Abstract
Pseudomonas aeruginosa is one of the major causative agents of mortality and morbidity in hospitalized patients due to a multiplicity of virulence factors associated with both chronic and acute infections. Acute P. aeruginosa infection is primarily mediated by planktonic bacteria expressing the type III secretion system (TTSS), a surface-attached needle-like complex that injects cytotoxins directly into eukaryotic cells, causing cellular damage. Lipopolysaccharide (LPS) is the principal surface-associated virulence factor of P. aeruginosa. This molecule is known to undergo structural modification (primarily alterations in the A- and B-band O antigen) in response to changes in the mode of life (e.g., from biofilm to planktonic). Given that LPS exhibits structural plasticity, we hypothesized that the presence of LPS lacking O antigen would facilitate eukaryotic intoxication and that a correlation between the LPS O-antigen serotype and TTSS-mediated cytotoxicity would exist. Therefore, strain PAO1 (A+ B+ O-antigen serotype) and isogenic mutants with specific O-antigen defects (A+ B−, A− B+, and A− B−) were examined for TTSS expression and cytotoxicity. A strong association existed in vitro between the absence of the large, structured B-band O antigen and increased cytotoxicity of these strains. In vivo, all three LPS mutant strains demonstrated significantly increased lung injury compared to PAO1. Clinical strains lacking the B-band O antigen also demonstrated increased TTSS secretion. These results suggest the existence of a cooperative association between LPS O-antigen structure and the TTSS in both laboratory and clinical isolates of P. aeruginosa.
Pseudomonas aeruginosa is one of the major causative agents of nosocomial illness and can elicit both chronic and acute infections in burn, immunocompromised, and cystic fibrosis (CF) patients (14, 15). The basis for this versatility stems from the bacterium's expression of a multitude of virulence factors, many of which are controlled by membrane-bound two-component response regulators (10, 27, 30). These regulators facilitate rapid responses to environmental conditions, including coordinated expression of specific virulence systems. Several of these regulators have been shown to be responsible for the reciprocal regulation of virulence genes specific to chronic (biofilm-associated) or acute (planktonic-mode-associated) infection by P. aeruginosa (10, 27, 29, 30). This suggests that embarking on either of these modes of infectivity represents a specific lifestyle choice for the species. For example, biofilm formation involves the generation of structured bacterial communities encased in a protective exopolysaccharide matrix. Cooperative gene regulation that enhances biofilm formation, e.g., increased exopolysaccharide production and reduced bacterial motility, has been shown to occur in chronic infections in the lungs of CF patients (26). Acute infection, on the other hand, usually involves motile bacteria and host cell contact so that cytotoxins may be injected through the needle-like type III secretion apparatus directly into host epithelial cells (22). These observations suggest that distinct cell surface physiologies exist for these two modes of infection and that changes in the surface-attached molecular composition may be required to facilitate each mode of pathogenicity.
The major P. aeruginosa surface-associated virulence factor, lipopolysaccharide (LPS), plays an important immunogenic and structural role, as well as mediating the interaction between the bacterial-cell surface and the external environment (21). Structurally, P. aeruginosa LPS is composed of a hydrophobic lipid A region, a central core oligosaccharide, and a variable-length repeating polysaccharide portion referred to as the O antigen (21). Lipid A modifications (e.g., addition of 2-hydroxylaurate, deacetylation of fatty acid, and addition of palmitate) are associated with an altered host-pathogen Toll-like receptor 4 signaling immune inflammatory response (6, 7, 11). Such modifications appear to be associated with chronic infection, since they are commonly observed in CF patients with long-term P. aeruginosa infections and have not been detected in cases of acute infection (5).
The O antigen represents another structurally plastic moiety of P. aeruginosa LPS. This molecule may comprise an A and/or B band composed of repeating sugar units and represents the main source of LPS structural and serotypic variation among isolates of P. aeruginosa. The LPS O-antigen composition is known to rapidly change when P. aeruginosa cells, initially grown as biofilms, are transferred into conditions that promote planktonic or free-swimming culture (2). It is widely believed that these rapid surface-associated changes have broad implications for pathogenesis, but to date, no detailed studies investigating the effect of the surface-attached LPS O-antigen composition on infection ability have been carried out.
Since cell-cell contact is key to effective type III secretion and acute infection, we hypothesized that structural changes in the LPS of P. aeruginosa would affect the ability of P. aeruginosa to efficiently secrete cytotoxins, affecting its capacity to elicit an acute infection. Specifically, we hypothesized that the presence or absence of P. aeruginosa LPS A-band or B-band O antigen would affect the expression of components of the type III secretion system (TTSS) and that a less structured LPS phenotype (lacking both O-antigen moieties) would promote cytotoxin production and secretion by the bacterium. Here, we report a strong association between the LPS O-antigen structural composition and TTSS expression in vitro and in vivo using isogenic wild-type (PAO1) and LPS mutant strains, as well as clinical isolates. These findings suggest that coregulation between these two disparate virulence systems exists and that loss of the O antigen, particularly the highly structured B band, facilitates type III cytotoxin secretion by P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study and their previously published O-antigen characteristics are listed in Table 1. P. aeruginosa strains were routinely grown in Luria-Bertani (LB) broth (Sigma-Aldrich, St. Louis, MO) with shaking at 250 rpm at 37°C for 18 h. For quantitative PCR (Q-PCR) experiments, laboratory strains were cultured overnight in inducing medium (16), and the cultures were diluted (optical density at 600 nm [OD600] = 0.1) in fresh inducing medium and reincubated to mid-exponential phase (OD600 = 0.7). As a control, strains were also cultured under noninducing conditions (inducing medium lacking EGTA). For analysis of TTSS protein production and secretion by P. aeruginosa laboratory strains, cultures were grown overnight in inducing medium (19), diluted to an OD600 of 0.1, and reincubated for a further 24 h in the same medium. For comparative assessment of gene expression and protein production by both planktonic and biofilm cultures of the same strains, each strain was cultured in 5 ml of TTSS-inducing MINS medium (19) overnight and diluted to an OD600 of 0.1 in the same medium. Half a milliliter of this diluted inoculum was plated on MINS agar plates and incubated statically at 37°C for 24 h (biofilm culture). The remaining 4.5 ml was incubated at 37°C for 24 h with vigorous shaking (planktonic culture).
TABLE 1.
Bacterial strains used in this study
| Strain | O antigena | Source | Reference |
|---|---|---|---|
| PAO1 | A+ B+ | Laboratory strain | 12 |
| PA103 | A+ B+ | Laboratory strain | 9 |
| gmd mutant | A− B+ | PAO1 isogenic mutant | 20 |
| wbpM mutant | A+ B− | PAO1 isogenic mutant | 21 |
| rmlC mutant | A− B− | PAO1 isogenic mutant | 3 |
| CIb 1 | Unknown | Wound | This study |
| CI 2 | Unknown | Sputum | This study |
| CI 3 | Unknown | Wound | This study |
A, A-band O-antigen phenotype; B, B-band O-antigen phenotype; +, present; −, absent.
CI, clinical isolate.
RNA isolation and quantitative PCR.
Total RNA was isolated from bacterial strains grown under the various conditions described above using Trizol (Invitrogen, California) according to the manufacturer's instructions. Specific primer sets for target genes (Table 2) were generated using Beacon Designer v.3.0 (Premier Biosoft International, California). Total RNA was checked for integrity and absence of DNA by PCR prior to cDNA synthesis using 1 μg of total RNA and the GeneAmp RNA PCR Kit (Applied Biosystems, California) according to the manufacturer's instructions. mRNA transcript concentrations were measured using SYBR Green PCR Master Mix (Applied Biosystems, California) according to the manufacturer's instructions. Reactions were carried out using the Mx3000P Real-Time PCR System (Stratagene, California) as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 58°C for 1 min, and 72°C for 30 s. The data acquisition step was set at 58°C with a final melting-curve analysis to ensure amplification of a single product. The transcript levels of each gene were normalized to that of the internal control, rpoD (23), and expression was calculated using the 2−ΔΔCT method (13).
TABLE 2.
Q-PCR primers used in this study
| Gene | Function | Sequence (5′ to 3′)a |
|---|---|---|
| exsA | TTSS positive regulator | F, AGTTGCTGATGCTCTTCG |
| R, GCGAAACCCCATAGACAC | ||
| exoS | TTSS cytotoxin | F, TCAATCGCTTCAGCAGAG |
| R, CGCTTGAACACGACGG | ||
| exoT | TTSS cytotoxin | F, TGTTCGCCGAGGTACTGCTC |
| R, AATCGCCGTCCAACTGCATGC | ||
| pcrV | TTSS secreted protein | F, GACAAGGTCAACGACAAG |
| R, TGACAATGTCGCGCAGGA | ||
| rpoD | Housekeeping gene | F, GGGCGAAGAAGAAATGGTC |
| R, CAGGTGGCGTAGGTGGAGAA |
F, forward; R, reverse.
Analysis of type III effector protein production and secretion.
P. aeruginosa laboratory and clinical strains were cultured as described above for biofilm and planktonic cultures. Biofilms were scraped from agar plates into microcentrifuge tubes and centrifuged to form a cell pellet. Planktonic cultures were harvested by centrifugation, and the supernatants were carefully removed and concentrated using Amicon Ultra centricon tubes (molecular weight cutoff, 10,000; Millipore, Massachusetts). The planktonic and biofilm cell pellets were resuspended in lysis buffer (1% sodium dodecyl sulfate and 50 mM Tris-HCl, pH 8.0) and boiled for 3 min. The protein concentrations of all samples were determined using the DC protein assay kit (Bio-Rad, California). Equal concentrations (10 μg) of each sample were separated on a sodium dodecyl sulfate (12.5% Tris-HCl)-polyacrylamide gel (Bio-Rad, California). The gels were transferred onto a polyvinylidene difluoride membrane (Bio-Rad, California) using a semidry electrotransfer unit (Bio-Rad, California). Immunoblotting was carried out using antibodies against ExoU, ExoS, and PcrV as previously described (22).
Cytotoxicity assay using human BEAS-2B lung epithelia.
A human bronchiolar epithelial cell line (BEAS-2B; ATCC CRL9609) was cultured in Dulbecco's modified Eagle medium with low glucose (DMEM H-16; Invitrogen, California) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, California). The cells were seeded into 96-well flat-bottom tissue culture plates at a density of 2.5 × 105 cells per well and incubated to confluence in a 5% CO2 chamber at 37°C for 18 h. The wells were rinsed twice with sterile phosphate-buffered saline (PBS), followed by the addition of 180 μl of RPMI 1640 containing l-glutamine (without phenol red, supplemented with 10% fetal bovine serum) to each well. Overnight P. aeruginosa 5-ml LB cultures were centrifuged, and the pellets were rinsed twice with Lactated Ringers (Baxter Healthcare Corporation, Illinois) to ensure the removal of exoproducts. The pellets were resuspended in sterile Lactated Ringers-saline (1:1) solution. Twenty microliters of Lactated Ringers-saline solution containing a standardized concentration of each P. aeruginosa strain (107 CFU ml−1) was added to triplicate wells. Negative controls received 20 μl of cell-free Lactated Ringers-saline solution. At various time intervals, 100 μl of supernatant from triplicate wells was removed, placed in a sterile 96-well flat-bottom tissue culture plate, and centrifuged at low speed (3,000 rpm). Lactate dehydrogenase activity assays were carried out on 50 μl of cell-free supernatant using the CytoTox96 Non-Radioactive Cytotoxicity Assay Kit (Promega, California) according to the manufacturer's instructions.
In vivo murine virulence.
Pathogen-free male BALB/c mice (12 weeks old) were obtained (Charles River Laboratories, Massachusetts). After inhaling anesthesia with sevoflurane, each mouse received an airspace instillate containing 2% bovine serum albumin, 2 mg of Evans blue dye, 0.12 μCi of 131I-labeled albumin, and P. aeruginosa (PAO1 or LPS mutants; 5 × 107 CFU ml−1; four mice per strain) in sterile PBS. A sample of the instillate was saved for radioactivity measurement (counts/min/g) using a γ-ray counter (Packard, Illinois) and quantitative bacterial culture on LB agar plates to confirm the inoculation CFU. The mice were returned to their cages and allowed access to food and water. Four hours after bacterial instillation, the mice were euthanized and exsanguinated. Lung injury was quantified by radioactivity counts (epithelial permeability) and lung weight (lung edema) as previously described (7, 24).
LPS B-band O-antigen serotyping.
All laboratory and clinical strains used in this study were grown as both biofilm and planktonic cultures as described above, and their B-band O-antigen serotypes were characterized in each mode of growth using a P. aeruginosa Serotyping Kit (ERFA Biotech, Canada) according to the manufacturer's instructions. Since the serotypes of PAO1 and isogenic mutants are known, these strains served as controls to ensure the accuracy of the kit. Three or four loopfuls of biofilm biomass per strain was resuspended in 100 μl PBS. Planktonic cultures were harvested by centrifugation and resuspended in 1 ml of PBS. Ten microliters of each cell suspension per strain was then mixed with 10 μl of each antibody solution (1× concentration) prior to being vortexed and incubated at room temperature for 20 to 30 min. Serotype-positive strains exhibited aggregation and settling to the bottom of the tube. Negative controls remained uniformly suspended in solution.
RESULTS
Quantitative PCR of exsA, exoS, exoT, and pcrV.
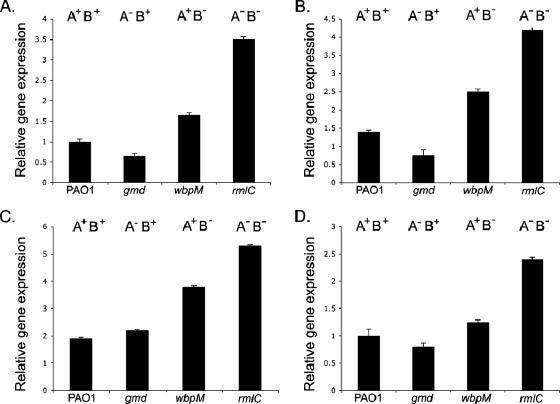
To determine whether the LPS structure affected TTSS gene expression, quantitative-PCR (Q-PCR) analysis was carried out on PAO1 and isogenic mutants (containing specific O-antigen mutations) (Table 1) to measure mRNA expression levels for genes involved in the TTSS (Table 2) under inducing and noninducing conditions. Figure 1A to D illustrates the relative expression levels observed for four key genes of the TTSS of PAO1; the positive regulator (exsA), two genes encoding secreted proteins (exoS and exoT) (cytotoxins), and pcrV (which facilitates cytotoxin injection). Under noninducing conditions, gene expression levels were identical for all four strains (data not shown). However, under TTSS-inducing conditions, wbpM (A+ B−) and rmlC (A− B−) mutant strains lacking the highly structured B-band O antigen consistently exhibited significantly increased (P = 0 for all for genes examined) TTSS gene expression relative to PAO1 (Fig. 1A to D). In addition, the two strains also demonstrated increased expression compared to wbpM, which exhibited TTSS gene expression levels similar to those of PAO1.
FIG. 1.
Relative expression levels of exsA (A), exoS (B), exoT (C), and pcrV (D). The results are representative of three independent experiments carried out in triplicate. The error bars indicate standard deviations.
Analysis of TTSS cytotoxin production and secretion.
Since measurement of mRNA expression may not directly correlate with concentrations of secreted effector proteins and therefore cytotoxicity, Western immunoblotting analysis was performed on PAO1 and isogenic LPS mutants to determine the production (intracellular) and secretion (extracellular fraction) of ExoS (the most potent of the cytotoxins of PAO1) and PcrV (crucial for effective cytotoxin secretion [17]). While all strains produced and secreted the ExoS cytotoxin, the three LPS mutant strains produced and secreted two- to fourfold more than the wild type (Fig. 2A). Wild-type PAO1 produced barely detectable concentrations of PcrV intracellularly and secreted only a low concentration of the protein. The LPS mutant strains, however, both produced and secreted approximately twofold more PcrV than PAO1 (Fig. 2B), suggesting that these strains would exhibit increased cytotoxicity compared to the wild type.
FIG. 2.
Analysis of ExoS (A) and PcrV (B) production (white bars) and secretion (black bars) from PAO1 and isogenic LPS mutant strains. The results are representative of three independent experiments. The error bars indicate standard deviations.
Cytotoxicity of PA01 and LPS mutants toward BEAS-2B epithelial cells.
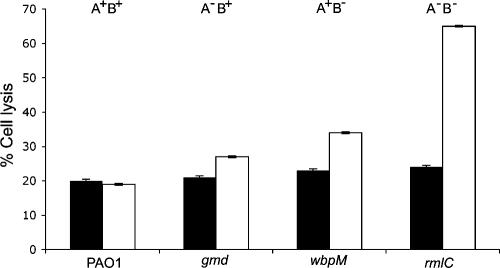
Delivery of effector proteins into target cells via the TTSS is central to acute infection by P. aeruginosa. To determine if observed differences in TTSS mRNA expression and protein production and secretion between PAO1 and isogenic mutants correlated with an increase in mammalian-cell death, cytotoxicity was determined as described in Materials and Methods. Increased cell death (compared to PAO1) was observed when the mutant strains were incubated with lung epithelial cells for 6 h. Consistent with Q-PCR and immunoblot analyses, the mutant wbpM and rmlC strains, which both lack the B-band O antigen, produced the greatest increases in cytotoxicity in vitro. Exposure of the BEAS-2B cell line to the gmd, wbpM, or rmlC mutant strain increased cytotoxicity by 8, 14, and 46%, respectively, compared to the wild-type PAO1 (Fig. 3).
FIG. 3.
Cytotoxicity assay using BEAS-2B lung epithelial cells and standardized inocula of PAO1 and isogenic LPS O-antigen mutant strains. Black bars, 4 h postinfection; white bars, 6 h postinfection. The results are representative of at least two independent experiments carried out in triplicate. The error bars indicate standard deviations.
In vivo murine study.
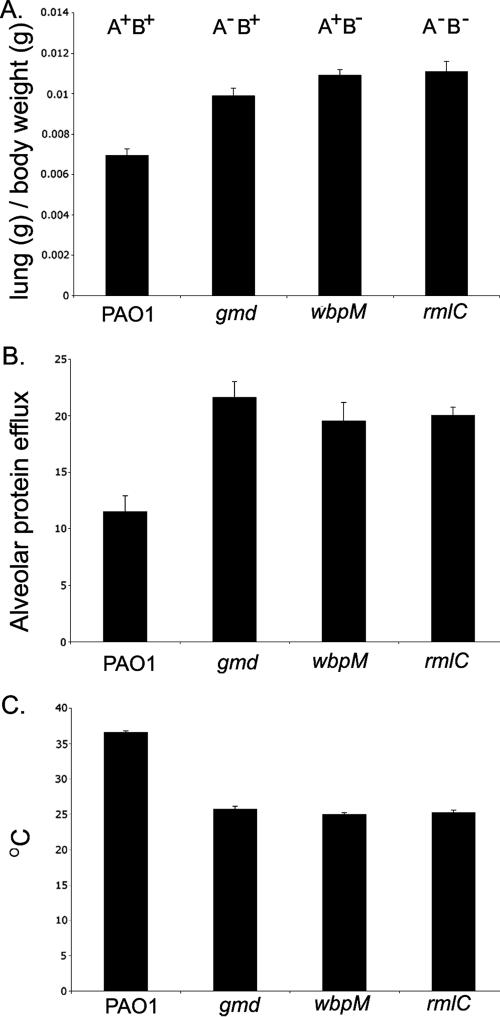
To assess if this dramatic difference in cytotoxicity between the strains was maintained in vivo, a murine study using BALB/c mice was carried out to evaluate lung injury. Two indicators of lung injury were measured, lung edema and epithelium permeability, as well as low body temperature (an indicator of bacteremia). Figure 4A demonstrates that the mice infected with LPS mutant strains demonstrated increased lung weight compared to those instilled with PAO1, suggesting that the mutant strains induced greater lung injury than the wild type. Consistent with these observations, measurement of epithelial permeability also demonstrated that the LPS mutants caused significantly more lung epithelial permeability than was elicited by PAO1 (Fig. 4B). All LPS mutant strains exhibited an approximately twofold increase in both lung edema and epithelial damage, suggesting that the alteration in the LPS O antigen resulted in increased bacterially induced lung injury. Mice instilled with the mutant strains also demonstrated considerably lower body temperatures than those infected with the wild-type strain, suggesting that these mice were also severely bacteremic (Fig. 4C).
FIG. 4.
In vivo evaluation of lung injury in BALB/c mice infected with PAO1 and an isogenic LPS mutant. (A) Measure of lung edema. (B) Epithelial permeability measurement. (C) Body temperature. The results represent the average measurement for four mice in each group. The error bars indicate standard deviations.
LPS characterization of P. aeruginosa clinical isolates.
From our initial studies, the B-band O antigen appeared to be a critical determinant affecting type III gene expression and protein secretion. To determine if loss of this O antigen also affected cytotoxin secretion by clinical isolates, three strains encoding the potent type III cytotoxin ExoU were isolated from patient respiratory samples and examined, in addition to the virulent laboratory strain PA103. As a control, the original laboratory strains used in this study were also analyzed. When the strains were cultured as biofilms and analyzed in a blind test, PAO1 and its iosogenic gmd mutant strain agglutinated with anti-O5 monoclonal antibody, while the other two LPS mutant strains lacking the B-band O antigen exhibited nontypeable phenotypes, confirming the ability of the test to differentiate between serotypes. The clinical isolates CI1 to -3, cultured as biofilms, each agglutinated with anti-O11 monoclonal antibody, similar to the virulent laboratory strain PA103 (Table 3). When each of these clinical isolates was cultured planktonically, it exhibited a nontypeable B-band serotype.
TABLE 3.
LPS B-band O-antigen serotypes of clinical isolates cultured under planktonic or biofilm conditions
| Strain | LPS O serotype
|
|
|---|---|---|
| Planktonic | Biofilm | |
| PAO1 | NT | O5 |
| gmd mutant | NT | O5 |
| wbpM mutant | NT | NTb |
| rmlCI mutant | NT | NT |
| PA103 | NT | O11 |
| CIa 1 | NT | O11 |
| CI 2 | NT | O11 |
| CI 3 | NT | O11 |
CI, clinical isolate.
NT, nontypeable.
Analysis of TTSS cytotoxin expression, production, and secretion by P. aeruginosa clinical isolates.
Since Western immunoblotting analysis had previously provided an excellent indication of cytotoxic potential, this approach was used to examine type III secretion by biofilm and planktonic cultures of each of the clinical strains. For planktonic cultures, intracellular and secreted fractions were separated, and analysis of the separated fractions was then performed. Planktonic strains (containing no B-band O antigen) produced (data not shown) and secreted (Fig. 5) both ExoU and PcrV proteins. Using samples normalized for total protein, immunoblot analysis was also performed on the clinical isolates cultured as biofilms (containing the 011 B-band O antigen). Under these conditions, all strains exhibited barely detectable or undetectable ExoU and PcrV proteins (Fig. 5).
FIG. 5.
Immunoblot analysis of ExoU (A) and PcrV (B) production and secretion by clinical isolates cultured as either planktonic or biofilm cultures. The results are representative of at least three independent experiments. The error bars indicate standard deviations.
DISCUSSION
This investigation provided evidence to show a correlation between the absence of A-band and B-band O antigen in LPS and the production and secretion of TTSS cytotoxins in P. aeruginosa. This relationship was documented for both laboratory strains and clinical isolates of the species. We specifically hypothesized that loss of O antigen (particularly the highly structured B band) would facilitate secretion of the TTSS and increase acute infectivity. Our results both in vitro and in vivo support this hypothesis and document an association between the LPS O-antigen structural composition and type III cytotoxin secretion and acute lung injury in this species.
Analysis of PAO1 (A+ B+) and isogenic mutants phenotypically deficient in specific O antigens (gmd [A+ B−], wbpM [A− B+], and rmlC [A− B−]) was carried out to support our hypothesis. Q-PCR analysis revealed that the mutant strains lacking B-band O antigen had significantly increased expression levels for all four type III secretion system genes examined (exoS, exoT, exsA, and pcrV) compared to the wild-type PAO1. Indeed, as the structural complexity of the O-antigen moiety of LPS decreased, expression of all four TTSS genes examined increased. This suggests that the LPS structural composition may act as a signal to control TTSS gene expression. Immunoblot analysis also supported this hypothesis; the concentration of ExoS and PcrV proteins produced intracellularly paralleled mRNA expression levels. Strains lacking the B-band O antigen produced and secreted considerably more ExoS and PcrV than the wild-type strain, suggesting that these strains are more cytotoxic than the wild type. Indeed, cytotoxicity assays using BEAS-2B lung epithelial cells confirmed this observation; all three LPS mutants exhibited increased cytotoxicity compared to PAO1 in the order PAO1 > gmd > wpmC > rmlC (O-antigen phenotypes, A+ B+ > A− B+ > A+ B− > A− B−). These results suggest that the loss of the LPS O-antigen structure may provide an intracellular signal to upregulate TTSS expression in P. aeruginosa. This effect appears to be dependent on the complexity of the O antigen, since cytotoxicity progressively increased as the lipid A portion putatively became more exposed to the external environment.
In vivo studies supported the in vitro results; the three isogenic mutant strains were associated with significantly increased lung injury compared to instillation of the wild-type PAO1. Interestingly, the distinction between specific O-antigen mutant strains, apparent in the in vitro tests, was not observed in vivo. This may be due the less sensitive nature of the measurements of lung injury compared to the in vitro measurements of cell cytotoxicity. Further, in vivo measurements, by necessity, reflect both the bacterially induced lung injury and the host response, which can also induce lung injury, masking the specific effect of the secreted cytotoxins. Nevertheless, all three isogenic mutant strains were associated with an approximately twofold increase in lung injury compared to the injury produced by the wild-type bacteria.
To determine whether the LPS compositions of P. aeruginosa clinical isolates also affected type III secretion, three strains that encoded the potent ExoU cytotoxin were examined. Immunoblot analysis of the type III components (ExoU and PcrV) was carried out on planktonic and biofilm cultures of the same strain. Planktonic cultures lacking the B-band O antigen secreted large quantities of both the potent cytotoxins ExoU and PcrV, while the same strains cultured as biofilms (containing B band O11) produced small or undetectable quantities of these proteins. These results suggest that for both the laboratory and clinical strains of P. aeruginosa, the mode of life (e.g., biofilm versus planktonic) affects the LPS O-antigen serotype and that the specific O-antigen composition affects the expression of the type III secretion system of P. aeruginosa. More structurally complex LPS (e.g., A+ B+) appears to reduce type III expression, while less complex LPS (e.g., A− B−) appears to promote the expression of this virulence system.
These data suggest that the switch from chronic to acute infectivity by P. aeruginosa (e.g., when cells detach from a biofilm) involves phenotypic remodeling of the LPS O antigen (e.g., loss of the B band) to facilitate free-swimming acutely infectious cells. The presence of particular O antigens on the surface of P. aeruginosa is known to affect the overall charge and physicochemistry of the bacterial cell; strains lacking the B-band O antigen have demonstrated greater ability to adhere to abiotic hydrophobic surfaces (2). It is also known that other bacterial species exhibiting a hydrophobic surface demonstrate enhanced adhesion to epithelial cells (4, 28). Therefore, it is likely that the loss of the B-band O antigen could facilitate acute infection via a number of cooperative mechanisms, including increased TTSS expression and increased host cell adherence. In addition, the sheer molecular bulk of the B-band O antigen (2) would likely hinder contact between the needle complex and the host cell membrane, reducing the efficiency of cytotoxin secretion. Thus, its loss could reduce steric hindrance and improve contact between the secretion apparatus and the host cell, improving acute infectivity.
It has recently been reported that the presence or absence of the Yersinia enterocolitica O:8 O antigen also affects virulence expression in this species (1). Together with the observations presented here, these data suggest that LPS plays a direct or indirect role in virulence gene expression in a number of pathogenic species. Future dissection of the regulatory circuitry underlying the cross talk that permits cooperation between these two disparate virulence systems could lead to an understanding of the environmental signals that cause this transition from chronic to acute infection by P. aeruginosa. Manipulation of these signals could then provide a means to convert antimicrobial-resistant chronic infections to susceptible planktonic cells. Such a strategy, coupled with immunotherapy (e.g., anti-PcrV immunoglobulin G [8, 18, 25]) to protect the host against the effects of type III secretion by this species, would be of immense utility in patient populations chronically infected with P. aeruginosa (e.g., CF patients).
Acknowledgments
We sincerely thank Joseph Lam for the PAO1 mutant strains used in this study and for all his helpful comments and advice. We also thank Eoin Brodie for assistance with designing Q-PCR primers for this study.
This research was supported by National Institutes of Health (NIH) grants PH50HL74005 and HL69809 to J.P.W.-K. D.K.A. was supported by NIH Bridge 5 R25 GM48972-06 and CSU LS-AMP HRD9802113 and by a minority supplement award, and S.V.L. was supported by a UCSF Academic Senate Independent Researcher Award.
Footnotes
Published ahead of print on 5 January 2007.
REFERENCES
- 1.Bengoechea, J. A., H. Najdenski, and M. Skurnik. 2004. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 52:451-469. [DOI] [PubMed] [Google Scholar]
- 2.Beveridge, T. J., S. A. Makin, J. L. Kadurugamuwa, and Z. Li. 1997. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 20:291-303. [DOI] [PubMed] [Google Scholar]
- 3.Burrows, L. L., R. V. Urbanic, and J. S. Lam. 2000. Functional conservation of the polysaccharide biosynthetic protein WbpM and its homologues in Pseudomonas aeruginosa and other medically significant bacteria. Infect. Immun. 68:931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding, H., C. Lammler, and R. S. Seleim. 1993. Adherence of Actinomyces pyogenes to HeLa cells mediated by hydrophobic surface proteins. Zentbl. Bakteriol. 279:299-306. [DOI] [PubMed] [Google Scholar]
- 5.Ernst, R. K., K. N. Adams, S. M. Moskowitz, G. M. Kraig, K. Kawasaki, C. M. Stead, M. S. Trent, and S. I. Miller. 2006. The Pseudomonas aeruginosa lipid A deacylase: selection for expression and loss within the cystic fibrosis airway. J. Bacteriol. 188:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst, R. K., A. M. Hajjar, J. H. Tsai, S. M. Moskowitz, C. B. Wilson, and S. I. Miller. 2003. Pseudomonas aeruginosa lipid A diversity and its recognition by Toll-like receptor 4. J. Endotoxin Res. 9:395-400. [DOI] [PubMed] [Google Scholar]
- 7.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 8.Faure, K., J. Fujimoto, D. W. Shimabukuro, T. Ajayi, N. Shime, K. Moriyama, E. G. Spack, J. P. Wiener-Kronish, and T. Sawa. 2003. Effects of monoclonal anti-PcrV antibody on Pseudomonas aeruginosa-induced acute lung injury in a rat model. J. Immune Based Ther. Vaccines 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleiszig, S. M., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman, A. L., B. Kulasekara, A. Rietsch, D. Boyd, R. S. Smith, and S. Lory. 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7:745-754. [DOI] [PubMed] [Google Scholar]
- 11.Hajjar, A. M., R. K. Ernst, J. H. Tsai, C. B. Wilson, and S. I. Miller. 2002. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 3:354-359. [DOI] [PubMed] [Google Scholar]
- 12.Holloway, B. W. 1978. Isolation and characterization of an R′ plasmid in Pseudomonas aeruginosa. J. Bacteriol. 133:1078-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 14.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 15.Matsui, H., V. E. Wagner, D. B. Hill, U. E. Schwab, T. D. Rogers, B. Button, R. M. Taylor II, R. Superfine, M. Rubinstein, B. H. Iglewski, and R. C. Boucher. 2006. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 103:18131-18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCaw, M. L., G. L. Lykken, P. K. Singh, and T. L. Yahr. 2002. ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol. Microbiol. 46:1123-1133. [DOI] [PubMed] [Google Scholar]
- 17.Nanao, M., S. Ricard-Blum, A. M. Di Guilmi, D. Lemaire, D. Lascoux, J. Chabert, I. Attree, and A. Dessen. 2003. Type III secretion proteins PcrV and PcrG from Pseudomonas aeruginosa form a 1:1 complex through high affinity interactions. BMC Microbiol. 3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neely, A. N., I. A. Holder, J. P. Wiener-Kronish, and T. Sawa. 2005. Passive anti-PcrV treatment protects burned mice against Pseudomonas aeruginosa challenge. Burns 31:153-158. [DOI] [PubMed] [Google Scholar]
- 19.Nicas, T. I., and B. H. Iglewski. 1984. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect. Immun. 45:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahim, R., L. L. Burrows, M. A. Monteiro, M. B. Perry, and J. S. Lam. 2000. Involvement of the rml locus in core oligosaccharide and O polysaccharide assembly in Pseudomonas aeruginosa. Microbiology 146:2803-2814. [DOI] [PubMed] [Google Scholar]
- 21.Rocchetta, H. L., L. L. Burrows, and J. S. Lam. 1999. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 63:523-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 23.Savli, H., A. Karadenizli, F. Kolayli, S. Gundes, U. Ozbek, and H. Vahaboglu. 2003. Expression stability of six housekeeping genes: a proposal for resistance gene quantification studies of Pseudomonas aeruginosa by real-time quantitative RT-PCR. J. Med. Microbiol. 52:403-408. [DOI] [PubMed] [Google Scholar]
- 24.Sawa, T., K. Kurahashi, M. Ohara, M. A. Gropper, V. Doshi, J. W. Larrick, and J. P. Wiener-Kronish. 1998. Evaluation of antimicrobial and lipopolysaccharide-neutralizing effects of a synthetic CAP18 fragment against Pseudomonas aeruginosa in a mouse model. Antimicrob. Agents Chemother. 42:3269-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shime, N., T. Sawa, J. Fujimoto, K. Faure, L. R. Allmond, T. Karaca, B. L. Swanson, E. G. Spack, and J. P. Wiener-Kronish. 2001. Therapeutic administration of anti-PcrV F(ab′)2 in sepsis associated with Pseudomonas aeruginosa. J. Immunol. 167:5880-5886. [DOI] [PubMed] [Google Scholar]
- 26.Tart, A. H., M. C. Wolfgang, and D. J. Wozniak. 2005. The alternative sigma factor AlgT represses Pseudomonas aeruginosa flagellum biosynthesis by inhibiting expression of fleQ. J. Bacteriol. 187:7955-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ventre, I., A. L. Goodman, I. Vallet-Gely, P. Vasseur, C. Soscia, S. Molin, S. Bleves, A. Lazdunski, S. Lory, and A. Filloux. 2006. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl. Acad. Sci. USA 103:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wibawan, I. T., C. Lammler, and F. H. Pasaribu. 1992. Role of hydrophobic surface proteins in mediating adherence of group B streptococci to epithelial cells. J. Gen. Microbiol. 138:1237-1242. [DOI] [PubMed] [Google Scholar]
- 29.Yahr, T. L., and E. P. Greenberg. 2004. The genetic basis for the commitment to chronic versus acute infection in Pseudomonas aeruginosa. Mol. Cell 16:497-498. [DOI] [PubMed] [Google Scholar]
- 30.Zolfaghar, I., A. A. Angus, P. J. Kang, A. To, D. J. Evans, and S. M. Fleiszig. 2005. Mutation of retS, encoding a putative hybrid two-component regulatory protein in Pseudomonas aeruginosa, attenuates multiple virulence mechanisms. Microbes Infect. 7:1305-1316. [DOI] [PubMed] [Google Scholar]