Abstract
Acid sphingomyelinase knockout mice are a model of the inherited human disorder types A and B Niemann-Pick disease. Herein, we show that heterozygous (ASMKO+/−) mice have two distinct sperm populations resembling those found in normal and mutant animals, respectively, and that these two populations could be distinguished by their morphology, ability to undergo capacitation or the acrosome reaction, and/or mitochondrial membrane potential (MMP). The abnormal morphology of the mutant sperm could be normalized by demembranation with detergents or by the addition of recombinant acid sphingomyelinase to the culture media, and the corrected sperm also had an enhanced fertilization capacity. Methods were then explored to enrich for normal sperm from the mixed ASMKO+/− population, and flow cytometric sorting based on MMP provided the best results. In vitro fertilization was performed using ASMKO+/− oocytes and sperm before and after MMP sorting, and it was found that the sorted sperm produced significantly more wild-type pups than nonsorted sperm. Sperm sorting is much less invasive and more cost-effective than egg isolation, and offers several advantages over the existing assisted reproduction options for Niemann-Pick disease carrier couples. It therefore could have a major impact on the prevention of this and perhaps other genetic diseases.
Mutations in the gene (SMPD1) encoding the lysosomal hydrolase acid sphingomyelinase (ASM) result in types A and B Niemann-Pick disease (NPD).1 Type A NPD is a severe, neurodegenerative form of the disorder that generally leads to death by ∼3 years of age. In contrast, patients with type B NPD have little or no neurological involvement and may survive into adolescence or adulthood. ASM belongs to a family of sphingomyelinases that degrade sphingomyelin (SPM) into ceramide and phosphorylcholine.2,3 Thus, the pathophysiology in NPD is primarily due to the accumulation of SPM and other metabolically related lipids (eg, cholesterol) within the cells and tissues of affected patients. However, because the product of ASM activity, ceramide, is an important signaling lipid, defects in ceramide-mediated signal transduction pathways also may contribute to the disease pathogenesis.1,2 In humans, ASM deficiency is inherited as a recessive trait, and carrier individuals are thought to be clinically normal.
The full-length human and murine cDNAs and genomic sequences encoding ASM have been isolated,4,5,6 and ASM knockout (ASMKO−/−) mice have been constructed.7,8 The ASMKO−/− animals have no residual ASM activity and present with a clinical and pathological phenotype that is intermediate between types A and B NPD. Heterozygous (ASMKO+/−) mice have no clinical abnormalities, although some pathological changes related to the disease have been documented.9 These models have been extremely valuable for the investigation of the pathophysiological mechanisms underlying types A and B NPD, as well as for the development and evaluation of various treatment approaches.10,11,12
Since constructing the ASM-deficient mouse model in 1995, our laboratory has observed reduced fecundity in the affected animals. Interestingly, reproductive impairment also has been documented in mouse models of Tay-Sachs and Sandhoff diseases,13,14,15 two related sphingolipid storage disorders that result from deficient β-hexosaminidase activity and the accumulation of GM2 ganglioside. Of particular relevance to the current study, the reproductive pathology and residual enzyme expression in male Tay-Sachs and Sandhoff mice has been characterized, and an increase in the size and number of lysosomes in the epithelia lining the efferent and epididymal ducts was found. Testis weight, morphology, and sperm counts seemed to be unaffected. A recent study also showed that in the mouse model of type C NPD (lacking functional NPC1 protein), various sperm defects occurred leading to a markedly reduced fertilization capacity,16 and Luddi and colleagues17 have demonstrated several sperm abnormalities in mice with Twitcher disease, deficient in galactosylceramidase activity.
Thus, the general importance of lysosomal proteins in male fertility has been well documented. Evidence also exists to suggest that the sperm acrosome is a modified lysosome with active acidic hydrolases, including sphingomyelinases.18,19 For example, ram sperm contain a Mg2+-dependent neutral sphingomyelinase in the plasma membrane and an acidic sphingomyelinase in the sperm homogenate.20,21 Because no acidic activity was found in the membrane fraction of rat sperm, it is presumed that ASM is a soluble component of the acrosome.
In a previous article, we reported the pathobiology of male gonadal tissue and sperm in ASMKO−/− mice and demonstrated the importance of ASM for normal sperm maturation and function.22 In the present study, we demonstrate that ASMKO+/− male mice have two distinct sperm populations that could be distinguished based on morphological, functional, and/or biochemical markers. These observations led us to develop methods to enrich for the normal sperm from the mixed population, providing proof of principle that sperm selection might be used as a prefertilization alternative for carrier couples who are at risk of having a child with this and perhaps other lethal genetic diseases. Our data also show that the sperm defects in ASMKO−/− mice can be corrected by in vitro treatment with recombinant ASM or by maintaining the sperm in conditioned media containing ASM, thus revealing that these abnormalities are a direct consequence of lipid storage.
Materials and Methods
Mouse Colony and Sperm Isolation
The ASMKO mouse colony was created as described previously,7 and maintained in a barrier facility on a set light cycle. Normal, ASMKO+/−, and ASMKO−/− mice between 4 and 6 months of age were sacrificed by cervical dislocation, and mature sperm were harvested from cauda epididymides (by mincing the tissue) and vas deferentia (by squeezing the duct). All mouse protocols were approved by the Mount Sinai Center for Comparative Medicine and Surgery and the Mount Sinai Institutional Animal Care and Use Committee.
Sperm Discrimination Assays Based on Physical Characteristics
Swim-Up Assay
Sperm were isolated from the epididymide of normal and ASMKO+/− mice at 6 months of age and harvested into CO2-equilibrated Medium 199 (M199) conditioned with 25 mmol/L NaHCO3, 4 mg/ml bovine serum albumin, and 30 μg/ml sodium pyruvate at 37°C and 5% CO2. After a 10-minute incubation, the sperm suspension (free of excess tissue and aggregates) was centrifuged at 800 × g for 3 minutes. The soft pellet was gently aspirated with a sterile, glass Pasteur pipette (Allegiance, McGaw Park, IL) and placed under media in a 2-ml round bottom tube (Cole-Palmer, Vernon Hills, IL) for 30 minutes at 37°C and 5% CO2. After this swim-out period, three aliquots of 1.2 ml (top), 400 μl (middle), and 400 μl (bottom) were isolated. The top two recovered (ie, swim-out) sperm fractions were combined and analyzed for sperm count (as was an aliquot of the original sperm suspension not used for the swim-up assay) and processed for DNA analysis (see below).
Sephadex Bead Columns
Sephadex bead columns were purchased and prepared as described by the manufacturer (SpermPrep I columns; ZDL, Inc., Lexington, KY). Sperm were isolated from the epididymide of normal and ASMKO+/− mice at 6 months of age and harvested into CO2-equilibrated Medium 199 conditioned with 25 mmol/L NaHCO3, 4 mg/ml bovine serum albumin, and 30 μg/ml sodium pyruvate at 37°C and 5% CO2. An aliquot of the sperm suspension was then transferred to prewarmed, hydrated Sephadex columns and filtered by gravity for 12 to 15 minutes at 37°C. During filtration, the top layer of beads was gently disturbed once to remove the film of dead or aggregated sperm, as per the manufacturer’s instructions. The original sperm suspension and filtrate were analyzed for sperm count and processed for DNA analysis as described below.
Glass Wool Fiber Columns
Glass wool (silica) fiber columns were purchased and prepared as described by the manufacturer (Cook Sperm Filter; Cook Ob/Gyn, Spencer, IN). Sperm samples were isolated and suspended as above. An aliquot of the sperm suspension was transferred to prewarmed, hydrated, glass wool columns and filtered by gravity until the entire suspension had passed through the wool. The original sperm suspension and filtrate were then analyzed for sperm count and processed for DNA analysis as described below.
Flow Cytometry to Assess Sperm Physiology
Analysis
Sperm from the cauda epididymides and vas deferentia of at least three normal, ASMKO+/−, and ASMKO−/− mice at 6 months of age were isolated into warmed, CO2-equilibrated M199 conditioned with NaHCO3 (25 mmol/L), bovine serum albumin (4 mg/ml), sodium pyruvate (30 μg/ml), and EGTA (4 mmol/L). Sperm were allowed to swim out for 10 minutes at 37°C and 5% CO2. The M199 lacked phenol red. The suspensions were then filtered with a 70-μm nylon mesh filter, diluted, and transferred to individual wells of a 12-well plate, and in situ assays were performed to assess capacitation, acrosome reaction, and mitochondrial membrane potential (MMP), as described previously.22 Fluorescence was acquired on a FACSCalibur cytometer (Becton, Dickinson and Company San Jose, CA). The fluorochromes were excited at 488 nm with an Argon laser. Cells (n = 100,000) were acquired after setting a threshold to exclude debris and compensating for spectral overlap. The percentage of these cells and the mean fluorescence channel (N) on a 1024-channel scale were derived. The latter parameter was converted into a mean fluorescence intensity value on a 4-decade (100 to 103) log scale. Analysis of the acquired data was performed with WinMDI 2.8 software (Scripps Institute, La Jolla, CA).
Sperm Sorting Based on MMP Status
Sperm were isolated and suspended as indicated above for 10 minutes in media containing JC-1 (10 μg/ml) at 37°C and 5% CO2. JC-1 is available commercially (Molecular Probes, Eugene, OR). Before cytometric analysis and sorting, the suspensions were filtered with a 70-μm nylon mesh filter (BD Biosciences, San Jose, CA) to remove aggregates. Thereafter, the suspensions were analyzed and sorted immediately on a Becton Dickinson FACSVantage SE cytometer. A two-way sort was performed using FL-1 (530/30 nm) and FL-2 (585/42 nm) band pass filters for JC-1 monomers and aggregates, respectively. Set-up parameters were as follows. An air-cooled 15 mW argon laser emitting at 488 nm was used to excite all fluorochromes. ASMKO+/− mice sperm were sorted at an event rate of ∼200 cells/second using a 100-μm orifice and a droplet formation rate of 49 kHz (Moflo; Cytomation, Fort Collins, CO) or 40 kHz (FACSVantage) with a sheath pressure of 30 or 25 psi, respectively. The sheath fluid was a modified phosphate-buffered saline (PBS) medium. Parameters were set using Summit (Moflo) or CellQuest software, version 3.3 (FACSVantage; Becton, Dickinson and Company) to analyze 50,000 cells. Instrument settings were adjusted so that all events were observed in the density dot plot. Gates were then set for the side and forward light scatter parameters in the linear mode. The percentage of these cells and the mean fluorescence channel (N) on a 1024-channel resolution scale were derived, and the latter parameter was converted into a mean fluorescence intensity value on a 4-decade (100 to 103) log scale. Compensation was not used, but cellular and some noncellular debris were excluded from analysis by setting a minimum threshold. Sperm cells were sorted into uncoated plastic tubes and processed for DNA analysis or used for assisted reproduction as described below. Analysis of the acquired data was performed with WinMDI 2.8 software (Scripps Institute).
Assisted Reproduction Procedures
Sperm for in vitro fertilization (IVF) were obtained from 5- to 6-month-old ASMKO+/− male mice as described above. Mature metaphase II oocytes were recovered from the oviducts of 6- to 8-week-old superovulated female ASMKO+/− mice 13 to 14 hours after injection of 10 IU of pregnant mares’ gonadotropin (PMSG; Syncro-Part, Sanofi, France) and 10 IU of human chorionic gonadotropin (hCG; Sigma, St. Louis, MO). Oocyte-cumulus cell complexes were liberated into warm HEPES-KSOM media containing 40 IU of bovine hyaluronidase (Sigma) for 3 to 5 minutes to separate cumulus cells from the oocytes. Cumulus-free oocytes were collected and washed in fresh HEPES-KSOM before culture in KSOM-AA medium. The medium was equilibrated at 37°C in 5% CO2 overnight before use.
For IVF, sperm were either used directly or subjected to MMP sorting as described above. Cumulus-free oocytes were transferred to the sperm solution (before or after sorting) for 3 hours, then washed twice, and cultured in KSOM-AA culture media under oil in 5% CO2 at 37°C. After insemination, oocytes were transferred to equilibrated drops of KSOM-AA culture medium. After 24 hours, two-cell cleavage stage embryos were transferred to pseudopregnant recipient normal female mice mated to vasectomized males. Recipients were allowed to give birth, and the pups were weaned and genotyped 4 weeks later.
For experiments to assess the effects of exogenous ASM on the fertilizing ability of ASMKO−/− sperm, IVF was performed as above except that 50 μg/ml of rASM was added to the culture media during the 3-hour insemination procedure. After 48 hours the number of four to eight cell blastocysts resulting from insemination of wild-type oocytes using ASMKO−/− sperm with and without ASM incubation were compared.
Genotype Confirmation
Semiquantitative polymerase chain reaction (PCR) genotyping was used to estimate the genotype ratio in the sorted sperm populations. DNA was extracted from sperm with a standard DNA isolation kit (Puregene; Gentra Systems, Minneapolis, MN). Fifty mmol/L dithiothreitol (Sigma) was added to the cell lysis solution to decondense the chromatin, and 33 μg/ml glycogen (Life Technologies, Rockville, MD) added to facilitate DNA precipitation. The wild-type and mutant alleles were both amplified with the same sense primer for exon 2 of murine ASM, in combination with specific anti-sense primers for exon 2 (wild-type allele) or the neomycin cassette (mutant allele).12 The PCR master mix contained all three primers at a final concentration of 100 nmol/L. Samples included the sorted sperm populations and an aliquot of the original unsorted ASMKO+/− sperm population for comparison. A negative control contained primers without template. PCR products were run on a 1% TAE agarose gel and quantified by densitometry (Scion Image Software, version β 4.0.2; Scion Corp., Frederick, MD). The ratio of the wild-type to mutant allele was obtained for each sorted sample and compared with the ratio of the same alleles in the unsorted original heterozygote sample. For each sorting experiment at least three independent amplification reactions were performed.
Cross-Correction Assay
At least two ASMKO−/− mice at 4 months of age were sacrificed and sperm harvested into CO2-equilibrated Medium 199 conditioned with 25 mmol/L NaHCO3, 4 mg/ml bovine serum albumin, and 30 μg/ml sodium pyruvate for 10 minutes at 37°C and 5% CO2. The suspension was aliquoted equally into individual wells of a 12-well plate and incubated in media containing the following concentrations (μg/ml) of recombinant ASM23: 3.8, 19, 38, and 76. An additional aliquot of mutant sperm was incubated in media containing ASM secreted from the sperm of age-matched normal mice. To obtain this conditioned media, normal mouse sperm were harvested and allowed to undergo the acrosome reaction for 2 hours. After brief centrifugation at high speed to pellet the sperm, an aliquot of this acrosome-reacted media (containing secreted ASM) was used to incubate an aliquot of ASMKO−/− mice sperm. As a negative control, ASMKO−/− mice sperm were incubated without recombinant ASM, or in media containing 0.1% Triton X-100 (Sigma). All suspensions were incubated for 40 minutes at 37°C and 5% CO2. The experiment was terminated when the sperm was collected, washed once in PBS lacking magnesium and calcium (Cellgro, Herndon, VA), and then homogenized and processed for in vitro enzyme analysis.22 Before processing, a small aliquot of each sample was removed, immobilized in a 65°C water bath for 2 minutes, and spotted onto a Superfrost/Plus precleaned microscope slide (Fisher Scientific, Fair Lawn, NJ). A coverslip was added and images acquired immediately. A ×40 objective was used on an Axiophot 2 (Nikon, Tokyo, Japan) microscope for differential-interference-contrast microscopy.
Results
Identification of Distinct Sperm Populations in ASMKO+/− Mice
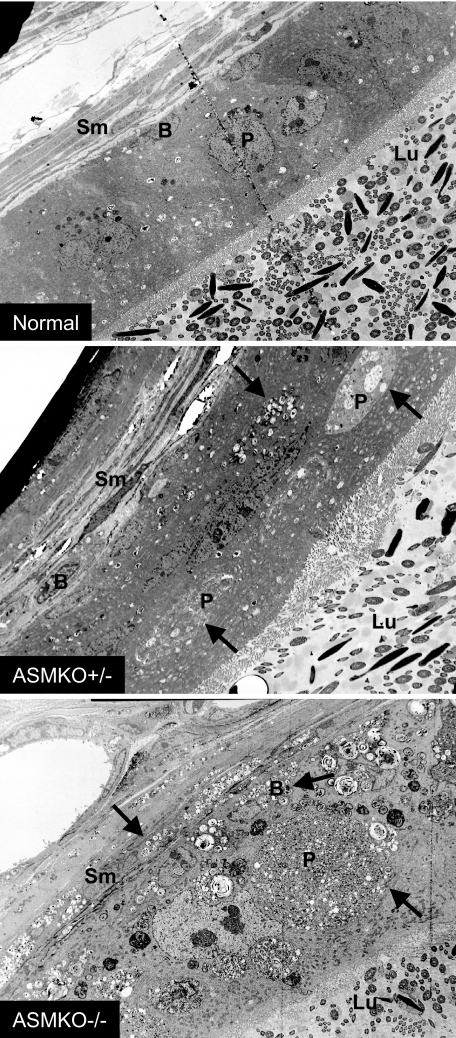
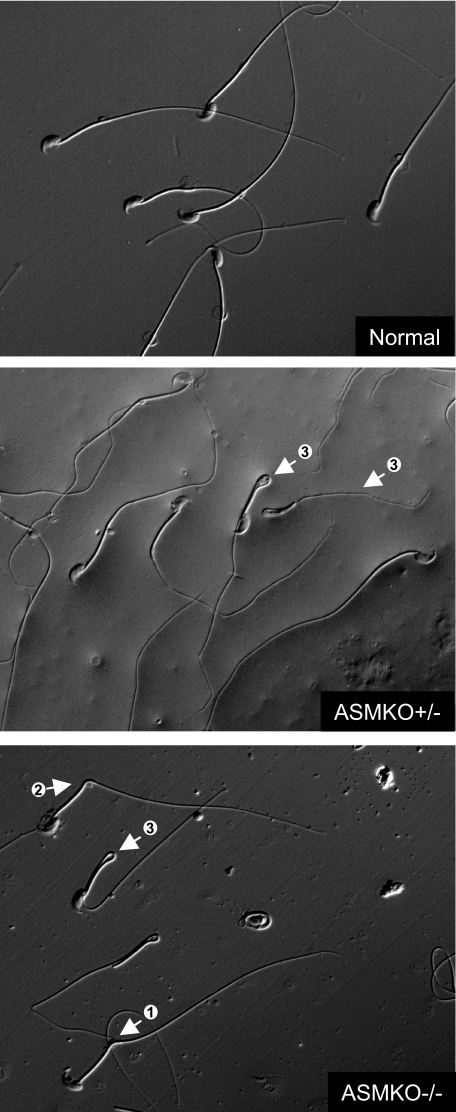
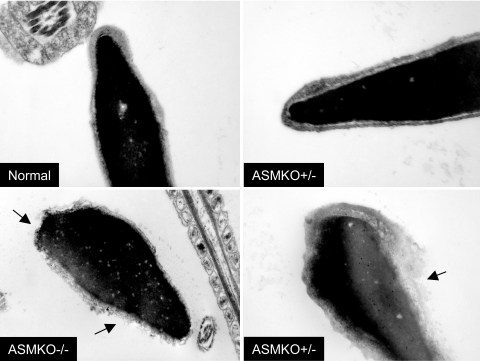
Sperm were isolated from normal, ASMKO−/−, and ASMKO+/− male mice. As shown in Table 1, although the total testis or epididymal sperm numbers were not different between these three groups of animals, the percentage of morphologically abnormal sperm in the ASMKO−/− and ASMKO+/− mice were clearly higher than that found in normal mice. Of direct relevance to this study, a large number of bent or hairpin sperm were found in the ASMKO+/− mice resembling those found in ASMKO−/− animals. This observation was supported by electron micrographs showing lipid accumulation in ASMKO+/− gonadal tissue (Figure 1), and light micrographs of individual sperm from the respective mice (Figure 2). Electron micrographs also revealed that individual sperm from ASMKO+/− mice exhibited intact or damaged sperm membranes, similar to normal or affected mice, respectively (Figure 3). Taken together, these data clearly revealed the presence of two sperm populations in ASMKO+/− mice.
Table 1.
Sperm Findings in Normal, ASMKO+/−, and ASMKO−/− Mice
| Normal* | ASMKO+/−* | ASMKO−/−* | |
|---|---|---|---|
| Testis sperm number (×106) | 39.6 ± 8.7 | 32.0 ± 7.9 | 39.1 ± 7.6 |
| Epididymal sperm number (×106) | 20.1 ± 4.4 | 18.6 ± 11.3 | 17.6 ± 10.8 |
| Morphology (%) | |||
| Straight† | 87.4 ± 3.3 | 69.2 ± 2.4 | 35.4 ± 6.0 |
| Bent† | 1.8 ± 0.6 | 6.2 ± 5.2 | 19.3 ± 4.6 |
| Hairpin† | 1.1 ± 0.5 | 12 ± 4.2 | 31.3 ± 6.1 |
| Other | 9.7 ± 2.7 | 12.7 ± 3.4 | 13.9 ± 4.9 |
Values represent means or percentage means ± SD.
P < 0.001 when comparing normal to ASMKO+/− or ASMKO−/−.
Figure 1.
Electron micrographs of normal, ASMKO+/−, and ASMKO−/− epididymides at 6 months of age. Note multilamellar vesicles (arrows) in ASMKO+/− and ASMKO−/− animals. Sm, smooth muscle cells; B, basal cells; P, principle cells; Lu, lumen. Original magnifications, ×2000.
Figure 2.
Nomarski micrographs showing morphological abnormalities in caudal spermatozoa of ASMKO−/− and ASMKO+/− mice at 6 months of age. Spermatozoon tail retroflexion: <90° (arrow 1), ∼90° (arrow 2), and 180° (arrow 3). Note the presence of both straight and bent sperm in the ASMKO+/− micrograph. Original magnifications, ×400.
Figure 3.
Membrane disruption in electron micrographs of ASMKO−/− and ASMKO+/− caudal spermatozoa at 6 months of age. Arrows indicate premature acrosome reaction or plasma membrane disruption. Note two populations of sperm in ASMKO+/− individuals resembling normal and mutant sperm. Original magnifications: top panels, ×36,000 (normal) and ×48,000 (ASMKO+/−); bottom panels, ×29,000 (ASMKO−/−) and ×36,000 (ASMKO+/−).
Enzymatic Cross Correction in ASMKO−/− Mice
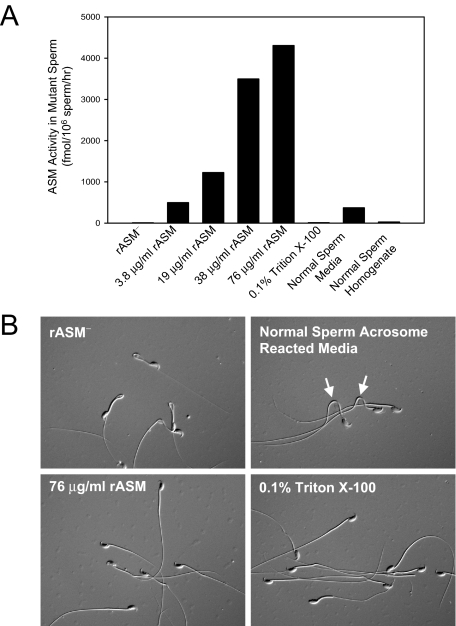
To assess the ability of ASM to be internalized by sperm, an experiment was performed in which mature sperm from ASMKO−/− mice was incubated with increasing concentrations of recombinant, human ASM. Figure 4A shows that recombinant ASM was taken up by the mutant sperm in a dose-dependent manner, as was ASM released from normal sperm into conditioned media (ie, normal sperm media). Micrographs of the mutant, hairpin sperm that had been incubated with recombinant ASM revealed that most had straightened and had normal morphology (Figure 4B). In addition, mutant sperm also began to straighten when exposed to the conditioned media containing ASM. Similar straightening was observed on lipid demembranation with the detergent Triton X-100.
Figure 4.
Analysis of caudal spermatozoa from 6-month-old ASMKO−/− mice incubated with exogenous recombinant ASM (rASM). A: ASM activity was assessed in vitro using sperm cell extracts (see Materials and Methods for details). The results indicate uptake of exogenous enzyme, as well as uptake of ASM from the conditioned media of acrosome reacted normal sperm (normal sperm media). Negative controls (rASM− and 0.1% Triton X-100) do not show ASM activity. B: Differential-interference-contrast microscopy illustrates that uptake of 76 μg/ml rASM (bottom left) straightens mutant sperm in a similar manner to lipid demembranation in 0.1% Triton X-100 (bottom right). Incubation of mutant sperm in media containing endogenous ASM from acrosome-reacted wild-type sperm of the same number begins to relax tail bends and kinks (top right, arrows).
We then performed IVF using ASMKO−/− sperm and wild-type oocytes either in the presence of rASM (50 μg/ml) or without. After insemination, we analyzed the number of four- to eight-cell embryos produced, and found that in the absence of exogenous enzyme, only two embryos were formed from 14 oocytes (14%). In contrast, in the presence of rASM, from 17 oocytes 11 embryos were formed (64%).
In sum, these data revealed for the first time that mature ASMKO−/− sperm can take up exogenous ASM and that uptake of the enzyme corrects the abnormal sperm morphology and fertilization capacity. These observations also demonstrated that the abnormal morphology of the mutant sperm was directly dependent on lipid accumulation. Finally, the data suggest that some mutant sperm in ASMKO+/− mice might undergo partial, phenotypic cross correction in vivo by ASM secretion from epithelial cells and/or ASM released from normal sperm. However, the fact that two distinct populations of sperm were found in these mice (Figures 2 and 3) also suggested that this process does not occur efficiently.
Physical Enrichment of Normal Sperm from ASMKO+/− Mice
To enrich for normal sperm from the mixed sperm population in ASMKO+/− mice, several methods were explored that favored motile sperm. However, although these swim-up techniques did partially enrich for the wild-type sperm, the results were not statistically significant, and the yield of viable sperm was low (data not shown). A second attempt to physically separate normal and mutant sperm was made based on acrosome status, since we had shown previously that many mutant sperm had lost their outer acrosome membrane.22 Acrosome-reacted sperm stick to glass wool, and commercial columns are available to enrich for acrosome-intact sperm.24 Although enrichment of genotypically normal sperm was greater in this assay than for the motility assays, the data also were not significant (not shown). Next, an attempt to enrich for normal sperm was undertaken using Sephadex (dextran polysaccharide) bead columns. On hydration, these beads become grooved, and it is theorized that these grooves hinder movement of nonlinear sperm as the flow-through passes.25 This seemed to be the case, because sperm containing the normal ASM allele were enriched 18.7% by these columns, with a percent yield of close to 40% (P < 0.05). However, although this enrichment was significant, more stringent methods were still needed to obtain a more highly enriched (or pure) population of normal sperm from heterozygous individuals. Hence, techniques for single cell sperm sorting by flow cytometry were investigated.
Flow Cytometric Analysis of Sperm from ASMKO+/− Mice
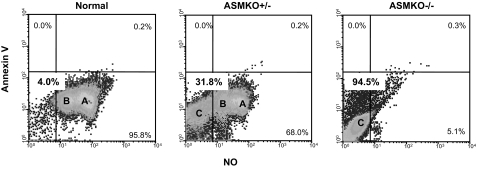
To assess capacitation in sperm from ASMKO+/− mice, flow cytometric analysis of annexin V binding and nitric oxide (NO) production was determined.26 As depicted in Figure 5, when subjected to this analysis normal mice had two populations (A and B) of sperm, whereas ASMKO−/− mice had a distinct, single sperm population (C). It is likely that this latter population in the mutant mice represents dead or fully capacitated sperm, whereas populations A and B in the normal mice either are partially capacitated or have not undergone capacitation at all. Notably, three distinct subpopulations of sperm were observed in the ASMKO+/− sample, including populations that corresponded to those found in normal (A and B) and ASMKO−/− (C) mice. The sperm population (C) corresponding to dead or fully capacitated sperm represented ∼32% of the total in the heterozygous animals. Importantly, these results confirmed the microscopic results mentioned above and further revealed distinct populations of sperm in heterozygous animals corresponding to those found in normal and mutant animals.
Figure 5.
Flow cytometric analysis of caudal spermatozoa from 6-month-old normal, ASMKO+/−, and ASMKO−/− mice based on capacitation status. A representative density-dot profile evaluating whether sperm were able to produce nitric oxide (NO) while undergoing phosphatidylserine translocation (Annex V), both required for capacitation. Note intermediate populations A and B in ASMKO+/− mice sperm (68%), which were also found in the majority of wild-type sperm (95.8%). Population C, which is the majority population in ASMKO−/− mice (95.5%), also was found in heterozygous mice (31.8%).
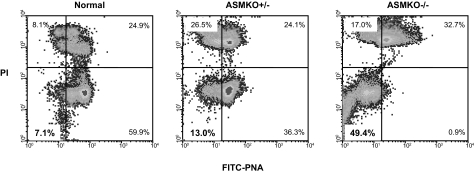
We next assessed the acrosome status of ASMKO+/− mouse sperm using a combination of fluorescein isothiocyanate-conjugated peanut agglutinin binding22 and PI labeling (Figure 6). As reported previously, ∼50% of sperm from ASMKO−/− mice had undergone the acrosome reaction,22 compared with only ∼7% from normal mice (compare lower left quadrants in Figure 6). In ASMKO+/− mice, the percentage of acrosome-reacted sperm was only somewhat higher (13%) than that found in normal mice.
Figure 6.
Flow cytometric analysis of caudal spermatozoa from 6-month-old normal, ASMKO+/−, and ASMKO−/− mice based on acrosome status. A representative density-dot profile showing sperm that had undergone the acrosome reaction as assessed by PI labeling and peanut agglutinin (PNA) binding (bottom left quadrants), and those with intact acrosome membranes (bottom right quadrant). Note that only 13% of sperm from ASMKO+/− mice had undergone the acrosome reaction, only slightly more than that in normal mice (∼7%).
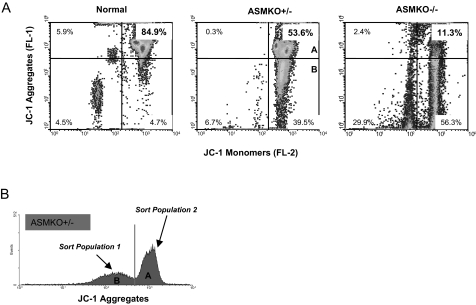
A third flow cytometry assay determined the polarized nature of mitochondria membranes in the sperm of ASMKO+/− mice (Figure 7). In healthy cells, where MMP is high, the lipophilic dye JC-1 aggregates in the mitochondria with a consequent increase in FL-2 fluorescence.27 If MMP is low, JC-1 does not aggregate and remains as monomers with a consequent increase in FL-1 fluorescence. As shown in Figure 7A, ASMKO−/− mice had a very small percentage of healthy sperm with high MMP (top right quadrant, 11.3%). In contrast, in normal mice ∼85% of the sperm had high MMP. Importantly, in ASMKO+/− mice, only approximately half (∼54%) of the sperm had high MMP. Taken together, each of these three analytical flow cytometry assays revealed distinct sperm populations in ASMKO+/− mice.
Figure 7.
Flow cytometric sorting of caudal spermatozoa from 6-month-old normal, ASMKO+/−, and ASMKO−/− mice based on MMP. A: A representative density-dot profile shows the decrease in MMP of mutant versus normal mice (JC-1 aggregates, top right quadrant; 11.3% versus 84.5%). Note that ASMKO+/− mice sperm had an intermediate population of healthy (high MMP) sperm (56.3%). B: A representative fluorescence histogram of ASMKO+/− mice sperm indicating the subpopulations that were sorted and analyzed by PCR. Densitometric quantitation of the PCR products for each population is found in Table 2.
Flow Cytometric Sorting of Sperm from ASMKO+/− Mice
The ability to phenotypically distinguish normal from mutant sperm by flow cytometry provided an impetus for sorting these populations. For the sorting experiments, we focused principally on MMP because the dye JC-1 is nontoxic and routinely used to sort live cells (see Discussion). It is important to note that live sperm sorting requires specially equipped flow cytometers, which we did not have access to. Thus, the sorting data presented was limited by the use of suboptimal equipment and conditions. None-the-less, we proceeded with these proof-of-principle experiments to evaluate whether there was a positive trend worthy of further investigation.
Sperm subpopulations A and B of the ASMKO+/− mouse (Figure 7B), corresponding to populations found in normal (Figure 7A, top right quadrant) and mutant mice (Figure 7A, bottom right quadrant), respectively, were sorted based on JC-1 staining. Genetic analysis of the sorted samples was then determined by semiquantitative PCR. Based on densitometric scanning of the wild-type and mutant PCR products, the percentage of wild-type alleles in the ASMKO+/− sperm before sorting was ∼52%, and the percentage of mutant alleles was ∼48%. This genotype analysis was consistent with other analyses described above demonstrating two populations of sperm in the ASMKO+/− animals. Table 2 shows that when compared with the presorted sample, genotypically normal sperm were significantly enriched in sorted population A (high MMP; healthy sperm). In contrast, in the sorted population B, with low MMP, there was a higher percentage of sperm with the mutant allele.
Table 2.
Genotype Analysis of Sperm Populations Sorted from ASMKO+/− Mice
| Sperm population | A* (corresponding to normal sperm) | B* (corresponding to mutant sperm) |
|---|---|---|
| Genotype | Wild type (70.6%) | Wild type (29.6%) |
| Mutant (30.7%) | Mutant (72.5%) |
Two sorts each of sperm from a single mouse were performed for MMP as shown in Figure 7, followed by PCR genotyping and densitometry. Data represent mean values from the two independent experiments. Population A corresponds to the upper right quadrant in Figure 7A, and population B to the lower right quadrant.
P < 0.05 when comparing the percentages of normal and mutant alleles in population A to population B.
We then used either unsorted ASMKO+/− sperm or sorted sperm population A for IVF with oocytes obtained from ASMKO+/− female mice. After IVF and reimplantation into pseudopregnant wild-type mice, we assessed the genotype of the pups born. As shown in Table 3, in the absence of sorting, sperm from ASMKO+/− mice produced ∼38% normal, 47% heterozygous, and 16% affected mice after fertilization of ASMKO+/− oocytes. The lower than expected percentage of affected mice was likely attributable to morphological and fertilization defects attributed to the mutant sperm in the unsorted ASMKO+/− population. In contrast, using sorted population A, the percentage of normal mice produced from this IVF procedure was increased to ∼52%, whereas the percentage of affected mice was reduced to ∼5%. Thus, MMP sorting provided functional enrichment of wild-type sperm that could result in a higher percentage of wild-type offspring (and less mutant offspring) after IVF. Further optimization of this approach may be achieved using cell sorters specifically designed for live sperm sorting and isolation.
Table 3.
Offspring Derived from IVF Using ASMKO+/− Sperm and Oocytes
| Normal* | ASMKO+/− | ASMKO−/−* | |
|---|---|---|---|
| Nonsorted | 17 (37.8%) | 21 (46.7%) | 7 (15.6%) |
| Sorted (population A) | 18 (51.4%) | 15 (42.9%) | 2 (5.7%) |
P < 0.05 when comparing the percentages of pups derived from sorted and nonsorted sperm. Population A was obtained by MMP sorting as described in Figure 7.
Discussion
Sperm defects have been found in several lysosomal disease animal models, including ASMKO−/− mice,22 and are likely related to the fact that the sperm acrosome is a modified lysosome. Herein, we reveal for the first time the presence of two distinct sperm populations in a heterozygous, lysosomal disease animal model (ie, ASMKO+/− mice), an observation that suggested to us that sperm sorting techniques might be used to enrich for normal sperm from these individuals. If successful, such enriched sperm could be used for in vitro fertilization or vaginal injection, with a reduced likelihood of producing an affected offspring.
Currently, carrier couples for most genetic disorders have few options to prevent the occurrence of affected offspring. For example, they may undertake natural pregnancies and subsequently undergo prenatal diagnosis. If an affected fetus is detected, termination may then be considered. Alternatively, they may undertake expensive and difficult assisted reproduction procedures in which eggs are obtained by superovulation, fertilized in vitro, and single cell assays are used to detect nonaffected blastocysts that are suitable for reimplantation. However, such single cell assays are not universally available and involve embryo manipulation. We have therefore explored sperm selection as an alternative approach that, if successful, would be considerably less invasive and costly than the currently available IVF techniques.
We first evaluated several approaches that were based on physical differences between normal and ASMKO−/− sperm. These approaches, which included swim-up assays, filtration columns, or density gradients, are used routinely in fertility and andrology clinics to enhance the fertilizing ability of ejaculated sperm. In general, they are extremely cost effective and easy to perform. Among these approaches, Sephadex bead columns provided the best results, with an average enrichment of normal sperm from the ASMKO+/− animals by ∼20% compared with the original sample. Although this is significant, it is surprising that similar or better results were not obtained, particularly using the swim-up approaches, since we have previously shown that sperm from ASMKO−/− mice do not exhibit linear movement.22 In the future it might be possible to improve these assays by decreasing the duration of the swim up or perhaps increasing the viscosity of the incubation media (ie, thereby offering more resistance).
In addition, it was surprising that glass wool filtration did not yield a greater percent enrichment because this too should select against immotile sperm. It should be noted that the glass wool columns used in this study were obtained commercially and optimized for human sperm. Because mouse sperm are approximately twice the size of human sperm and contain a distinctively shaped head,28 it is perhaps not surprising that the percent yield and enrichment were statistically insignificant. Data for optimized mouse sperm filtration using glass wool columns exist,29 although these columns are not commercially available.
During the course of these experiments, it was found that sperm incubated in HEPES-buffered media (in contrast to media buffered with sodium bicarbonate) produced slightly more meaningful enrichment data (data not shown). HEPES is known to induce changes in cell volume that may influence kinks or bends in the midpiece of the sperm tail.30 Because sperm are considered linear osmometers,31 and ASMKO−/− sperm have defects in regulatory volume decrease,22 sperm osmolarity also may be used in the future for better enrichment of normal sperm.
Percoll density centrifugation is another potential method of sperm selection. Although the relevant assays were not performed for this study, the buoyant density and sedimentation velocity of normal mouse and human sperm are known.32,33,34 Empirical observations by our group that sperm from ASMKO mice appear to pellet faster and harder at the same centrifugal force than sperm from wild-type mice suggest that selection of distinct sperm populations may be possible by discontinuous or continuous Percoll centrifugation. Notably, this approach has been used previously to distinguish sperm populations from infertile males that have differing phospholipid compositions.35 Given the accumulation of lipid by ASMKO−/− sperm,22 it would be interesting to pursue this method as a means of enrichment.
At best, the methods described above are likely to result in enrichment of normal sperm, not pure populations. For this reason, we also evaluated flow cytometric (FC) sorting. However, to obtain optimal purity of sperm by FC sorting, numerous modifications to the standard cell sorter are necessary to compensate for the unique properties of this cell. Such modifications include properly orienting the asymmetric sperm as it passes the quantifying detector (by changing the shape of the stream from cylindrical to ribbon and improving the nozzle design such that the detection system is closer to the oriented sperm). In addition, installation of a detector to specifically determine the orientation of the passing sperm is important.36 Another modification is the use of slit-scan procedures to determine overlap in the distributions of signal strength for sperm in the ideal orientations.37,38 Unfortunately, such specialized sperm sorters are not available widely, and we did not have access to one during the course of these studies. However, we felt that it was important to pursue these proof-of-principle experiments to establish the feasibility of this approach and to provide a basis for further investigation.
For the FC studies, we first evaluated the use of a fluorescently (BODIPY)-conjugated SPM to identify and distinguish normal versus mutant sperm based on ASM activity (data not shown). However, the fluorescent SPM failed to localize to the acrosome of normal sperm, presumably where most of the ASM is found. This may be attributable to the lipophilic nature of this substrate, particularly because sperm contain a double membrane (plasma membrane and outer acrosome membrane) and a midpiece ensheathed in lipid. Furthermore, the BODIPY-SPM may be sequestered by cholesterol, which is enriched in the sperm membranes. Second, bovine serum albumin, a necessary component of all sperm media, also sequesters lipids and prevents their entry into cells.39
Because BODIPY-SPM failed to localize to the sperm acrosome, fluorochromes assessing various aspects of sperm physiology were used as a basis for ASMKO+/− mouse sperm FC analysis and sorting. With the exception of MMP, all assays used two fluorochromes to determine the physiological health of the sperm. Of these assays, sorting of sperm based on acrosome status was focused on initially because of the importance of this function on fertility. However, these attempts were mostly unsuccessful because normal mouse sperm underwent the acrosome reaction rapidly in vitro. Moreover, even if this obstacle could be overcome, as mentioned previously that fluorescein isothiocyanate-conjugated lectin binds to the exposed outer acrosome membrane and because binding of sperm to the zona pellucida is a receptor-ligand interaction necessary for acrosome exocytosis, lectin binding may preclude fertilization. In addition, even if fertilization does occur, it is possible that residual, bound (fluorescein isothiocyanate-labeled) molecules may endocytose into the egg and disrupt or arrest zygotic development. Thus, live sperm sorting based on acrosome status may not be a feasible approach.
We therefore turned to the sorting of ASMKO+/− sperm based on MMP. The discrimination of healthy sperm based on MMP has been previously used successfully, and a positive correlation was found between MMP and sperm motility.40,41 Indeed, analysis of MMP seems to be the most reliable way to determine the health of human sperm for fertility42 and is performed with a single dye (JC-1) that is nontoxic to cells. The dye specifically labels mitochondria and is both excited and detectable in the visible range of the electromagnetic spectrum (the latter is in contrast to X- and Y-sperm analysis, which requires UV excitation of the fluorochrome). Because mitochondria are located in the midpiece of sperm, do not take part in sperm-egg binding, and are highly ubiquitinated and rapidly degraded in the zygote soon after fertilization,43,44,45 this assay may be used most successfully to discriminate and select subpopulations of human sperm. Indeed, sorting of sperm from ASMKO+/− mice for MMP provided very encouraging results, and although the genotypic enrichment we obtained using this technique was only ∼20%, similar to that obtained with Sephadex beads, as noted above our flow cytometry equipment was not optimized for sperm sorting, and we expect that this can be markedly improved. Importantly, when we used sperm sorted in this manner for IVF, we also observed a significant increase in the number of wild-type pups produced and a concordant decrease in the number of affected pups (Table 3).
Another important result reported in this study is the ability of exogenous ASM to induce a normal morphological appearance and increase in the fertilization capacity of mutant sperm (see text and Figure 4). This deserves some discussion because it may provide insights into the sperm processing that takes place in the intraluminal environment of the ASMKO+/− epididymis. It is generally accepted that the phenotype of sperm entering the epididymis is uniform and does not vary with the haploid genotype. The reasons for this presumption are that 1) before entering the epididymis, there are intercellular bridges that connect sperm and allow for sharing of transcriptional/translational products (such as ASM) between neighboring cells of different genotype; 2) gene expression is markedly repressed during the later stages of spermiogenesis as chromatin condensation occurs; and 3) there is a uniform coating of all mature sperm with macromolecules that occurs during and after spermiogenesis. Hence, it has been proposed that early in development cross correction of genetically mutant sperm by products transcribed from either normal sperm or normal Sertoli cells occurs and that this results in a primarily uniform population of mature sperm within the epididymis.46,47,48,49 Some evidence for these conclusions has come from repeated failures in separating X- and Y-chromosome-bearing sperm,50 which are reported to be phenotypically identical despite the 50:50 genotype ratio.50
It was therefore of particular interest to note differences in mature epididymal sperm from normal and ASMKO−/− males, and to identify two distinct sperm populations in heterozygous mice. Clearly, our in vitro cross correction studies show that exogenous ASM can be taken up by mature ASMKO−/− sperm and that this uptake leads to a normal appearance. However, the fact that morphologically distinct sperm populations were observed in heterozygous mice also shows that this cross-correction process does not occur very efficiently in vivo because, if it did, distinct sperm populations would not have been found.
Thus, in summary the data presented in this article reveal for the first time that haploinsufficiency of a lysosomal enzyme can lead to germ cell abnormalities, perhaps explaining, at least in part, the reduced fecundity observed in the breeding colonies for several lysosomal disease animal models, including ASMKO mice. These findings have important implications for heterozygous NPD individuals, because such individuals have historically been considered phenotypically normal. In addition, we provide proof-of-principle that the novel approach of prefertilization sperm sorting might be used in the future to prevent the birth of individuals with fatal genetic diseases, such as ASM-deficient NPD. Last, the data within also document for the first time that sperm can internalize exogenous lysosomal enzymes, an observation that provides important insights into the mechanism of enzymatic cross correction during sperm maturation.
Footnotes
Address reprint requests to Edward H. Schuchman, Ph.D., Department of Human Genetics, Mount Sinai School of Medicine, 1425 Madison Ave., Room 14-20A, New York, NY 10029. E-mail: edward.schuchman@mssm.edu
Supported by the National Institutes of Health (grant R01 HD 28607).
References
- Schuchman E, Desnick R. Niemann-Pick disease types A and B: acid sphingomyelinase deficiencies. Scriver C, Beaudet A, Sly W, Valle D, editors. New York: McGraw-Hill,; The Metabolic and Molecular Basis of Inherited Disease. (ed 8) 2001:p 3589. [Google Scholar]
- Levade T, Jaffrezou JP. Signaling sphingomyelinases: which, where, how and why? Biochim Biophys Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Tomiuk S, Zumbansen M, Stoffel W. Characterization and subcellular localization of murine and human magnesium-dependent neutral sphingomyelinase. J Biol Chem. 2000;275:5710–5717. doi: 10.1074/jbc.275.8.5710. [DOI] [PubMed] [Google Scholar]
- Schuchman EH, Suchi M, Takahashi T, Sandhoff K, Desnick RJ. Human acid sphingomyelinase. Isolation, nucleotide sequence, and expression of the full-length and alternatively spliced cDNAs. J Biol Chem. 1991;13:8531–8539. [PubMed] [Google Scholar]
- Schuchman EH, Levran O, Pereira LV, Desnick RJ. Structural organization and complete nucleotide sequence of the gene encoding human acid sphingomyelinase (SMPD1). Genomics. 1992;12:197–205. doi: 10.1016/0888-7543(92)90366-z. [DOI] [PubMed] [Google Scholar]
- Horinouchi K, Sakiyama T, Pereira L, Lalley PA, Schuchman EH. Mouse models of Niemann-Pick disease: mutation analysis and chromosomal mapping rule out the type A and B forms. Genomics. 1993;18:450–451. doi: 10.1006/geno.1993.1497. [DOI] [PubMed] [Google Scholar]
- Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandoff K, Desnick RJ, Stewart CL, Schuchman EH. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nat Genet. 1995;10:288–293. doi: 10.1038/ng0795-288. [DOI] [PubMed] [Google Scholar]
- Otterbach B, Stoffel W. Acid sphingomyelinase-deficient mice mimic the neurovisceral form of human lysosomal storage disease (Niemann-Pick disease). Cell. 1995;81:1053–1061. doi: 10.1016/s0092-8674(05)80010-8. [DOI] [PubMed] [Google Scholar]
- Dhami R, He X, Gordon RE, Elleder M, Schuchman EH. Analysis of the lung pathology and alveolar macrophage function in the acid sphingomyelinase deficient mouse model of Niemann-Pick disease. Lab Invest. 2001;81:987–999. doi: 10.1038/labinvest.3780311. [DOI] [PubMed] [Google Scholar]
- Miranda SRP, Erlich S, Friedrich VL, Haskins ME, Gatt S, Schuchman EH. Biochemical, pathological, and clinical response to transplantation of normal bone marrow cells into acid sphingomyelinase deficient mice. Transplantation. 1998;65:884–892. doi: 10.1097/00007890-199804150-00005. [DOI] [PubMed] [Google Scholar]
- Miranda SRP, Erlich S, Visser JWM, Gatt S, Dagan A, Friedrich VL, Schuchman EH. Bone marrow transplantation in acid sphingomyelinase deficient mice: engraftment and cell migration into the brain as a function of radiation, age, and phenotype. Blood. 1997;90:444–452. [PubMed] [Google Scholar]
- Erlich S, Miranda SRP, Visser JWM, Dagan A, Gatt S, Schuchman EH. Fluorescence-based selection of gene-corrected hematopoietic stem and progenitor cells from acid sphingomyelinase-deficient mice: implications for Niemann-Pick disease gene therapy and the development of improved stem cell gene transfer procedures. Blood. 1999;93:80–86. [PubMed] [Google Scholar]
- Trasler J, Saberi F, Somani IH, Adamali HI, Huang JQ, Fortunato SR, Ritter G, Gu M, Aebersold R, Gravel RA, Hermo L. Characterization of the testis and epididymis in mouse models of human Tay Sachs and Sandhoff diseases and partial determination of accumulated gangliosides. Endocrinology. 1998;139:3280–3288. doi: 10.1210/endo.139.7.6117. [DOI] [PubMed] [Google Scholar]
- Adamali HI, Somani IH, Huang JQ, Mahuran D, Gravel RA, Trasler JM, Hermo LI. Abnormalities in cells of the testis, efferent ducts, and epididymis in juvenile and adult mice with beta-hexosaminidase A and B deficiency. J Androl. 1999;20:779–802. [PubMed] [Google Scholar]
- Kapur DK, Gupta GS. Immunocytochemical localization of β-N-acetyl glucosaminidase in human reproductive organs. Biol Reprod. 1988;38:373–376. doi: 10.1095/biolreprod38.2.373. [DOI] [PubMed] [Google Scholar]
- Fan J, Akabane H, Graham SN, Richardon LL, Zhu G-Z. Sperm defects in mice lacking a functional Niemann-Pick C1 protein. Mol Reprod Dev. 2006;73:1284–1291. doi: 10.1002/mrd.20559. [DOI] [PubMed] [Google Scholar]
- Luddi A, Strazza M, Carbone M, Moretti E, Costantino-Ceccarini E. Galactosylceramidase deficiency causes sperm abnormalities in the mouse model of globoid cell leukodystrophy. Exp Cell Res. 2005;304:59–68. doi: 10.1016/j.yexcr.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Tulsiani DR, Abou-Haila A, Loeser CR, Pereira BM. The biological and functional significance of the sperm acrosome and acrosomal enzymes in mammalian fertilization. Exp Cell Res. 1998;240:151–164. doi: 10.1006/excr.1998.3943. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Harper MJK, de Kretser DM, Kerr JB. Mammalian fertilization, gamete and zygote transport, and cytology of the testis. Knobil E, Neill JD, editors. New York: Raven Press,; The Physiology of Reproduction. (ed 2) 1994:pp 189–206. [Google Scholar]
- Hinkovska VT, Petkova DH, Koumanov KS. A neutral sphingomyelinase in spermatozoal plasma membranes. Biochem Cell Biol. 1987;65:525–528. doi: 10.1139/o87-067. [DOI] [PubMed] [Google Scholar]
- Hinkovska-Galcheva VTS, Petkova DH, Nikolova MN. Sphingomyelin and ceramide: phosphoethanolamine synthesis in ram spermatozoa plasma membrane. Int J Biochem. 1989;21:1153–1156. doi: 10.1016/0020-711x(89)90058-x. [DOI] [PubMed] [Google Scholar]
- Butler A, He X, Gordon RE, Wu HS, Gatt S, Schuchman EH. Reproductive pathology and sperm physiology in acid sphingomyelinase-deficient mice. Am J Pathol. 2002;161:1061–1075. doi: 10.1016/S0002-9440(10)64267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Miranda SR, Dagan A, Gatt S, Schuchman EH. Characterization of human acid sphingomyelinase purified from the media of overexpressing Chinese hamster ovary cells. Biochim Biophys Acta. 1999;1432:251–264. doi: 10.1016/s0167-4838(99)00069-2. [DOI] [PubMed] [Google Scholar]
- Sterzik K, De Santo M, Uhlich S, Gagsteiger F, Strehler E. Glass wool filtration leads to a higher percentage of spermatozoa with intact acrosomes: an ultrastructural analysis. Hum Reprod. 1998;13:2506–2511. doi: 10.1093/humrep/13.9.2506. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Saji F, Wakimoto A, Kato M, Tsutsui T, Tanizawa O. Preparation of oligozoospermic and/or asthenozoospermic semen for intrauterine insemination using the SpermPrep semen filtration column. Fertil Steril. 1992;57:866–870. [PubMed] [Google Scholar]
- Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Gravance CG, Garner DL, Miller MG, Berger T. Fluorescent probes and flow cytometry to assess rat sperm integrity and mitochondrial function. Reprod Toxicol. 2001;15:5–10. doi: 10.1016/s0890-6238(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Cummins JM, Woodall PF. On mammalian sperm dimensions. J Reprod Fert. 1985;75:153–175. doi: 10.1530/jrf.0.0750153. [DOI] [PubMed] [Google Scholar]
- Kholkute SD, Rodriguez J, Rawlins RG, Dukelow WR. Glass wool column filtration: effects on motility, viability, and fertilizing ability of epididymal spermatozoa from mice. Lab Anim Sci. 1994;44:537–539. [PubMed] [Google Scholar]
- Lo C, Ferrier J, Tenenbaum HC, McCulloch CA. Regulation of cell volume and intracellular pH in hyposmotically swollen rat osteosarcoma cells. Biochem Cell Biol. 1995;73:535–44. doi: 10.1139/o95-059. [DOI] [PubMed] [Google Scholar]
- Willoughby CE, Mazur P, Peter AT, Critser JK. Osmotic tolerance limits and properties of murine spermatozoa. Biol Reprod. 1996;55:715–727. doi: 10.1095/biolreprod55.3.715. [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Meistrich ML, Furrer R, Bruce WR. Physical characteristics of mouse nuclei. Biophys J. 1976;16:811–825. doi: 10.1016/S0006-3495(76)85730-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio S. Apparent densities of spermatozoa of various mammalian species. Gamete Res. 1988;20:159–164. doi: 10.1002/mrd.1120200206. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Oshio S, Kobayashi T, Mohri H, Iizuka R. Buoyancy and sedimentation of human X- and Y-bearing sperm. Arch Androl. 1987;19:211–215. doi: 10.3109/01485018708986818. [DOI] [PubMed] [Google Scholar]
- Zalata AA, Christophe AB, Depuydt CE, Schoonjans F, Comhaire FH. The fatty acid composition of phospholipids of spermatozoa from infertile patients. Mol Hum Reprod. 1998;4:111–118. doi: 10.1093/molehr/4.2.111. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Welch GR. Sex preselection: high-speed flow cytometric sorting of X and Y sperm for maximum efficiency. Theriogenology. 1999;52:1323–1341. doi: 10.1016/s0093-691x(99)00220-4. [DOI] [PubMed] [Google Scholar]
- Benaron DA, Gray JW, Gledhill BL, Lake S, Wyrobek AJ, Young IT. Quantification of mammalian sperm morphology by slit-scan flow cytometry. Cytometry. 1982;2:344–349. doi: 10.1002/cyto.990020512. [DOI] [PubMed] [Google Scholar]
- van Munster EB. Interferometry in flow to sort unstained X- and Y-chromosome-bearing bull spermatozoa. Cytometry. 2002;47:192–199. doi: 10.1002/cyto.10064. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol. 1999;214:429–443. doi: 10.1006/dbio.1999.9428. [DOI] [PubMed] [Google Scholar]
- Troiano L, Granata AR, Cossarizza A, Kalashnikova G, Bianchi R, Pini G, Tropea F, Carani C, Franceschi C. Mitochondrial membrane potential and DNA stainability in human sperm cells: a flow cytometry analysis with implications for male infertility. Exp Cell Res. 1998;241:384–393. doi: 10.1006/excr.1998.4064. [DOI] [PubMed] [Google Scholar]
- Imai H, Suzuki K, Ishizaka K, Ichinose S, Oshima H, Okayasu I, Emoto K, Umeda M, Nakagawa Y. Failure of the expression of phospholipid hydroperoxide glutathione peroxidase in the spermatozoa of human infertile males. Biol Reprod. 2001;64:674–683. doi: 10.1095/biolreprod64.2.674. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17:1257–1265. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol Reprod. 2000;63:582–590. doi: 10.1095/biolreprod63.2.582. [DOI] [PubMed] [Google Scholar]
- Cummins JM, Kishikawa H, Mehmet D, Yanagimachi R. Fate of genetically marked mitochondrial DNA from spermatocytes microinjected into mouse zygotes. Zygote. 1999;7:151–156. doi: 10.1017/s0967199499000519. [DOI] [PubMed] [Google Scholar]
- Shitara H, Hayashi JI, Takahama S, Kaneda H, Yonekawa H. Maternal inheritance of mouse mtDNA in interspecific hybrids: segregation of the leaked paternal mtDNA followed by the prevention of subsequent paternal leakage. Genetics. 1998;148:851–857. doi: 10.1093/genetics/148.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337:373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Morales CR, Hake LE, Hecht NB. Cellular localization of the mRNAs of the somatic and testis-specific cytochromes c during spermatogenesis in the rat. Mol Reprod Dev. 1993;34:196–205. doi: 10.1002/mrd.1080340212. [DOI] [PubMed] [Google Scholar]
- Thomas KH, Wilkie TM, Tomashefsky P, Bellve AR, Simon MI. Differential gene expression during mouse spermatogenesis. Biol Reprod. 1989;41:729–739. doi: 10.1095/biolreprod41.4.729. [DOI] [PubMed] [Google Scholar]
- Amann RP, Seidel GE. Boulder: Colorado Associated University Press,; Prospects for Sexing Mammalian Sperm. 1982 [Google Scholar]
- Fisher RA. Oxford: Oxford University Press,; The Genetical Theory of Natural Selection. 1930 [Google Scholar]