Abstract
In Shewanella sp. strain ANA-3, utilization of arsenate as a terminal electron acceptor is conferred by a two-gene operon, arrAB, which lacks a gene encoding a membrane-anchoring subunit for the soluble ArrAB protein complex. Analysis of the genome sequence of Shewanella putrefaciens strain CN-32 showed that it also contained the same arrAB operon with 100% nucleotide identity. Here, we report that CN-32 respires arsenate and that this metabolism is dependent on arrA and an additional gene encoding a membrane-associated tetraheme c-type cytochrome, cymA. Deletion of cymA in ANA-3 also eliminated growth on and reduction of arsenate. The ΔcymA strains of CN-32 and ANA-3 negatively affected the reduction of Fe(III) and Mn(IV) but not growth on nitrate. Unlike the CN-32 ΔcymA strain, growth on fumarate was absent in the ΔcymA strain of ANA-3. Both homologous and heterologous complementation of cymA in trans restored growth on arsenate in ΔcymA strains of both CN-32 and ANA-3. Transcription patterns of cymA showed that it was induced under anaerobic conditions in the presence of fumarate and arsenate. Nitrate-grown cells exhibited the greatest level of cymA expression in both wild-type strains. Lastly, site-directed mutagenesis of the first Cys to Ser in each of the four CXXCH c-heme binding motifs of the CN-32 CymA nearly eliminated growth on and reduction of arsenate. Together, these results indicate that the biochemical mechanism of arsenate respiration and reduction requires the interactions of ArrAB with a membrane-associated tetraheme cytochrome, which in the non-arsenate-respiring Shewanella species Shewanella oneidensis strain MR-1, has pleiotropic effects on Fe(III), Mn(IV), dimethyl sulfoxide, nitrate, nitrite, and fumarate respiration.
Metal-reducing bacteria can significantly impact the fate and transport of arsenic in sediments and groundwater (2, 10-12, 35). Reduction of iron oxides containing arsenate [HAsO42−; As(V)] can liberate arsenite [H3AsO3; As(III)] into porewaters, leading to the contamination of aquifers and groundwater (4). Arsenate reduction under these conditions is most likely due to As(V)-respiring prokaryotes, which are known to be diverse and to utilize a variety of electron acceptors, including Fe(III) and Mn(IV) (hydr)oxides (17, 24, 25).
In the metal-reducing bacterium Shewanella sp. strain ANA-3, two genes, arrA and arrB, are required for arsenate respiration (28). The gene products, ArrA, a ∼95-kDa molybdenum-containing arsenate reductase subunit, and ArrB, an ∼26-kDa Fe-S-containing subunit, are soluble and localized to the periplasm (1, 15, 16). Based on several biochemical features (e.g., size, Mo cofactor, amino acid sequence similarity, and cofactor binding motifs), ArrA is part of a large family of molybdenum-containing oxidoreductases (e.g., dimethyl sulfoxide, nitrate, polysulfide, and trimethylamine N-oxide [TMAO] reductases) (18). Other prokaryotic molybdenum cofactor-containing oxidoreductases employ a membrane protein, which serves as a site for anchoring the soluble Mo and Fe-S subunits to the cytoplasmic membrane (18). In some cases, the membrane-anchoring subunit also functions to transfer electrons from the quinone pool to the catalytic subunits (6). Several of these membrane-associated subunits have been shown to be c-type cytochromes (16, 20, 26). In the non-As(V)-respiring Shewanella species Shewanella oneidensis strain MR-1, a 21-kDa membrane-associated periplasmic c-type cytochrome, CymA, is required for respiration of fumarate, nitrate, Fe(III), dimethyl sulfoxide, and nitrite (20, 23, 32). CymA is part of the NapC/NirT family of tetraheme cytochromes, which, unlike CymA, provide more specialized functions in other bacteria (20). For example, in Paracoccus denitrificans, the NapC protein transfers electrons from the quinols to the periplasmic nitrate reductase (26). In contrast to most members of the NapC/NirT tetraheme cytochromes, CymA acts as a common branching point in the electron transport chain of Shewanella species by serving as a redox intermediary between the quinone pool and multiple terminal reductases (32).
Because the arr operon of Shewanella sp. strain ANA-3 lacks a gene encoding a membrane subunit for ArrAB, it was predicted that CymA would be involved in the As(V) respiratory pathway in As(V)-respiring Shewanella species. Here, we investigate the functional role of cymA in the As(V) respiratory reduction pathways of several Shewanella species.
MATERIALS AND METHODS
Strains and plasmids.
All Escherichia coli and Shewanella strains and plasmids used in this study are described in Table 1. Shewanella sp. strain W3-18-1 was a kind gift from Jizhong Zhou at the University of Oklahoma.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or markers, characteristics, and uses | Source or reference |
|---|---|---|
| E. coli strains | ||
| TOPO Top 10 | E. coli host for cloning; F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| UQ950 | E. coli DH5α λ(pir) host for cloning; F− Δ(argF-lac)169 φ80dlacZ58(ΔM15) glnV44(AS) rfbD1 gyrA96(NalR) recA1 endA1 spoT1 thi-1 hsdR17 deoR λpir+ | 28 |
| WM3064 | Donor strain for conjugation; thrB1004 pro thi rpsL hsdS lacZΔM15 RP4-1360 Δ(araBAD)567 ΔdapA1341::[erm pir(wt)] | 28 |
| Shewanella strains | ||
| Shewanella sp. ANA-3 | Isolated from an As-treated wooden pier piling in a brackish estuary (Eel Pond, Woods Hole, MA) | 27 |
| AN-CYMA | Shewanella sp. strain ANA-3; ΔcymA; does not respire As(V) or fumarate | This study |
| S. putrefaciens CN-32 | Isolated from anaerobic subsurface core sample, New Mexico | 8, 34 |
| CN-CYMA | S. putrefaciens CN-32; ΔcymA; does not respire As(V) | This study |
| Shewanella sp. W3-18-1 | Isolated from Pacific Ocean marine sediments at 630 m | 19 |
| Plasmids/vectors | ||
| pCR4-TOPO | 4-kb cloning vector; lacZ-ccdB: Kmr Ampr | Invitrogen |
| pSMV10 | 9.1-kb mobilizable suicide vector; oriR6K mobRP4 sacB Kmr Gmr | 28 |
| ANpΔcymA | 1.2-kb fusion PCR fragment containing ΔcymA from ANA-3 cloned into the SpeI site of pSMV10; used to make the AN-CymA ΔcymA strain. | This study |
| CNpΔcymA | 2-kb fusion PCR fragment containing ΔcymA from S. putrefaciens cloned into the SpeI site of pSMV10; used to make the CN-CymA ΔcymA strain. | This study |
| pBBR1MCS-2 | 5.1-kb broad-host-range plasmid; KmrlacZ | 14 |
| pANcymA | ANA-3 cymA PCR fragment, including the promoter region, cloned into the SpeI site of pBBR1MCS-2 | This study |
| pCNcymA | S. putrefaciens cymA PCR fragment cloned into the SpeI sites of pBBR1MCS-2 | This study |
| pH 1(C46S) | pCNcymA plasmid with SXXCH of heme 1 | This study |
| pH 2(C78S) | pCNcymA plasmid with CXXSH of heme 2 | This study |
| pH 3(C136S) | pCNcymA plasmid with SXXCH of heme 3 | This study |
| pH 4(C173S) | pCNcymA plasmid with SXXCH of heme 4 | This study |
Growth conditions.
Standard culturing of ANA-3, CN-32, and E. coli strains was done in Luria-Bertani medium (31) or a minimal medium (described below). The ANA-3 and CN-32 strains were grown at 30°C, and liquid cultures were shaken at 250 rpm. Anaerobic culturing was done in a minimal medium (TME, pH 7) consisting of 1.5 g liter−1 NH4Cl, 0.6 g liter−1 NaHPO4, 0.1 g liter−1 KCl, 0.5 g liter−1 yeast extract, 10 mM HEPES, 20 mM lactate, and 10 ml liter−1 (each) trace mineral and vitamin solutions (13). The medium was boiled under a stream of N2, anaerobically dispensed into N2-flushed Balch tubes, sealed with butyl rubber stoppers, and autoclave sterilized. Media were supplemented with 10 mM fumarate, nitrate, TMAO, or arsenate or 25 mM hydrous ferrous oxide from sterile anaerobic stock solutions.
Growth experiments.
Aerobic cultures were grown overnight in TME medium. The optical density (OD) at 600 nm of each culture was brought below 0.6, and they were standardized to each other by the addition of TME medium to ensure that the inoculation levels for all strains were equal. The cells were then inoculated at 1/100 dilution into anaerobic tubes. Growth was monitored by measuring the OD at 600 nm using a Spectronic 20. Control cultures were also grown and monitored in anaerobic TME without added electron acceptors.
The ferrozine assay was used to monitor Fe(III) reduction. Under anaerobic conditions, 50 μl of cell culture was added to 450 μl 0.5 M HCl, mixed, and filtered through a 0.2-μm filter (Spin-X column); 10 μl of this mixture was then added to an additional 90 μl 0.5 M HCl in a 96-well microtiter dish. Ferrozine solution (100 μl of 0.1% ferrozine dissolved in 50% [wt/vol] ammonium acetate solution) was added, and the absorbance at 540 nm was read after 10 min. Sample absorbances were compared to standard curves of known ferrous chloride concentrations [10, 100, and 500 μM Fe(II)] prepared in HCl.
Some growth experiments also measured the level of oxidation of lactate to acetate and reduction of arsenate to arsenite. This was done by removing 0.5 ml from each culture, filtering it through a 0.2-μm filter (Spin-X column), and quantifying the sample by high-performance liquid chromatography as described previously (27). All growth experiments were done in triplicate.
Sequencing of ANA-3 cymA.
The set of degenerate primers (CymA-F, 5′-TGG CGT GCA CTA TTY AAA CC-3′, and CymA-R, 5′-TAR GGG TGA GCR ACR CCT TT-3′) used to amplify the cymA gene in ANA-3 was designed by comparing the cymA sequences (with corresponding accession numbers in parentheses) in S. putrefaciens CN-32 (ZP_00815799), S. oneidensis MR-1 (NP_720107), S. amazonensis SB2B (ZP_00584682), S. frigidimarina (YP_748973), S. loihica PV4 (ZP_00837170), and S. baltica (ZP_00582221) using MacVector and selecting the regions of highest DNA similarity. The amplified product from ANA-3 genomic DNA was cloned into E. coli using the TA TOPO Cloning Kit (Invitrogen). Plasmids from three Kmr colonies were sequenced using M13 primers. The resulting sequence was then used to design primers for inverse PCR and sequencing (ANAcymAiPCR-F1, 5′-AGA ACC AGC CAG ACA CTA TG-3′, and ANAcymAiPCR-R1, 5′-GTG TGG TAA GTG GCA GTC TT-3′) of BamHI-digested and self-ligated ANA-3 genomic DNA. This PCR product was cloned using the TA TOPO cloning kit, and the resulting plasmids were sequenced using ANAcymAiPCR-F1 and ANAcymAiPCR-R1. Primer walking and sequencing were done using the primers ANAcymAiPCR-F2, 5′-GCT GCA CGA GAA TAA TAG GT-3′, and cymAiPCR-R2, 5′-TAA TAC GAC AAC TCG CTC AA-3′.
Mutagenesis.
In-frame, nonpolar deletions of cymA were generated using previously developed methods (28) with primers X-CNcymA-A (5′-GGA CTA GTG TCA AAC CGC CAA AAA TAA A-3′), X-CNcymA-B (5′-[TGT TTA AGC ATG CTG GAT GGG] AAA GGA TAA TAG GTT TTA GCG-3′), X-CNcymA-C (5′-[CCC ATC CAG CAT GCT TAA ACA] CCA GTT CAT TAC TCT ATC TCC-3′), and X-CNcymA-D (5′-GGA CTA GTC TGA CGA TAA GGC ACC ACA A-3′) for cymA in CN32 and X-ANAcymA-A (5′-GGA CTA GTG TGT ACC TGT TCC TAA AGA CC-3′), X-ANAcymA-B (5′-[CCC ATC CAG CAT GCT TAA ACA] CCA GTT CAT CAC TCT ATC TCC-3′), X-ANAcymA-C (5′-[TGT TTA AGC ATG CTG GAT GGG] AAA GGA TAA GGT TTA GCG CTT-3′), and X-ANAcymA-D (5′-GGA CTA GTG ATA CGA CTC AGG GTG AG GT-3′) for cymA in ANA-3 (SpeI sites are underlined; the bracketed areas are the 21-bp PCR linkers, with SphI sites underlined). Because of the limited sequence data at the time of this work, two ∼400-bp DNA fragments flanking the cymA gene were amplified (the complete ANA-3 genome sequence is now available).
The complementation plasmids pCNcymA and pANcymA were generated by cloning PCR products of the CN-32 and ANA-3 cymA genes into the SpeI site of pBBR1MCS-2. The following primers were used to generate the genes: CNcymAcomp-F1 (5′-GGA CTA GTG TTT ATC GTC AGC GAG TGA T-3′), CNcymAcomp-R1 (5′-GGA CTA GTT GGC AAT TTT GGA GAT AGA G-3′), ANcymAcomp-F1 (5′-GGA CTA GTG GAG ATA GAG TGA TGA ACT GG-3′), and ANcymAcomp-R1 (5′-GGA CTA GTC AAG CAT TTA CTG TTA TGT GG-3′) (SpeI sites are underlined). Transformation of the complementation vectors into Shewanella sp. strain ANA-3 and CN-32 was performed as previously described (28).
Mutations to the heme binding regions in CN-32 cymA were generated using the following modified protocol of the QuikChange Site-Directed Mutagenesis method (Stratagene). Primers CNcymAheme-F1 (5′-T ACG GAT CAG TTC agc ATG TCC TGT CAC A-3′), CNcymAheme-F2 (5′-ACC GTT CAA TGT CAA GAC agc CAC TTA CCA CA-3′), CNcymAheme-F3 (5′-AAT GAC TCC GCT AAC agc CAA CAC TGC CAT ACT-3′), and CNcymAheme-F4 (5′-GAA GCC AGA AAA ACC agc GTT GAC TGC CAT AAA-3′) were designed with a cysteine-to-serine substitution (in lowercase); the primers are numbered F1 to F4 to correspond to hemes 1 to 4, respectively. Each heme motif was mutated separately in 25-μl reaction mixtures containing 2.5 μl 10× Pfu-Turbo Hotstart Buffer (Strategene), 0.2 mM deoxynucleoside triphosphate mix, 125 ng primer, 50 ng pCNcymA plasmid, 1 μl Pfu-Turbo Hotstart DNA polymerase (Stratagene), and nuclease-free water. Samples were incubated with the following cycle profile: 95°C for 30 s and 18 cycles of 95°C for 30 s, 55°C for 1 min, and 68°C for 11 min. The reaction mixtures were cooled to room temperature, and 1 μl DpnI (10 units) was added to each reaction mixture and incubated for 1 h at 37°C. Each reaction mixture was transformed into a DH5α-λpir strain (UQ950) (28). Plasmids were extracted and sequenced to confirm the correct mutation. The resulting heme-mutated pCNcymA vectors were then transformed into CN-CYMA by conjugation as previously described. The plasmid strains were denoted pH 1(C46S), pH 2(C78S), pH 3(C136S), and pH 4(C173S) for mutations in the heme 1 through the heme 4 motif, respectively.
Quantification of cymA transcription.
The methods for quantitative reverse transcription-PCR have been described previously (29). ANA-3 cells were grown anaerobically in triplicate 10-ml TME cultures amended with 10 mM nitrate, 10 mM fumarate, or 10 mM As(V). Aerobically prepared cells were grown in 15 ml TME in 250-ml flasks with constant sparging of filtered air. Cells were harvested in mid-exponential growth phase (OD at 600 nm, 0.1). One milliliter of the cultures was centrifuged at 14,000 rpm at 4°C for 10 min. The cell pellets were stored at −80°C until further analysis was performed. RNA was extracted from the cell pellets using the QIAGEN RNeasy miniprep kit according to the manufacturer's instructions, with a final elution volume of 40 μl. DNA contamination was removed by DNase (Promega RQ1 DNase) digestion. cDNA was synthesized as described previously (29).
The real-time PCRs (30 μl) consisted of 15 μl 2× SybrGreen Taq Mix (Applied Biosystems), 300 nM of primers q-ANAcymA-F1 (5′-GAG AAC CAG CCA GAC ACT AT-3′) and q-ANAcymA-R1 (5′-ACA ATC CAC ACA GGT CTT TC-3′), and 4 μl RNA sample diluted 1/4 in nuclease-free water. Each sample was run in duplicate using the MJ Research Opticon2. The thermocycle profile consisted of 95°C for 10 min, 40 cycles of 95°C for 30 s and 60°C for 1 min, and a final denaturing cycle to examine the DNA melting curves of PCR products.
The cycle threshold was calculated for each sample and converted to a DNA nanogram equivalent by comparing the cycle threshold values to a standard curve of known ANA-3 genomic DNA concentrations (1, 0.1, 0.01, 0.001, and 0.0001 ng), which had a slope of 3.6 and an R2 (calculated from a linear regression of the data points) of 0.999. The resulting DNA concentrations were normalized to the original amount of RNA template in each sample.
RESULTS
Shewanella putrefaciens strain CN-32 grows on arsenate.
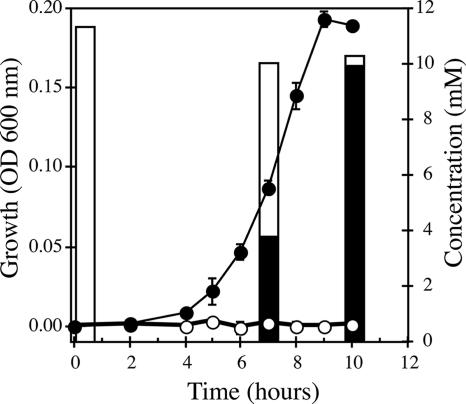
Sequence analysis of the recently completed genome of Shewanella putrefaciens strain CN-32 showed that it contained an arrAB gene cluster that was identical to the arrAB of Shewanella sp. strain ANA-3. Moreover, the CN-32 arr-containing contig 160 was 100% identical to a 20-kb region of the ANA-3 genome sequence containing arrAB. We observed a similar genome structure in Shewanella sp. strain W3-18-1 contig 292; however, the region had numerous base pair differences (83% over ∼8 kb). Neither CN-32 nor W3-18-1 has been shown to respire As(V), and we were interested in testing whether these strains could use arsenate as an electron acceptor. Figure 1 shows the growth curve of CN-32 in anaerobic medium containing arsenate. Growth reached stationary phase after 10 h of incubation. Analysis of substrates and products in culture filtrates collected during early- and mid-log and stationary phases of growth confirmed that all As(V) was reduced to As(III) (Fig. 1), with nearly 2:1 molar stoichiometry of As(V) reduction compared to lactate oxidation. All the As(V) (11 mM) was reduced to As(III), while ∼6 mM lactate was oxidized to acetate (∼4 mM final concentration). These results are consistent with previous growth studies with ANA-3 (27). We confirmed the involvement of arrA in arsenate respiration by generating an arrA null mutation in CN-32. Growth on arsenate was eliminated but could be restored by complementation of the wild-type arrA gene in trans to the CN-32 arrA null mutant (data not shown). Strain W3-18-1 also grew with arsenate as a terminal electron acceptor (data not shown). Based on these observations, we concluded that both ANA-3 and CN-32, and most likely W3-18-1, respire arsenate via very similar mechanisms.
FIG. 1.
Growth on arsenate (10 mM) and reduction to arsenite by Shewanella putrafaciens strain CN-32. ○, arrA null mutant; •, wild type; open bars, arsenite; dark bars, arsenate. The data points and error bars represent the means and standard deviations of triplicate cultures, respectively.
Identification of cymA in ANA-3.
Based on genome sequence data in other arsenate-respiring bacteria (e.g.., Desulfitobacterium hafniense and Wolinella succinogenes), their arr operons contain a third gene, arrC, which encodes a putative membrane protein that most likely anchors the ArrAB to the cytoplasmic membrane. However, in the ANA-3, CN-32, and W3-18-1 genomes, no ArrC gene homologs were found. We hypothesized that CymA, a membrane-bound tetraheme c-type cytochrome, would be required for utilization of arsenate as a terminal electron acceptor in arsenate-respiring Shewanella. This predication was based on several studies that demonstrated that cymA was required for anaerobic respiration of many electron acceptors in Shewanella oneidensis strain MR-1 (20, 32).
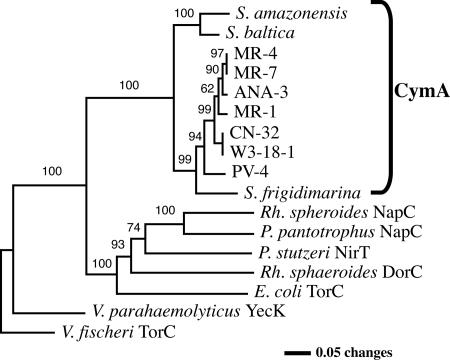
We obtained the sequence of ANA-3 cymA prior to the recent release of the full genome sequence of ANA-3. PCR primers were designed from conserved regions of DNA multisequence alignments of cymA homologs found in other Shewanella genomes. The consensus primers generated an ∼500-bp PCR product, which was sequenced. The predicted protein exhibited a high degree of similarity to other CymAs from various Shewanella species and was 100% identical to the CymA found in the ANA-3 genome sequence that is now available. Figure 2 illustrates the phylogenetic relationship of CymAs and other members of the NapC/NirT family tetraheme cytochromes. Shewanella CymAs were highly conserved and formed a distinct cluster within the tree of the tetraheme cytochrome family.
FIG. 2.
Phylogenetic analysis of CymA using distance analysis (neighbor joining). The analysis included CymAs of various Shewanella species and selected tetraheme cytochromes within the NirT/NapC family. Bootstrap values are labeled at nodes with ≥50% occurrences. Accession numbers are as follows: Shewanella oneidensis MR-1, NP_720107; Shewanella sp. strain ANA-3, ZP_00849215; Shewanella sp. strain MR-4, YP_735897; Shewanella sp. strain MR-7, ABI40840; Shewanella putrefaciens CN-32, ZP_00815799; Shewanella sp. strain W3-18-1, ZP_00905699; Shewanella sp. strain PV-4, ZP_00837170; Shewanella frigidimarina, YP_748973; Shewanella amazonensis, ZP_00584682; Shewanella baltica, ZP_00582221; Pseudomonas stutzeri, TP24038; Vibrio fischeri, YP_206147; Vibrio parahaemolyticus, Q53178; Paracoccus pantotrophus, Q56352; E. coli, P33226; Rhodobacter sphaeroides, AAB94872; Vibrio parahaemolyticus, NP_799637.
Physiological effects of deleting cymA.
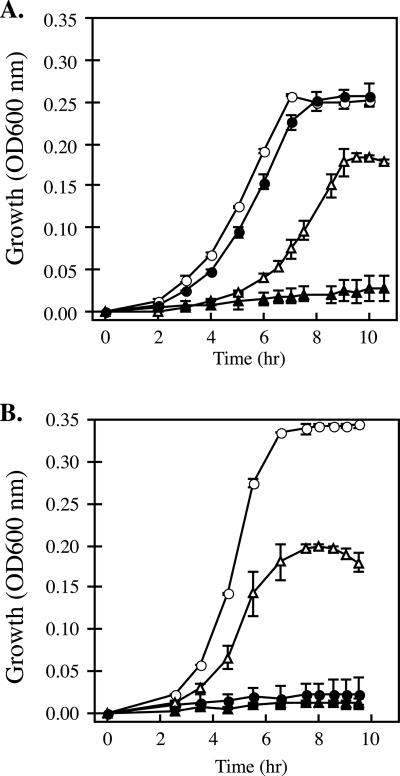
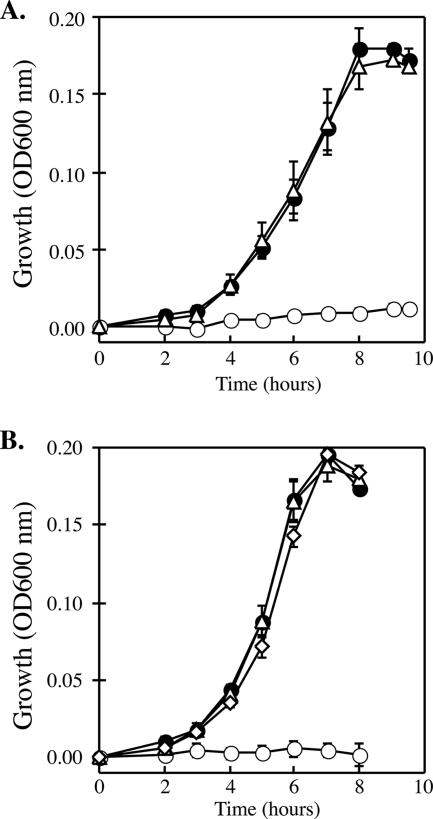
Because cymA has such a diverse roll in the respiration pathways of S. oneidensis, we predicted that similar roles for cymA would be observed in both ANA-3 and CN-32 and in arsenate respiration. To test this prediction, we constructed nonpolar deletions of the cymA gene in ANA-3 and CN-32. To generate a cymA null mutant in ANA-3, additional DNA flanking the cymA gene was sequenced from an inverse PCR product of a genome fragment containing the cymA gene. This was not necessary for CN-32, because the genome sequence was available at the time of this study. Strains lacking cymA were grown anaerobically on a variety of electron acceptors. The results are summarized in Table 2. Compared to wild-type strains of ANA-3 and CN-32 that normally reach stationary phase within 10 h, neither deletion strain was able to grow when As(V) was added as an electron acceptor (Fig. 3A and B). Additionally, AN-CYMA was unable to respire fumarate; however, CN-CYMA showed growth on fumarate similar to that of the wild-type strain when grown under similar conditions. No growth defects were observed in AN-CYMA compared to the wild type when grown on TMAO. The wild-type CN-32 did not utilize TMAO as an electron acceptor. To confirm that the phenotypes of the cymA null mutants were due to the loss of cymA, both deletion strains, CN-CYMA and AN-CYMA, were complemented in trans with pCNcymA (Fig. 4A) or pANcymA (Fig. 4B), respectively. When grown anaerobically on the same substrates, the complemented strains regained the ability to respire As(V). Moreover, heterologous complementation of pCNcymA in AN-CYMA and pANcymA in CN-CYMA showed full recovery of growth on all substrates. Strains harboring only the vector (pBBR1MCS-2) exhibited the same growth characteristics as their respective genetic backgrounds without the vector. These results provide evidence that cymA is essential to arsenate respiration and support the role of CymA as a universal redox mediator to various terminal electron acceptors in Shewanella.
TABLE 2.
Growth characteristics of AN-CYMA and CN-CYMA on known terminal electron acceptors
| Substrate | Growtha
|
|||||
|---|---|---|---|---|---|---|
| ANA-3
|
CN-32
|
MR-1b
|
||||
| ΔcymA | Wild type | ΔcymA | Wild type | ΔcymA | Wild type | |
| Arsenate | − | + | − | + | − | − |
| Fumarate | − | + | + | + | − | + |
| DMSO | − | − | ND | ND | − | + |
| Nitrate | + | + | + | + | − | + |
| TMAO | + | + | − | − | + | + |
| Thiosulfate | + | + | + | + | + | + |
| Oxygen | + | + | + | + | + | + |
| Fe(III) | − | + | − | + | − | + |
| Mn(IV) | − | + | − | + | − | + |
Plus indicates growth on or reduction [Fe(III) and Mn(IV)] of the substrate similar to the wild type. Minus indicates lack of growth on the corresponding substrate. Lactate (20 mM) served as the electron donor and carbon source. Terminal electron acceptors were included in the anaerobic medium at 10 mM. ND, not determined.
FIG. 3.
Anaerobic growth on arsenate or fumarate of wild-type and cymA null mutant (ΔcymA) (A) Shewanella putrafaciens strain CN-32 and CN-CYMA and (B) Shewanella sp. strain ANA-3 and AN-CYMA, respectively. The time course for growth was inferred from the optical density at 600 nm. •, ΔcymA grown on fumarate; ○, wild type grown on fumarate; ▴, ΔcymA grown on arsenate; ▵, wild-type grown on arsenate. The data points and error bars represent the means and standard deviations of triplicate cultures, respectively.
FIG. 4.
Restoration of growth on arsenate (10 mM) of Shewanella (A) CN-32 and (B) ANA-3 cymA null mutants (ΔcymA). The time course for growth was inferred from the optical density at 600 nm. •, wild-type strain; ○ ΔcymA null mutant with the vector (pBBR1MCS-2); ▵, ΔcymA null mutant with cymA on a plasmid; ⋄, heterologous complementation of AN-CYMA (ΔcymA) with pCNcymA. The data points and error bars represent the means and standard deviations of triplicate cultures, respectively.
cymA transcription patterns.
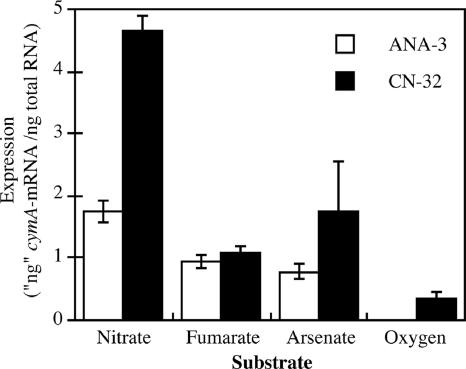
Having established that cymA is necessary for respiration of As(V) and fumarate (ANA-3 only), we investigated various growth conditions that would most likely affect the transcription of cymA in ANA-3 and CN-32. Total RNA was extracted from cultures grown to mid-log phase (OD at 600 nm, 0.1) on oxygen (vigorously sparged with air) and anaerobically with nitrate, fumarate, or As(V) as the terminal electron acceptor. cymA expression was higher in cells grown anaerobically than in cells grown aerobically (Fig. 5). When anaerobic growth conditions were compared, cymA expression was greater in samples grown in nitrate than in samples grown on fumarate or As(V). It was concluded that cymA expression is regulated on several levels, first by oxygen and secondly by the presence of specific terminal electron acceptors, mainly nitrate.
FIG. 5.
Effects of different electron acceptors on cymA transcription in Shewanella putrefaciens strain CN-32 and Shewanella sp. strain ANA-3. CN-32 and ANA-3 were grown on different electron acceptors to the mid-exponential phase of growth (OD at 600 nm, 0.1). The nanogram genomic equivalent of mRNA for cymA was normalized to the total RNA used in the cDNA reaction. The data points and error bars represent the means and standard deviations of triplicate cultures, respectively.
Mutations in heme motifs and their effects on arsenate respiration.
The mechanism of electron transfer from CymA to terminal reductases is unknown. Presumably, redox reactions involve electron transfer from heme cofactors to other redox mediators in the terminal reductases (e.g., Fe-S clusters or Mo cofactor). To further understand this mechanism, we investigated the role of each heme cofactor in growth on and reduction of arsenate. Four cymA alleles were generated by site-directed mutagenesis of one of the cysteine residues in the four CXXCH motifs. These were replaced with a codon encoding serine (Fig. 6A). The four plasmids containing different Cys-to-Ser mutations were transformed into CN-CYMA, and growth and reduction of arsenate were monitored over time. Growth and reduction of arsenate were affected to varying degrees in each mutant cymA strain (Fig. 6B). Wild-type CN-32 cultures containing only the vector reached stationary phase within 9 h, while CN-CYMA carrying various cymA heme mutations exhibited little growth during that time. During extended incubation, growth was observed in the heme variants at a reduced capacity compared to the wild type (Fig. 6B). Mutations in Cys-46 to Ser [pH 1(C46S)] had the least effect on growth compared to the other the three Cys-to-Ser mutations. The strain carrying this heme variant had a lower growth rate than the wild-type cymA; however, it eventually reached stationary phase by 28 h. The three other Cys-to-Ser mutations resulted in dramatically lower growth rates and decreased OD at 600 nm in stationary-phase growth. By the end of the 52 h, some growth was detected in CN-CYMA+pH 4(C173S), CN-CYMA+pBBR1MCS-2, CN-CYMA+pH 2(C78S), and CN-CYMA+pH 3(C136S). Reduction of arsenic occurred rapidly over the course of 10 h in CN-CYMA with a wild-type cymA gene. However, in the CN-CYMA strains harboring the various cymA heme mutations, arsenate reduction was considerably less than in the wild-type cymA plasmid strain (Table 3).
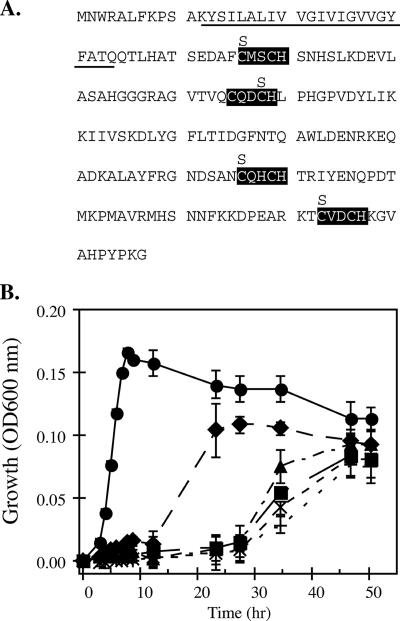
FIG. 6.
The locations of heme motifs, cysteine-to-serine substitutions, and the predicted alpha helix membrane-anchoring domain (underlined) in CymA (A) and growth on arsenate of four cymA heme mutants containing single cysteine-to-serine substitutions (B). The time course for growth was inferred from the optical density at 600 nm. •, CN-32 plus vector; ▪, CN-CYMA plus vector; ⧫, pH 1(C46S); ×, pH 2(C78S); +, pH 3(C136S); ▴, pH 4(C173S). The data points and error bars represent the means and standard deviations of triplicate cultures, respectively.
TABLE 3.
Physiological effects of S. putrefaciens CN-32 with cymA heme variants on the reduction of arsenatea
| Strain | Time (h) | Arsenate (mM) | Arsenite (mM) |
|---|---|---|---|
| All | 0 | 9.9 ± 0.22 | 0.23 ± 0.32 |
| CN-32 + pBBR1MCS-2 | 12.5 | 0.7 ± 0.11 | 9.7 ± 0.00 |
| 50.5 | 0 | 9.7 ± 0.06 | |
| CN-CYMA + pBBR1MCS-2 | 12.5 | 9.7 ± 0.14 | 0.12 ± 0.16 |
| 50.5 | 1.4 ± 0.21 | 8.6 ± 0.01 | |
| CN-CYMA + pH 1(C46S) | 12.5 | 9.2 ± 0.30 | 1.0 ± 0.27 |
| 27.5 | 0 | 10.2 ± 0.50 | |
| 50.5 | 0 | 9.8 ± 0.26 | |
| CN-CYMA + pH 2(C78S) | 12.5 | 10.1 ± 0.12 | 0.10 ± 0.12 |
| 50.5 | 1.5 ± 0.21 | 8.1 ± 0.13 | |
| CN-CYMA + pH 3(C136S) | 12.5 | 9.8 ± 0.03 | 0.18 ± 0.16 |
| 50.5 | 2.5 ± 0.36 | 7.2 ± 0.11 | |
| CN-CYMA + pH 4(C173S) | 12.5 | 9.9 ± 0.22 | 0.07 ± 0.05 |
| 50.5 | 1.4 ± 1.98 | 9.9 ± 0.61 |
The cultures represented in Fig. 6B were sampled at the indicated times, and the filtrates were analyzed by high-performance liquid chromatography for arsenate and arsenite. The data and errors represent the means and standard deviations of triplicate cultures, respectively.
DISCUSSION
Previous work with Shewanella sp. strain ANA-3 showed that arsenate respiration was conferred by arrA and arrB (28). The recent identification of arrAB operons in the genome sequences of two other Shewanella species strains, strains CN-32 and W3-18-1, suggested that these species should also respire arsenate, which should also be conferred by the arr operon. This was confirmed in CN-32 by deleting arrA, which eliminated growth on and reduction of arsenate (Fig. 1). Shewanella sp. strain W3-18-1 was also able to utilize arsenate (data not shown) as an electron acceptor, and it is predicted that deleting its arrA homolog would eliminate growth on arsenate.
One striking feature of the Shewanella arr operon is the lack of a gene encoding a membrane-anchoring subunit for ArrAB, “ArrC”; evidence for an ArrC is present in the genomes of several bacteria that contain arr genes (e.g., Wolinella succinogenes [locus tag, WS0763; accession no. NP_906979] and Desulfitobacterium hafniense strain DCB-2 [locus tag, Dhaf_0249; accession no. ZP_01372403]). This observation raised questions about how the soluble arsenate respiratory reductase, ArrAB, interacts with components of the electron transport chain. In Shewanella, this interaction is most likely mediated by the tetraheme c-type cytochrome CymA, which is tethered to the membrane by an N-terminal alpha helix (Fig. 6A). This conclusion is supported by gene deletion and complementation of cymA in two arsenate-respiring Shewanella strains, CN-32 and ANA-3 (Fig. 3 and 4). Null mutations of cymA showed that this gene was necessary for As(V) respiration. Heterologous complementation of the ANA-3 cymA null mutant with a CN-32 wild-type cymA gene (and vice versa) restored utilization of arsenate as a terminal electron acceptor in these strains. This observation is not surprising, because the genes are nearly identical; the nucleotide identity is ∼90% between the two cymA genes.
Consistent with previous studies of cymA (20, 23, 32), the substrate utilization patterns of Shewanella cymA null mutants exhibited pleiotropic effects on Fe(III) and Mn(IV) reduction (Table 2). However, deficiencies in fumarate and nitrate utilization differed among various Shewanella cymA null mutants (Table 2). Unlike in ANA-3 and MR-1, deleting cymA in CN-32 did not affect its growth on fumarate. A similar pattern was observed for nitrate respiration. ANA-3 and CN-32 cymA null mutant strains were capable of growing on nitrate; in contrast, MR-1 cymA null mutants cannot respire nitrate (23). A Blast search for the periplasmic nitrate reductase Nap showed that the CN-32, ANA-3, and W3-18-1 genomes contain two nap loci, whereas MR-1 had only one. This additional nap locus may not involve CymA as an intermediate electron carrier for that particular nitrate reductase. In MR-1, deletion of cymA also resulted in an inability to grow on fumarate (23), but this effect was not observed in CN-32. Additional genome analyses of ANA-3, CN-32, MR-1, and W3-18-1 revealed that CN-32 and W3-18-1 contain additional orthologs of fumarate reductases/succinate dehydrogenases, unlike ANA-3 and MR-1. Similar to the additional nap locus in several Shewanella, it is likely that reduction of fumarate by one of these additional fumarate reductases may not involve CymA.
Because cymA is required for growth on arsenate and other terminal electron acceptors, we investigated the environmental conditions that affected its expression. Previous studies had shown that the CymA protein, along with a number of other heme-containing proteins, was present in Shewanella membranes isolated under anaerobic growth conditions (7, 21-23). Quantification of cymA gene expression in ANA-3 confirmed that cymA was induced under anaerobic conditions (Fig. 5). The transcription patterns suggest that cymA is regulated in response to several environmental signals; oxygen, which causes repression; and the availability of specific electron acceptors, leading to induction. cymA mRNA was most abundant in nitrate-grown cells of ANA-3 and CN-32, even though cymA is not essential to nitrate reduction in these strains.
The functional roles of the four hemes of CymA were addressed by examining the involvement of each heme group in arsenate respiration (Fig. 6). It was hypothesized that mutations in any of the four hemes would significantly alter arsenate respiration in Shewanella. One of the cysteines of the CXXCH heme c binding motif was changed to serine. In each of the single heme mutations, growth on arsenate was significantly diminished but not entirely eliminated. We observed an increased lag in growth on arsenate (∼25 h) if the mutations occurred in the three distal CXXCH motifs nearest the C terminus of CymA. A mutation in the first CXXCH motif (heme 1) resulted in the least severe growth defect on arsenate (an ∼12-h lag) (Fig. 6B). Mutational studies with the CXXCH motifs in monoheme cytochromes have shown varying outcomes for physiology, which were generally attributed to the specific cytochrome maturation machinery employed by the cell (3). In Ccm-based maturation (biogenesis system I), monoheme cytochromes usually do not mature if one of the cysteines in the CXXCH motif is replaced (3, 30). Generally, the physiological effects of a cysteine mutation in CXXCH often eliminate the particular respiration in mutant backgrounds. However, the physiological outcomes of single cysteine mutations in CXXCH in multiheme cytochromes are not well studied. In the Wolinella succinogenes nitrite reductase, NrfH, seven out of eight individual cysteine replacements resulted in elimination of nitrite respiration (33). Interestingly, one of the heme mutants (C66S) contained a heme covalently linked to the apocytochrome. This was attributed to the function of cytochrome biogenesis system II, which is present in several gram-positive bacteria and several epsilon-proteobacteria, including Wolinella succinogenes. Mutations to the histidine in the CXXCH heme binding motifs eliminate heme attachment, which usually eliminates the particular respiration (5, 9). In multiheme cytochromes, mutations in either of the cysteine or histidine residues of one CXXCH motif are likely to affect the structure and conformation of the protein. The absence of one heme could cause distortion in the packing of neighboring hemes, which could lead to secondary effects, such as disrupting a quinone-binding site and/or altering the midpoint potentials of the remaining hemes. It is doubtful that the Ccm biogenesis system (type I) of Shewanella can still attach hemes to variants of CymA containing single cysteine-to-serine mutations (italic) in the heme c binding motif (SXXCH or CXXSH). However, the unaltered CXXCH motifs in CymA may still contain covalently linked hemes. Further investigations should uncover these molecular details.
The interactions of CymA with ArrAB most likely extend to other terminal reductases (e.g., periplasmic nitrate and fumarate reductases). Such interactions may require conserved domains on both CymA and the cognate redox partner of the terminal reductase. Whether these same interactions and domains occur among similar terminal reductases in non-arsenate-respiring Shewanella species strains (e.g., ANA-3 versus MR-1) and more broadly in arsenate-respiring non-Shewanella prokaryotes remains unknown. The conservation of CymA at the sequence and molecular levels (Fig. 2) and its physiological role in anaerobic respiration suggest that Shewanella has evolved to streamline a number of respiratory pathways by simplifying the branching point of the electron transport chain. The origin of CymA and its mechanisms of interaction with other redox partners are largely unknown.
Supplementary Material
Acknowledgments
We thank the three anonymous reviewers for their constructive comments on the manuscript during the review process. We also thank Jizhong Zhou at the University of Oklahoma for providing us with Shewanella sp. strain W3-18-1.
Footnotes
Published ahead of print on 5 January 2007.
REFERENCES
- 1.Afkar, E., J. Lisak, C. Saltikov, P. Basu, R. S. Oremland, and J. F. Stolz. 2003. The respiratory arsenate reductase from Bacillus selenitireducens strain MLS10. FEMS Microbiol. Lett. 226:107-112. [DOI] [PubMed] [Google Scholar]
- 2.Ahmann, D., L. R. Krumholz, H. F. Hemond, D. R. Lovley, and F. M. Morel. 1997. Microbial mobilization of arsenic from sediments of the Aberjona watershed. Environ. Sci. Technol. 31:2923-2930. [Google Scholar]
- 3.Allen, J. W., and S. J. Ferguson. 2006. What is the substrate specificity of the System I cytochrome c biogenesis apparatus? Biochem. Soc. Trans. 34:150-151. [DOI] [PubMed] [Google Scholar]
- 4.Appelo, C. A., M. J. Van Der Weiden, C. Tournassat, and L. Charlet. 2002. Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic. Environ. Sci. Technol. 36:3096-3103. [DOI] [PubMed] [Google Scholar]
- 5.Cartron, M. L., M. D. Roldan, S. J. Ferguson, B. C. Berks, and D. J. Richardson. 2002. Identification of two domains and distal histidine ligands to the four haems in the bacterial c-type cytochrome NapC; the prototype connector between quinol/quinone and periplasmic oxido-reductases. Biochem. J. 368:425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich, W., and O. Klimmek. 2002. The function of methyl-menaquinone-6 and polysulfide reductase membrane anchor (PsrC) in polysulfide respiration of Wolinella succinogenes. Eur. J. Biochem. 269:1086-1095. [DOI] [PubMed] [Google Scholar]
- 7.Field, S. J., P. S. Dobbin, M. R. Cheesman, N. J. Watmough, A. J. Thomson, and D. J. Richardson. 2000. Purification and magneto-optical spectroscopic characterization of cytoplasmic membrane and outer membrane multiheme c-type cytochromes from Shewanella frigidimarina NCIMB400. J. Biol. Chem. 275:8515-8522. [DOI] [PubMed] [Google Scholar]
- 8.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, H. L. Dong, T. C. Onstott, N. W. Hinman, and S. M. Li. 1998. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 62:3239-3257. [Google Scholar]
- 9.Gross, R., R. Eichler, and J. Simon. 2005. Site-directed modifications indicate differences in axial haem c iron ligation between the related NrfH and NapC families of multihaem c-type cytochromes. Biochem. J. 390:689-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey, C. F., C. H. Swartz, A. B. Badruzzaman, N. Keon-Blute, W. Yu, M. A. Ali, J. Jay, R. Beckie, V. Niedan, D. Brabander, P. M. Oates, K. N. Ashfaque, S. Islam, H. F. Hemond, and M. F. Ahmed. 2002. Arsenic mobility and groundwater extraction in Bangladesh. Science 298:1602-1606. [DOI] [PubMed] [Google Scholar]
- 11.Islam, F. S., A. G. Gault, C. Boothman, D. A. Polya, J. M. Charnock, D. Chatterjee, and J. R. Lloyd. 2004. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68-71. [DOI] [PubMed] [Google Scholar]
- 12.Kneebone, P. E., P. A. O'Day, N. Jones, and J. G. Hering. 2002. Deposition and fate of arsenic in iron- and arsenic-enriched reservoir sediments. Environ. Sci. Technol. 36:381-386. [DOI] [PubMed] [Google Scholar]
- 13.Kostka, J., and K. H. Nealson. 1998. Isolation, cultivation and characterization of iron- and manganese-reducing bacteria, p. 58-78. In R. S. Burlage, R. Atlas, D. Stahl, G. Geesey, and G. Sayler (ed.), Techniques in microbial ecology. Oxford, New York, NY.
- 14.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop, and K. M. Peterson. 1994. pBBR1MCS—a broad-host-range cloning vector. BioTechniques 16:800. [PubMed] [Google Scholar]
- 15.Krafft, T., and J. M. Macy. 1998. Purification and characterization of the respiratory arsenate reductase of Chrysiogenes arsenatis. Eur. J. Biochem. 255:647-653. [DOI] [PubMed] [Google Scholar]
- 16.Macy, J. M., J. M. Santini, B. V. Pauling, A. H. O'Neill, and L. I. Sly. 2000. Two new arsenate/sulfate-reducing bacteria: mechanisms of arsenate reduction. Arch. Microbiol. 173:49-57. [DOI] [PubMed] [Google Scholar]
- 17.Malasarn, D., C. W. Saltikov, K. M. Campbell, J. M. Santini, J. G. Hering, and D. K. Newman. 2004. arrA is a reliable marker for As(V) respiration. Science 306:455. [DOI] [PubMed] [Google Scholar]
- 18.McEwan, A. G., J. P. Ridge, C. A. Mcdevitt, and P. Hugenholtz. 2002. The DMSO reductase family of microbial molybdenum enzymes; molecular properties and role in the dissimilatory reduction of toxic elements. Geomicrobiol. J. 19:3-21. [Google Scholar]
- 19.Murray, A. E., D. Lies, G. Li, K. Nealson, J. Zhou, and J. M. Tiedje. 2001. DNA/DNA hybridization to microarrays reveals gene-specific differences between closely related microbial genomes. Proc. Natl. Acad. Sci. USA 98:9853-9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers, C. R., and J. M. Myers. 1997. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J. Bacteriol. 179:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers, C. R., and J. M. Myers. 1992. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers, C. R., and J. M. Myers. 1997. Outer membrane cytochromes of Shewanella putrefaciens MR-1: spectral analysis, and purification of the 83-kDa c-type cytochrome. Biochim. Biophys. Acta 1326:307-318. [DOI] [PubMed] [Google Scholar]
- 23.Myers, J. M., and C. R. Myers. 2000. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J. Bacteriol. 182:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oremland, R. S., and J. F. Stolz. 2005. Arsenic, microbes and contaminated aquifers. Trends Microbiol. 13:45-49. [DOI] [PubMed] [Google Scholar]
- 25.Oremland, R. S., and J. F. Stolz. 2003. The ecology of arsenic. Science 300:939-944. [DOI] [PubMed] [Google Scholar]
- 26.Roldan, M. D., H. J. Sears, M. R. Cheesman, S. J. Ferguson, A. J. Thomson, B. C. Berks, and D. J. Richardson. 1998. Spectroscopic characterization of a novel multiheme c-type cytochrome widely implicated in bacterial electron transport. J. Biol. Chem. 273:28785-28790. [DOI] [PubMed] [Google Scholar]
- 27.Saltikov, C. W., A. Cifuentes, K. Venkateswaran, and D. K. Newman. 2003. The ars detoxification system is advantageous but not required for As(V)-respiration by the genetically tractable Shewanella species, strain ANA-3. Appl. Environ. Microbiol. 69:2800-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saltikov, C. W., and D. K. Newman. 2003. Genetic identification of a respiratory arsenate reductase. Proc. Natl. Acad. Sci. USA 100:10983-10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saltikov, C. W., R. A. Wildman, Jr., and D. K. Newman. 2005. Expression dynamics of arsenic respiration and detoxification in Shewanella sp. strain ANA-3. J. Bacteriol. 187:7390-7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambongi, Y., R. Stoll, and S. J. Ferguson. 1996. Alteration of haem-attachment and signal-cleavage sites for Paracoccus denitrificans cytochrome C550 probes pathway of c-type cytochrome biogenesis in Escherichia coli. Mol. Microbiol. 19:1193-1204. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Schwalb, C., S. K. Chapman, and G. A. Reid. 2003. The tetraheme cytochrome CymA is required for anaerobic respiration with dimethyl sulfoxide and nitrite in Shewanella oneidensis. Biochemistry 42:9491-9497. [DOI] [PubMed] [Google Scholar]
- 33.Simon, J., R. Eichler, R. Pisa, S. Biel, and R. Gross. 2002. Modification of heme c binding motifs in the small subunit (NrfH) of the Wolinella succinogenes cytochrome c nitrite reductase complex. FEBS Lett. 522:83-87. [DOI] [PubMed] [Google Scholar]
- 34.Zachara, J. M., J. K. Fredrickson, S. M. Li, D. W. Kennedy, S. C. Smith, and P. L. Gassman. 1998. Bacterial reduction of crystalline Fe3+ oxides in single phase suspensions and subsurface materials. Am. Mineral. 83:1426-1443. [Google Scholar]
- 35.Zobrist, J., P. R. Dowdle, J. A. Davis, and R. S. Oremland. 2000. Mobilization of arsenite by dissimilatory reduction of adsorbed arsenate. Environ. Sci. Technol. 34:4747-4753. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.