extensively (Table 1). The final products for these strains were VA for the Roseburia and B. fibrisolvens isolates, a mixture of CLA (mainly cis-9,trans-11-18:2, trans-9,trans-11-18:2, and trans-10,cis-12-18:2) for Propionibacterium freudenreichii subsp. shermani, and cis-9,trans-11-18:2 and trans-9,trans-11-18:2 for Bifidobacterium breve (Table 1). Thus, it appears that whereas other species produced a mixture of CLA products, bacteria belonging to Clostridium cluster XIVa formed one product, either VA or HFA. Thirteen strains, belonging to both phylogenetic groups, metabolized LA (range, 26% to 88%) despite their low linoleate isomerase activity (Table 1). Gas chromatography traces indicated that all these strains produced the same compound, which was subsequently identified by gas chromatography-mass spectrometry (Fig. 1A). The main fragmentation in mass spectrometry led to the formation of ions at m/z 169 and 201, which are characteristic of a 10-HFA with the first 10 bonds saturated. When R. intestinalis L1-952 was grown in the presence of 50 μg/ml LA and deuterium oxide, the two major peaks were shifted to ions at m/z 171 and 203 (Fig. 1B). This shift corresponded to an addition of deuterium atoms at the double bond between carbons 9 and 10, suggesting that hydration occurred on this double bond (Fig. 1B). The location of the double bond could not be determined by analysis of the FAME. However, the signature masses at m/z 201 and 294 identified in 10-hydroxy-cis-12-18:1 by Schroepfer et al. (37) were present in the FAME spectrum. This information, together with purely biochemical considerations and comparison of the elution time of the FAME with that of ricinoleic acid (12-hydroxy-cis-9-18:1), indicated that the product was most likely 10-hydroxy-cis-12-18:1.
FIG. 1.
Mass spectra of the methyl ester of the HFA produced by 13 of the 30 bacterial strains studied here when they were incubated with linoleic acid. (A) Mass spectrum obtained with the unlabeled FAME derivative. (B) Mass spectrum obtained with the FAME derivative obtained from cultures containing deuterium oxide, showing peaks at m/z 160 to 210, where the two characteristic fragments of HFA are present.
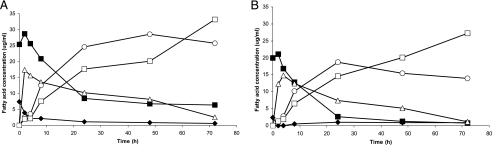
Modified M2 medium prepared from the culture supernatant of R. intestinalis L1-952 initially contained 5 μg/ml LA and 25 μg/ml HFA. The main fatty acids produced during incubation with diluted feces were cis-9,trans-11-18:2, VA, and stearic acid (Fig. 2). After a few hours, LA was almost completely metabolized, but the synthesis of CLA, VA, and stearic acid continued and corresponded to the disappearance of HFA (Fig. 2). Thus, we concluded that HFA is a precursor of cis-9,trans-11-18:2 in the mixed community. None of the pure cultures examined metabolized the HFA further.
FIG. 2.
Metabolism of HFA (▪) and LA (⧫) and formation of the products CLA (▵), VA (○), and stearic acid (□) by mixed fecal flora from volunteer 1 (A) and from volunteer 2 (B). The values are the means of duplicates and are expressed in μg of fatty acid/ml of fecal suspension.
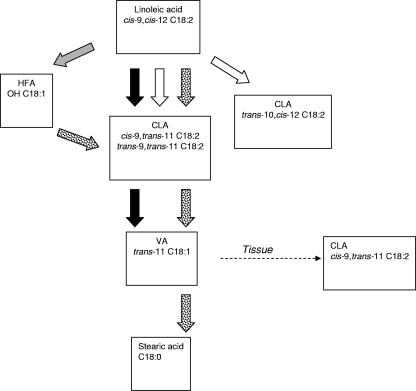
Animal studies and clinical trials have indicated that CLA may be useful in improving human health (5, 27, 31). The uptake of CLA formed in the intestine seems to be minor (17). However, local effects on gut tissue might be anticipated. It is now well established that CLA have antiproliferative and anti-inflammatory effects on colonocytes (4, 19), so provision of CLA in the intestinal lumen could be considered beneficial, particularly for inflammatory bowel diseases, such as ulcerative colitis and Crohn's disease (10). Bacteria from other ecosystems and from food products which are also found in the human gut, including strains of Lactobacillus, Propionibacterium, and Bifidobacterium (1, 6, 15, 23, 33, 34), have been known for some time to possess the ability to generate CLA. For the first time, we found here that the more abundant bacterial species belonging to clostridial clusters IV and XIVa also metabolize LA at some of the highest rates of all bacteria investigated, forming products that can be precursors of CLA (Fig. 3). Given the greater abundance of Clostridium-like bacteria in the human intestinal microbiota (9)—the numbers of lactobacilli, propionibacteria, and bifidobacteria are low, less than 5% of the total microbiota (2, 21)—it may be deduced that LA metabolism by this major group is quantitatively more important than LA metabolism by the Lactobacillus, Propionibacterium, and Bifidobacterium groups.
FIG. 3.
Proposed pathways of LA metabolism by bacterial species isolated from the human gut. The open arrows represent the bacterial activity of Lactobacillus, Propionibacterium, and Bifidobacterium species leading to the formation of CLA. The shaded arrows represent the bacterial activity of some Lactobacillus, Propionibacterium, and Bifidobacterium species and some Clostridium-like bacteria belonging to clusters IV (e.g., Eubacterium siraeum) and XIVa (e.g., R. intestinalis and Roseburia faecis) leading to the formation of HFA. The solid arrows represent the bacterial activity of Clostridium-like bacteria belonging to cluster XIVa leading to the formation of VA (e.g., Roseburia hominis and R. inulinivorans). The dotted arrows represent activities observed in fecal microbiota for which the responsible bacterial species are still unknown.
The discovery that HFA is a precursor of cis-9,trans-11-18:2 in the mixed intestinal community is also new, leading to the likely scheme of CLA formation shown in Fig. 3. Similar importance of HFA was proposed for a Lactobacillus sp., involving a hydration/dehydration process (30). No similar role for HFA has been postulated for bacteria in the rumen, where biohydrogenation is a quantitatively very important activity (12, 32).
The final product of LA metabolism by mixed fecal microbiota was shown here to be stearic acid, as shown previously by Howard and Henderson (14), yet none of the strains tested here produced stearate from LA (Fig. 3). Searching for stearate producers in the rumen has been difficult, largely because these organisms are extraordinarily sensitive to the toxic effects of unsaturated fatty acids (25, 42). The same may be true of human intestinal bacteria. Thus, stearate producers and the species that convert HFA to CLA, both potentially very important reactions in the mixed ecosystem of the human intestine, remain to be identified.
Acknowledgments
The Rowett Research Institute receives funding from the Scottish Executive Environmental and Rural Affairs Department.
We thank David Brown, Graham Calder, and Maureen Annand for technical help and expertise. We thank Kevin Shingfield and William Christie for advice on fatty acid analysis. We are grateful to Harry Flint for helpful criticism of the manuscript.
Footnotes
Published ahead of print on 5 January 2007.
REFERENCES
- 1.Alonso, L., E. P. Cuesta, and S. E. Gilliland. 2003. Production of free conjugated linoleic acid by Lactobacillus acidophilus and Lactobacillus casei of human intestinal origin. J. Dairy Sci. 86:1941-1946. [DOI] [PubMed] [Google Scholar]
- 2.Aminov, R. I., A. W. Walker, S. H. Duncan, H. J. M. Harmsen, G. W. Welling, and H. J. Flint. 2006. Molecular diversity, cultivation, and improved fluorescent in situ hybridization detection of a dominant group of human gut bacteria related to Roseburia and Eubacterium rectale. Appl. Environ. Microbiol. 72:6371-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barcenilla, A., S. E. Pryde, J. C. Martin, S. H. Duncan, C. S. Stewart, C. Henderson, and H. J. Flint. 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassaganya-Riera, J., K. Reynolds, S. Martino-Catt, Y. Cui, L. Hennighausen, F. Gonzalez, J. Rohrer, A. U. Benninghoff, and R. Hontecillas. 2004. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 127:777-791. [DOI] [PubMed] [Google Scholar]
- 5.Belury, M. A. 2002. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu. Rev. Nutr. 22:505-531. [DOI] [PubMed] [Google Scholar]
- 6.Coakley, M., R. P. Ross, M. Nordgren, G. Fitzgerald, R. Devery, and C. Stanton. 2003. Conjugated linoleic acid biosynthesis by human-derived Bifidobacterium species. J. Appl. Microbiol. 94:138-145. [DOI] [PubMed] [Google Scholar]
- 7.Devillard, E., F. M. McIntosh, C. J. Newbold, and R. J. Wallace. 2006. Rumen ciliate protozoa contain high concentrations of conjugated linoleic acids and vaccenic acid, yet do not hydrogenate linoleic acid or desaturate stearic acid. Br. J. Nutr. 96:697-704. [PubMed] [Google Scholar]
- 8.Duncan, S. H., R. I. Aminov, K. P. Scott, P. Louis, T. B. Stanton, and H. J. Flint. 2006. Proposal of Roseburia faecis sp. nov., Roseburia hominis sp. nov., and Roseburia inulinivorans sp. nov., based on isolates from human feces. Int. J. Syst. Evol. Microbiol. 56:2437-2441. [DOI] [PubMed] [Google Scholar]
- 9.Eckburg, P. B., E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 308:1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greicius, G., V. Arulampalam, and S. Pettersson. 2004. A CLA's act: feeding away inflammation. Gastroenterology 127:994-996. [DOI] [PubMed] [Google Scholar]
- 11.Ha, Y. L., N. K. Grimm, and M. W. Pariza. 1987. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis 8:1881-1887. [DOI] [PubMed] [Google Scholar]
- 12.Harfoot, C. G., and G. P. Hazlewood. 1997. Lipid metabolism in the rumen, p. 348-426. In P. N. Hobson and C. S. Stewart (ed.), The rumen microbial ecosystem. Chapman & Hall, London, United Kingdom.
- 13.Hobson, P. N. 1969. Rumen bacteria. Methods Microbiol. 3B:133-149. [Google Scholar]
- 14.Howard, F. A. C., and C. Henderson. 1999. Hydrogenation of polyunsaturated fatty acids by human colonic bacteria. Lett. Appl. Microbiol. 29:193-196. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, J., L. Bjorck, and R. Fonden. 1998. Production of conjugated linoleic acid by dairy starter cultures. J. Appl. Microbiol. 85:95-102. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, J. L., and C. S. Cummins. 1972. Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. J. Bacteriol. 109:1047-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamlage, B., L. Hartmann, B. Gruhl, and M. Blaut. 1999. Intestinal microorganisms do not supply associated gnotobiotic rats with conjugated linoleic acid. J. Nutr. 129:2212-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandler, O., K. O. Stetter, and R. Köhl. 1980. Lactobacillus reuteri sp. nov., a new species of heterofermentative lactobacilli. Zentbl. Bakteriol. Hyg. Abt. I Orig. Reihe C 1:264-269. [Google Scholar]
- 19.Kemp, M. Q., B. D. Jeffy, and D. F. Romagnolo. 2003. Conjugated linoleic acid inhibits cell proliferation through a p53-dependent mechanism: effects on the expression of G1-restriction points in breast and colon cancer cells. J. Nutr. 133:3670-3677. [DOI] [PubMed] [Google Scholar]
- 20.Kepler, C. R., K. P. Hirons, J. J. McNeill, and S. B. Tove. 1966. Intermediates and products of the biohydrogenation of linoleic acid by Butyrivibrio fibrisolvens. J. Biol. Chem. 241:1350-1354. [PubMed] [Google Scholar]
- 21.Lay, C., M. Sutren, V. Rochet, K. Saunier, J. Dore, and L. Rigottier-Gois. 2005. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human fecal microbiota. Environ. Microbiol. 7:933-946. [DOI] [PubMed] [Google Scholar]
- 22.Lin, H., T. D. Boylston, M. J. Chang, L. O. Luedecke, and T. D. Shultz. 1995. Survey of the conjugated linoleic acid contents of dairy products. J. Dairy Sci. 78:2358-2365. [DOI] [PubMed] [Google Scholar]
- 23.Lin, T. Y., C. W. Lin, and C. H. Lee. 1999. Conjugated linoleic acid concentration as affected by lactic cultures and added linoleic acid. Food Chem. 67:1-5. [Google Scholar]
- 24.Louis, P., S. H. Duncan, S. I. McCrae, J. Millar, M. S. Jackson, and H. J. Flint. 2004. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 186:2099-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maia, M. R. G., L. C. Chaudhary, L. Figueres, and R. J. Wallace. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Leeuwenhoek, in press. [DOI] [PubMed]
- 26.McIntosh, F. M., R. J. Wallace, and E. Devillard. 2006. Variations in intermediates and products of linoleic acid biohydrogenation by two different human mixed colonic flora. Reprod. Nutr. Dev. 46(Suppl. 1):S52. [Google Scholar]
- 27.Mensink, R. P. 2005. Metabolic and health effects of isomeric fatty acids. Curr. Opin. Lipidol. 16:27-30. [DOI] [PubMed] [Google Scholar]
- 28.Miller, A., E. McGrath, C. Stanton, and R. Devery. 2003. Vaccenic acid (t11-18:1) is converted to c9,t11-CLA in MCF-7 and SW480 cancer cells. Lipids 38:623-632. [DOI] [PubMed] [Google Scholar]
- 29.Moore, W. E. C., J. L. Johnson, and L. V. Holdeman. 1976. Emendation of Bacteroidaceae and Butyrivibrio and descriptions of Desulfomonas gen. nov. and ten new species of the genera Desulfomonas, Butyrivibrio, Eubacterium, and Ruminococcus. Int. J. Syst. Bacteriol. 26:238-252. [Google Scholar]
- 30.Ogawa, J., K. Matsumura, S. Kishino, Y. Omura, and S. Shimizu. 2001. Conjugated linoleic acid accumulation via 10-hydroxy-12-octadecaenoic acid during microaerobic transformation of linoleic acid by Lactobacillus acidophilus. Appl. Environ. Microbiol. 67:1246-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pariza, M. W. 2004. Perspective on the safety and effectiveness of conjugated linoleic acid. Am. J. Clin. Nutr. 79:1132S-1136S. [DOI] [PubMed] [Google Scholar]
- 32.Polan, C. E., J. J. McNeill, and S. B. Tove. 1964. Biohydrogenation of unsaturated fatty acids by rumen bacteria. J. Bacteriol. 88:1056-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rainio, A., M. Vahvaselka, T. Suomalainen, and S. Laakso. 2001. Reduction of linoleic acid inhibition in production of conjugated linoleic acid by Propionibacterium freudenreichii ssp. shermanii. Can. J. Microbiol. 47:735-740. [PubMed] [Google Scholar]
- 34.Rosberg-Cody, E., R. P. Ross, S. Hussey, C. A. Ryan, B. P. Murphy, G. F. Fitzgerald, R. Devery, and C. Stanton. 2004. Mining the microbiota of the neonatal gastrointestinal tract for conjugated linoleic acid-producing bifidobacteria. Appl. Environ. Microbiol. 70:4635-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schleifer, K. H., J. Kraus, C. Dvorak, R. Kilpper-Baelz, M. D. Collins, and W. Fischer. 1985. Transfer of Streptococcus lactis and related streptococci to the genus Lactococcus gen. nov. Syst. Appl. Microbiol. 6:183-195. [Google Scholar]
- 36.Schleifer, K. H., R. Plapp, and O. Kandler. 1968. Glycine as crosslinking bridge in the ll-diaminopimelic acid containing murein of Propionibacterium peterssonii. FEBS Lett. 1:287-290. [DOI] [PubMed] [Google Scholar]
- 37.Schroepfer, G. J., Jr., W. G. Niehaus, Jr., and J. A. McCloskey. 1970. Enzymatic conversion of linoleic acid to 10D-hydroxy-Δ12-cis-octadecenoic acid. J. Biol. Chem. 245:3798-3801. [PubMed] [Google Scholar]
- 38.Scollan, N. D., N. J. Choi, E. Kurt, A. V. Fisher, M. Enser, and J. D. Wood. 2001. Manipulating the fatty acid composition of muscle and adipose tissue in beef cattle. Br. J. Nutr. 85:115-124. [DOI] [PubMed] [Google Scholar]
- 39.Sharpe, M. E. 1955. A serological classification of lactobacilli. J. Gen. Microbiol. 12:107-122. [DOI] [PubMed] [Google Scholar]
- 40.van de Vossenberg, J. L., and K. N. Joblin. 2003. Biohydrogenation of C18 unsaturated fatty acids to stearic acid by a strain of Butyrivibrio hungatei from the bovine rumen. Lett. Appl. Microbiol. 37:424-428. [DOI] [PubMed] [Google Scholar]
- 41.Wahle, K. W., S. D. Heys, and D. Rotondo. 2004. Conjugated linoleic acids: are they beneficial or detrimental to health? Prog. Lipid Res. 43:553-587. [DOI] [PubMed] [Google Scholar]
- 42.Wallace, R. J., L. C. Chaudhary, N. McKain, N. R. McEwan, A. J. Richardson, P. E. Vercoe, N. D. Walker, and D. Paillard. 2006. Clostridium proteoclasticum: a ruminal bacterium that forms stearic acid from linoleic acid. FEMS Microbiol. Lett. 265:195-201. [DOI] [PubMed] [Google Scholar]
- 43.Wąsowska, I., M. R. G. Maia, K. M. Niedzwiedzka, M. Czauderna, J. M. C. Ramalho Ribeiro, E. Devillard, K. J. Shingfield, and R. J. Wallace. 2006. Influence of fish oil on ruminal biohydrogenation of C18 unsaturated fatty acids. Br. J. Nutr. 95:1199-1211. [DOI] [PubMed] [Google Scholar]