Abstract
Vasculogenesis, the formation of blood vessels in embryonic or fetal tissue mediated by immature vascular cells (ie, angioblasts), is poorly understood. We report the identification of a population of vascular progenitor cells (hVPCs) in the human fetal aorta composed of undifferentiated mesenchymal cells that coexpress endothelial and myogenic markers. Under culture conditions that promoted cell differentiation, hVPCs gave rise to a mixed population of mature endothelial and mural cells when progenitor cells were stimulated with vascular endothelial growth factor-A or platelet-derived growth factor-ββ. hVPCs grew as nonadherent cells and, when embedded in a three-dimensional collagen gel, reorganized into cohesive cellular cords that resembled mature vascular structures. hVPC-conditioned medium contained angiogenic substances (vascular endothelial growth factor-A and angiopoietin-2) and strongly stimulated the proliferation of endothelial cells. We also demonstrate the therapeutic efficacy of a small number of hVPCs transplanted into ischemic limb muscle of immunodeficient mice. hVPCs markedly improved neovascularization and inhibited the loss of endogenous endothelial cells and myocytes, thus ameliorating the clinical outcome from ischemia. We conclude that fetal aorta represents an important source for the investigation of the phenotypic and functional features of human vascular progenitor cells.
Vasculogenesis, the primary differentiation of endothelial cells (ECs) from angioblasts, was considered to be restricted to early embryogenesis.1,2 Recently, this belief has been challenged by the demonstration that endothelial progenitor cells (EPCs) persist into adulthood.3,4,5,6 Progenitor cells are identified by their capacity to express specific cellular membrane epitopes or nuclear proteins. Circulating CD34+ EPCs carry hematopoietic antigens,7 which suggests that hematopoietic cells and ECs may share a common progenitor.8 This hypothesis is also supported by the observation that mature ECs are obtained from AC133/CD133+ bone marrow cells, a subset of CD34+ hematopoietic progenitors.7,9 Given that blood vessel wall-composing ECs and mural cells may originate from a common mesenchymal precursor,10 it has been hypothesized that different subsets of circulating EPCs exist, which either are committed to the endothelial cell lineage only or have the capacity for myogenic differentiation.11,12,13 The latter possibility is supported by the observation that, in postnatal life, murine bone marrow-derived circulating progenitors differentiate into skeletal muscle, thus indicating that the fate of adult mesodermal progenitors is unexpectedly complex.14 Furthermore, a common mesenchymal progenitor (mesoangioblast) coexpressing both endothelial and myogenic markers was identified in the rat dorsal aorta at early embryonic stages.15 We previously identified cells phenotypically similar to EPCs in the outer layer of the aortic stroma of 11- to 12-week-old human fetuses. These cells express CD34 and vascular endothelial growth factor (VEGF) receptor-2 (KDR) but are negative for mature endothelial markers.16 The potential of these cells to differentiate into mural cells was not investigated. In this study, we addressed the following questions: 1) Do human fetal aorta (hFA)-derived cells possess the characteristics of embryonic mesoangioblasts, and if so, are they capable of forming mature and functionally competent vessels? 2) Are hFA-derived vascular progenitor cells (hVPCs) able to produce angiogenic factors? 3) Are hVPCs exploitable for clinical purposes, namely to induce reparative neovascularization and to protect and/or regenerate skeletal muscles damaged by ischemia?
Materials and Methods
Human Fetal Tissues
Samples of 11- to 12-week-old hFA, were obtained according to local ethical and biohazard regulations and following the ethical guidelines of the Network for European CNS Transplantation and Restoration. The experimental protocol was approved by the ethics committee of the National Neurological Institute “Carlo Besta” (Milan, Italy) and Fondazione IRCCS Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena.
Flow Cytometry
After collagenase (collagenase D; Roche, Mannheim, Germany) digestion (2 hours at 37°C) of human fetal aorta, the coexpressions of CD133+, CD34+, and KDR+ cells were analyzed by flow cytometry (FACS). The analysis was performed by incubating cells with phycoerythrin-conjugated anti-CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany), fluorescein isothiocyanate-conjugated anti-KDR (BD Pharmingen, Franklin Lakes, NJ), and Cy5-conjugated anti-CD34 (BD Pharmingen) monoclonal antibodies.
FACS was also performed on freshly immunoselected CD133+ cells and during their culture to determine their phenotypic profile. Anti-CD45 and anti-CD105 antibodies were purchased from BD Pharmingen, fluorescein isothiocyanate-labeled anti-CD146 was purchased from Cabru (Milan, Italy), anti-CD31 was from BD Pharmingen, and tetramethylrhodamine B isothiocyanate-Ulex europaeus agglutinin-1 lectin (UEA-1) was from Sigma (St. Louis, MO).
For each mouse antibody analyzed, isotype-matched mouse immunoglobulin (BD Pharmingen) served as control. Single- and three-color flow cytometric analyses were performed, using a FACS scan flow cytometer and Cell Quest software (BD Pharmingen).
CD133+ Cell Isolation
Specimens of hFA were excised under a dissection microscope. After several washes, the aortas were mechanically minced with scissors and then dissociated for 2 hours at 37°C in Dulbecco’s medium (Gibco, Grand Island, NY) containing 0.2% bovine serum albumin, 0.2% collagenase, and 0.01% DNase (Sigma). On dissociation, cells were washed twice in phosphate-buffered saline (PBS) by centrifugation at 1000 rpm × 10 minutes at 4°C. Cellular suspensions were passed through 10-μm pore size filters to remove cell aggregates. The cells were first incubated with anti-CD31 conjugated to magnetic beads (Dynal Biotech, Hamburg, Germany) (5 μg of anti-human CD31/5 × 107 beads) to remove the presence of tissue-resident mature ECs. After removing the beads, CD31-negative cells were further incubated with anti-CD133-conjugated superparamagnetic microbeads (CD133 Isolation kit; Miltenyi Biotec), washed, and processed to obtain purified CD133+ cells. FACS analysis after separation with anti-CD133-coated magnetic beads showed that the purity ranged from 95.4 ± 1.5%.
Cell Cultures and hVPC Differentiation
hVPCs were cultured in serum-free human medium (HM-GM) consisting of Dulbecco’s modified Eagle’s medium/F12 (1:1) (Gibco) supplemented with 10 ng/ml epidermal growth factor (Peprotech Inc., Princeton, NJ), 20 ng/ml basic fibroblast growth factor (Tebu Bio, Le-Perray en Yvelines Cedex, France), and growth supplement as previously described.17 Under these culture conditions, hVPCs grew as nonadherent cells and formed spheroids. On spheroid dissociation, cells were passaged with collagenase D at a split ratio 1:2 once a week. They showed a doubling time of approximately 1 week, maintaining their initial phenotypic profile over a period of 2 to 3 weeks. Therefore, in vitro and in vivo studies were performed with cells cultured during this period of time.
Human fetal aorta-derived mature ECs (hFAECs) and HUVECs were isolated as described previously.16,18 Both EC lines were cultured in endothelial basal medium growth medium (EBM-GM) (EBM Bullet kit; Biowhittaker Cambrex, Milan, Italy) and passaged every 3 to 4 days at a split ratio of 1:3. Both EC cultures were used until five in vitro passages. Human adult EPCs were prepared from the blood of healthy volunteers by density gradient centrifugation (Histopaque-1077; Sigma) as previously described.4 EPCs at 4 days of culture were used for transplantation.
Vascular differentiation of the hVPCs was induced as previously reported.16 To enhance myogenic or endothelial differentiation, 50 ng/ml platelet-derived growth factor (PDGF)-ββ or 10 ng/ml VEGF-A (both purchased from Sigma) was added to EBM-GM. The cells were grown for up to 10 days, and the expression of mature endothelial cells [CD31, von Willebrand factor (vWf), and Tie-2] and of mural cell markers [α–smooth muscle actin (α-SMA), desmin, nerve/glial antigen 2 (NG2), and smooth muscle 22α] was analyzed by immunocytochemistry.
Evaluation of VEGF and Angiopoietin-2 mRNA Level by Reverse Transcriptase-Polymerase Chain Reaction
Total RNA from differentiated hVPCs and hVPCs was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. RNA from each sample was reverse transcribed with random hexamer primers and Superscript III reverse transcriptase (Invitrogen) following the manufacturer’s instructions. Specific primers for human VEGF were reported previously.19 Primers for human Angiopoietin-2 (ANG2) were as follows: sense, 5′-GATGGCAGCGTTGATTTTCA-3′; and antisense, 5′-ACATGCATCAAACCACCAGC-3′. Glyceraldehyde-3-phosphate dehydrogenase20 was used as internal control.
A PCR-Express (Thermo Electron Corporation, Waltham, MA) was programmed as follows: one cycle at 94°C for 2 minutes, 35 cycles at 94°C for 30 seconds, followed by a specific melting temperature for 30 seconds for each gene analyzed (at 72°C for 30 seconds) and finally one cycle at 72°C for 10 minutes.
Evaluation of Angiogenic Factors in hVPC Culture Supernatant
Approximately 2 × 104 hVPCs or differentiated hVPCs (as described above) resuspended in HM-GM were seeded into each well of an uncoated 24-multiwell plate. The conditioned culture medium (CCM) was aspirated at 24, 48, and 72 hours and rapidly frozen until used. The concentration of VEGF-A, stromal-derived factor-1 (SDF-1), and Ang-2 in CCM of hVPCs before and after differentiation was evaluated by enzyme-linked immunosorbent assay cytokines kit assay (R&D Systems, Minneapolis, MN). Because differentiated hVPCs grow faster than undifferentiated hVPCs, the absolute values of growth factors released in the CCM were normalized for an equivalent number of cells (105) for each sample.
Proliferation Assay and Cloning Experiment
hFAECs and HUVECs were diluted in EBM-GM to obtain a final concentration of 2 × 105 cells/ml that were seeded onto collagen-fibronectin-coated plates. EC growth response to hVPC-conditioned medium was evaluated in EBM (not supplemented with standard growth factors) plus 10% fetal calf serum, diluted 1:1, 1:2, and 1:4 with hVPC-conditioned medium. Seven days after incubation, cells were detached by trypsin and counted. Each test was run in triplicate and repeated with three different batches of hVPC-conditioned medium preparations. To evaluate that the cultured hVPCs were stem cells with the capacity to give rise to both endothelial and mural cell lineages, a cloning procedure was performed as previously described.17
Capillary-Like Structure Formation on Collagen Gel
As previously described for fetal aorta rings,16 we used a tridimensional collagen gel culture assay to investigate the capacity of the hVPCs to form capillary-like structures in vitro. After formation of hVPC spheroids (2 weeks), the cells were kept on ice before being added to collagen at a concentration of approximately 20 to 30 spheroids per 50 μl of collagen solution. After collagen gelification, 0.5 ml of EBM alone or supplemented with growth factors (VEGF-A, PDGF-ββ, and basic fibroblast growth factor) was added to each well. The formation of cords or capillary-like structures was followed for up to 10 days, and pictures were taken every 24 hours.
Immunocytochemistry
The cells plated on collagen-fibronectin-coated chamber slides (Nunc, Naperville, IL) were fixed for 10 minutes in cold 4% paraformaldehyde, washed with PBS, and incubated with PBS plus 0.1% Triton X-100 containing 10% normal goat serum (Gibco) and the appropriate dilution of rabbit anti-human vWf (1:80), mouse anti-human CD146 (1:100) (Cabru), mouse anti-human CD31 (1:100) (Sigma), anti-desmin (1:20) (Signet Laboratories, Dedham, MA), UEA-1 (1:100) (Sigma), anti-Tie-2 (1:100) (Chemicon, Temecula, CA), anti-NG2 (1:200) (Chemicon), or smooth muscle 22α (1:50) (Novocastra Laboratories, Newcastle, UK) overnight at 4°C.
Cells were then washed twice with PBS and incubated with a secondary antibody of the appropriate species, anti-rabbit IgG with a 1:1000 dilution (Cy2; Jackson Immunoresearch Laboratories Inc., West Grove, PA) or anti-mouse IgG with a 1:500 dilution (Cy3; Jackson Immunoresearch), for 45 minutes at room temperature. After washing, cells were incubated with 4,6-diamidino-2-phenylindole (1 μg/ml) diluted 1:15 for 20 minutes at room temperature in the dark. The cells were then washed twice with PBS and mounted after air-drying with Fluorsave (Calbiochem, La Jolla, CA). The coexpressions of desmin with Tie-2 and UEA-1 binding were analyzed as described above. In brief, tetramethylrhodamine B isothiocyanate-labeled UEA-1, anti-Tie-2, and anti-Desmin antibodies were diluted 1:100, 1:100, and 1:20, respectively. On the other hand, the cells were processed using the standard avidin-biotin peroxidase complex as previously described.17
In Vivo Studies
Immunodeficient CB-17/1crHsd-scid-bg mice21,22,23 (Harlan, Milan, Italy) were housed at constant room temperature (24 ± 1°C) and humidity (60 ± 3%). With mice under the effect of 2,2,2-tribromoethanol anesthesia (880 mmol/kg body weight intraperitoneally; Sigma), the left femoral artery was exposed, dissected free, and electrocoagulated in its upper part, as previously described.21 During the same procedure, undifferentiated hVPCs (2 × 104 cells, n = 16) were intramuscularly injected (total injected volume of 30 μl) into three different sites of the ischemic adductor muscle. Controls consisted of cell culture medium (CM, n = 16) or cells derived from the differentiation of hVPCs in EBM-GM for 10 days (n = 10) or of hEPCs (n = 7).
Postischemic Hemodynamic Recovery and Clinical Outcome from Ischemia
Animals received analgesic medication on application of ischemia, with treatment continued for the following 3 days after surgery. At 7 and 14 days from ischemia induction, a clinical score was calculated, based on the number of necrotic toes and occurrence of autoamputation of the foot. Mice showing no necrotic toe scored zero. One point was attributed for each necrotic toe. Five points were scored to mice with all toes necrotic or with foot amputation.
Mice were anesthetized and humanely sacrificed at first signs of amputation. At 7 and 14 days postischemia, the superficial blood flow (BF) of both feet was measured in anesthetized mice by color laser Lisca Doppler (Peri-med AB, Järfälla, Sweden), and the ratio of perfusion between ischemic to nonischemic foot was calculated as described previously.22 In addition, the deep BF of adductors was measured (at 14 days after Lisca Doppler analysis) by inserting an OxyLite/OxyFlow probe into the muscle (Oxford Optronic, Oxford, UK), as previously published.24
Histological Analysis
At 14 days from ischemia, anesthetized mice were perfusion fixed. Both adductor muscles were processed for paraffin embedding. Histological and immunohistochemical analyses were performed blinded. The percentages of transplant-derived cells incorporated into blood vessels and into myofibers of ischemic muscles were determined (n = 5 ischemic adductors for each of the EPC and hVPC groups). To this aim, a monoclonal mouse antibody targeting the human anti-nucleus antigen (hNAg) (Chemicon) was used (1:50 in PBS, 3% bovine serum albumin, and 0.05% Tween 20) on 3-μm-thick tissue sections. Sections were incubated at the same time with primary and secondary antibody (DAKO, Glostrup, Denmark), stained with streptavidin-conjugated peroxidase/diaminobenzidine (SCP/DAB), and counterstained with hematoxylin. Negative controls consisted of sections from muscles not receiving cell transplantation and sections from transplanted muscles in which a nonimmune mouse serum was used instead of the primary antibody. Positive controls consisted of human breast cancer samples obtained from biopsies. Sections were evaluated at ×1000 magnification. The number of hNAg-positive (hNAG+) ECs and hNAG+ myoblasts/myocytes per square millimeter of section was calculated. On the other hand, tissue sections were processed in the same way to analyze the expression of human CD31 and to evaluate the capacity of hVPCs to differentiate into mature ECs.
Capillary, arteriole, and myofiber density of ischemic and contralateral adductors was determined (n = at least 6 per group), as described previously.25 The percentage of sectional area occupied by myocytes was determined (n = at least 6 per group) by computer-assisted (Image ProPlus software; Media Cybernetics, Inc., Bethesda, MD) morphometric evaluation of muscular sections. From each section, at least 15 fields were randomly captured at ×200 magnification by using a Pixera videocamera (Pixera Corp., Los Gatos, CA). Finally, apoptosis in the ischemic muscles (at least n = 5 per group) was assessed by terminal deoxynucleotidyl transferase dUTP nick-end labeling assay as previously described.22
Immunofluorescence Analysis and Confocal Microscopy
To investigate the differentiation fate of transplanted hVPCs, we performed immunohistochemical staining of 5-μm-thick sections from adductor muscles (n = 5 hVPC-receiving ischemic adductors and their contralaterals plus n = 2 of their contralateral and n = 2 not transplanted ischemic muscle for control). Deparaffinized sections were heated for antigen unmasking in a microwave. Slides were then allowed to equilibrate at room temperature for 20 minutes. Successively, cells were permeabilized in PBS with 0.1% Triton X-100 for 10 minutes. After a gentle rinse in PBS, sections were exposed for 1 hour at 37°C to lectin I (FL-1201, fluorescein Griffonia simplicifolia lectin I, isolectin B4; Vector Laboratories, Burlingame, CA) or to mouse monoclonal anti-α-actin (1A4), mouse monoclonal anti-desmin (DE-U-10), rabbit polyclonal anti-laminin, or isotype matched controls. Each antibody was diluted in PBS, 3% bovine serum albumin, and 0.05% Triton X-100. After two washes in PBS, sections were incubated for 1 hour at 37°C with secondary antibodies against rhodamine red (goat anti-mouse IgG (H+L) or goat anti-rabbit IgG (Molecular Probes, Leiden, The Netherlands) and with fluorescein-conjugated anti-hNAg antibody (Chemicon) diluted 1:50 in PBS, 3% bovine serum albumin, and 0.05% Tween 20.
After two washes with PBS, nuclei were counterstained using membrane-permeant Hoechst 33342 (Molecular Probes). Slides were mounted using fluorescent mounting medium and then observed and analyzed by confocal laser-scanning microscope (Olympus, Hamburg, Germany).
Real-Time Quantitative Polymerase Chain Reaction
Total RNA and c-DNA from 40 to 50 mg of frozen adductor muscles containing undifferentiated hVPCs, differentiated hVPCs, or control (injected with vehicle) were performed as described above.
The relative expression of human MyoD and human MYF5 was evaluated by real-time quantitative polymerase chain reaction (PCR) using a GeneAmp 5700 Sequence Detection System (Applied Biosystems, Foster City, CA) in a PCR volume of 20 μl containing cDNA, the TaqMan Universal PCR Master Mix (with AmpliTaq Gold DNA polymerase), and the Target Assay Mix (all from Applied Biosystems) for 35 to 40 cycles. The glyceraldehyde-3-phosphate dehydrogenase gene was used as the endogenous control gene. The PCR profile consisted of 2 minutes at 50°C, 10 minutes at 95°C, and 45 cycles of amplification for 15 seconds at 95°C and 1 minute at 60°C. To ensure accuracy, each reaction was repeated three times.
The relative amount of mRNA from each gene was expressed as the ratio of each mRNA to that of glyceraldehyde-3-phosphate dehydrogenase and calculated according to the formula 2−ΔΔCT as described in the manufacturer’s instructions (Applied Biosystems).
Statistical Analysis
All results are expressed as mean ± SD. One-way analysis of variance for multiple comparisons was performed. Comparisons between two groups were then done by t-test. The comparative incidence of toe necrosis was evaluated by one-way analysis of variance for multiple comparisons followed by Kruskal-Wallis or Mann-Whitney tests (rank sums). A P value <0.05 was interpreted to denote statistical significance.
Results
FACS Analysis on Human Fetal Aorta Cells
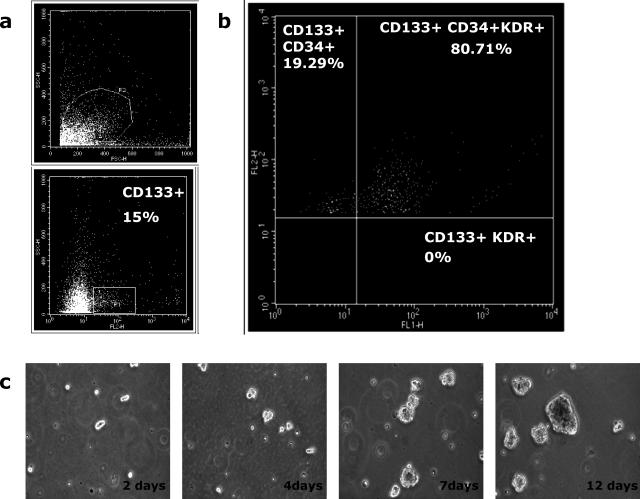
Adult endothelial progenitors are mostly characterized by the expression of CD34, CD133, and KDR. Thus, using FACS analysis, we explored the coexpression of these markers on freshly digested cells from 10 hFAs. Consistent with our previous observations,16 we found that the CD34+ relative abundance in hFA is routinely very high (38 to 48%) (data not shown). Using fluorescein isothiocyanate-labeled monoclonal antibodies, we found that KDR+ was also abundant in hFA (14 to 21%) (data not shown) and that CD133, a marker associated with neural stem cells,26 was from 13 to 16.6% (Figure 1a), whereas the expression of CD31 ranged from 9 to 13% of the total hFA cells.
Figure 1.
Coexpression of CD133, CD34, and KDR in human fetal aorta was analyzed on freshly isolated cells after collagenase dissociation of the tissues. Coexpression of markers was measured by activated cell sorting analysis. a: Live freshly digested CD133+ cells. b: Percentages of triple-positive cells are reported in the top right panel. Representative panels are of at least three independent experiments. c: Time-course live culture of immunoselected CD133+CD34+KDR+ cells derived from hFA. Cells in HM-GM grown as floating cluster of cells forming spheroids. Magnification, ×10.
Next, we analyzed the coexpression of CD34, CD133, and KDR. CD133+ cells were gated and analyzed for the expression of KDR and CD34: triple-positive cells were 80.71 ± 3% (Figure 1b). We previously reported that KDR and CD34 are strongly associated with a specific cell subset of aortic progenitor cells.16 The present study expands these observations, demonstrating that most CD133+cells are also CD34+KDR+. On removal of CD31+ cells to discard potential contamination of pre-existing mature ECs, CD133+ cells were immuno-selected by the use of magnetic microbeads from a pool of five hFAs and further analyzed by FACS. As shown in Table 1, the selected CD133+ cells confirmed the high level of CD34/KDR coexpression. Most of the cells were also positive for Tie-2, VE-cadherins, and EC markers of either immature or mature ECs27,28,29 and were able to bind UEA-1. By contrast, markers of mature ECs, such as CD31, vWf, CD146, and CD105, were never greater than 1 to 3% of total CD133+. The hematopoietic marker CD45 was undetectable. Interestingly, 38.9% of CD133+ cells were positive for desmin, a mesenchymal marker expressed by skeletal and smooth muscle cells.30
Table 1.
Antigenic Characterization of Immunomagnetically Separated hFA CD133+ Cells
| Antigens | Positive cells (%) |
|---|---|
| CD133* | 95.4 ± 1.5 |
| CD34* | 81.5 ± 12.4 |
| KDR* | 64.8 ± 15.9 |
| UEA-1* | 92.4 ± 6.8 |
| CD146* | 2.8 ± 2.3 |
| CD105* | 2.7 ± 1.9 |
| CD31* | 0.4 ± 0.2 |
| CD45* | Not detected |
| vWf† | Not detected |
| Ve-cad† | 75.4 ± 7.9 |
| Desmin† | 38.9 ± 5.7 |
| Tie-2† | 88.5 ± 7.9 |
CD133+ cells were immunoselected from a pool of five different aortas and analyzed by FACS (*) or immunocytochemistry (†). Numbers are the means ± SD of three independent experiments and represent the percentage of positive cells of total cells tested.
Culture of hFA-Derived CD133+CD34+KDR+
From a single hFA, 1.8 to 2.5 × 105 CD133+CD34+KDR+ cells were isolated. Cultures were maintained in HM-GM, a serum-free medium devised to generate multipotent stem cells from human brain and skeletal muscle.28,31 Under these culture conditions, CD133+CD34+KDR+ cells were floating and rounded, sometimes forming small clusters (Figure 1c). Ten to 15 days after seeding, the cells grew as floating spheroids composed of cell aggregates. The doubling time was around 5 to 7 days during the initial 2 to 3 weeks but increased to 7 to 10 days on 1 month of culture. This result was routinely seen with different CD133+CD34+KDR+ preparations. Interestingly, under the same serum-free culture conditions, the CD133− cell subset was unable to grow, thus confirming the severe selective culture condition imposed by HM-GM medium. The time course of phenotypic modification in culture was performed by FACS analysis. At 21 days from seeding in HM-GM, around 49% of cells maintained the original phenotype, which decreased to 39% after 42 days. The substitution of growth factors contained in HM-GM with 10% fetal calf serum induced cell adhesion to plastic and significantly accelerated phenotypic changes. In particular, CD133 expression fell to 20 to 30% of the initial level in 2 to 3 weeks.
hFA-Derived CD133+CD34+KDR+ Cells Identify a Population of Putative hVPCs
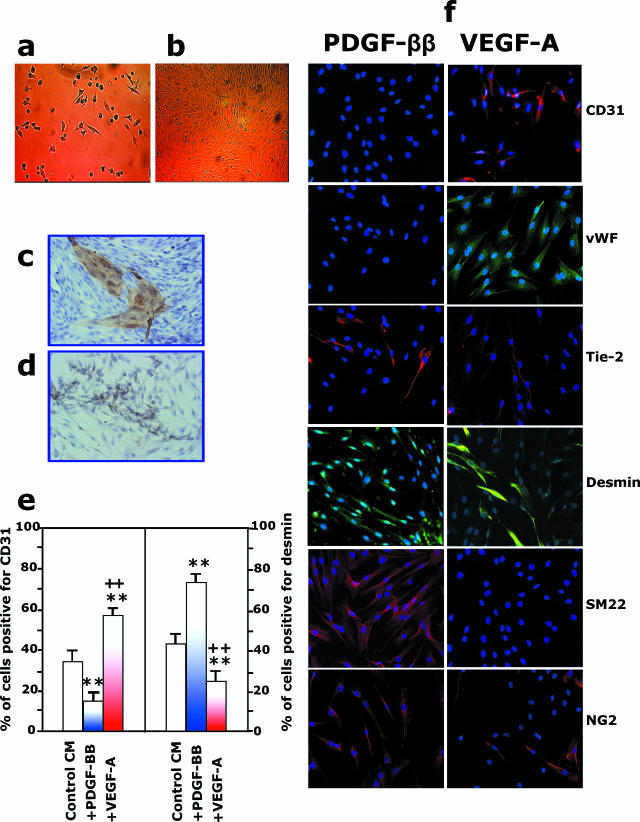
The next step was to determine the ability of CD133+CD34+KDR+ cells to differentiate into components of vascular tissue. CD133+CD34+KDR+ cells were tested for their capacity to generate mature ECs in vitro. Cells were cultured in EBM-GM medium, which supports the long-term development of human ECs.16 In such conditions, a few hours after seeding, CD133+CD34+KDR+ cells developed into rounded refractive cells that became more adherent after 24 hours (Figure 2a). Endothelial-like cells and cobblestone areas were visible from day 3 up to day 7. Light microscopy analysis of the adherent cells revealed a heterogeneous cell population comprised of small-sized flat cells and large-sized spindle-shaped cells with endothelial morphology that formed colonies in some areas of the dish. After reaching confluence, mature ECs could be purified from the culture by selection with magnetic beads coated with anti-CD31 antibodies; these cells formed a typical EC monolayer (Figure 2b). The formation of mature EC colonies was confirmed by CD31 (Figure 2c), CD146, and vWf immunostaining (data not shown). The differentiation of hFA-derived CD133+CD34+KDR+ cells into mature ECs was quantified by FACS analysis, showing that 30 to 40% of the cells became CD31+vWf+ within 10 days (data not shown). Because approximately 30 to 40% of the CD133+ cells were also desmin+, we tested the possible coexistence of both endothelial and muscular progenitors in hFA-derived CD133+CD34+KDR+. Cells were maintained in EBM-GM supplemented either with 50 ng/ml PDGF-ββ or with 10 ng/ml VEGF-A. As shown by Figure 2, d–f, after 7 days, PDGF-ββ reduced the expression of endothelial markers while enhancing the expression of mural markers (desmin, SM22, NG2, and α-SMA). In contrast, VEGF-A reduced the expression of mural markers and enhanced the expression of endothelial markers (CD31, vWf, and Tie-2).
Figure 2.
CD133+CD34+KDR+ cells derived from hFA differentiate into vascular endothelial and mural cells. a and b: Freshly immunoselected CD133+CD34+KDR+ cells seeded on collagen type I-plus fibronectin-coated dishes were cultured under differentiated culture medium EBM-GM.16 a: A few hours after seeding, cells started to adhere. At 7 days of culture, the formation of mature EC colonies was observed, and at confluence, 10 to 14 days after seeding, mature ECs could be separated from the culture by using immunomagnetic beads coated with anti-CD31. The newly formed mature ECs could be grown in EBM-GM to obtain a typical endothelial monolayer in b. Magnification, ×10. The formation of EC colonies on confluent culture was also confirmed by immunoperoxidase staining with anti-CD31 antibody (c), whereas the presence of mural cells was shown by their immunoreactivity with anti-α-SMA antibody (d). e and f: Addition of PDGF-ββ or VEGF-A to EBM-GM enhanced the formation of mural and endothelial cells, respectively. Quantification is shown in e. **P < 0.01 versus control medium, ++P < 0.01 versus PDGF-ββ addition. f: Immunostaining for CD31, vWF, Tie-2, desmin, smooth muscle 22α, and NG2 of CD133+CD34+KDR+ cells 10 days on stimulation with PDGF-ββ or VEGF-A.
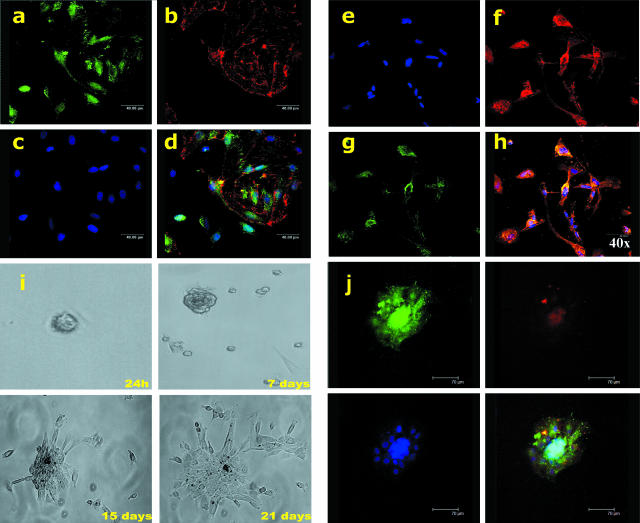
Altogether, the above findings indicate that the 12-week-old hFA contains a pool of CD133+CD34+KDR+ progenitor cells named hVPCs that coexpress endothelial and myogenic markers in their more immature phenotype and can differentiate into ECs or muscular cells when exposed to appropriate environmental stimuli. To gain further insight into the differentiation potential of these cells, we next investigated the coexpression of desmin and UEA-1 binding as well as Tie-2 and desmin. We found that the hVPC population contains a mix of progenitor cells positive for either desmin or UEA-1 or for both markers (Figure 3, a–d). Similar results were obtained by analyzing Tie-2 and desmin coexpression (Figure 3, e–h).
Figure 3.
Multilineage potential of hVPCs: coexpression of myogenic and endothelial markers. a–d: Expression of desmin (green) and binding to UEA-1- (red). Cells are stained for desmin+ (a) or UEA-1+ cells (b). Nuclei are shown in c. Cells expressing desmin and UEA-1-binding are shown in d. It is possible to recognize the presence of cells that stained individually for desmin or UEA-1 and cells positive for both markers. e–h: Coexpression of Tie-2 (red) and desmin (green). Nuclei are shown in e, single Tie-2-positive cells are shown in f, desmin staining is shown in g, and cells coexpressing Tie-2 and desmin are shown in h. Note that cells coexpressing Tie-2 and desmin are colored in yellow: in this case, all of the desmin-positive cells are also Tie-2 positive, but not vice versa. i and j: Clone of hVPCs expressing both myogenic and endothelial markers. i: Limiting dilution was used to test self-renewal of hVPCs; the position of a single cell/well was identified by the use of a marker; the cell was routinely observed during the time under inverted microscopy. j: After 28 days, the clones were aspirated and transferred into larger wells and analyzed by immunocytochemistry for the expression of endothelial (Tie-2, in red) and myogenic (desmin, in green) markers. Note that most of the cells coexpressed both markers.
The multilineage potential of hVPCs was unequivocally demonstrated by cloning. Limiting dilution was used to demonstrate the self-renewal of hVPCs, with single-cell clones examined every 24 hours (Figure 3i). By 28 days, as shown in Figure 3j, most clones derived from hVPC populations demonstrated the coexpression of both endothelial (tie-2) and mural (desmin) markers. It was interesting to observe that compared with the uncloned cell population, all of the analyzed clones were positive for both endothelial and myogenic markers. We further evaluated the capacity of hVPCs to form vascular-like structures in vitro when embedded into collagen gel.16 At 24 hours after seeding, hVPC spheroids started to produce cord-like structures of different morphological appearance, some of these cords were very thin and appeared to arise from a single hVPC sphere (Figure 4a). Interestingly, the addition of PDGF-ββ alone or in association with basic fibroblast growth factor enhanced cord thickness and branching, whereas VEGF-A induced a preferential pattern of very long and fine capillary-like structures (data not shown).
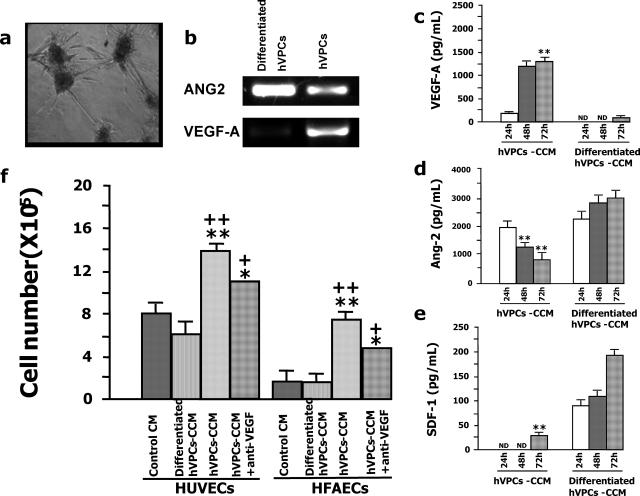
Figure 4.
Angiogenic potential of hFA-derived hVPCs. a: The capacity of hVPCs to organize into vascular-like structures was studied by embedding the cells in collagen gel. b: Total RNAs were extracted from hVPCs and differentiated hVPCs; ANG2 and VEGF-A expression were determined by RT-PCR, and transcript levels were unequal. c–e: HVPCs or differentiated hVPCs were cultured in HM-GM to detect the release of angiogenic factors in the culture supernatant (c); undifferentiated hVPCs produced a significant amount of VEGF-A, whereas hVPCs stopped producing VEGF-A on their differentiation (**P < 0.01 versus differentiated hVPCs at the same time of collection. ND, not detected by the assay). d: Ang-2 was rapidly produced by undifferentiated hVPCs, but its secretion by these cells declined over time; in contrast, Ang-2 was continuously released by hVPCs on their differentiation (**P < 0.01 versus undifferentiated hVPCs at the same time of collection). e: SDF-1 was released by hVPCs only after their maturation (**P < 0.01 versus differentiated hVPCs). f: CCM from hVPCs enhanced HUVEC and hFAEC proliferation. **P < 0.01 versus CCM from differentiated hVPCs, ++P < 0.01 versus control medium. Note that the addition of anti-VEGF significantly reduced (+P < 0.05 versus CCM, *P < 0.05 versus control medium) EC proliferation but did not completely block CCM stimulation.
Angiogenic Potential of FA-Derived hVPCs
The reorganization of hVPCs into vascular-like structures occurred regardless of VEGF-A, PDGF-ββ, or basic fibroblast growth factor supplementation. We therefore hypothesized that hVPCs themselves might be able to produce the growth factors necessary for their own needs. Thus we verified and determined the expression of ANG2 and VEGF-A at the mRNA level by reverse transcriptase-PCR (RT-PCR). As shown in Figure 2b, ANG2 is more expressed in differentiated hVPCs than hVPCs, whereas VEGF is more expressed in hVPCs than differentiated hVPCs. To investigate and quantify Ang-2 and VEGF-A presence in supernatants, we performed enzyme-linked immunosorbent assay analysis. Thereby, the CCM of hVPCs was collected and analyzed. Undifferentiated hVPCs produced large amounts of immunoreactive VEGF-A (Figure 4c) and Angiopoietin-2 (Figure 4d). However, although VEGF-A concentration in CCM increased for up to 72 hours, the levels of Ang-2 progressively declined in a time-dependent fashion. SDF-1, a chemokine that acts as an attractant for stem cells,32 was detected at very low levels only after 72 hours of culture (Figure 4e). Interestingly, the pattern described above was profoundly altered on serum-induced hVPC differentiation. In fact, in the differentiated cell CCM, the VEGF-A amount was negligible, whereas Ang-2 and SDF-1 reached 3.5- and 7.0-fold higher levels than in the CCM of undifferentiated cells. These results indicate that hVPCs produce angiogenic factors and that the concentration of these factors in the CCM varies according to cell maturation. The angiogenic properties of hVPC CCM were confirmed in an in vitro proliferation assay with human ECs. As shown in Figure 4f, CCM from hVPCs stimulated the growth of both HUVECs and hFAECs, whereas CCM produced by hVPCs on differentiation was ineffective. The complex angiogenic composition of hVPC-derived CCM was confirmed because its proangiogenic effect was significantly reduced, but not abrogated, by the addition of anti-VEGF-A antibodies (Figure 4f).
Altogether, these data indicate that hFA-derived hVPCs possess provasculogenic (ie, they differentiate into mature ECs) and proangiogenic properties (ie, they accelerate the proliferation of mature ECs). Thus, to respond to our two initial questions, we can conclude that hFA contains niches of progenitor cells endowed with self-renewal potential and is capable of regenerating all of the components of the vessel wall in a way that is reminiscent of embryonic vessel formation. These cells also have the capacity to stimulate resident ECs.
In Vivo Effect of hVPCs in a Murine Model of Peripheral Ischemia
Progenitor cells of fetal origin have been successfully used to replace bone marrow in hematological disease. Likewise, naïve hVPCs could provide a new way to treat ischemic diseases. Thus, the final point addressed was whether the myogenic and vasculogenic properties of hVPCs could be clinically exploited to treat ischemic disease.
At 14 days from hVPC transplantation into ischemic muscles, we found 96 ± 14 hNAg+ ECs/mm2 and 209 ± 42 hNAg+ myocytes/mm2. After adult EPC transplantation, we found a similar number of hNAg+ ECs (66 ± 9 hNAg+ ECs/mm2, P = NS), whereas the number of hNAg+ myocytes tended to be lower (87 ± 3 hNAg+ myocytes/mm2, P = 0.07 versus hVPCs) compared with hVPC-transplanted muscles.
Confocal microscopy pictures of Figure 5 show that hVPC-derived hNAg+ cells were detected between ECs, identified by lectin staining (Figure 5a, left) and by the presence of cells associated to muscle microvessels within skeletal muscle, which stained positively to anti-CD31 specific for human (Figure 5d, left); furthermore, hVPC-derived hNAg+ cells were identified also between vascular smooth muscle cells (positive staining to α-SMA; Figure 5b, left) of the arteriolar wall and myofibers (identified by laminin staining; Figure 5c, left), thus suggesting that once transplanted, hVPCs might differentiate into or fuse with vascular and muscular cells.
Figure 5.
In vivo studies: histological analysis. Panels a through d are related to the confocal microscopy analysis and immunohistochemistry performed on adductor muscles harvested at 14 days from induction of ischemia and the local injection of 2 × 104 hVPCs (left panels) or control culture medium (right panels) (original magnification, ×400). In a, endothelial cells were detected by fluorescence lectin (green) and transplanted human cells by their positivity to human nuclear antigen (hNAg+ in red). Nuclei were counterstained by Hoechst (blue). Arrow in left panel indicates an area containing a hNAg+ cells belonging to a blood capillary vessel. The same area is shown at ×1000 in the upper right corner. A microvessel negative to hNAg is shown for reference in the right panel. b: Sections stained for α-SMA (red) and hNAg (green). In the left panel, hNAg+ cells are incorporated into the arteriolar wall of mouse adductor muscles injected with hVPCs. As expected, the CM-injected muscle (negative control) does not show the presence of human cells. Nuclei are counterstained by Hoechst (blue). c: Sections stained for laminin (a basal lamina marker, red) and hNAg (green). Transplanted hNAg+ cells are incorporated in myofibers (left panel). The CM-injected muscle does not show hNAg+ cells. Nuclei are counterstained by Hoechst (blue). These figures are representative of three independent experiments. d: Immunohistochemical staining on 3-μm sections using an anti-CD31 antibody (brown, peroxidase). The arrow in the left panel points to a cell associated with muscle microvessels within skeletal muscle, which stained positively to human-specific anti-CD31. As expected, the CM-injected muscle (negative control) does not show the presence of human cells. e: Images of the hindlimb region of mice that had been submitted to left limb ischemia and injected with hVPCs or CM 14 days in advance. f: Real-time PCR was performed with primers specific for MyoD and MYF5 using total RNA from adductor muscles injected with hVPCs, differentiated hVPCs, or control. MYF5 is approximately 80-fold up-regulated in the hVPC-transplanted muscles with respect to differentiated hVPC-injected muscles. Error bars represent SDs calculated on three different replicas. g: Distribution of necrotic toes in the left foot of mice that were exposed to ipsilateral ischemia. Zero means absence of necrotic toes, and 5 indicates all toes being necrotic or absent. One point was scored for every necrotic toe. Each circle is representative of a single mouse. Colored transverse lines represent the median for each group. Blue, CM-injected mice; green, EPCs; yellow, differentiated hVPCs; red, hVPCs.
Absence of hNAg+ and human CD31+ cells in contralateral muscles (data not shown) or CM-injected muscles (Figure 5, a–d, right panels) confirmed the specificity of the reactions. On the other hand, we evaluated the expression of human MyoD and MYF5 by real-time PCR to verify the possible differentiation of hVPCs into skeletal muscle cells. As shown in Figure 5f, the MYF5 expression in hVPCs increased by approximately 80-fold compared with the differentiated hVPCs; MyoD expression, instead, was not detected in either sample.
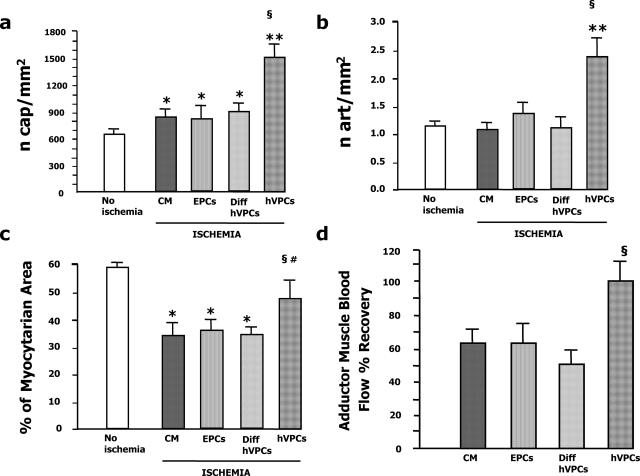
Then, we evaluated microvessel density of transplanted ischemic muscles. As shown in Figure 6, hVPCs potentiated the angiogenic and arteriogenic responses to muscular ischemia. In fact, the density of both capillaries (Figure 6a) and arterioles (Figure 6b) was higher in the ischemic adductors injected with hVPCs (for both parameters: P < 0.01 versus CM and P < 0.05 versus either EPCs or differentiated hVPCs). Moreover, the muscle area section occupied by myocytes was conserved after hVPC transplantation (Figure 6c), thus suggesting that hVPCs protected the ischemic limb from the loss of myocytes. It is possible that a paracrine antiapoptotic effect of hVPCs may have contributed to their therapeutic benefit. In fact, myocyte apoptosis was reduced in hVPC-transplanted muscles (0.78 ± 0.19 terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive myocytes/mm2 of muscular sections versus 1.47 ± 0.12 in CM and 1.93 ± 0.35 in differentiated hVPCs, P < 0.05 for both comparisons). Likewise, EC apoptosis was reduced by hVPCs (0.69 ± 0.19 terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive ECs/mm2 of muscular sections versus 1.19 ± 0.19 in CM, P < 0.05 and versus 1.64 ± 0.13 in differentiated hVPCs, P < 0.01). Alternatively or complementarily, apoptosis could be diminished as a consequence of improved muscle reperfusion (vide infra).
Figure 6.
In vivo studies: clinical outcome. Graphs in a and b show the capillary density and arteriole numerical density, respectively, expressed as number of capillaries or the number of arterioles per square millimeter of transverse muscular section. Values of normoperfused (no ischemia, open column) and ischemic adductor muscles injected with CM or transplanted with 2 × 104 hVPCs are shown. Values are mean ± SD. **P < 0.01 versus no ischemia; §P < 0.05 versus CM, EPCs, and differentiated hVPCs; *P < 0.05 versus no ischemia. c: The percentage of sectional area occupied by myocytes was determined by computer-assisted morphometric evaluation of muscular sections. hVPC transplantation significantly reduced the loss of myocytes. Values are mean ± SD. §P < 0.05 versus CM, EPCs, and differentiated hVPCs; #P < 0.05 hVPCs versus no ischemia; *P < 0.05 versus no ischemia. d: hVPCs improved the perfusion of adductor muscles subjected to left limb surgical ischemia. BF of left (ischemic) and right (contralateral normoperfused control) adductors was measured by the OxyLite/OxyFlo probe at 14 days postischemia. BF recovery was calculated as the ratio of left to right BF multiplied per 100. Mice transplanted with hVPCs showed improved perfusion recovery versus all of the control groups. Values are mean ± SD. §P < 0.05 versus CM, EPCs, and differentiated hVPCs.
From a clinical point of view, the finding that the number of necrotic/autoamputated toes was significantly reduced in mice transplanted with hVPCs is significant (Figure 5g). Pictures representative of the better outcome from ischemia for those mice transplanted with hVPCs are shown in Figure 5e. In line, as showed by Figure 6d, the BF recovery of the ischemic adductor was significantly ameliorated after hVPC transplantation. In contrast, the superficial BF in the most distal part of the hindlimb was not ameliorated by cell therapy (recovery of hVPC, 55 ± 6%; EPCs, 50 ± 9%; CM, 55 ± 3%; P = NS for all comparisons). It is interesting to observe that to obtain a positive clinical outcome by injecting EPCs, 10-fold more (2 × 105) cells were required (data not shown). In summary, our in vivo studies suggest that undifferentiated hVPCs stimulate the healing of ischemic limbs, possibly through direct participation in ongoing regeneration of vascular/muscular cells and paracrine release of angiogenic and antiapoptotic factors.
Discussion
In this study, we performed experiments to identify, isolate, and culture vascular progenitor cells derived from hFA, to define their phenotypic and functional characteristics, and to evaluate their therapeutic potential in a model of peripheral ischemia. In our previous work, we reported that hFA-derived endothelial progenitors coexpress CD34 and KDR markers.16 Here, we report for the first time that hFA contains a population of vascular progenitors able to generate mature microvessels. Rafii and colleagues33 reported that fetal liver may contain multipotential vascular progenitors with a phenotype similar to that described here. However, fetal liver specimens were hard to obtain from abortive fetus; thus in this study, we were unable to make any comparative study between liver and aorta vascular progenitors.
These cells are characterized by the expression of early vascular markers (CD133, CD34, KDR, and Tie-2) and by the absence of mature endothelial markers (CD31, vWf, and CD146). Almost all hVPCs were UEA-1+ and VE-cadherin+. Because around 40% of hVPCs were also positive for the myogenic marker desmin, we reasoned that hFA may contain progenitors capable of both endothelial and mural cell differentiation. This interpretation was corroborated by the following additional findings: 1) Under differentiating culture conditions, VEGF-A or PDGF-ββ enhanced differentiation of hVPCs into ECs or myogenic cells, respectively, thus confirming the previous observation by Yamashita et al10 on embryonic stem cells. 2) hVPCs organized vascular-like structures when embedded in collagen gel. 3) Immunostaining of hVPCs showed the presence of cells coexpressing endothelial (UEA-1 and Tie-2) and myogenic (desmin) markers. 4) More importantly, clones of hVPCs were able to differentiate into ECs and myocytes. Until now, there has not been any evidence for the existence of mesenchymal progenitors able to generate vascular and extravascular mesodermal cells in hFA. Data provided by Cossu and colleagues15,34 suggest the presence of similar cells in mouse in the very early embryonic dorsal aorta.
Our findings indicate for the first time that hFA contains a population of immature vascular progenitors at different levels of differentiation that secrete a complex combination of angiogenic factors like VEGF-A and Ang-2 and that, through an autocrine/paracrine loop, may render hVPCs resistant to apoptosis and promote their reorganization into capillary-like structures. Through VEGF-A secretion and probably other angiogenic factors, as demonstrated by the incomplete blockade by anti-VEGF antibodies, hVPCs may also stimulate the proliferation of mature ECs, thus favoring angiogenesis. On hVPC differentiation into ECs and muscular cells, Ang-2 increased or remained constant while VEGF-A rapidly declined, thus suggesting that Ang-2 may be involved in terminal vascular differentiation, probably through the Tie-2 receptor, which is expressed also on mural precursor cells.35 SDF-1, a chemoattractant for EPCs, neuroectodermal and mesenchymal stem cells,36,37 was produced only on differentiation, thus suggesting that SDF-1 released by stem/progenitor cells undergoing differentiation may contribute to the amplification of stem cell recruitment, thereby promoting further vasculogenesis and angiogenesis. Thus, stem cells may function either as a target or producer of angiogenic/chemotactic factors. Interestingly, a very small number of hVPCs transplanted into the ischemic limb muscles of immunodeficient mice is established to have a therapeutic effect. When compared with control differentiated cells, with EPCs, and with culture medium, hVPCs markedly improved neovascularization, inhibited the apoptotic loss of resident ECs and myocytes, and ameliorated the clinical outcome from ischemia. Under our experimental conditions, EPCs failed to produce therapeutic effects: this result, which seemed to diverge from other studies,36,38 was really due to the very low dose of injected EPCs used here. A 15-fold higher dose of injected EPCs (3 × 105 cells) did produce a significant positive therapeutic effect (data not shown).
We conclude that hVPCs are endowed with a significantly higher therapeutic effect if compared with those evoked by postnatal circulating EPCs in the hindlimb ischemia model. The prevention of necrotic/autoamputated toes by hVPC treatment represents very impressive clinical results. However, the mechanism of action of hVPCs in vivo is difficult to explain. For example, the increment of arteriolar microvessels density did not suffice to increase superficial BF, possibly because the implanted cells did not make collaterals that could bypass the vessel cut off proximally to the site of implantation, suggesting that the major improvement of BF was at the level of the tight. In any case, we think that the therapeutic efficacy of hVPCs may result from the combination of different positive effects that work together synergistically to obtain this remarkable final clinical outcome.
HVPCs may ameliorate ischemic tissue outcome as a result of a synergic combination of their capacity to amplify spontaneous neovascularization through paracrine mechanisms, with a direct integration and differentiation into vessels and muscular fibers, as suggested by the positive staining with anti-human CD31 and by the expression of human Myf5. During a normal skeletal muscle developmental program leading to terminal differentiation, cells express Myf-5 and/or MyoD.39 Thus the presence of Myf5 in hVPC-transplanted tissues confirmed our findings; namely, a direct integration and differentiation of hVPCs in muscular fibers. The absence of MyoD could be due to a specific myogenic Myf5-dependent program involved in muscle regeneration40; otherwise, MyoD-/Myf5+ cells could represent progenitors at a distinct developmental stage in the regenerative myogenic program.41 Finally, by releasing SDF-1, hVPCs may attract host circulating endothelial progenitors to the site of ischemia.36,37
In conclusion, this is the first study to demonstrate the existence of immature vascular cells coexpressing endothelial and myogenic markers in hFA. The absence of a CD45 marker suggests that hVPCs may not be of hematopoietic origin but may derive from immature mesenchymal stem cells residing in niches located in the fetal paraortic membrane or at the periphery of aorta wall parenchyma.16 These cells can be expanded in culture and are endowed with a strong regenerative capacity; both of these characteristics are instrumentally exploitable for the treatment of experimental vascular and muscular diseases. At present, the potential therapeutic application of these cells is unlikely. However, recent observations have shown that postnatal artery walls contain mesenchymal cells with multilineage differentiation potential.42,43,44,45 Whether the same phenotype of fetal hVPCs persists during postnatal life remains to be determined.
Acknowledgments
We thank Dr. Andrea Smith for help with the English translation.
Footnotes
Address reprint requests to Giulio Alessandri, Ph.D., Neurobiology and Neuroregenerative Therapies Unit, Carlo Besta Neurological Institute, Milan 20133, Italy. E-mail: giulio.alessandri@istituto-besta.it.
Supported by Giorgio Fattori-Pupi Solari Foundation; Cariplo (grant nos. 2002.1915/10 and 4898-06-0062-2002); Italian Education, University, and Research Ministry (grant no. RBNEO1R4MJ); Juvenile Diabetes Foundation Research (grant no. 1-2004-124); the Italian Health Ministry (the National Program on Stem Cells); and the Italian Health Institute (Ricerca Finalizzata 2003). National Institute of Biostructures and Biosystems laboratories are partners of the European Vascular Genomic Network of Excellence.
G.I. and C.E. equally contributed to this work.
References
- Risau W. Differentiation of endothelium. FASEB J. 1995;9:926–933. [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expression of post-natal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for post-natal vasculogenesis in physiological and pathological neovascolarization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Malcom AS, Moore RF, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGF-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Pelosi E, Valtieri M, Coppola S, Botta R, Gabbianelli M, Lulli V, Marziali G, Masella B, Muller R, Sgadari C, Testa U, Bonanno G, Peschle C. Identification of the hemangioblast in postnatal life. Blood. 2002;100:3203–3208. doi: 10.1182/blood-2002-05-1511. [DOI] [PubMed] [Google Scholar]
- Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitors cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Le Ricousse-Roussanne S, Barateau V, Contreres JO, Boval B, Kraus-Berthier L, Tobelem G. Ex vivo differentiated endothelial and smooth muscle cells from human cord blood progenitors home to the angiogenic tumor vasculature. Cardiovasc Res. 2004;62:176–184. doi: 10.1016/j.cardiores.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Pesce M, Orlandi A, Iachininoto MG, Straino S, Torella AR, Rizzuti V, Pompilio G, Bonanno G, Scambia G, Capogrossi MC. Myoendothelial differentiation of human umbilical cord blood-derived stem cells in ischemic limb tissues. Circ Res. 2003;93:51–62. doi: 10.1161/01.RES.0000090624.04507.45. [DOI] [PubMed] [Google Scholar]
- Simper D, Stalboerger PG, Panetta CJ, Wang S, Calice NM. Smooth muscle progenitor cells in human blood. Circulation. 2002;106:1199–1204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri G, Girelli M, Taccagni G, Colombo A, Nicosia R, Caruso A, Baronio M, Pagano S, Cova L, Parati E. Human vasculogenesis ex vivo: embryonal aorta as a tool for isolation of endothelial cell progenitors. Lab Invest. 2001;81:875–885. doi: 10.1038/labinvest.3780296. [DOI] [PubMed] [Google Scholar]
- Alessandri G, Pagano S, Bez A, Benetti A, Pozzi S, Iannolo G, Baronio M, Invernici G, Caruso A, Muneretto C, Bisleri G, Parati E. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet. 2004;364:1872–1883. doi: 10.1016/S0140-6736(04)17443-6. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from human umbilical veins. Identification of morphologic and immunologic criteria. J Clin Invest. 1973;32:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüssel J, Behr B, Hirchenhain J, Wen Y, Milki AA, Cupisti S, Bielfeld P, Polan ML. Expression of vascular endothelial growth factor mRNA in human preimplantation embryos derived from tripronuclear zygotes. Fertil Steril. 2000;74:1220–1226. doi: 10.1016/s0015-0282(00)01581-8. [DOI] [PubMed] [Google Scholar]
- Parati EA, Bez A, Ponti D, de Grazia U, Corsini E, Cova L, Sala S, Colombo A, Alessandri G, Pagano SF. Human neural stem cells express extra-neural markers. Brain Res. 2002;925:213–221. doi: 10.1016/s0006-8993(01)03291-7. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Graiani G, Salis M-B, Gadau S, Desortes E, Madeddu P. Prophylactic gene therapy with human tissue kallikrein ameliorates limb ischemia recovery in type 1 diabetic mice. Diabetes. 2004;53:1096–1103. doi: 10.2337/diabetes.53.4.1096. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Salis M-B, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arterogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- Madeddu P, Emanueli C, Pelosi E, Salis MB, Cerio AM, Bonanno G, Patti M, Stassi G, Condorelli G, Peschle C. Transplantation of low dose CD34+KDR+ cells promotes vascular and muscular regeneration in ischemic limbs. FASEB J. 2004;18:1737–1739. doi: 10.1096/fj.04-2192fje. [DOI] [PubMed] [Google Scholar]
- Gadau S, Emanueli C, Van Linthout S, Graiani G, Todaro M, Meloni M, Campesi I, Invernici G, Spillmann F, Ward K, Madeddu P. Benfotiamine accelerates the healing of ischaemic diabetic limbs in mice through protein kinase B/Akt-mediated potentiation of angiogenesis and inhibition of apoptosis. Diabetologia. 2006;49:405–420. doi: 10.1007/s00125-005-0103-5. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Salis MB, Van Linthout S, Meloni M, Desortes E, Silvestre JS, Clergue M, Figueroa CD, Gadau S, Condorelli G, Madeddu P. Akt/protein kinase B and endothelial nitric oxide synthase mediate muscular neovascularization induced by tissue kallikrein gene transfer. Circulation. 2004;110:1638–1644. doi: 10.1161/01.CIR.0000142051.36244.83. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junction differential association of plakoglobin, beta-catenin, and alpha catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CJ, Garbett PK, Nissen B, Schrieber L. Binding of human endothelium to Ulex Europaeus coated dynalbeads. J Cell Sci. 1990;96:257–262. doi: 10.1242/jcs.96.2.257. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite of angiopoietin 1, a ligand for the Tie-2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301:1–7. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, Frolichsthal-Schoeller P, Cova L, Arcellana-Panlilio M, Colombo A, Galli R. Isolation and cloning of multipotent from the embryonic human CNS and establishment of transplantable human neural stem cells lines by epigenetic stimulation. Exp Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1-mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Meeus S, Moussazadeh N, Kermani P, Rashbaum WK, Rabbany SY, Hanson MA, Lane WJ, St. Clair R, Walsh KA, Dias S, Jacobson JT, Hempstead BL, Edelberg JM, Rafii S. Cytokine preconditioning promotes codifferentiation of human fetal liver CD133+ stem cells into angiomyogenic tissue. Circulation. 2005;111:1175–1183. doi: 10.1161/01.CIR.0000157155.44008.0F. [DOI] [PubMed] [Google Scholar]
- Cossu G, Bianco P. Mesangioblasts: vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Iurlaro M, Scatena M, Zhu WH, Fogel E, Wieting SL, Nicosia RF. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–3643. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Wynn RF, Hart CA, Corradi-Perini C. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci. 1999;112:2895–2901. doi: 10.1242/jcs.112.17.2895. [DOI] [PubMed] [Google Scholar]
- Seale P, Ishibashi J, Holterman C, Rudnicki MA. Muscle satellite cell-specific genes identified by genetic profiling of MyoD-deficient myogenic cell. Dev Biol. 2004;275:287–300. doi: 10.1016/j.ydbio.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- Abedin M, Tintut Y, Demer LL. Mesenchymal stem cells and the artery wall. Circ Res. 2004;95:671–976. doi: 10.1161/01.RES.0000143421.27684.12. [DOI] [PubMed] [Google Scholar]
- Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]