Abstract
Arrays of octameric peptide libraries on cellulose paper were screened by using 32P-autophosphorylated cGMP-dependent protein kinase Iα (cGPK) to identify peptide sequences with high binding affinity for cGPK. Iterative deconvolution of every amino acid position in the peptides identified the sequence LRK5H (W45) as having the highest binding affinity. Binding of W45 to cGPK resulted in selective inhibition of the kinase with Ki values of 0.8 μM and 560 μM for cGPK and cAMP-dependent protein kinase (cAPK), respectively. Fusion of W45 to membrane translocation signals from HIV-1 tat protein (YGRKKRRQRRRPP-LRK5H, DT-2) or Drosophila Antennapedia homeo-domain (RQIKIWFQNRRMKWKK-LRK5H, DT-3) proved to be an efficient method for intracellular delivery of these highly charged peptides. Rapid translocation of the peptides into intact cerebral arteries was demonstrated by using fluorescein-labeled DT-2 and DT-3. The inhibitory potency of the fusion peptides was even greater than that for W45, with Ki values of 12.5 nM and 25 nM for DT-2 and DT-3, respectively. Both peptides were still poor inhibitors of cAPK. Selective inhibition of cGPK by DT-2 or DT-3 in the presence of cAPK was demonstrated in vitro. In pressurized cerebral arteries, DT-2 and DT-3 substantially decreased NO-induced dilation. This study provides functional characterization of a class of selective cGPK inhibitor peptides in vascular smooth muscle and reveals a central role for cGPK in the modulation of vascular contractility.
Keywords: protein kinase inhibitor, combinatorial libraries, SPOT method, membrane translocation signal, smooth muscle
The cGMP-dependent protein kinases type Iα and Iβ (cGPK) act directly downstream in the NO-mediated signaling pathway, controlling a variety of cellular responses, ranging from smooth muscle cell relaxation to neuronal synaptic plasticity (1, 2). The structural similarity of cGPK and its closest relative, the cAMP-dependent protein kinase (cAPK), has made it difficult to study cGPK pathways independent of those mediated by cAPK, primarily because of the lack of potent and selective cGPK inhibitors. Because recent studies have suggested that cAMP and cGMP are each able to cross-activate either cGPK or cAPK under physiological conditions, the specific role for cGPK within the NO/cGMP-mediated signaling pathway remains obscure (for a review see ref. 1). However, recent advances have clearly identified specific intracellular targets for the cGPK isozymes Iα and Iβ (3, 4). Also, inactivation of the genes for cGPK Iα/Iβ and cGPK II showed that the cGPK isozymes regulate distinct cellular functions by pathways separate from those mediated by cAPK (5, 6).
Attempts to identify cGPK-selective inhibitor peptides based on the autoinhibitory domain of the enzyme or in vivo substrates have been tedious at best, because of the lack of a well defined consensus sequence. Only a relative preference for basic residues surrounding the phosphate acceptor site has been established (7). Various synthetic peptides have been used to analyze the sequence requirements for cGPK substrates (8–11). Recently, we developed an iterative approach using phosphorylation of peptide libraries on cellulose paper to determine a priori the substrate specificity of cGPK versus cAPK. Consequently, we identified the cGPK substrate sequence TQAKRKKSLAMFLR, in which the serine represents the phosphate-acceptor site (12, 13). Substitution of this serine by alanine yielded cGPK inhibitors with Ki values of 7.5–22 μM (13) and improved cGPK/cAPK selectivity, as has been reported with other synthetic peptide derivatives (14, 15). However, all cGPK peptide inhibitors known so far lack satisfactory potency and selectivity.
Here we report a peptide library screen specifically designed to select for tight binding peptides rather than substrate peptides. First, we took advantage of the autophosphorylation properties of cGPK, which provides the means to study the transient enzyme–peptide interactions. Second, we used peptide libraries that lack the phosphate acceptor residues serine and threonine to select for peptide binding over phosphorylation. Linking the best sequence from this screen to membrane translocation signals (MTS) for intracellular delivery resulted in the highly effective cGPK Iα inhibitors DT-2 and DT-3. Finally, we have demonstrated that both peptides are powerful tools for studying the specific functional roles of cGPK in smooth muscle.
Methods
Enzyme Preparations.
cAPK-Cα was expressed and purified from Escherichia coli (16), and cGPK type Iα was expressed and purified from SF9-insect cells as described in ref. 13. 32P-labeling of cGPK was accomplished by incubating the enzyme in buffer A (50 mM Mops, pH 6.9/0.4 mM EDTA/1 mM Mg-acetate/200 mM NaCl/1 mg/ml BSA/10 mM DTT) and [γ-32P]-ATP (0.1 mM; specific activity 1,600 cpm/pmol) for 2 h at room temperature. Excess label was removed by using G-25 Sepharose chromatography, and SDS/PAGE and Western blotting were performed (17).
Synthesis of Peptide Libraries and cGPK Screening.
The peptide arrays on paper (SPOTs) were generated as described in refs. 12, 13, and 18. Ser and Thr were omitted from the defined and the randomized positions resulting in arrays of 18 × 18 sublibrary spots. The following arrays were generated and used successively in the iterative approach: 1, XXX12XXX; 2, XXXRK12X; 3, XRKKK12X; 4, 1RKKKKK2; 5, LRKKKKKH12; 6, 12LRKKKKKH (with “1” and “2” denoting defined amino acids and “X” denoting the mixture of natural amino acids excluding Ser and Thr). Sublibraries from each generation with the highest binding affinity were assembled in 5 to 10 copies each, which were then used to verify the previous results. The library membranes were blocked with BSA (13). After addition of 1 nmol 32P-labeled cGPK, the libraries were agitated for 12 h at room temperature in buffer A and 0.1 mM ATP. The papers were washed 5–10 times with buffer A and analyzed by using a 32P-imaging system (12, 13).
Synthesis of Soluble Peptides and Determination of Inhibition Constants.
Synthesis, purification, and characterization of soluble peptides for kinetic determinations were carried out as described in refs. 12 and 13. Cysteine-containing peptides were prepared by using fluorenylmethoxycarbonyl-Cys(Trt)-OH. All peptides were prepared as C-terminal amides. The concentrations of the stock solutions were determined by quantitative amino acid analyses. Fluorescein peptide labeling was carried out by incubating 10 mg of peptide, containing an extra Cys followed by a β-Ala at the N terminus, in 1 ml of 1 M phosphate buffer (pH 7.4) with 60 μl of a 0.1 M stock solution of fluorescein-5-maleimide (Molecular Probes) in DMSO at 4°C overnight in the dark. The mono-labeled products were purified from excess reagent, and multiple labeled compounds were separated by preparative HPLC (acetonitrile/0.5% trifluoroacetic acid gradients). Peptide analysis was carried out by matrix-assisted laser desorption ionization-MS. Kinetic constants (Km, Vmax, Ki) were determined by an adapted phospho-cellulose assay (19) as described (13).
cGPK Assays in Cultured Vascular Smooth Muscle Cells.
Human aortic smooth muscle cells (HuASM; Clonetics, San Diego) were cultured in smooth muscle cell basal medium (SmBM; Clonetics) containing 0.5 μg/ml hEGF, 2.5 μg/ml insulin, 0.5 ng/ml hFGF, 50 μg/ml gentamicin, 50 μg/ml amphotericin-B, 1% FBS. Cells were used at passage 2–5. To increase endogenous cGPK activity, HuASM cells (75% confluent) were transfected by using 5 μg pMT3-cGPK Iα vector and 15 μl of Fugene (Roche Molecular Biochemicals) in 800 μl of serum-free medium per 10-cm plate for 15–30 min. Cells were harvested after 48 h. For each experiment, two dishes were incubated with 50 μM W45, DT-2, DT-3, DT-5, DT-6, or buffer for 60 min; the cells washed, trypsinized, pelleted, and homogenized in cold lysis buffer (50 mM KPO4, pH 6.5/10 mM DTT/10 mM benzamidin/5 mM EDTA/5 mM EGTA/5 mg/ml Nα-(p-tosyl)lysine chloromethyl ketone/10 mg/ml l-1-tosylamido-2-phenylethyl chloromethyl ketone/17 mg/ml PMSF/2 mg/ml soybean trypsin inhibitor/25 μg/ml antipain). The homogenate was then centrifuged and kinase activity in the supernatant was quickly determined. Endogenous cAPK activity was suppressed by the addition of 70 nM protein kinase inhibitor (PKI)(15–24) in all assays and assay cyclic nucleotide concentrations were 1 μM.
Fluorescence Imaging of Vascular Smooth Muscle Cells in Intact Arteries.
Isolated intact cerebral arteries were incubated with fluorescein-labeled peptides (2 μM) in Hepes buffer (10 mM Hepes, pH 7.4/6 mM KCl/140 mM NaCl/2 mM CaCl2/1 mM MgCl2/10 mM glucose) for 3–30 min at room temperature. Arteries were washed 3 to 5 times and examined with a Bio-Rad MRC 1024ES confocal scanning laser microscopy system. Fluorescein excitation was imaged with the 488-nm line of a krypton–argon laser. All images were captured with a 100× oil immersion objective lense (NA = 1.3) mounted on an Olympus BX50 (New Hyde Park, NJ) upright microscope. Optical thickness was approximately 1.5 μm. Single optical sections were acquired with seven Kalman averages.
Diameter Measurements in Isolated Pressurized Cerebral Arteries.
Small posterior cerebral arteries (internal diameter ≈150 μm) from euthanized rats were isolated in cold bicarbonate buffered physiological salt solution (PSS) and denuded of endothelium by placing an air bubble in the lumen for 2 min. Artery segments were cannulated on glass pipettes, pressurized to 20 mmHg (1 mmHg = 133 Pa) and superfused with warmed (37°C), gassed (95% O2/5% CO2) PSS. Arterial diameter was measured by using a video dimension analyzer as described in refs. 20 and 21. The vessels were subsequently pressurized to 80 mmHg, which induced sustained myogenic tone. Additional agents (peptides, vasodilators) were applied to the arteries via the superfusate. For these experiments, endothelium removal was confirmed by the lack of 10 μM acetylcholine-mediated vasodilation, and maximal arterial diameters were obtained via treatment with the Ca2+ channel blocker nisoldipine (10 μM).
Results
Affinity Selection of Inhibitors for cGPK from Peptide Library Arrays.
We constructed peptide libraries without Ser and Thr and monitored the binding of 32P-autophosphorylated cGPK Iα as described in Methods (Fig. 1). By using this approach, we took advantage of the autophosphorylation properties of cGPK, which does not alter the catalytic constant (kcat) of the enzyme (22). The octameric library array XXX12XXX revealed strong binding to the kinase with the amino acid combinations RR, KR, and RK at positions 1 and 2. The RK motif gave the strongest signal (Fig. 1A). For this library and all subsequent libraries, we reexamined and verified the strongest binding motifs by synthesizing 5–10 copies for each combination (data not shown). The second-generation library with the structure XXXRK12X identified unambiguously the combination KK as the strongest binding motif (Fig. 1B). In the third library XRKKK12X, again KK was favored, although other lysine-containing combinations (AK, WK, KF) were also selected by cGPK (Fig. 1C). To demonstrate that the phosphorescence imaging signals we observed were caused by 32P-labeled cGPK and not 32P-ATP contamination, individual sublibrary spots (red numbers in Fig. 1C) were excised from the membrane, and bound material eluted with SDS/PAGE sample buffer. Western blot analysis of this material by using an anti-cGPK Iα/Iβ antibody (ref. 23; Fig. 1D) confirmed the presence of the enzyme. In addition, phosphorescence imaging of the same blot identified the kinase as 32P-labeled (Fig. 1E). In the fourth library, 1RKKKKK2, cGPK strongly selected Leu at position 1 and His at position 2 (Fig. 1F). C- and N-terminal extended libraries identified more hydrophobic residues surrounding the cluster of positive residues (data not shown), with the dodecamer FLLRKKKKKHHK as the longest peptide included in our search.
Figure 1.
Evolution of peptide libraries for the iterative screening of cGPK binding sequences. A, B, C, and F show phosphorescence images of consecutive generations of the library screens after binding of 32P-labeled cGPK. Each library membrane carries 18 × 18 spots, which resulted from substitutions of 18 amino acids (Ser and Thr were omitted) at positions 1 and 2 in the peptides, both positions being varied in alphabetical order according to the single letter code. The best combinations are indicated with arrows. (D) Western blot of material eluted from excised sublibrary spots (red numbers in C) by using a cGPK I-specific antibody. (E) PhosphorImage of the blot shown in D.
Kinetic Analysis of Synthetic Peptide Inhibitors and Fusion to Membrane Translocation Sequences.
Dixon plot analysis for cGPK inhibition by the synthetic peptides was performed by using the peptide TQARKKSLAMA as substrate (13). A summary of the inhibition constants is shown in Table 1. The octamer LRK5H (W45) derived from the fourth-generation library (Fig. 1E) was a potent and selective competitive inhibitor of cGPK. Furthermore, W45 showed clearly enhanced inhibitory potency when compared with WW21, a dodecameric peptide derived from cGPK substrate libraries (13). N- and C-terminal extensions in fifth- and sixth-generation libraries did not provide peptides with improved Ki values (W74 and W75, Table 1). Ala scanning analysis of W45 demonstrated that all five lysines are required for optimal inhibition. However, the peptide LRKKKAKH (9W31) showed the smallest reduction of inhibitory potency (Ki = 5.3 μM), suggesting that the fourth Lys in W45 occupies the phosphorylation site of cGPK. To probe further the apparent need of cGPK for lysines, we synthesized the peptide polylysine (K9) and found that it still inhibits cGPK but with diminished inhibitory potency (Ki ≈ 10 μM). Also, we tested polycations such as spermine and spermidine and found that they did not inhibit cGPK.
Table 1.
Inhibition constants (Ki) of synthetic peptides for cAPK and cGPK
| No. | Sequence | cGPKKi, μM | cAPKKi, μM | cAPK/cGPK |
|---|---|---|---|---|
| WW21 | TQAKRKKALAMA* | 7.5 | 750 | 1.0 × 102 |
| W45 | LRKKKKKH | 0.82 ± 0.33 (10) | 559 ± 108 (4) | 6.8 × 102 |
| 9W28 | LRAKKKKH | 14.5 ± 4.9 (3) | ND | — |
| 9W29 | LRKAKKKH | 11.3 ± 2.6 (3) | ND | — |
| 9W30 | LRKKAKKH | 9.9 ± 2.6 (3) | ND | — |
| 9W31 | LRKKKAKH | 5.3 ± 1.9 (3) | ND | — |
| 9W32 | LRKKKKAH | 46.1 ± 5.5 (3) | ND | — |
| W74 | FLLKKKKKKGHK | 1.2 ± 0.16 (6) | ND | — |
| W75 | FLLKKKKKKHHK | 1.2 ± 0.38 (5) | ND | — |
| DT-3 | RQIKIWFQNRRMKWKK-LRKKKKKH | 0.025 ± 0.009 (8) | 493 ± 86 (3) | 1.97 × 104 |
| DT-5 | RQIKIWFQNRRMKWKK | 0.97 ± 0.24 (4) | 107 ± 32 (4) | 1.10 × 102 |
| DT-2 | YGRKKRRQRRRPP-LRKKKKKH | 0.0125 ± 0.003 (5) | 16.5 ± 3.8 (4) | 1.32 × 103 |
| DT-6 | YGRKKRRQRRRPP | 1.1 ± 0.22 (3) | 26 ± 4 (4) | 2.36 × 101 |
The number of experiments for each Ki are given in brackets. ND, not determined.
Taken from ref. 13.
To allow internalization of the highly charged peptide W45 into live cells, we used two MTS sequences, one from the HIV-1 Tat protein (amino acids 47–59; refs. 24 and 25) and the other from the Drosophila Antennapedia homeodomain (amino acids 43–58; ref. 26). N-terminal fusion of either MTS sequences to W45 resulted in the competitive inhibitors DT-2 and DT-3. Both peptides showed potentiated inhibitory potencies with Ki values 40 to 80 fold lower than W45 (Table 1). Interestingly, the fusion peptides alone had significant cGPK inhibitory activity (Table 1).
Intracellular Delivery into Smooth Muscle Cells and Intact Arteries.
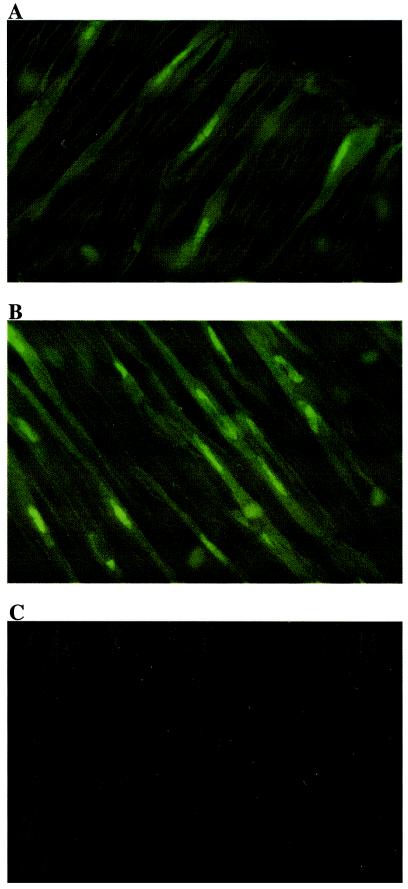
The internalization of DT-2 and DT-3 into living cells was monitored by using fluorescein-labeled peptides (Fluo-DT-2/3). Fig. 2 shows segments of a cerebral artery from rat, stained for 30 min with Fluo-DT-2 (Fig. 2A), Fluo-DT-3 (Fig. 2B), or Fluo-W45 (Fig. 2C). The labeled peptide analogs of DT-2 and DT-3 were rapidly internalized and distributed through the cytosol and nuclei in a time-dependent manner, whereas Fluo-W45 did not noticeably penetrate through the plasma membrane.
Figure 2.
Translocation of fluorescein-labeled MTS fusion peptides (Fluo-DT-2 and Fluo-DT-3) in smooth muscle cells from cerebral arteries. The arteries were isolated from rat and incubated for 30 min with Fluo-DT-2 (A), Fluo-DT-3 (B), or Fluo-W45 (C). Confocal images (100×) of tissue samples were taken after washing arteries with physiological salt solution. Fluorescein excitation was imaged at 488 nm. Images show the smooth muscle layer of the arteries (optical thickness = 1.5 μm).
Selective Inhibition of cGPK Over cAPK in Vitro.
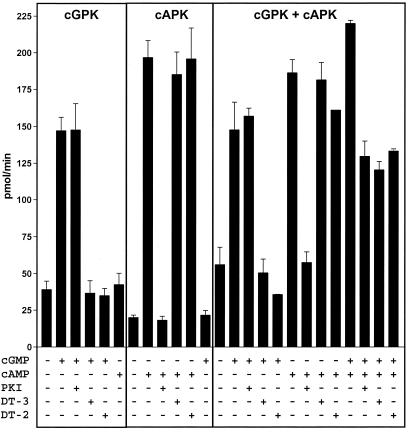
To demonstrate that DT-2 and DT-3 are both capable of inhibiting cGPK under conditions where the cAPK-selective inhibitor PKI(5–24) and/or cAPK are present, we established an in vitro reconstitution assay (Fig. 3). In this assay, we used purified recombinant cAPK and cGPK at low concentrations (1 nM) and chose cyclic nucleotide concentrations of 1 μM, conditions under which cAMP will activate only cAPK and cGMP will activate only cGPK. Also, we selected concentrations for the inhibitors DT-2, DT-3, and PKI that should selectively inhibit only cGPK or cAPK, respectively. Fig. 3 shows that cGPK and cAPK are stimulated only by their specific agonists (cGMP or cAMP) and inhibited only by their specific inhibitors (DT-2/3 or PKI). In a mixture of both enzymes, this result could be verified, which means that cGMP-stimulated cGPK was inhibited only by DT-2/3 and cAMP stimulated cAPK only by PKI. When both enzymes were activated with cGMP and cAMP in the mixture, a differential response of approximately 50% inhibition was observed with either DT-2/3 or PKI.
Figure 3.
Differential inhibition of recombinant cGPK and cAPK by DT-3, DT-2, and PKI(5–24). The assays contained 1 nM enzyme, 16 μM substrate peptide TQAKRKKSLAMA (13), 1 μM cAMP/cGMP, 70 nM PKI(5–24), and 200 nM DT-2 or DT-3, as indicated. Kinase activity was determined for 1.5 min at 30°C in a final volume of 100 μl as described (13).
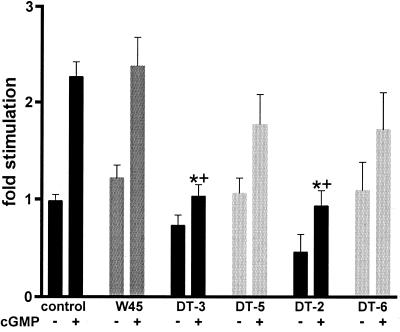
To establish the ability of DT-2 and DT-3 to inhibit cGPK in intact cells, human aortic smooth muscle cells were incubated with DT-2, DT-3, or control peptides W45, DT-5, and DT-6 for 60 min. Cells were then harvested, washed, and homogenized, and a phosphoryltransferase assay was performed. Fig. 4 shows that only preincubation with DT-3 or DT-2 caused inhibition of cGMP-stimulated cGPK activity. Preincubation with W45 or the control peptides DT-5 and DT-6 showed no significant effect, demonstrating that the MTS sequence was necessary for efficient uptake into the cells leading to inhibition of endogenous cGPK activity. To observe the effects shown in Fig. 4, we had to use high concentrations (50 μM) of peptides, because the cytosolic fractions were diluted 500-fold in the enzyme assay buffer. In addition, endogenous cAPK activity was inhibited by the addition of 70 nM PKI(5–24).
Figure 4.
Inhibition of endogenous cGPK activity in human aortic smooth muscle cells by internalization of exogenous MTS-fusion peptides DT-2 and DT-3. Cells were preincubated with either W45, DT-5, DT-3, DT-6, or DT-2, and then cell extracts were assayed for kinase activity (n = 3–6). Endogenous cAPK activity was blocked with PKI(5–24). * and + indicate significant differences (P < 0.05, ANOVA followed by Bonferroni post hoc test) from the untreated and W45 treated control groups, respectively.
Functional Antagonism of NO-Mediated Vasodilation.
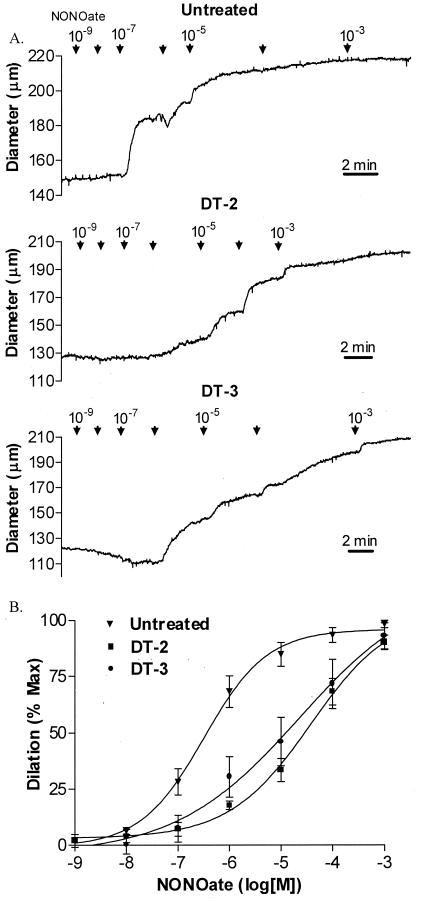
To evaluate the physiological effects of DT-2 and DT-3 as selective cGPK inhibitors in smooth muscle further, we studied their effects on NO-induced vasodilation in intact cerebral arteries. The NO donor NONOate elicited concentration-dependent vasodilation. Pretreatment of arteries with the cGPK inhibitors DT-2 or DT-3 for 20 min substantially impaired NO-mediated vasodilation (Fig. 5). DT-2 (1 μM) significantly (P < 0.05) increased the EC50 for NONOate (47 ± 9 μM, n = 6) compared with untreated vessels (0.43 ± 0.15 μM, n = 11) or vessels treated with the HIV tat carrier sequence DT-6 alone (0.92 ± 0.67 μM, n = 5). Similarly, DT-3 (0.1 μM) significantly (P < 0.05) increased the NONOate EC50 (34 ± 18 μM, n = 6) compared with untreated vessels or vessels treated with the Antennapedia carrier sequence DT-5 alone (0.13 ± 0.09 μM, n = 5). NO responses in arteries treated with the carrier sequences (DT-6 and DT-5) were not different from those in untreated arteries. In all cases, the fusion peptides or carrier peptides alone caused slight to moderate increases (<10%) in vascular tone. Table 2 summarizes the effects of peptide treatment on NO-induced vasodilation. Collectively, these data indicate that the combination peptides DT-2 and DT-3 translocate into smooth muscle cells in intact arteries and effectively inhibit NO-induced vasodilation, presumably by inhibiting cGPK.
Figure 5.
Inhibition of NO-mediated vasodilation of intact cerebral arteries by DT-2 and DT-3. Pressurized segments of rat posterior cerebral artery were dilated with the NO donor NONOate (1 nM to 1 mM, indicated by arrows) in the presence or absence of the fusion peptides DT-2 and DT-3 for 25 min. (A) Continuous diameter tracings are shown for untreated, DT-2 treated, and DT-3 treated vessels. (B) NONOate dose–response curves in untreated arteries (n = 11) or arteries exposed to DT-2 (n = 6) or DT-3 (n = 6).
Table 2.
Summary of peptide effects on NONOate mediated vasodilation
| Peptide | NO EC50 (μM) |
|---|---|
| Untreated | 0.43 ± 0.15 |
| DT-2 | 47 ± 9*† |
| DT-3 | 34 ± 18*‡ |
| DT-6 | 0.92 ± 0.67 |
| DT-5 | 0.13 ± 0.09 |
*, Indicates significant difference (P < 0.05) from the untreated group, whereas
and
indicate significant difference (P < 0.05) from DT-6 and DT-5, respectively.
Discussion
The SPOT method has been used successfully to identify substrate sequence motifs for a number of protein kinases (27, 28). In this study, we have expanded the practical utility of the SPOT method and demonstrated that peptide libraries specifically designed to screen for binding combinations lead to peptides that are potent inhibitors of cGPK. Furthermore, the peptide with the highest inhibitory potency (W45) showed unprecedented selectivity for cGPK over cAPK. Interestingly, the cGPK isoform Iβ was inhibited by W45 in much the same way as the Iα isoform, whereas cGPK II was relatively insensitive (≈100 fold) to these inhibitors (data not shown). These results clearly demonstrate the power of the strategies and approaches we have used to develop novel cGPK inhibitors.
To deliver the highly charged peptide W45 into living cells, we synthesized two fusion peptides, DT-2 and DT-3, with MTS sequences from the tat protein and from the antennapedia homeodomain. Cellular internalization of the fusion peptides was extensive (Fig. 2). Interestingly, the inhibitory potencies of DT-2 and DT-3 were profoundly enhanced compared with the inhibitory potency of W45 alone. However, the explanation for this synergism is presently unclear. The MTS sequences (DT-5 and DT-6), perhaps because they are positively charged themselves, inhibited cGPK and cAPK (Table 1). The nature of the cGPK inhibition by these peptides was of a linear mixed competitive/noncompetitive type (Dixon plot analysis, data not shown), involving two different and mutually exclusive binding sites. This finding suggests that the membrane translocation peptides can bind and inhibit cGPK at a different site from the catalytic cleft. Thus, fusion of the MTS sequences to W45 may yield the observed synergistic and competitive inhibition by linking the MTS and W45 affinity binding motifs in a single peptide sequence. Irrespective of the mechanism, the observed synergism improves the utility of these compounds in biological systems.
Inhibitors of cGPK with nanomolar inhibition constants have not been reported, and the observed selectivities over cAPK of 20,000 (DT-3) and 1,300 (DT-2) are exceptional among protein kinase inhibitors. We also observed highly selective cGPK inhibition by the fusion peptides in mixtures of recombinant cGPK/cAPK, as well as in intact cells. Clearly more work is needed to investigate the mechanism of uptake, cellular distribution, temporal dynamics, and proteolytic stability of these peptides. We are currently addressing these issues by the use of retro-inverse sequences of DT-2 and DT-3.
NO has a central role in vascular biology, but its mechanisms of action have not yet been fully elucidated (1). NO-induced relaxation of vascular smooth muscle does seem to involve activation of cGPK, leading to alterations in [Ca2+]i and effects on myosin light chain kinase and phosphatase activities (1, 3). It was reasonable, therefore, to look for functional effects of the cGPK-inhibitor peptides in intact arteries by using a NO donor as a vasodilator agent. DT-2 and DT-3 decreased the dilator potency of NONOate by 50- to 100-fold when applied to intact cerebral resistance arteries, indicating a substantial translocation of functionally active inhibitors into the vascular smooth muscle cells.
The parallel shift in dose–response curves suggests that a competitive inhibition of cGPK by the inhibitor peptides, as observed for the purified enzyme, also occurs in vascular smooth muscle cells in situ. In contrast to the inhibitory effects of the carrier peptides DT-5 and DT-6 on purified cGPK in vitro, we observed no appreciable effects of the carriers alone on the NO-induced dilations of intact arteries. We infer from this observation that, at the extracellular concentrations used in the intact artery experiments, the carrier sequences do not reach sufficient intracellular levels (i.e., >50–100 nM) to inhibit cGPK. We did note some direct contractile action of DT-2 or DT-3 when applied in concentrations greater than 2 μM or 500 nM, respectively. This observation underscores the need for careful titration of these inhibitors in the particular cellular system under study. A possible explanation for the contractile activity of the inhibitors is that basally active cGPK may be important in regulating cerebral arterial diameter, but further studies are required to verify this action.
The present study has resulted in the discovery of selective inhibitors of cGPK and has defined an effective means for intracellular delivery of these compounds. Further, we have demonstrated the ability of the inhibitors to alter NO-induced cerebral vasodilation and substantiated a central role of cGPK as a mediator of this response. The development of these membrane-permeable, selective cGPK inhibitors should allow much clearer dissection of the roles of cGPK in living cells than has heretofore been possible.
Acknowledgments
We thank B. Kornak and S. Daenicke for excellent technical assistance with the peptide synthesis and Drs. Mark Nelson and Karen Lounsbury for their helpful reviews. This work was supported by Deutsche Forschungsgemeinschaft Grants Do329/3-3 and Do329/4-1, the Lake Champlain Cancer Research Organization and the Totman Medical Research Trust (to W.R.G.D.), and National Institutes of Health Grants HL44455 (to M.S.T.) and HL58231 (to J.E.B.).
Abbreviations
- cAPK
cAMP-dependent protein kinase
- cGPK
cGMP-dependent protein kinase
- MTS
membrane translocation signal
- PKI
protein kinase inhibitor
References
- 1.Lincoln T M, Komalavilas P, Boerth N J, Mac-Millan-Crow L A, Cornwell T L. Adv Pharmacol (San Diego) 1995;34:305–322. doi: 10.1016/s1054-3589(08)61094-7. [DOI] [PubMed] [Google Scholar]
- 2.Pfeifer A, Ruth P, Dostmann W, Sausbier M, Klatt P, Hofmann F. Rev Physiol Biochem Pharmacol. 1999;135:105–149. doi: 10.1007/BFb0033671. [DOI] [PubMed] [Google Scholar]
- 3.Surks H K, Mochizuki N, Kasai Y, Georgescu S P, Tang K M, Ito M, Lincoln T M, Mendelsohn M E. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 4.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang G-X, Allescher H D, Korth M, Wilm M, et al. Nature (London) 2000;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- 5.Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- 6.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang G X, Korth M, Aszodi A, Andersson K E, et al. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennelly P J, Krebs E G. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- 8.Glass D B, Cheng H C, Mende-Mueller L, Reed J, Walsh D A. J Biol Chem. 1989;264:8802–8810. [PubMed] [Google Scholar]
- 9.Mitchell R D, Glass D B, Wong C W, Angelos K L, Walsh D A. Biochemistry. 1995;34:528–534. doi: 10.1021/bi00002a018. [DOI] [PubMed] [Google Scholar]
- 10.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- 11.Yan X, Corbin J D, Francis S H, Lawrence D S. J Biol Chem. 1996;271:1845–1848. doi: 10.1074/jbc.271.4.1845. [DOI] [PubMed] [Google Scholar]
- 12.Tegge W, Frank R, Hofmann F, Dostmann W R. Biochemistry. 1995;34:10569–10577. doi: 10.1021/bi00033a032. [DOI] [PubMed] [Google Scholar]
- 13.Dostmann W R, Nickl C, Thiel S, Tsigelny I, Frank R, Tegge W. J Pharmacol Ther. 1999;82:373–387. doi: 10.1016/s0163-7258(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 14.Wood J S, Yan X, Mendelow M, Corbin J D, Francis S H, Lawrence D S. J Biol Chem. 1996;271:174–179. doi: 10.1074/jbc.271.1.174. [DOI] [PubMed] [Google Scholar]
- 15.Lev-Ram V, Jiang T, Wood J, Lawrence D S, Tsien R Y. Neuron. 1997;18:1025–1038. doi: 10.1016/s0896-6273(00)80340-2. [DOI] [PubMed] [Google Scholar]
- 16.Slice L W, Taylor S S. J Biol Chem. 1989;264:20940–20946. [PubMed] [Google Scholar]
- 17.Dostmann W R G, Koep N, Endres R. FEBS Lett. 1996;398:206–210. doi: 10.1016/s0014-5793(96)01242-2. [DOI] [PubMed] [Google Scholar]
- 18.Frank R. Tetrahedron. 1992;48:9217–9232. [Google Scholar]
- 19.Ruth P, Landgraf W, Keilbach A, May B, Egleme C, Hofmann F. Eur J Biochem. 1991;202:1339–1344. doi: 10.1111/j.1432-1033.1991.tb16509.x. [DOI] [PubMed] [Google Scholar]
- 20.Brayden J E, Nelson M T. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 21.Conway M A, Nelson M T, Brayden J E. Am J Physiol. 1994;266:H1322–H1326. doi: 10.1152/ajpheart.1994.266.4.H1322. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann F, Gensheimer H P, Gobel C. Eur J Biochem. 1985;147:361–365. doi: 10.1111/j.1432-1033.1985.tb08758.x. [DOI] [PubMed] [Google Scholar]
- 23.Keilbach A, Ruth P, Hofmann F. Eur J Biochem. 1992;208:467–473. doi: 10.1111/j.1432-1033.1992.tb17209.x. [DOI] [PubMed] [Google Scholar]
- 24.Fawell S, Seery J, Daikh Y, Moore C, Chen L L, Pepinsky B, Barsoum J. Proc Natl Acad Sci USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vivès E, Brodin P, Lebleu B. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 26.Derossi D, Chassaing G, Prochiantz A. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- 27.Tegge W J, Frank R. Methods Mol Biol. 1998;87:99–106. doi: 10.1385/0-89603-392-9:99. [DOI] [PubMed] [Google Scholar]
- 28.Toomik R, Ek P. Biochem J. 1997;322:455–460. doi: 10.1042/bj3220455. [DOI] [PMC free article] [PubMed] [Google Scholar]