Abstract
Eosin is a probe for the Na pump nucleotide site. In contrast to previous studies examining eosin effects on Na only ATPase, we examined Na,K ATPase and K activated pNPPase activity in red blood cell membranes and purified renal Na,K ATPase. At saturating ATP (3mM) the eosin IC50 for Na pump inhibition was 19uM. Increasing ATP concentrations (0.2 – 2.5 mM) did not overcome eosin-induced inhibition thus eosin is a mixed-type inhibitor of ATPase activity. To test if eosin can bind to the high affinity ATP site, purified Na,K ATPase was labeled with 20 uM FITC. With increasing eosin concentrations (0.1 uM – 10 uM) the incorporation of FITC into the ATP site significantly decreases suggesting that eosin prevents FITC reaction at the high affinity ATP site. Eosin was a more potent inhibitor of K activated phosphatase activity than of Na,K ATPase activity. At 5mM pNPP the eosin IC50 for Na pump inhibition was 3.8 ± 0.23 uM. Increasing pNPP concentrations (0.45 – 14.5 mM) did not overcome eosin-induced inhibition thus eosin is a mixed-type inhibitor of pNPPase activity. These results can be fit by a model in which eosin and ATP bind only to the nucleotide site; in some pump conformations, this site is rigid and the binding is mutually exclusive and in other conformations, the site is flexible and able to accommodate both eosin and ATP (or pNPP). Interestingly, eosin inhibition of pNPPase became competitive after the addition of C12E8 (0.1%) but the inhibition of ATPase remained mixed.
Introduction
The Na,K-ATPase (aka Na pump) is a nearly ubiquitous transmembrane protein in animal cells that actively counter-transports Na+ and K+ across the plasma membrane in a 3Na+out / 2K+in ratio, energized by the hydrolysis of one ATP molecule. The disequilibrium in ion transport makes the Na pump electrogenic. The Na+ gradient created by the Na,K-ATPase is coupled to a multitude of secondary active transporters which mediate the uptake of amino acids, glucose, HCO3-, and neurotransmitters [1], as well as the extrusion of Ca++ and H+ [2]. Moreover, the maintenance of the Na+ and K+ gradients, in combination with selective ion channels, gives rise to resting membrane potential in most animal cells [for review see 3, 4]. Indeed, the homeostatic role of the Na pump is so critical that Na,K-ATPase activity accounts for approximately 23% of ATP hydrolysis in humans during rest [5] and is the major user of ATP in red blood cells from most species.
The Na pump reaction cycle is conveniently described by a model proposed independently by Albers and Post [6, 7], which describes two major conformations: the first has cation accessibility from the cytoplasm (Ein) and the second has cation access from the extracellular space (Eout). Briefly, the binding of 3 intracellular Na+ ions shifts the pump to a conformation where it binds one molecule of ATP to a high affinity nucleotide binding site. The γ phosphate of ATP is transferred to the protein forming a phosphorylated intermediate E-P; this covalent phosphointermediate distinguishes P-type ATPases from the V-type and F0F1 ATPases [8]. A conformational shift accompanies the release of ADP and the translocation of Na+ to the extracellular space. Two K+ ions bind to the extracellular face and initiate the hydrolysis of the phosphoenzyme releasing the inorganic phosphate (Pi). A subsequent conformational change of the protein once again flips the cation binding site to the intracellular side and concomitantly releases K+ ions. In the absence of K+, the Na pump mediates a slower rate of ATP hydrolysis termed Na (only) ATPase activity. This Na (only) ATPase activity is activated by ATP with high affinity. The Na,K ATPase activity response to ATP shows both high and low affinity ATP effects; the low affinity ATP effect is thought to be the result of ATP stimulation of K+ deocclusion. A kinetic model which generates both ATP effects but which requires only 1 structural ATP site remains the most popular description of the pump cycle (3, 4).
Fluorescein Inhibition
From the topology of the Na,K-ATPase, many of the initial studies regarding cation binding, ATP binding, and phosphorylation focused on the large cytoplasmic domain between transmembrane segments 4 and 5 (M4 and M5) of the α-subunit. This cytoplasmic segment is 430 amino acids in length of which, 180 residues comprise the ATP binding domain [9]. As hypothesized, the M4M5 cytoplasmic loop contains the nucleotide-binding site as evidenced by several studies utilizing chemical modification to inhibit ATP hydrolysis. It was first reported in 1980 that covalent modification with fluorescein 5′-isothiocyanate (FITC) abolished Na,K-ATPase activity in a 1:1 stoichiometry and that ATP protected against FITC labeling [10, 11]. Nevertheless, the FITC-conjugated protein still bound inorganic phosphate and underwent Na+ and K+-induced conformational shifts [10]. Similarly, FITC inhibited ATPase activity in the sarcoplasmic reticulum Ca-ATPase in a 1:1 stoichiometry [12]. It was subsequently determined that FITC covalently attached to Lys501 of the canine Na,K-ATPase within a conserved sequence (i.e. KAGP/SER) [13]. The equivalent residue in SERCA (i.e. Lys515) has been shown to be an ATP contact residue via crystallography [14].
Eosin, i.e.tetrabromofluorescein, is a reversible inhibitor that is competitive with ATP for the Na (only) ATPase [15]. Na (only) ATPase activity was measured in the absence of K+ ions. Similarly, increasing ATP concentration overcame eosin inhibition of the gastric H,K-ATPase [16], consistent with eosin interacting with the ATP binding site. Indeed, the nucleotide site binding efficiency of eosin remains an exploited tool for real-time fluorescent monitoring of nucleotide binding site conformational changes [17, 18, 19]. Thus, it was surprising when we observed that eosin non-competitively inhibited ATP hydrolysis and ATP-dependent 45Ca++ transport in the red cell plasma membrane Ca-ATPase [20]. Yet, like other P-type ATPases, FITC was an irreversible, ATP-protectable, inhibitor of PMCA, which modified the same conserved lysine residue (i.e. K591GASE) [21]. Consequently, it seems inappropriate to assume that eosin is an ATP site probe for all P-type ATPases in the absence of further experimentation.
In this study, we reexamined the inhibitory properties of eosin on Na,K-ATPase. Interestingly, we found that eosin appears to be a mixed-type inhibitor with respect to ATP for porcine red cell Na,K-ATPase and purified sheep kidney Na,K-ATPase. In addition, eosin did not compete with para-nitrophenylphosphate for K activated phosphatase activity in the kidney enzyme. However, when the purified renal Na,K-ATPase preparation was treated with a non-ionic detergent (i.e. 0.1% C12E8), eosin did appear to be a mutually exclusive inhibitor of pNPPase activity. Our results show that eosin inhibition of the Na pump is more complicated than mere binding to the high affinity ATP site.
Materials and Methods
Materials
[32P]ATP was from Perkin-Elmer Life Sciences. All other chemicals were reagent grade purchased either from Sigma (St. Louis, MO) or from Fisher Scientific (Pittsburgh, PA). Sheep kidneys and porcine red blood cells were purchased from Pel-Freez Biologicals (Rogers, AR). Purified Na,K-ATPase was obtained as described previously [22]. Red cell membranes were prepared as described previously [20]. Protein concentrations were determined by the method of Lowry et al. [23].
Na,K-ATPase Assay
Red Cell Na,K-ATPase activity was measured as reported previously for the red cell Ca pump [20], with minor modifications for Na,K-ATPase. Briefly, 0.5 mg of red cell membrane protein was diluted into 30 mL of assay media containing (in mM): 140 NaCl, 34 KCl, 33 HEPES (pH = 7.4), 4 MgCl2, and 0.06 EGTA. Reactions tubes received 400ul of the above medium and were run at a total volume of 500ul in the presence or absence of 5uM eosin at the [32P]ATP concentrations indicated in figure 1. Reactions were incubated at 37oC for 80 min and stopped by adding 5mL of 5% TCA/charcoal. The charcoal was spun down in a microfuge and 175uL aliquots were taken from the supernatant and the liberated 32PO4 determined via liquid scintillation spectroscopy. All tube were run in the presence and absence of 0.5mM ouabain and the differences were reported as the specific Na,K-ATPase activity.
Figure 1. ATP-dependence of eosin inhibition of the pig red cell Na,K-ATPase.
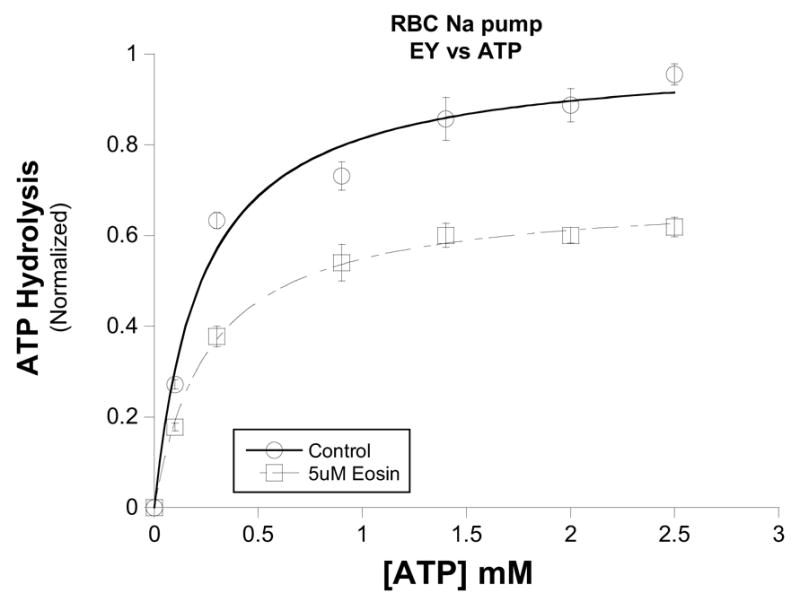
Ouabain-sensitive ATPase activity was measured in the presence of saturating substrates and the indicated amount of ATP. Increasing concentrations of ATP in the presence of 5μM eosin (□) were unable to displace eosin and attain control (○) Vmax levels. Control Vmax= 1.00 ± 0.04, K1/2 = 0.23 ± 0.04 mM; Eosin Vmax = 0.69 ± 0.01, K1/2 = 0.26 ± 0.02 mM. Data from three different experiments were normalized to maximal velocity of ATP hydrolysis in the absence of inhibitor. Points represent means ± SEM.
Purified kidney Na,K-ATPase activity was measured as reported previously [24]. Briefly, Na,K-ATPase activity was measured in a standard assay medium containing (in mM): 1 EGTA, 130 NaCl, 20 KCl, 3 MgCl2, 3 Na2ATP, 50 imidazole (pH 7.2, 25oC), and 0.5 μg/ml purified enzyme. The suspension was incubated at 37oC for 15 min and the liberation of PO4 measured as described by Brotherus et al. [25]. The specific Na,KATPase activity was the difference between the ATP hydrolysis measured in the absence and presence of 0.5 mM ouabain.
para-nitrophenylphosphatase activity. All pNPPase assays were conducted essentially as described in [26] using 2-4ug of Na,K-ATPase from dog kidney preparations. Briefly, assay medium contained (in mM) 50 MOPS/Tris, 5 KCl, 3 MgCl2 (pH 7.4) with a final concentration of 5mM di-tris pNPP and 100 Choline-Cl. For pNPP competition experiments, eosin concentration was fixed while varying the concentration of pNPP as indicated in the respective figure. Each reaction tube was incubated at 37oC for 15 minutes and the reaction stopped by the addition of 200ul of “ice-cold” 200mM NaOH and the reaction tubes placed in an ice bath for 10 min. Absorbance at 410nm was then recorded utilizing a Beckman DU-530 spectrophotometer and absorbance was converted to activity based on a pNP standard curve.
Eosin protection against FITC labeling of the Na,K-ATPase
Na,K-ATPase (50 μg) was mixed with either 20 μM FITC (diluted from 10 mM stock in DMSO), 20 μM FITC and 5 mM ATP, or 20 μM FITC and eosin (concentration range, 0.1 – 10 μM, diluted from 10 mM stock in DMSO). A FITC concentration of 20 μM was chosen to reduce activity to less than 10% of controls. The reactions were incubate at room temperature for 30 minutes and stopped with 150 μl of ice-cold stopping solution (i.e. 50 mM Tris/100 mM NaCl/50 mM β–mercaptoethanol, pH 6.5). A 5 μg quantity of protein from each reaction was mixed with Laemmli sample buffer (1:1:1 v/v mixture of 8 M Urea, 10% SDS, 125 mM Tris Buffer) and loaded onto a 12% Laemmli gel [27]. The fluorescent protein bands were visualized on a UVP Transilluminator Gel Documentation System with Image Store 7500 version 7.21 software. Aliquots from each FITC incubation were used to determine Na,K-ATPase activity as described above.
Results
The ATP-dependence of eosin inhibition of the red cell Na pump is shown in figure 1. In these experiments on red cell Na pump, the inhibition produced by 5uM eosin was not overcome by increasing ATP concentration. Similarly, increasing the ATP concentration from 0.1mM to 2.5mM also did not appear to diminish eosin inhibition of purified renal Na,K-ATPase (Fig. 2A). Clearly, the Vmax in the absence of eosin (1.11 ± 0.02) was significantly different than either the Vmax in the presence of 20uM (0.77 ± 0.02) or 50uM (0.53 ± 0.01) eosin.
Figure 2. Eosin inhibition of purified renal Na,K-ATPase.
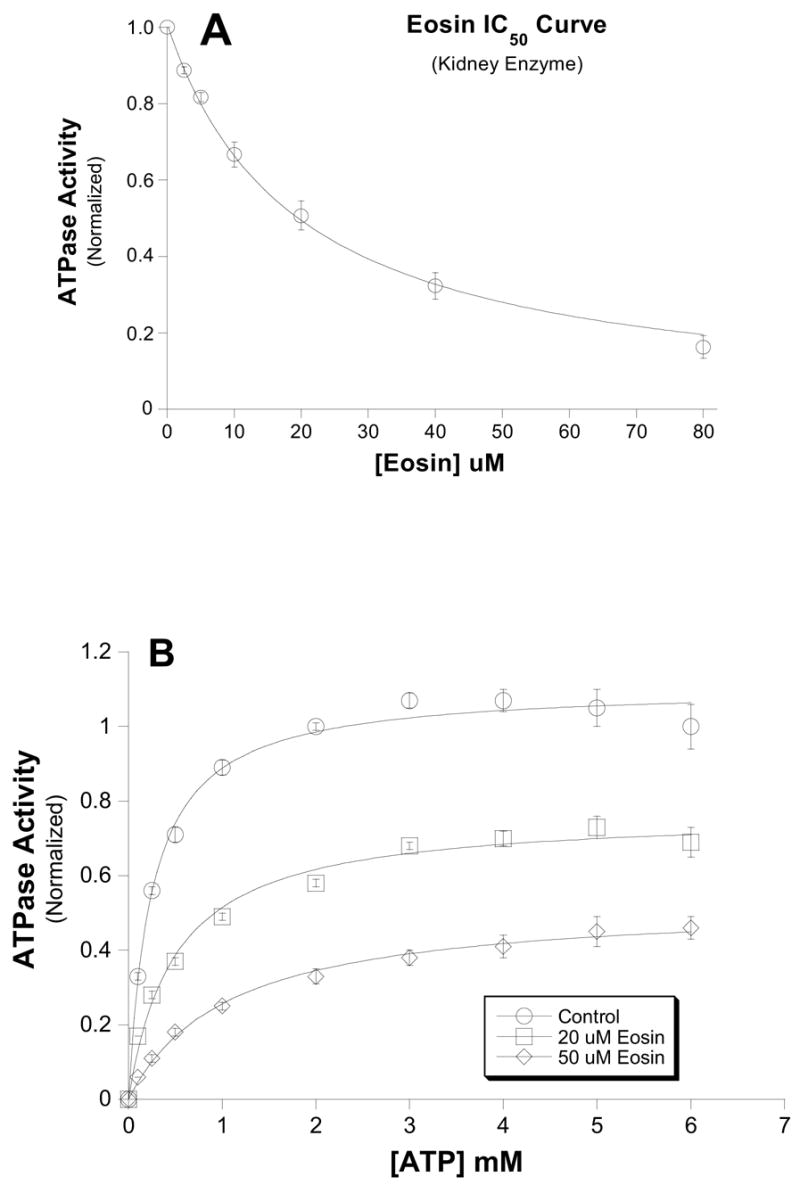
Ouabain-sensitive ATPase activity was measured in the presence of saturating substrates and the indicated amount of ATP. A.) ATP-dependence of eosin inhibition of kidney Na,K-ATPase. Similar to the red cell Na pump (Fig. 1), increasing concentrations of ATP in the presence of either 20μM (□) or 50μM eosin (○) were unable to displace eosin and attain control Vmax levels. Control Vmax= 1.11 ± 0.02, K1/2 = 0.25 ± 0.02mM; 20uM Eosin Vmax = 0.77 ± 0.02, K1/2 = 0.49 ± 0.06mM; 50uM Eosin Vmax = 0.53 ± 0.01, K1/2 = 1.0 ± 0.10mM. Data from three different experiments were normalized to maximal velocity of ATP hydrolysis in the absence of inhibitor. Points represent means ± SEM. B.) Dose-dependence of eosin inhibition on Na,K-ATPase. The inhibitory effect of eosin was measured on ouabain-sensitive Na,K-ATPase from purified sheep kidney enzyme. The data were fit to the equation v = V * IC50/ (IC50+I) where V is the maximal velocity in the absence of eosin (i.e. I=0), and I is [eosin]. The IC50 was 19.2 ± 1.01μM. Data were normalized to the activity in the absence of eosin.
The concentration dependence of eosin inhibition of purified Na,K-ATPase (sheep kidney) at saturating ATP shows that the eosin IC50 for Na pump inhibition was 19uM and is consistent with only 1 eosin binding site for inhibition. Interestingly, eosin is nearly 500-times more potent at inhibiting PMCA [20, 28, 29].
One test of whether eosin can bind to the high affinity ATP site is to see if it can prevent chemical modification of Lys-501 by FITC; lysine-501 is an ATP coordinating residue. We incubated purified Na,K-ATPase with 20uM FITC in the presence of eosin concentrations ranging from 0.1uM – 10uM (Fig. 3). The incorporation of FITC into the a-subunit significantly decreases with increasing [eosin] as one would predict if eosin bound to the ATP site. In addition, aliquots from the FITC-treated enzyme were used to measure ATPase activity to ensure that modification was at the critical lysine residue (Fig. 3B). When the eosin concentration is plotted against remaining activity, it reveals a Kapp for eosin of 0.83 ± 0.06uM. The fact that the apparent affinity drops >20-fold in the absence of ATP is also consistent with the two sharing a common binding site. However, eosin and ATP binding simply binding to the same site would predict mutually exclusive binding, which is not what was observed (Figs. 1 and 2B).
Figure 3. Eosin Protects against covalent modification by FITC.
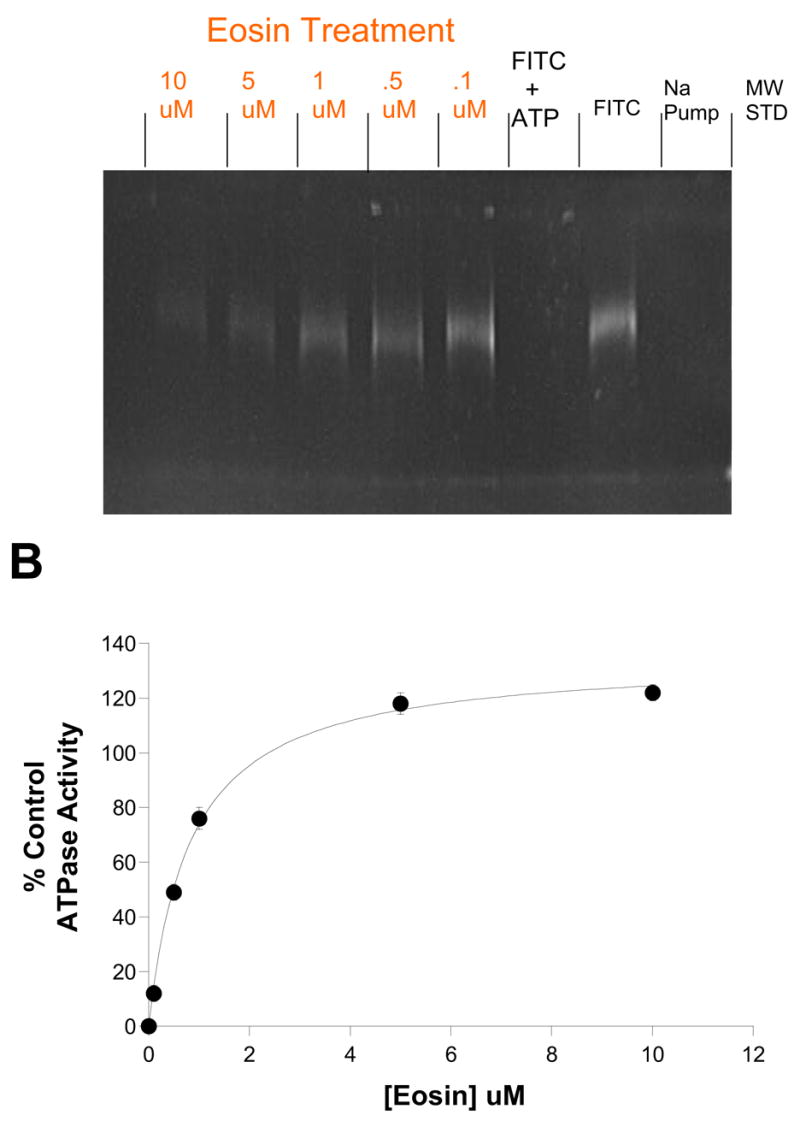
A.) FITC covalently labels Lys-501 of the Na,K-ATPase nucleotide binding domain of Na,K-ATPase. The FITC-modified alpha subunit can be detected by UV illumination on SDS-PAGE (FITC lane). Moreover, the simultaneous presence of 3mM ATP protects against FITC (FITC +ATP lane). FITC labeling was inhibited by eosin in a dose-dependent fashion (Eosin Treatment lanes, [eosin] range = 0.1 – 10 μM as indicated). B.) Eosin dose-response curve for protection against FITC-inactivation of Na,K-ATPase Activity. Aliquots from the FITC-labeled enzyme shown in A were diluted 120-fold and ouabain-sensitive ATPase activity measured and compared to the non-FITC modified purified sheep renal Na,K-ATPase. The K1/2 for eosin protection was 0.82 ±0.6μM. Data points are means and bars are standard deviation of a single experiment, representative of two separate experiments.
We wanted to investigate the effect of eosin on an alternative mode of action of the Na pump, namely, the K activated phosphatase activity. Eosin also inhibited the pNPPase activity with an IC50 of 3.8 ± 0.23uM (Fig. 4A). Interestingly, it appears that eosin was unable to completely block this activity as maximal inhibition appears to plateau at about 80% (Fig. 4A).
Figure 4. Dose-dependence of eosin inhibition on K+-dependent phosphatase activity.
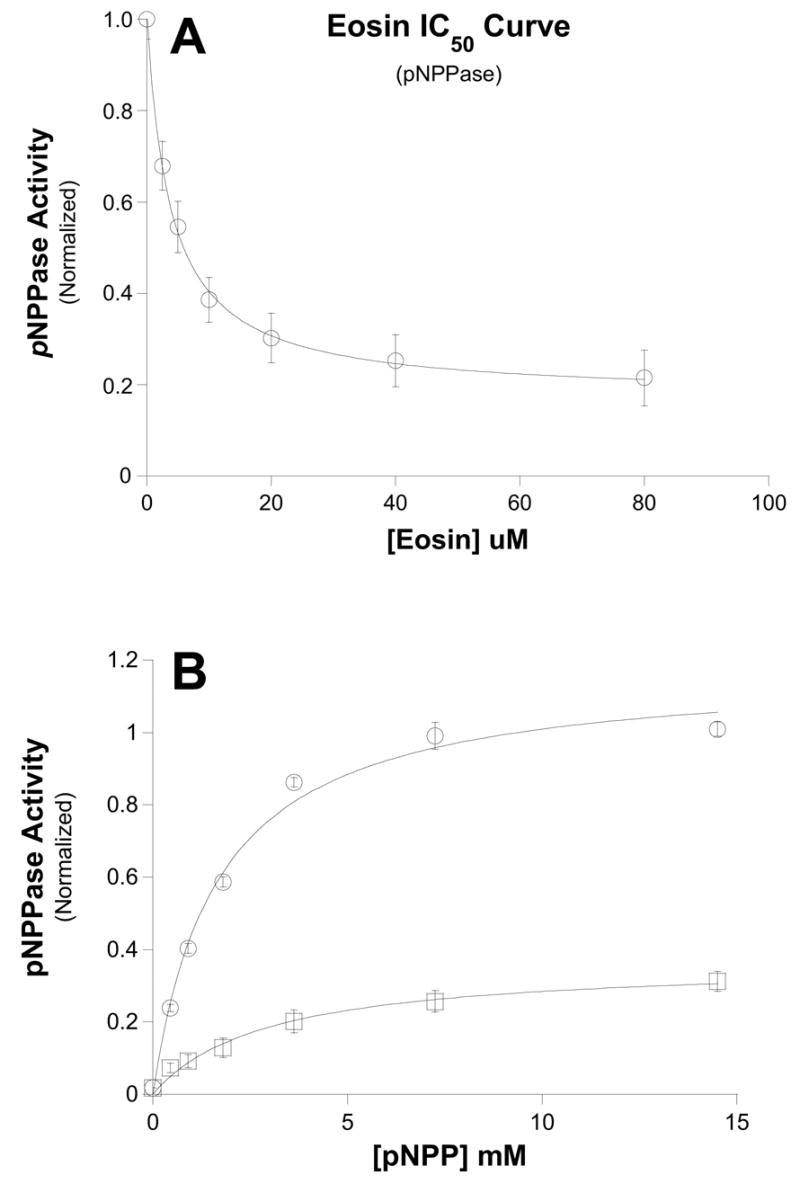
Ouabain-sensitive, K+-dependent pNPPase activity was measured in the presence of the indicated concentrations of eosin (see Methods). Data were fit to the same equation show in Fig. 1. Interestingly, the IC50 curve appears to plateau with a residual noneosin-sensitive 20% activity. The IC50 for eosin-sensitive component was 3.8 ± 0.23 μM. Data points are means and bars are standard error of six separate experiments. B.) pNPP dose-dependence of eosin inhibition. K+-dependent phosphatase activity was measured with increasing [pNPP] in the absence (○) and presence (□) of 25μM eosin. Data were fit to the Michaelis-Menten equation. Control Vmax = 1.17 ± 0.05; K1/2 = 1.65 ± 0.21 μM; 25 μM eosin Vmax = 0.38 ± 0.05; K1/2 = 2.91 ± 0.54 μM. These data suggest that eosin does not compete with pNPP. Data points are means and bars are standard error of three separate experiments.
Similar to Na,K-ATPase, when eosin inhibition was determined in the presence of increasing pNPP concentration the two compounds did not appear to be mutually exclusive (Fig. 4B). In constrast, when the purified enzyme was further treated with the nonionic detergent C12E8 (0.1%), the inhibition by eosin appears to become competitive with pNPP (Fig. 5). Increasing [pNPP] was able to displace eosin and the enzyme is able to attain the same maximal velocity (easily observed as all lines intersect the y-axis at the same point in the double-reciprocal plot shown in Fig. 5B). Na,K-ATPase treatment with C12E8 has been suggested to ensure that the enzyme is a single functional protomer (i.e. a single α/β complex) [30, 31]. We also reexamined eosin and ATP competition for Na,K-ATPase on C12E8-treated enzyme, but even in this case eosin appeared to be a mixed-type rather than a competitive inhibitor (data not shown).
Figure 5. pNPP concentration-dependence of eosin inhibition of K+-dependent Phosphatase activity.
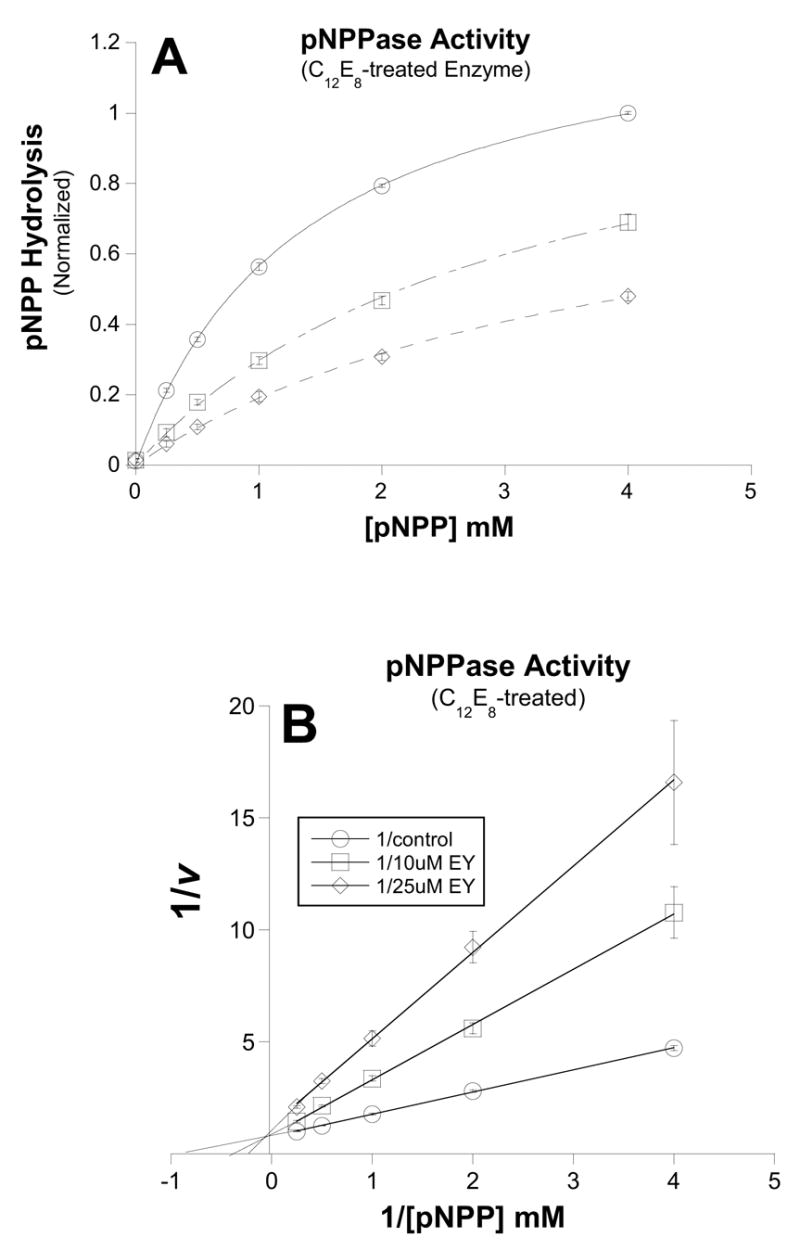
pNPP-activation of phosphatase activity was measured in the absence (○) or presence of 10uM (□) or 25uM (◇) eosin. A.) Michaelis-Menten plot of data. In contrast to its effects on the phosphatase reaction of untreated enzyme, eosin appears to compete with pNPP for C12E8-treated enzyme. Control Vmax = 1.3 ± 0.02; K1/2 = 1.4 ± 0.04 μM; 10 μM eosin Vmax = 1.2 ± 0.06; K1/2 = 3.1 ± 0.26 μM; 25 μM eosin Vmax = 1.0 ± 0.07; K1/2 = 4.0 ± 0.51 μM. B.) Double-Reciprocal plot of the data shown in fig. A. Here it is clear that the three lines intersect at the y-axis, consistent with eosin and pNPP binding in a mutually exclusive manner. Data points are means and bars are standard error of three separate experiments.
Discussion
Because eosin has been used as an ATP site probe in Na pump studies for the past 25 years, we expected that eosin would compete with ATP and pNPP during the Na,K-ATPase reaction and the K activated phosphatase activity. However, we found that eosin was a mixed-type inhibitor for both of these reactions. In general mixed inhibition implies that both substrate (ATP or pNPP) and eosin can be bound to the pump, at least in one conformation and therefore that their binding is not mutually exclusive.
In this discussion we review the previous evidence in support of a model in which ATP and eosin bind in a mutually exclusive manner at the high affinity nucleotide site. We then discuss an unconventional model that stretches the mutually exclusive model to accommodate the mixed inhibition. Finally we discuss potential explanations for the change in kinetics observed in the presence of C12E8.
Previous work
Skou and Esmann [15] have shown that eosin and ATP compete for Na (only) ATPase consistent with their binding to the same site in a mutually exclusive manner. In contrast, we found that eosin was a mixed inhibitor. However the present study differs from Skou and Esmann’s work in that here Na,K ATPase activity or K activated pNPPase was measured, whereas the earlier study focused on Na (only) ATPase activity. One obvious difference between these different enzymatic measurements is that during Na,K-ATPase or K activated pNPPase, there is a considerable contribution to kcat by a low affinity ATP binding site that does not occur in the Na (only) ATPase measurement. Thus our results are not in conflict with those of Skou and Esmann, but by examining additional parts of the pump cycle, we have revealed a conformation that behaves differently than those involved in Na (only) ATPase.
Following the initial findings by Skou and Esmann that eosin competes with ATP for Na (only) ATPase activity [15], eosin has been used as a fluorescent probe to monitor conformational changes at the high affinity ATP site. Specifically, fluorescence fluctuations during ligand-induced conformational changes have been used to deduce coordinated movements between specific domains of P-type ATPases [16, 18, 19, 33, 34]. Interestingly, initial investigations did not observe any measurable eosin binding (as monitored by fluorescence changes) to the K+-dependent portions of the catalytic cycle for either the Na,K-ATPase or H,K-ATPase suggesting that eosin was only a high affinity ATP binding site probe for P-type ATPases [15, 16], but recent work suggests that eosin does bind to K+ liganded conformations, but it cannot be easily detected by fluorescence changes [19]. Additional support for eosin binding to the ATP site comes from our observation that eosin protects Lys-501 from covalent modification by fluorescein isothiocyanate (FITC) (Fig. 3), which is not surprising since eosin is tetrabromofluorescein. Lysine-501 is a conserved residue among P-type ATPases that has been shown to coordinate ATP binding in high resolution structures of SERCA [14, 37].
Eosin, in addition to inhibiting the Na pump, also inhibits the plasma membrane Ca pump from red cells [20] and smooth muscle [35]. Interestingly, those studies found that eosin was not competitive with ATP, similar to the results presented here for the Na pump. Because the Ca pump does Ca++/nH+ exchange, it is likely that those experiments were measuring analogous conformations to those observed during Na, K ATPase. Indeed, we are unaware of any attempts to measure a Ca ATPase activity analogous to Na only ATPase. Obviously, one cannot have H+ = 0, and at very low H+, there may be nonspecific affects on the Ca pump.
Earlier we suggested that the mixed type inhibition observed for red cell PMCA may be simply do the fact that eosin can bind the enzyme in E1 as well as E1P (see scheme 1), whereas ATP only binds to E1 with high affinity [28]. In this case, some of the amino acid contact residues for eosin remain the same, but the two enzyme conformations are kinetically distinct. One prediction of this model would be that eosin should compete with ADP for the dephosphorylation of ADP-sensitive EP as shown in Fig. 6A. This model is consistent with eosin acting as a mixed-type inhibitor even though it resides within the high affinity ATP site. However, this model would predict that eosin should be a mixed-type inhibitor of Na (only) ATPase and that was not observed [15], so this model does not apply to the Na pump.
Figure 6. Potential kinetic schemes for eosin inhibition of the Na pump.
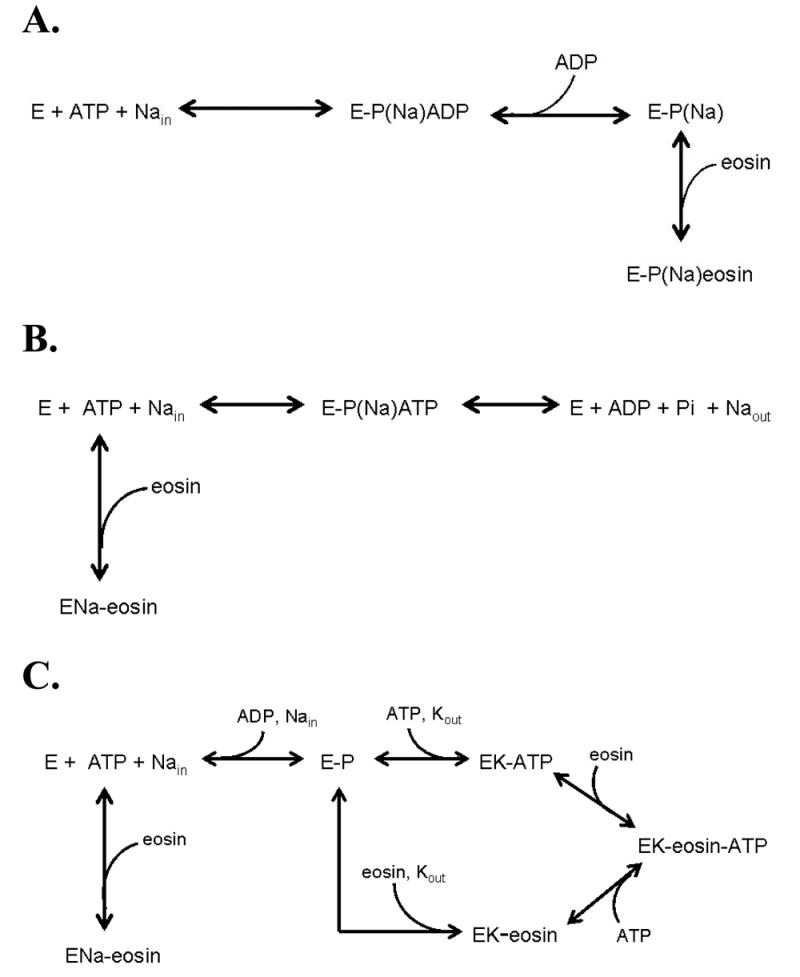
A. Eosin and ADP compete which is consistent with eosin being a mixed inhibitor of ATP for Na,K ATPase. It is not consistent with eosin being a competitive inhibitor of Na (only) ATPase.
B. Eosin and ATP compete for Na (only) ATPase.
C. Eosin and ATP can both bind for Na,K ATPase.
Eosin Inhibition of K+ activated Phosphatase Activity
In addition to ATP, the P-type pumps can hydrolyze other phosphate-containing substrates such as para-nitrophenyl phosphate [26, 38, 39, 40], 3-O-methylfluorescein phosphate [41, 42], and acetylphosphate [43, 44]. We observed that eosin was a potent inhibitor of pNPPase activity (IC50 = 3.8 ± 0.23 μM; Fig. 4A). Indeed, this appears to be the first report directly measuring eosin inhibition of pNPPase activity in native Na pump, aside from one report showing that eosin blocks the subtle residual pNPPase activity associated with a bacterially expressed M4M5 loop from rat Na,K-ATPase [17].
Eosin inhibition of pNPPase may seem surprising, given that FITC-labeled enzyme remains capable of phosphatase activity (40, 41). However, previous work has also established that ATP still inhibits pNPPase in FITC treated Na pump. One explanation is that during the conformational change from E1 to E2, the tethered FITC molecule is swung out of the active site which allows pNPPase activity [41], but because eosin is not tethered, it is either bound at the site or not attached to the enzyme. An alternative model for the response of the FITC modified pump proposed by Martin and Sachs [40] is that an additional low affinity site for TNP-ADP and erythrosin isothiocyanate appears only after FITC-modification and likely represent a non-physiological allosteric inhibition site. This hypothesis is supported by the dramatically reduced affinities for both TNP-ADP and erythrosin isothiocyanate inhibition of the pNPPase activity in the FITC modified pump compared to the native enzyme [40]. Indeed, our finding that eosin inhibits pNPPase in the non-modified enzyme with high affinity is consistent with this hypothesis. However, Martin and Sachs suggest that pNPPase occurs at the catalytic site (and we tend to agree), yet we find that eosin inhibition of pNPPase is not competitive with pNPP in the native Na,K-ATPase (Fig. 4B).
Model of eosin inhibition of the Na pump
Mixed inhibition typically is explained with models in which the inhibitor binds to a separate site from the substrate. However, because of the substantial evidence that eosin binds to the ATP site, we sought to develop a model that encompasses all the key data and that has eosin and ATP sharing the same site. Specifically, the following key features of eosin inhibition of intact Na pump are included:
eosin competes with ATP on Na (only) ATPase (15)
eosin is a mixed inhibitor with respect to ATP on Na,K-ATPase (Figures 1 and 2)
eosin is a mixed inhibitor with respect to pNPP for K activated phosphatase (Figure 5)
eosin protects the pump from reaction with FITC (Figure 3)
eosin stimulates K+ deocclusion and high eosin prevents the higher stimulation by ATP (18)
eosin does not completely inhibit pNPPase activity (Figure 4).
The simplest model that we can develop which is consistent with the data and has eosin and ATP sharing the same site, is a model in which this site is flexible. In certain conformations this site is able to bind both eosin and ATP (Fig. 6B). In other conformations, the site is unable to bind both, thus eosin and ATP are mutually exclusive (Fig. 6C.) It should be pointed out that Esmann and Fedosova [45] have shown that the site can simultaneously accommodate nitrate and eosin (and presumably nitrate and ADP) which supports the idea that at least sometimes, the site can stretch. In the presence of Na+ and the absence of K+, the conformation is such that eosin and ATP are mutually exclusive [45]. This is consistent with the earlier competition for Na (only)ATPase data, and the fact that ATP prevents the increase in eosin fluorescence due to binding to the pump [15]. However, there exists one or more conformations during the Na, K ATPase cycle and during the pNPPase cycle in which the site can stretch and accommodate both eosin and ATP, thus explaining why high ATP or pNPP, respectively, cannot overcome the inhibition by eosin under these conditions.
A candidate for a conformation that allows the nucleotide site to stretch and accommodate both ATP (or pNPP) and eosin is the occluded state. However, the recent work by Rossi [18, 19] seems to suggest that in the presence of K+, eosin and ATP would be mutually exclusive as eosin prevents the extra stimulation of deocclusion when ATP is present. They found that while eosin can accelerate Rb+ deocclusion, it was not as large an acceleration as observed with ATP [19]. At high eosin, ATP was not able to stimulate the rate, suggesting that eosin prevented ATP from binding. But an alternative explanation is that the site could stretch in this conformation and allow both ATP and eosin to bind, but that this stretched conformation cannot deocclude K+ as fast as the “normal” conformation with ATP present. Thus, this conformation remains a candidate as one of the conformation(s) of the pump which allow the site to stretch and accommodate both eosin and ATP (or pNPP) to bind.
C12E8 treated pump
The Na pump behavior changes in the presence of C12E8: the addition of C12E8 changes the inhibition by eosin from mixed to competitive with pNPP. However a difficult constraint to the modeling is that eosin remains a mixed inhibitor for Na,K-ATPase.
C12E8 treatment shifts the equilibrium of pump from a diprotomer state to a monoprotomer state. C12E8 may also have additional effects. Clearly, all of the ion binding, transport, and enzymatic properties of the Na,K-ATPase can be catalyzed by a single functioning protomer consisting of one α and one β. Nonetheless, there continues to be discussions of and evidence for diprotomeric (or high oligoprotomeric) functioning Na,K-ATPase. Both camps have valid arguments. The single protomeric camp is justified in questioning the need to invoke oligomers when a single protomer can explain the vast majority of the existing data. However, since higher oligomers can clearly be isolated from membrane factions, the oligomeric camp is justified in questioning why oligmers exist if they are physiologically irrelevant. In an attempt to satisfy both camps we propose two possibilities for our pNPPase observations.
Diprotomer model
In this model, in the absence of C12E8, eosin binds to the catalytic pNPP binding site on one protomer, and the contacts with the second protomer allow both eosin and pNPP to fit within the pocket in one or more (but not all) conformations. This change in extensibility could reflect contacts between the P domain on one monomer and the N domain on the other as seen in the first high resolution SERCA crystal structure (14). Treatment with C12E8 separates the diprotomer to a predominate population of monoprotomers. Protomers are fully capable of hydrolyzing pNPP, but the site is not able to stretch to accommodate eosin and pNPP because the contact points on the other protomer are no longer close by. However, in a conformation involved in Na,K ATPase, but not pNPPase, even the monoprotomer is able to stretch and bind both ATP and eosin. While this model is appealing, we cannot explain why the stretch still occurs during Na,K-ATPase but does not during pNPPase.
Single protomer model
In this model, C12E8 causes a mild unfolding of the protein such that, while the enzyme can still stretch in C12E8, the rate of the rate limiting step for pNPPase activity is the same when eosin and pNPP are both bound or when just pNPP is bound. (The effect of eosin is merely to lower the affinity for pNPP, so that, at low pNPP eosin inhibits.) In contrast, in the native state, the pNPPase rate is slower with both bound to the stretched substrate site. One must also assume that the rate limiting step for Na,K-ATPase is slower when eosin and ATP are bound compared to just ATP bound, in both the native and C12E8 structure. Since the Na,K-ATPase cycle involves additional conformations compared to pNPPase, this is plausible.
Although neither model is completely satisfactory, they both emphasize that eosin inhibition of the Na pump appears to be more complicated than merely binding to the high affinity ATP site. Consequently, experiments which utilize eosin as a real-time fluorescent indicator of ATP site conformational changes are more safely interpreted if they include the possibility that ATP and eosin are not always mutually exclusive.
There are some potential tests to distinguish these models. The key difference between the models is whether the effect of C12E8 on the type of inhibition by eosin is due to the shift from diprotomer to monoprotomer or whether it is due to a slight unfolding of the pump. Since the Na pump in red blood cells is a monoprotomer of Na pump, if pNPP and eosin compete in the red cell, this would support the diprotomer model. On the other hand, if pNPP and eosin are mixed in the red cell, this would suggest that the shift in inhibition type by C12E8 on the kidney enzyme is due to it causing a slight unfolding. However, such data would not rule out the diprotomer model if band 3 associates with the monoprotomer and mimics the effect of the other Na pump in the diprotomer. This possibility could be tested in band 3 knockout animals which lack band 3 in their red cells, if the Na pump in these red cells remains in its monoprotomer state.
Acknowledgments
We would like to thank Dr. Charles J. Costa and Neil Johnson for initial investigations into the C12E8 experiments. This work was supported by: NIH grant GM061583 to CG and NIH DK37512 grant to MAM. This paper is based on a presentation at the Red Cell Conference held at the Yale University in New Haven Connecticut November 3-4, 2006.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Freel RW, Goldner AM. Sodium-coupled nonelectrolyte transport across epithelia: emerging concepts and directions. Am J Physiol. 1981;241:G451–G460. doi: 10.1152/ajpgi.1981.241.6.G451. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res. 2003;57:897–912. doi: 10.1016/s0008-6363(02)00656-9. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen PL, Hakansson KO, Karlish SJD. Structure and Mechanism of Na,K-ATPase: Functional sites and their interactions. Annu Rev Physiol. 2003;65:817–849. doi: 10.1146/annurev.physiol.65.092101.142558. [DOI] [PubMed] [Google Scholar]
- 5.DeWeer P. Renal Na,K-ATPase. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. Raven Publishing; New York: 1985. pp. 31–55. [Google Scholar]
- 6.Albers RW. Biochemical aspects of active transport. Annu Rev Biochem. 1967;36:727–756. doi: 10.1146/annurev.bi.36.070167.003455. [DOI] [PubMed] [Google Scholar]
- 7.Post RL, Kume S, Tobin T, Orcutt B, Sen AK. Flexibility of an active center in sodium-plus-potassium adenosine triphosphatase. J Gen Physiol. 1969;54:306–326. doi: 10.1085/jgp.54.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutsenko S, Kaplan JH. Organization of P-type ATPase: Significance of structural diversity. Biochem. 1995;34:15607–15613. doi: 10.1021/bi00048a001. [DOI] [PubMed] [Google Scholar]
- 9.Taylor WR, Green NM. The predicted secondary structures of the nucleotide-binding sites of six cation-transporting ATPases lead to a probable tertiary fold. Eur J Biochem. 1989;179:241–248. doi: 10.1111/j.1432-1033.1989.tb14547.x. [DOI] [PubMed] [Google Scholar]
- 10.Karlish SJD. Characterization of conformational changes in (Na,K) ATPase labeled with fluorescein at the active site. J Bioenerg Biomembr. 1980;12:111–136. doi: 10.1007/BF00744678. [DOI] [PubMed] [Google Scholar]
- 11.Carilli CT, Farley RA, Perlman DM, Cantley LC. The active site structure of Na+- and K+-stimulated ATPase. J Biol Chem. 1982;257:5601–5606. [PubMed] [Google Scholar]
- 12.Andersen JP, Moller JV, Jorgensen PL. The functional unit of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 1982;257:8300–8307. [PubMed] [Google Scholar]
- 13.Farley RA, Tran CM, Carilli CT, Hawke D, Shively JE. The amino acid sequence of a fluorescein-labeled peptide from the active site of (Na,K)-ATPase. J Biol Chem. 1984;259:9532–9535. [PubMed] [Google Scholar]
- 14.Toyoshima C, Nakasako M, Nomura H, Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum as 2.6 Å resolution. Nature. 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 15.Skou JC, Esmann M. Eosin, a fluorescent probe of ATP binding to the (Na+ +K+)-ATPase. Biochim Biophys Acta. 1981;647:232–240. doi: 10.1016/0005-2736(81)90251-0. [DOI] [PubMed] [Google Scholar]
- 16.Helmich-de Jong ML, Duynhoven JPM, Schuurmanns Stekhoven FMAH, De Pont JJHHM. Eosin, a fluorescent marker for the high-affinity ATP site of (K+ +H+)-ATPase. Biochim Biophys Acta. 1986;858:254–262. doi: 10.1016/0005-2736(86)90330-5. [DOI] [PubMed] [Google Scholar]
- 17.Krumscheid R, Ettrich R, Sovova Z, Susankonva K, Lansky Z, Hofbauerova K, Linnertz H, Teisinger J, Amler E, Schoner W. The phosphatase activity of the isolated H4-H5 loop of Na,K-ATPase resides outside its ATP binding stie. Eur J Biochem. 2004;271:3923–3936. doi: 10.1111/j.1432-1033.2004.04330.x. [DOI] [PubMed] [Google Scholar]
- 18.Montes MR, Gonazalez-Lebrero RM, Garrahan PJ, Rossi RC. Quantitative analysis of the interaction between the fluorescent probe eosin and the Na,K-ATPase studied through Rb+ occlusion. Biochemistry. 2004;43:2062–2069. doi: 10.1021/bi0351763. [DOI] [PubMed] [Google Scholar]
- 19.Montes MR, Gonazalez-Lebrero RM, Garrahan PJ, Rossi RC. Eosin Fluorescence changes during Rb+ occlusion in the Na,K-ATPase. Biochemistry. 2006;45:13093–13100. doi: 10.1021/bi060778i. [DOI] [PubMed] [Google Scholar]
- 20.Gatto C, Milanick MA. Inhibition of the red blood cell calcium pump by eosin and other fluorescein analogues. Am J Physiol. 1993;264:C1577–C1586. doi: 10.1152/ajpcell.1993.264.6.C1577. [DOI] [PubMed] [Google Scholar]
- 21.Filoteo A, Gorski JP, Penniston JT. The ATP-binding site of the erythrocyte membrane Ca2+ pump. J Biol Chem. 1987;262:6526–6530. [PubMed] [Google Scholar]
- 22.Gatto C, Helms JB, Prasse M, Arnett KL, Milanick MA. Tetrapropylammonium, an exclusive extracellular cation site probe in Na,K-ATPase, reveals how ATP and Pi alter access to the transport site. Am J Physiol – Cell Physiol. 2005;289:C302–C311. doi: 10.1152/ajpcell.00043.2005. [DOI] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Gatto C, Lutsenko S, Kaplan JH. Chemical modification with H2DIDS reveals the distance between K480 and K501 in the ATP-binding domain of the Na,K-ATPase. Arch Biochem Biophys. 1997;340:90–100. doi: 10.1006/abbi.1997.9879. [DOI] [PubMed] [Google Scholar]
- 25.Brotherus JR, Moller JV, Jorgensen PL. Soluble and active renal Na,K-ATPase with maximum protein molecular mass 170,000 +9,000 daltons; formation of larger units by secondary aggregation. Biochem Biophys Res Commun. 1981;100:146–154. doi: 10.1016/s0006-291x(81)80075-7. [DOI] [PubMed] [Google Scholar]
- 26.Drapeau P, Blostein R. Interactions of K+ with (Na,K)-ATPase orientation of K+-phosphatase sites studied with inside-out red cell membrane vesicles. J Biol Chem. 1980;255:7827–7834. [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Gatto C, Milanick MA. In: Does Eosin Treat all P-type ATPases Equally? The Sodium Pump: Structure Mechanism, Hormonal Control and its Role in Disease. Bamberg E, Schoner W, editors. Dietrich Steinkopff; Darmstadt, Germany: 1994. pp. 609–612. [Google Scholar]
- 29.Gatto C, Hale CC, Xu W, Milanick MA. Eosin, a potent inhibitor of the plasma membrane Ca pump, does not inhibit the cardiac Na-Ca exchanger. Biochemistry. 1995;34:965–972. doi: 10.1021/bi00003a031. [DOI] [PubMed] [Google Scholar]
- 30.Brotherus JR, Jacobsen L, Jorgensen PL. Soluble and enzymatically stable (Na+ + K+)- ATPase from mammalian kidney consisting predominantly of protomer alpha beta-units. Preparation, assay and reconstitution of active Na+, K+ transport. Biochim Biophys Acta. 1983;731:290–303. doi: 10.1016/0005-2736(83)90021-4. [DOI] [PubMed] [Google Scholar]
- 31.Ward DG, Cavieres JD. Solubilized alpha beta Na,K-ATPase remains protomeric during turnover yet shows apparent negative cooperativity toward ATP. Proc Natl Acad Sci. 1993;90:5332–5336. doi: 10.1073/pnas.90.11.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa CJ, Gatto C, Kaplan JH. Interactions between Na,K-ATPase α-subunit ATP- binding domains. J Biol Chem. 2003;278:9176–9184. doi: 10.1074/jbc.M212351200. [DOI] [PubMed] [Google Scholar]
- 33.Skou JC, Esmann M. Effects of ATP and protons on the Na:K selectivity of the (Na+ +K+)-ATPase studied by ligand effects on intrinsic and extrinsic fluorescence. Biochim Biophys Acta. 1980;601:386–402. doi: 10.1016/0005-2736(80)90543-x. [DOI] [PubMed] [Google Scholar]
- 34.Skou JC, Esmann M. Effect of magnesium ions on the high affinity binding of eosin to the (Na+ +K+)-ATPase. Biochim Biophys Acta. 1983;727:101–107. doi: 10.1016/0005-2736(83)90373-5. [DOI] [PubMed] [Google Scholar]
- 35.Slinchenko NN, Bratkova NF, Kosterin SA, Zimina VP, Chernysh IG. Effects of eosin Y on the catalytic and functional activities of Mg2+,ATP-dependent calcium pump of smooth muscle cell plasma membrane. Biochemistry (Mosc) 1998;63:685–90. [PubMed] [Google Scholar]
- 36.Skou JC, Esmann M. The effects of Na+ and K+ on the conformational transitions of (Na+ +K+)-ATPase. Biochim Biophys Acta. 1983b;746:101–113. doi: 10.1016/0167-4838(83)90016-x. [DOI] [PubMed] [Google Scholar]
- 37.Toyoshima C, Nomura H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature. 2002;418:605–611. doi: 10.1038/nature00944. [DOI] [PubMed] [Google Scholar]
- 38.Mignaco JA, Lupi OH, Santos FT, Barrabin H, Scofano HM. Two simultaneous binding sites for nucleotide analogs are kinetically distinguishable on the sarcoplasmic reticulum Ca2+-ATPase. Biochem. 1996;35:3886–3891. doi: 10.1021/bi9518353. [DOI] [PubMed] [Google Scholar]
- 39.Ward DC, Cavieres JD. Affinity labeling of two nucleotide sites on Na,K-ATPase using 2’(3’)-O-(2,4,6-trinitrophenyl)8-axidoadenosine 5’-[α-32P] diphosphate (TNP-8N3-[α-32 P]ADP) as a photoactivatable probe. J Biol Chem. 1998b;273:33759–33765. doi: 10.1074/jbc.273.50.33759. [DOI] [PubMed] [Google Scholar]
- 40.Martin DW, Sachs JR. Ligands presumed to label high affinity and low affinity ATP binding sites do not interact in an (αβ)2 diprotomer in duck nasal gland Na+,K+-ATPase, nor do the sites coexist in native enzyme. J Biol Chem. 2000;275:24512–24517. doi: 10.1074/jbc.M003179200. [DOI] [PubMed] [Google Scholar]
- 41.Davis RL, Robinson JD. Substrate sites of the (Na++K+)-ATPase: pertinence of the adenine and fluorescein binding sites. Biochim Biophys Acta. 1988;953:26–36. doi: 10.1016/0167-4838(88)90006-4. [DOI] [PubMed] [Google Scholar]
- 42.Freire MM, Mignaco JA, de Carvalho-Alves BH, Scofano HM. 3-O-methylfluorescein phosphate as a fluorescent substrate for plasma membrane Ca2+-ATPase. Biochim Biophys Acta. 2002;1553:238–48. doi: 10.1016/s0005-2728(01)00245-6. [DOI] [PubMed] [Google Scholar]
- 43.Swann AC, Albers RW. Na,K-ATPase of mammalian brain: differential effects on cation affinities of phosphorylation by ATP and acetylphosphate. Arch Biochem Biophys. 1980;203:422–7. doi: 10.1016/0003-9861(80)90195-2. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki A, Kaya S, Tsuda T, Araki Y, Hayashi Y, Taniguchi K. An extra phosphorylation of Na+,K(+)-ATPase by paranitrophenylphosphate (pNPP): evidence for the oligomeric nature of the enzyme. J Biochem (Tokyo) 1994;116:1360–9. doi: 10.1093/oxfordjournals.jbchem.a124688. [DOI] [PubMed] [Google Scholar]
- 45.Esmann M, Fedosova NU. Anion interactions with Na,K-ATPase: simultaneous binding of nitrate and eosin. Eur Biophys J. 2004:683–690. doi: 10.1007/s00249-004-0411-6. [DOI] [PubMed] [Google Scholar]


