Abstract
The potential presence of maternal cell contamination (MCC) in chorionic villus or amniotic fluid samples poses a serious preanalytical risk for prenatal misdiagnosis. The aim of this study was to identify current diagnostic practices in the absence of comprehensive practice guidelines. Thirty-five clinical molecular laboratories that conduct prenatal testing agreed to participate in a clinical practice survey. The survey included questions about sample requirements, test indications, assay type, test performance and limitations, criteria and management of uninformative test results, reporting, and billing. Sixty percent of participating laboratories performed testing on direct and cultured amniotic fluid, whereas forty percent tested cultured cells only. Most also accepted chorionic villus samples. Although MCC testing of fetal samples is recommended in guidelines by the American College of Medical Genetics, only 60% of surveyed laboratories performed it without exception. Commercially available assays were used by 75% of participating laboratories, and at least five identity markers were evaluated at 87% of the laboratories. The reported lower limit of MCC detection ranged from 1 to 20% but was not determined in all laboratories. MCC testing was performed in the majority of molecular diagnostic laboratories, but guidelines for standardization are needed to ensure optimal and accurate prenatal patient care.
Increasing knowledge of inherited genetic conditions, the characterization of associated genes, and continuing advances in diagnostic techniques have enabled genetic testing of prenatal samples in cytogenetic and molecular laboratories. Prenatal diagnosis of genetic disorders, however, is often not straightforward. On the one hand, amniotic fluid (AF) or a chorionic villus sample (CVS) is obtained by the obstetrician/gynecologist during an invasive procedure that is associated with potential pregnancy loss. Thus, the samples are essentially irreplaceable, and often only small amounts or suboptimal specimens are submitted. The laboratory testing, on the other hand, depends on the certainty that the tested material is of fetal origin. It also requires robust assay performance with unambiguous and accurate test results, because a positive result for a genetic condition may result in termination of the pregnancy. In addition, laboratories face demands of fast turn-around-time to minimize anxiety in the prospective parents and to allow for counseling with informed decision making during the ongoing pregnancy.
The potential presence of maternal cells in CVS or AF samples poses a significant preanalytical risk for prenatal misdiagnosis. This is particularly of concern with sensitive polymerase chain reaction (PCR)-based molecular assays that may lead to a positive result based on the presence of a very small amount of mutation-positive maternal cells. Even though the risk of maternal cell contamination (MCC) in CVS or AF may not be entirely avoidable, the magnitude of this risk depends on several variables. MCC is more common with clinicians who perform less than 50 amniocenteses annually, and these physicians also have a higher rate of fetal loss after the procedure. Thus, routine performance of this procedure seems paramount to achieving and maintaining competence.1 Another factor is the technique used during sample collection. If the first 2 to 5 ml of an AF sample are discarded together with the first syringe and the actual sample is obtained after attachment of a second syringe, the risk of MCC will be smaller. The risk of MCC may increase with the number of needle passes, penetration of the placenta, and lack of ultrasound guidance during the procedure.1,2,3 Sample processing also ultimately influences this risk. Uncultured (direct) AF cells have a higher chance of MCC than cultured cells because during the culturing process itself, growth of amniocytes is enhanced, but that of peripheral blood cells is not.4 In a CVS tissue specimen, however, both cell types are cultured when chorionic villi are not well separated from the maternal decidua. Thus, CVS cultures present the highest level of potential MCC.5
The only practice guidelines available for the assessment of MCC during prenatal testing are described in the 2006 edition of Standards and Guidelines for Clinical Genetics Laboratories at http://www.acmg.net/Pages/ACMG_Activities/stds-2002/g.htm (prenatal testing section G19, first added in 2003). Considering the potential risk for misdiagnosis in the event of co-amplification of maternal sequences with the prenatal sample, we endeavored to investigate current national testing practices. We report our findings of a large national survey of 35 laboratories that offer prenatal genetic services and provide suggestions for standardization and quality assurance.
Materials and Methods
Laboratories in the United States were selected for the study based on their Genetests (http://www.genetests.org/) entries for prenatal diagnostic testing of the relatively common inherited disorders cystic fibrosis and α-thalassemia. Thirty-five of the 46 clinical molecular laboratories initially approached agreed to participate in the clinical practice survey. The survey included questions about sample type tested (direct AF, cultured AF, direct CVS, cultured CVS), the testing algorithm used if more than one sample type was accepted for testing in the laboratory, sample requirements, test indications, type of assay used, test performance and limitations, criteria for and management of uninformative test results, reporting, and billing. The questionnaire is summarized in Figure 1. Interviews with the medical directors or technical supervisors of the participating laboratories were conducted during one or more phone conversations.
Figure 1.
Clinical practice questionnaire, used in phone interviews.
Results
Samples
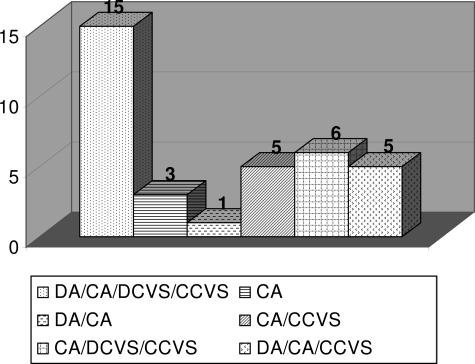
All participating molecular diagnostic laboratories offered AF testing. Sixty percent (21 of 35) performed diagnostic prenatal testing on direct and cultured AF, whereas 40% (14 of 35) used only cultured AF cells. The majority of participating laboratories also offered CVS testing (88.6%, 31 of 35). Direct, as well as cultured CV samples were tested in 67.7% of those facilities (21 of 31). Ten laboratories tested CVS cultures but not direct CVS. Four did not offer CVS testing at all (Figure 2). The laboratories that offered testing on both direct and cultured cells made their decision about which sample type to use based on the following considerations: 1) sample amount requirements for PCR-based assays versus Southern blots, 2) gestational age and urgency of diagnostic testing, and 3) presence or absence of evidence for MCC in a direct prenatal specimen. Five laboratories indicated that they always perform a follow-up cell culture, to be used as a backup for the direct specimen and for confirmatory diagnostic testing. Two of these offered testing on all four specimen types (Figure 2), two offered testing on cultured AF as well as direct and cultured CVS, and one performed testing on direct and cultured AF only.
Figure 2.
Distribution of accepted specimen types in 35 molecular diagnostic laboratories. DA, direct amniotic fluid; CA, cultured amniotic fluid; DCVS, direct chorionic villus sample; CCVS, cultured chorionic villus sample.
Of the 35 participating laboratories, 24 performed MCC testing in-house at the time of the survey. Four were in the process of setting up the assay and had already decided on the sample type they would accept, and three other laboratories sent their samples to a reference laboratory for MCC evaluation. Four laboratories did not perform MCC testing at all. The quantity of material requested for direct AF testing ranged from 2 to 25 ml, whereas the sample requirements for direct CVS testing ranged from no minimum to 30 mg (Figure 3, a and b). None of the participating laboratories, however, rejected samples smaller than the requested amount. If the volume was insufficient to perform the ordered assay, those laboratories that had the capability would proceed to culturing the specimen. Specimens were typically cultured to a stage of one to two T25 tissue culture flasks, although one laboratory routinely cultured to four T25 flasks, and another laboratory required no more than a single confluent coverslip to proceed to PCR-based assays.
Figure 3.
Sample requirements. a: Tissue sample requirements for direct CVS testing (n = 21 laboratories). b: Sample volume requirements for direct AF testing (n = 21 laboratories).
MCC Testing Practices
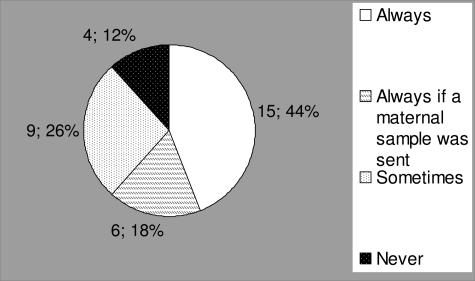
Testing for MCC in prenatal samples is considered the standard of care as described in the 2006 edition of Standards and Guidelines for Clinical Genetics Laboratories at http://www.acmg.net/Pages/ACMG_Activities/stds-2002/g.htm (prenatal testing section G19, first added in 2003). One laboratory in the process of developing an MCC assay remained undecided about their future MCC testing practice at the time of the survey. Thus, only 34 laboratories are represented in Figure 4. However, only 15 of 34 (44.1%) of surveyed laboratories reported a testing practice of performing it without exception, and 6 of 34 (17.7%) performed it always except if no maternal sample was received. In such cases, the prenatal testing was completed but reported with a disclaimer. Twelve percent (4 of 34) of surveyed laboratories never performed MCC testing. Those who answered that they only performed MCC testing “sometimes” had a variety of reasons for their policies. These included testing for MCC only when there was a questionable diagnostic result that would indicate to the laboratory that MCC might have been present, performing MCC testing only if a dissection of the maternal decidua was felt to have been inadequate, or when a sample appeared visually bloody. These reasons assume that an occurrence of MCC can be reliably assessed during one or more stages of diagnostic sample processing or interpretation. Another set of deciding factors to perform MCC analysis was when a fetal sample demonstrated a positive molecular result or in the case of an autosomal recessive disorder when the maternal mutation was present in the fetal sample. In these examples, MCC would not be performed when only the paternal mutation was identified in the fetus. These reasons assume that a negative contaminating maternal sample would not interfere with a positive result, if present, in the fetus. In some other laboratories, however, MCC testing was performed for a fetal sample with a negative result, or if the fetus was female and cytogenetic testing was to follow. Other laboratories reported that MCC was not thought to be an issue when male fetuses were tested for X-linked disorders or because the laboratory had never received feedback about occurrence of an incorrect prenatal result.
Figure 4.
MCC testing practice for 34 US laboratories. Four laboratories never test for MCC, nine perform MCC testing on occasion, six performed it whenever a maternal sample was available, and 15 performed it without exception.
MCC Assays
One survey question addressed the type of MCC assay used in each laboratory. This question was answered by the 24 laboratories that currently performed MCC testing in-house. In addition, three of the four laboratories that were developing MCC testing had decided on their approach, and one of three laboratories that sent out their samples for MCC testing was readily familiar with the method used by their reference laboratory. A variety of commercially available DNA typing assays (by Applied Biosystems, Foster City, CA, and Promega, Madison, WI) were used by 71.4% (20 of 28) of participants, whereas 28.6% (8 of 28) had developed a method in their own laboratory. The number of identity markers included in individual tests was comparable between commercial and homebrew MCC assays with 4 to 16, and 2 to 13, respectively. Overall, however, fewer markers were used in homebrew assays. Only three laboratories used four markers or fewer (10.7%).
Assay Sensitivity
The reported lower limit of detection for the MCC assays included in this study ranged from 1 to 20% but was not determined in all laboratories (Figure 5). This question (number 6 in Figure 1) was answered by 27 laboratories, excluding the four laboratories that never performed MCC testing and the four laboratories that were in the process of setting up the assay. In Figure 5, the categories of reported lower limits of detection are reflected by the individual columns in the histogram, with the number of laboratories representing each category listed on top of the columns. The one laboratory that indicated a sensitivity of 20% stated that a 20% control was included in the assay because alleles at one or more markers were sometimes lost in their commercial assay at lower levels of contamination.
Figure 5.
The reported lower limit of detection for MCC in US diagnostic laboratories. The lower limit of detection is indicated in the legend on the right and by the columns of the histogram, whereas the number of laboratories for each category is listed on top of the columns. Each individual column represents a reported sensitivity category of the MCC assays in use. This question was answered by 27 laboratories. Individual methods for determination of the lower limit of detection were not disclosed.
Assay Interpretation and Reporting
As a consequence of allelic identity between mother and fetus, an MCC assay can be uninformative. The number of informative markers between the maternal and fetal samples deemed necessary for an overall informative test interpretation was reported to range from one to four for validated assays. One laboratory, using three to eight identity markers as needed, reported a percentage (75 to 80%) of informative markers instead of a number. If the assay was uninformative by their own criteria, additional markers were added by two thirds of the laboratories, whereas one third did not expand the assay. Not surprisingly, the latter testing facilities all used nine markers or more (up to 16). Uninformative results had not been encountered by 12 laboratories. All but one of these, however, required only two markers to be informative. The single laboratory that required four informative markers used an assay developed in-house with up to 10 markers, adding them as needed to reach a status of informative results.
The 19 laboratories that provided a report for MCC assays with uninformative results and with no evidence of MCC in any of the markers tested, reported to sign out such results as “uninformative” or “equivocal.” A comment was added reflecting that MCC was unlikely but could not be excluded or stating that the results are consistent with fetally derived cells and that no MCC was detected. Of the 28 laboratories that were offering MCC in-house or would offer it very soon and had decided on the content of their reports, four provided only a written report and 23 offered a written report with verbal consultation, as necessary. Verbal communication was most often used for complex cases, or performed routinely for all cases positive for MCC. One laboratory provided MCC testing but did not issue a report because this assay was not considered part of the diagnostic testing ordered by the clinician. In this laboratory, MCC testing was primarily interpreted for internal QA/QC purposes. Of note, however, this laboratory had only recently initiated MCC testing and had not yet encountered MCC in a prenatal sample.
Billing Practices for MCC Assays
Twenty-nine laboratories commented on their billing practice. Of these, 58.6% (17 of 29) billed separately, and 27.6% (8 of 29) billed for the MCC test as an integral part of the performed prenatal test. Four laboratories provided MCC testing at no cost at all. Of the 17 laboratories that charged for MCC testing separately, 15 provided information about pricing. Twelve laboratories charged less than $300, and one laboratory, which uses a variable number of markers (depending on informativeness), charged less than $300 when less than eight markers were used and between $300 and $500 when 13 markers were used. One additional laboratory was in this price range. Finally, at the time of the survey, a single laboratory charged between $500 and $800.
Discussion
Amniocentesis is the most frequently applied procedure for prenatal diagnosis of inherited conditions.1 It is a minor surgical intervention typically performed in the middle trimester to ensure the presence of adequate numbers of fetal cells.6,7 One way of measuring successful outcome of this process is by evaluation of procedure-related fetal loss rate, which is ∼0.6% for amniocenteses performed by obstetricians who use concurrent ultrasound guidance.2 The quality of the amniotic specimen itself, however, is another important component of successful outcome. Contamination with maternal cells can result in interpretation errors of diagnostic tests, including mutation analyses and detection of aneuploidy by fluorescent in situ hybridization. Even low levels of MCC may interfere with correct molecular diagnoses because PCR can, under optimized circumstances, detect a subpopulation of cells at levels of 0.1%. This level is routinely achieved in quantitative diagnostic assays used for the determination of chimerism or of minimal residual leukemic disease after bone marrow transplantation. MCC may also affect cytogenetic diagnoses. One major issue of concern is that fluorescent in situ hybridization alone will not detect the presence of MCC when the fetus is female. Fluorescent in situ hybridization results may seem mosaic whereas in fact all fetal cells are abnormal. In addition, false-negative fluorescent in situ hybridization results, caused by MCC of uncultured amniocytes, have been reported in up to 7.5% of trisomic fetuses.8,9,10 Unfortunately, MCC cannot be reliably assessed by eye because the visual presence of erythrocytes does not necessarily mean that these cells are of maternal origin, and, conversely, the visual absence of peripheral blood in the sample does not mean that the sample is exclusively fetal. Even entirely clear direct AF has been reported to be affected by MCC.4 One very important measure to reduce the risk of MCC during the collection of AF is to discard the first 2 to 5 ml of the sample to remove maternal lymphocytes or tissue fibroblasts.1,11 Finally, it is worth mentioning that MCC can be an issue in settings other than prenatal molecular or cytogenetic testing: with the use of human umbilical cord blood in transplantation, maternal T cells might elicit graft-versus-host disease after cord blood transplantation.12
Compared with AF, CVS is performed at ∼11 weeks of gestation and has a higher overall risk of MCC (<5%), because it is difficult to thoroughly remove the maternal decidua from the fetal cells.11 CVS is used for prenatal diagnosis of genetic disorders, as well as for cytogenetic analysis of tissue from pregnancy losses, providing important medical information and answers to couples affected by spontaneous abortion. In such samples, Bell and colleagues13 described erroneous 46, XX karyotypes with a frequency of 29% and 5% in the first and second trimesters, respectively. However, because only those male fetuses that were misdiagnosed as females were detected, the incidence of MCC could potentially be twice as high. The correct karyotype, which may have been aneuploid, might not be identified as a reason for the pregnancy loss. Molecular analysis of MCC, therefore, is a valuable adjunct to cytogenetic testing of products of conception. Factors that can contribute to an erroneous karyotype include the amount and preservation of the villi, the amount of residual maternal tissue, and perhaps characteristics of the conceptus such as the growth rate of the cells and genetic composition.13 The risk of MCC in CVS may be minimized by optimal sampling procedures and careful dissection.11
AF and CVS have their respective advantages and disadvantages, reflected in the spectrum of accepted sample types by the surveyed diagnostic laboratories (Figure 2). For AF samples, direct testing may reduce turn-around-time by ∼2 weeks and avoids the additional cost of culture. AF cell culture, however, allows a preferential expansion of fetal cells. At the same time, it decreases contamination with maternal hematopoietic cells, which senesce sooner and are not expected to proliferate.7 Prolonged culture, however, may overcome the initial limitation and allow growth of maternal fibroblasts and epithelial cells, potentially leading to detectable MCC.6 For CVS, culture actually increases the risk of detectable MCC because both maternal and fetal cells are part of the same tissue sample. In general, the risk of MCC is greater early in gestation, because of the nature of the specimen obtained, but also because of the relative paucity of cells, in which the admixture of maternal cells may be relatively larger.7
Even though MCC has been recognized as a potential cause of misdiagnosis in prenatal testing, our survey of 35 clinical diagnostic laboratories indicated that these laboratories use different assays and MCC testing practices. One example of variation between laboratories was in the number of markers in the MCC assay, which ranged from 2 to 16. Although the number of markers used is somewhat arbitrary, the inclusion of a low number of markers may provide a false sense of security if MCC is not detected. Especially when the percentage of maternal cells contaminating the prenatal sample is very low (∼1%), the maternal allele may not be detectable in every single marker. Thus, more information can be gleaned from an expanded assay with multiple markers associated with a requirement of several informative markers. The vast majority of participating laboratories used two or three markers to determine that the assay was informative. Another example of variation between laboratories was in the reported lower level of detection for MCC assays, which varied from 1 to 20% (Figure 5). Red blood cell contamination with just a few percent of cells is readily visible. Although the presence of red blood cells in an AF sample could represent either maternal or fetal cells, this finding should raise a suspicion and prompt testing for MCC. Hence, the lower level of detection is especially relevant in those samples in which MCC is not immediately obvious. In an experiment of contamination with foreign nucleated cells, trace detection was evident in a PCR-based assay at 0.1%.7 This percentage, however, will necessarily depend on individual assay conditions and primer sets. It has been suggested that misinterpretation of a diagnostic prenatal test becomes a more likely possibility at 1 to 2% MCC.7 In a very simple variable number of tandem repeats-PCR-based method, a level of 2% MCC could unequivocally be identified with two variable number of tandem repeats and identification by regular gel electrophoresis.10 Thus, even without expensive instruments, this level of detection should be achievable for robust assays. The practical aim of MCC assays should be to determine how often one can reliably detect MCC instead of how often one will detect it with 100% of the markers.
The different modes of inheritance associated with genetic conditions deserve brief consideration because they may be impacted by MCC in different ways. For conditions with autosomal recessive inheritance, one quarter of the tested fetuses are expected to carry the maternal mutation and cannot be distinguished from the mother by mutation analysis alone. It has been proposed to perform the diagnostic assay first and only to proceed to MCC testing if the fetal sample appears positive for the known maternal mutation. This would reduce the number of MCC assays in cases of testing for autosomal recessive conditions by almost 50%, if all cases of multiple gestation and those in which both parents carry the same mutation would still receive MCC testing.11 If the prenatal sample was a mutation-positive direct or cultured CVS tested for cystic fibrosis, however, then it was suggested to combine the MCC test with an assay for uniparental disomy if none of the MCC markers was located on chromosome 7. This would allow identification of trisomy 7, which is one of the most common aneuploidies in CVS.11,14 For dominant disorders, MCC testing can be performed to exclude that a positive result is attributable to MCC. For both autosomal dominant and autosomal recessive disorders, however, the possibility of a negative result attributable to maternal nonaffected status may not be negligible, although this risk may be most prominent for CVS.
General standards and guidelines for prenatal testing are available from the American College of Medical Genetics (2006 Edition of Standards and guidelines for clinical genetics laboratories, http://www.acmg.net/Pages/ACMG_Activities/stds-2002/g.htm), but these may not have been taken into consideration by all participating laboratories. Surprisingly, 12% of laboratories in our survey never performed MCC testing on prenatal samples. Although MCC testing was performed by the majority of molecular diagnostic laboratories, more specific guidelines and suggestions for standardization would, in our opinion, benefit the quality of both diagnostic testing and patient care. Opinions on MCC testing are likely to be diverse, and recommendations about the standard of practice are best determined by professional practice organizations. In the interim, however, we present a practical testing algorithm based on the results of our study, the literature, and currently available guidelines (Figure 6).
Figure 6.
A practical testing algorithm for prenatal samples.
To eliminate errors in prenatal testing as much as possible, we recommend that MCC testing be performed on all prenatal samples and for all modes of inheritance. With this approach, diagnostic laboratories obtain the greatest amount of information to address optimally the pitfalls of prenatal testing. Although some laboratories request samples from both the mother and the father, only the maternal sample is strictly required to perform MCC testing, also avoiding issues of potential nonpaternity. For any prenatal sample, a larger volume or tissue amount, older gestational age, and highly robust prenatal assays are preferable. The laboratory should be familiar with the band intensities of specific primers and be alert to novel bands or changes in band intensities.7 They should also have determined the sensitivity of their MCC assay. For every sample, a back-up culture should be maintained. This is essential in cases in which MCC was detected, when the sample amount or quality of a direct sample did not suffice for testing, or to confirm positive results. This need was illustrated by a prenatal sample of poor quality received by the Stanford Molecular Pathology Laboratory. It demonstrated weak general amplification and the presence of a band for the South East Asian deletion in a multiplex assay for α-globin deletions. The band representing the normal α-globin gene, expected to be present in unaffected and heterozygous samples, was absent even though a control amplicon of higher molecular weight had amplified appropriately. The sample was tested again after cultured cells were available, demonstrating unequivocally that the fetus was heterozygous and that the selective amplification of the normal α-globin fragment had previously failed. Thus, the cultured specimen was essential to a correct diagnosis. In this survey, only five laboratories indicated that they always perform a follow-up culture for purposes of backing up the direct specimen and for confirmatory diagnostic testing.
The utility and efficiency of the algorithm proposed in Figure 6 can be illustrated with a scenario similar to the described α-thalassemia case. Using the algorithm, a culture is started from a direct AF sample on receipt, whereas MCC testing proceeds concurrently on the direct AF itself. When MCC testing is negative, the diagnostic assay is performed on the DNA extracted from the direct AF sample. If the diagnostic test is positive, it is repeated with the cultured AF cells for confirmation. Only then is the result finalized and reported.
Although the presence of MCC does not always lead to diagnostic errors, if such errors do occur, the consequences in the prenatal setting could result in an inappropriate termination of a pregnancy. This large survey of 35 US diagnostic laboratories that perform prenatal testing demonstrates that the majority perform MCC testing in conjunction with the diagnostic test. However, it also illustrates that clinical practice is not yet standardized. This emphasizes the need for specific guidelines to ensure consistent and optimal prenatal patient care.
Acknowledgments
We thank all participating laboratories for their participation in this study.
References
- Welch RA, Salem-Elgharib S, Wiktor AE, Van Dyke DL, Blessed WB. Operator experience and sample quality in genetic amniocentesis. Am J Obstet Gynecol. 2006;194:189–191. doi: 10.1016/j.ajog.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Seeds JW. Diagnostic mid trimester amniocentesis: how safe? Am J Obstet Gynecol. 2004;191:607–615. doi: 10.1016/j.ajog.2004.05.078. [DOI] [PubMed] [Google Scholar]
- Steed HL, Tomkins DJ, Wilson DR, Okun N, Mayes DC. Maternal cell contamination of amniotic fluid samples obtained by open needle versus trocar technique of amniocentesis. J Obstet Gynaecol Can. 2002;24:233–236. doi: 10.1016/s1701-2163(16)30223-7. [DOI] [PubMed] [Google Scholar]
- Winsor EJ, Silver MP, Theve R, Wright M, Ward BE. Maternal cell contamination in uncultured amniotic fluid. Prenat Diagn. 1996;16:49–54. doi: 10.1002/(SICI)1097-0223(199601)16:1<49::AID-PD808>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Saura R, Roux D, Taine L, Maugey B, Laulon D, Laplace JP, Horovitz J. Early amniocentesis versus chorionic villus sampling for fetal karyotyping. Lancet. 1994;344:825–826. doi: 10.1016/s0140-6736(94)92384-1. [DOI] [PubMed] [Google Scholar]
- Smith GW, Graham CA, Nevin J, Nevin NC. Detection of maternal cell contamination in amniotic fluid cell cultures using fluorescent labelled microsatellites. J Med Genet. 1995;32:61–64. doi: 10.1136/jmg.32.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson RM, Wang HS, Surh LC. Some caveats in PCR-based prenatal diagnosis on direct amniotic fluid versus cultured amniocytes. Prenat Diagn. 1999;19:113–117. doi: 10.1002/(sici)1097-0223(199902)19:2<113::aid-pd475>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Witters I, Devriendt K, Legius E, Matthijs G, Van Schoubroeck D, Van Assche FA, Fryns JP. Rapid prenatal diagnosis of trisomy 21 in 5049 consecutive uncultured amniotic fluid samples by fluorescence in situ hybridisation (FISH). Prenat Diagn. 2002;22:29–33. doi: 10.1002/pd.225. [DOI] [PubMed] [Google Scholar]
- Batanian JR, Ledbetter DH, Fenwick RG. A simple VNTR-PCR method for detecting maternal cell contamination in prenatal diagnosis. Genet Test. 1998;2:347–350. doi: 10.1089/gte.1998.2.347. [DOI] [PubMed] [Google Scholar]
- Ward BE, Gersen SL, Carelli MP, McGuire NM, Dackowski WR, Weinstein M, Sandlin C, Warren R, Klinger KW. Rapid prenatal diagnosis of chromosomal aneuploidies by fluorescence in situ hybridization: clinical experience with 4,500 specimens. Am J Hum Genet. 1993;52:854–865. [PMC free article] [PubMed] [Google Scholar]
- Antoniadi T, Yapijakis C, Kaminopetros P, Makatsoris C, Velissariou V, Vassilopoulos D, Petersen MB. A simple and effective approach for detecting maternal cell contamination in molecular prenatal diagnosis. Prenat Diagn. 2002;22:425–429. doi: 10.1002/pd.325. [DOI] [PubMed] [Google Scholar]
- Tsang KS, Wong AP, Cheung MS, Tang SH, Leung Y, Li CK, Lau TT, Ng MH, Yuen PM. Implication of maternal-cell contamination in the clinical banking of umbilical cord blood. Cytotherapy. 2002;4:375–383. doi: 10.1080/146532402760271163. [DOI] [PubMed] [Google Scholar]
- Bell KA, Van Deerlin PG, Haddad BR, Feinberg RF. Cytogenetic diagnosis of “normal 46, XX” karyotypes in spontaneous abortions frequently may be misleading. Fertil Steril. 1999;71:334–341. doi: 10.1016/s0015-0282(98)00445-2. [DOI] [PubMed] [Google Scholar]
- Le Bris MJ, Giovangrandi Y, Audrezet MP, Journel H, Ferec C. Confined placental trisomy 7: pitfall for cystic fibrosis prenatal diagnosis. Lancet. 1994;344:556. doi: 10.1016/s0140-6736(94)91955-0. [DOI] [PubMed] [Google Scholar]