Abstract
Leukocyte infiltration into inflamed tissues is considered to involve sequential steps of rolling over the endothelium, adhesion, and transmigration. In this model, the leukocyte adhesion molecule L-selectin and its ligands expressed on inflamed endothelial cells are involved in leukocyte rolling. We show that upon experimental and human renal ischemia/reperfusion, associated with severe endothelial damage, microvascular basement membrane (BM) heparan sulfate proteoglycans (HSPGs) are modified to bind L-selectin and monocyte chemoattractant protein-1. In an in vitro rolling and adhesion assay, L-selectin-binding HSPGs in artificial BM induced monocytic cell adhesion under reduced flow. We examined the in vivo relevance of BM HSPGs in renal ischemia/reperfusion using mice mutated for BM HSPGs perlecan (Hspg2Δ3/Δ3), collagen type XVIII (Col18a1−/−), or both (cross-bred Hspg2Δ3/Δ3×Col18a1−/−) and found that early monocyte/macrophage influx was impaired in Hspg2Δ3/Δ3×Col18a1−/− mice. Finally, we confirmed our observations in human renal allograft biopsies, showing that loss of endothelial expression of the extracellular endosulfatase HSulf-1 may be a likely mechanism underlying the induction of L-selectin- and monocyte chemoattractant protein-1-binding HSPGs associated with peritubular capillaries in human renal allograft rejection. Our results provide evidence for the concept that not only endothelial but also (microvascular) BM HSPGs can influence inflammatory responses.
Upon tissue damage, leukocytes are recruited to the site of inflammation to exert their functions. This process is generally considered to involve an initial phase of tethering of leukocytes to activated endothelium, mediated by leukocyte-expressed L-selectin and endothelium-expressed E- and P-selectin and their ligands, followed by activation and firm adhesion, mediated by chemokines and integrins, respectively.1,2 Finally, leukocytes transmigrate through the endothelium and vascular basement membrane (BM) to the site of inflammation.
Although this multistep model of leukocyte extravasation is well established, there are some clinically important situations in which additional factors may come into play. For example, it is well known that the endothelium of the heart and kidney is severely damaged as a result of prolonged ischemia and subsequent reperfusion (I/R),3,4,5,6,7,8 which is particularly important in transplantation settings. Upon renal I/R, endothelial cell swelling, loss of endothelial cell-cell attachment, and even complete loss of endothelial lining has been reported in peritubular capillaries as early as 24 hours after reperfusion.4,5 In this environment, leukocytes encounter a damaged endothelial layer with exposed vascular BM, over which they will transmigrate.
Vascular BM is composed of various matrix components including laminins, collagens, and proteoglycans.9,10 Proteoglycans are glycoconjugates consisting of extended linear carbohydrate side chains (glycosaminoglycans; GAGs) linked to a protein core.11 A specific subset of proteoglycans, heparan sulfate proteoglycans (HSPGs), is especially well known for its ability to bind a number of proteins important for the inflammatory response, including the leukocyte-expressed adhesion molecule L-selectin, and various chemokines.12 Binding of these proteins to HSPGs is dependent on the presence of specific binding domains on the HS GAG chain.13,14 We and others have previously shown that for L-selectin binding to HSPGs a number of determinants, including 6-O-sulfation of the GAG chain, are crucial.15,16 Because the presence or absence of these domains on HS GAG chains determines whether HSPGs will bind molecules like L-selectin and chemokines, active regulation of these domains, eg, during biosynthesis and/or by later modification, could clearly affect the inflammatory response. Interestingly, extracellular endosulfatases that specifically cleave 6-O-linked sulfate residues of HSPGs have been identified in humans (HSulf-1 and HSulf-2),17 and although a change in expression pattern of these enzymes has not been reported in inflammatory settings to date, differential expression of HSulf-1 has been described in carcinomas.18,19
In the normal kidney, HSPGs are abundantly present in glomeruli, tubular BM (TBM), microvascular BM, vasa recta bundles, papilla, and on epithelial cells. However, only a subset of HSPGs, present in medullary TBMs, vasa recta bundles, and papilla, are able to bind L-selectin under normal conditions.15 In this study, we examine the expression and relevance of HSPGs that bind L-selectin and chemokine upon renal I/R, a model used to study the early inflammatory processes involved in kidney transplantation. Because we are interested in HSPG alterations that can have functional consequences for the inflammatory response, we chose to directly detect binding of L-selectin and monocyte chemoattractant protein-1 (MCP-1) to HSPGs in tissue sections,20 rather than using antibodies directed against structurally well defined, but not necessarily functional, HS epitopes. We show that upon renal I/R, microvascular BM HSPGs in the renal interstitium are modified to bind L-selectin and MCP-1. Using an in vitro model for leukocyte rolling and adhesion, we provide evidence for a functional role of L-selectin-binding HSPGs in monocytic cell adhesion, and we show that in vivo monocyte influx is impaired in kidneys of mice that lack functional BM HSPGs perlecan and collagen type XVIII. Finally, we extrapolate our findings to human renal transplant biopsies and show that down-regulation of endothelial-expressed HSulf-1 could be a mechanism for the observed HSPG alterations.
Materials and Methods
Animals and Renal I/R
Adult male Wistar rats (300 to 350 g) were obtained from Harlan CPB (Zeist, The Netherlands). Adult male wild-type mice, Hspg2Δ3/Δ3 mutant mice,21 Col18a1−/− mice,22 and crossbred Hspg2Δ3/Δ3×Col18a1−/− mutant mice21 were all on C57BL/6 background and ranging from 10 to 18 weeks old. Unilateral renal warm I/R was performed by clamping left renal pedicle (mice) or renal artery (rats) of isoflurane-anesthetized animals for 45 minutes while keeping the abdominal cavity moist with saline, after which the clamp was released, reperfusion of the kidney was visually checked, and wounds were closed. During and after the operation, animals were kept warm until they regained consciousness, after which they had free access to food and water. Animals were sacrificed at t = 24 hours or t = 48 hours after reperfusion; both contralateral and I/R kidneys were removed and either snap-frozen or formalin-fixed and paraffin-embedded according to routine histology protocol. Animal housing and experiments were approved by local animal experimentation ethics committees.
Proteins, Enzymes, and Antibodies
L-selectin-IgM chimeric protein, consisting of the extracellular domain of human L-selectin linked to an IgM Fc-tail, was produced as described.20,23 Heparitinase I from Flavobacterium heparinum (EC4.2.2.8) was from Seikagaku Corp., Tokyo, Japan. Anti-rat CD31, anti-rat CD68 (ED1), and anti-mouse F4/80 were from Serotec, Oxford, UK. Anti-rat perlecan (10B2) was kindly provided by Dr. Couchman, Division of Biomedical Sciences, Imperial College, London, UK. Anti-rat agrin (GR14) and anti-human MCP-1 (5D3-F7) were previously described.24,25 Recombinant human MCP-1 was from Peprotech, London, UK. Anti-collagen XVIII NC11 was kindly provided by Dr. T. Sasaki, Max-Planck-Institut für Biochemie, Martinsried, Germany. DREG-56, MECA-79, and HECA-452 were from BD PharMingen (Erembodegem, Belgium); and anti-human CD31, CD34, and von Willebrand factor were from DAKO (Heverlee, Belgium). Alexa Fluor-labeled anti-human IgM, anti-mouse IgG, anti-rabbit IgG, anti-sheep IgG, anti-rat IgG, and streptavidin were from Molecular Probes (Invitrogen, Breda, The Netherlands) and biotinylated anti-rat IgG + IgM from Jackson ImmunoResearch (Cambridgeshire, UK).
Immunofluorescence
In situ L-selectin binding was performed as described on either formalin-fixed cryostat tissue sections or formalin-fixed, paraffin-embedded tissue sections.20 MCP-1 binding was performed accordingly on formalin-fixed, paraffin-embedded sections, incubating MCP-1 (2.5 μg/ml) overnight at 4°C. Specific digestion of HS GAG chains was performed by preincubation of tissue sections with 0.05 U/ml heparitinase I in acetate buffer (50 mmol/L C2H3O2Na, 5 mmol/L CaCl2·2H2O, and 5 mmol/L MgCl2·6H2O; pH 7.0) for 1 hour at 37°C in a humidified chamber or with HNO2 at pH 1.5 for 10 minutes at room temperature.26 Double staining was performed using endothelial markers including rhodamine-labeled Ulex europaeus agglutinin I (Vector, Burlingame, CA). Sections were examined by two independent investigators using a Nikon Eclipse E800 fluorescence microscope (Tokyo, Japan) or Leica AOBS SP2 confocal laser-scanning microscope system (Wetzlar, Germany). For specific detection of monocytes/macrophages, acetone-fixed (10 minutes room temperature) tissue sections were rehydrated in phosphate-buffered saline (PBS), blocked with PBS containing 5% normal goat serum (10 minutes at room temperature), and incubated with anti-monocyte/macrophage F4/80 (1 hour at room temperature) and subsequently with anti-rat Alexa Fluor 488-labeled secondary antibody. Quantification of monocyte/macrophage influx was based on five digital photographs (×200 magnification) of renal medulla per kidney, using four mice per group per time point. The percentage of positive area relative to total area was determined using AnalySis software (Soft Imaging System GmbH, Münster, Germany). Fold induction of influx in I/R kidneys was calculated compared with contralateral kidneys of the same animals.
Histology
Formalin-fixed kidney sections (4 μm) were stained with periodic acid-Schiff’s reagent and hematoxylin according to routine protocol. Leukocyte counts and the percentage of tubules showing tubular necrosis (defined by the loss of nuclei) were determined in 10 nonoverlapping fields (×400 magnification) of the outer medulla region per kidney/biopsy.
Flow Experiments
Rolling and adhesion of monocytic U937 cells over coated coverslips were determined under continuous flow conditions in a perfusion chamber with well-defined rheological characteristics as described.27 Thermanox coverslips (Nunc, Rochester, NY) were coated overnight at 37°C with laminin (human placenta; 2 μg/ml) and BSA (10 μg/ml) or laminin (2 μg/ml) and heparin-albumin (10 μg/ml), all from Sigma-Aldrich, Zwijndrecht, The Netherlands. U937 cells were cultured in RPMI 1640 medium plus 10% fetal bovine serum and used at 2 × 106 cells/ml for flow experiments. After rinsing coated coverslips within the perfusion chamber, a fixed volume of cells was allowed to interact (rolling/adhesion) at indicated shear stress, and coverslips were again rinsed to remove nonbound or loosely attached cells (adhesion). Rolling was evaluated by analyzing 20-second recordings of six fields (×200 magnification) per coverslip. Adhesion was determined by analyzing at least 50 fields (×200 magnification) per coverslip. Data represented are based on six experiments. In two experiments, L-selectin binding sites on heparin-albumin were blocked by preincubating coated coverslips with excess L-selectin-IgM chimeric protein for 3 hours at room temperature.
Human Renal Biopsies
Formalin-fixed, paraffin-embedded archival renal transplant biopsies were used. Acute renal allograft rejection was defined based on histology by an experienced pathologist, according to Banff 97 classification.28 Normal tissue from kidneys removed because of renal adenocarcinoma served as controls. All samples were used with local ethics committee approval.
In Situ Hybridization
hSulf1 or hSulf2 cDNA fragments (gi29789063, 2986 to 3601; and gi38327656, 2151 to 2801, respectively) were amplified by polymerase chain reaction using sense and anti-sense primers containing SP6 and T7 RNA polymerase binding sequences, respectively, and were cloned in pCR2.1-TOPO (Invitrogen). Digoxigenin-labeled anti-sense or control sense riboprobes were synthesized by in vitro transcription of the purified inserts using T7 or SP6 RNA polymerases and digoxigenin-conjugated UTP (Roche Diagnostics, Almere, The Netherlands). In situ hybridization was performed on 6-μm sections of formalin-fixed, paraffin-embedded tissue. Digoxigenin was detected using alkaline phosphatase-conjugated anti-digoxigenin antibodies and NBT/BCIP (Roche Diagnostics) yielding a blue precipitate. To provide a counterstain not interfering with cellularly localized in situ hybridization signals, immunohistochemistry was performed using anti-collagen type IV (Euro-Diagnostica, Arnhem, The Netherlands), poly-horseradish peroxidase anti-rabbit IgG (Powervision; Immunovision Technologies, Brisbane, CA), and 3,3′-diaminobenzidine plus hydrogen peroxide, yielding a brown precipitate.
Statistics
The number of mice and data generated for each experiment are provided in the figure legends. Statistical analysis was performed using the Mann-Whitney test or Kruskal-Wallis test as indicated, with P < 0.05 considered to be statistically significant.
Results
Interstitial Microvascular BM HSPGs Bind L-Selectin upon Renal I/R
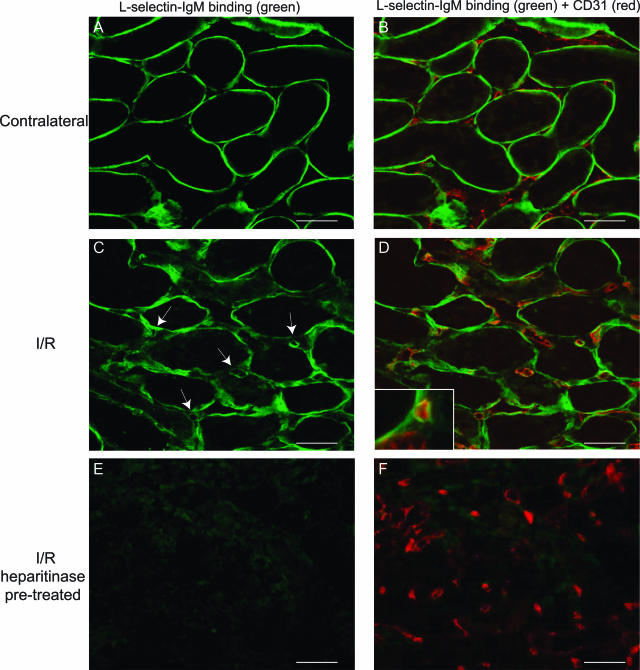
We performed unilateral renal warm I/R in rats, after which the nonischemic contralateral and the I/R kidneys were analyzed for the presence of L-selectin-binding HSPGs. Clear binding of L-selectin to TBMs in the medulla was observed in the contralateral kidneys at 24 hours after reperfusion (Figure 1A). In addition, L-selectin binding was detected in the large vasa recta bundles and renal papilla (not shown). Thereby, L-selectin binding in contralateral kidneys was identical to binding in kidneys of nonoperated control rats.15 No L-selectin binding to interstitial capillaries, identified using double-staining for CD31, was observed in contralateral kidney (Figure 1B). In contrast, L-selectin binding to interstitial capillaries was detected in I/R kidneys at 24 hours after reperfusion, in addition to binding to TBMs (Figure 1, C and D). These L-selectin-binding capillaries were most abundantly found in the outer medulla of the I/R kidneys. Interestingly, high-power magnification suggests that L-selectin binding occurs directly underneath, rather than at the luminal side of the endothelial cells (Figure 1D, inset). Binding to both TBMs and capillaries was completely abrogated by pretreatment with heparitinase I, an enzyme that specifically degrades HS GAG chains (Figure 1, E and F), as well as by pretreatment with HNO2 at pH 1.5, which chemically cleaves HS GAG chains (not shown). These data show that L-selectin-binding HSPGs are specifically induced in renal capillaries upon I/R. No L-selectin binding in glomeruli of either contralateral or I/R kidneys was observed at the examined time points (not shown).
Figure 1.
Interstitial microvascular BM HSPGs are induced to bind L-selectin upon renal I/R. L-selectin ligands (A–F, green) were detected using the L-selectin-IgM chimeric protein (see Materials and Methods) on tissue sections of either contralateral rat kidney (A, B) or kidney 24 hours after I/R (C–F) by confocal laser-scanning microscopy. In B, D, and F, double staining is shown to identify endothelium using anti-CD31 antibody (red). Arrows indicate the presence of L-selectin ligands associated with interstitial capillaries, and high-power magnification indicates that these ligands are localized in the microvascular BM (D, inset). L-selectin ligands were shown to be HSPGs using heparitinase I pretreatment of sequential I/R tissue sections (E, F). Result shown is representative of four animals. Scale bars = 50 μm. Original magnification, ×1000 (D, inset).
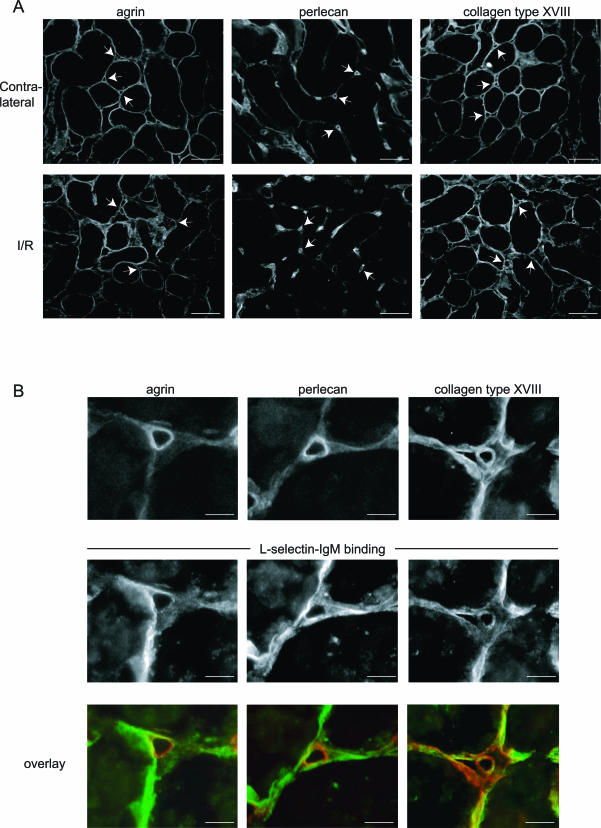
Because it seemed that the HSPG-dependent microvascular L-selectin binding upon renal I/R occurred in a subendothelial pattern, we performed double staining to detect both L-selectin binding and the presence of the three dominant BM HSPGs, agrin, perlecan, and collagen type XVIII, in I/R kidneys. Both in contralateral and I/R kidneys, agrin, perlecan, and collagen type XVIII were present to a similar extent and in a similar pattern, showing no evident up-regulation of either of the three in the microvascular BM upon I/R (Figure 2A). The I/R-induced microvascular L-selectin binding co-localized to various degrees with agrin, perlecan, and collagen type XVIII (Figure 2B). Taken together, these data show that microvascular BM HSPGs are induced to bind L-selectin upon renal I/R.
Figure 2.
The BM HSPGs agrin, perlecan, and collagen type XVIII are equally present in contralateral and I/R kidneys and co-localize with induced microvascular L-selectin ligands. A: Agrin, perlecan, and collagen type XVIII were detected using specific antibodies in both contralateral and I/R (t = 24 hours) kidney tissue sections to show similar distribution under both conditions. B: High-power magnification showing co-localization of agrin, perlecan, and collagen type XVIII (top) with L-selectin ligands (bottom) in microvascular structures at 24 hours after renal I/R. Overlays of the same structures are provided showing, respectively, agrin, perlecan, or collagen type XVIII in red, and L-selectin binding in green. Scale bars: 50 μm (A); 10 μm (B).
Induction of L-Selectin-Binding HSPGs Coincides with Monocyte/Macrophage Influx and Induces Monocytic Cell Adhesion under Reduced Flow Conditions
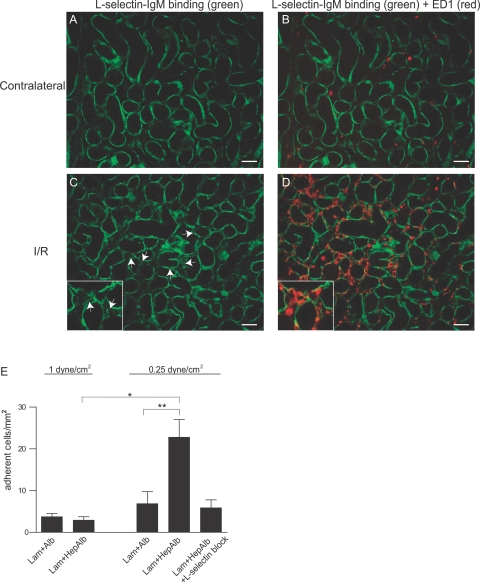
Because L-selectin is a leukocyte adhesion receptor involved in extravasation, we wondered whether the induced L-selectin-binding HSPGs could be associated with leukocyte influx observed upon renal I/R. Although neutrophil extravasation occurs within a few hours after I/R, monocytes are expected to start extravasating around the time point of 24 hours after I/R, and we therefore focused on these cells. As expected, although a minimal number of monocytes/macrophages (identified using the ED1 marker) were detected in contralateral kidneys (Figure 3, A and B), a prominent influx of these cells was observed in the outer medulla of I/R kidneys at 24 hours after reperfusion (Figure 3, C and D). Interestingly, the L-selectin-binding capillaries identified in single stain (Figure 3C, arrows) were present in close proximity to the monocyte/macrophage influx found in this region (Figure 3D). High-power magnification showed ED1-positive cells directly surrounding the L-selectin-binding capillaries (Figure 3, C and D; inset).
Figure 3.
Monocyte influx coincides with microvascular L-selectin-binding HSPGs and monocytic cells specifically adhere to L-selectin-binding HSPGs in artificial BM under flow. A–D: L-selectin ligands (green) were detected using the L-selectin-IgM chimeric protein in contralateral rat kidney (A, B) or I/R kidney (C, D; t = 24 hours). Arrows indicate the presence of L-selectin ligands associated with the renal microvasculature. In B and D, influx of ED1-positive monocytes (red, overlay) is shown to occur in close proximity to induced L-selectin ligands in the I/R kidney. High-power magnification is provided in C and D. Result shown is representative of four animals. E: U937 monocytic cells were allowed to adhere under physiological (1.0 dyne/cm2) or reduced shear stress (0.25 dyne/cm2) to artificial BM composed of either laminin and albumin (Lam + Alb) or laminin and heparin-albumin (Lam + HepAlb) (n = 6). L-selectin binding sites in the artificial BM were blocked by preincubation with an excess of L-selectin-IgM chimeric protein (n = 2). Bars represent mean ± SEM. *P = 0.0095, **P = 0.017 using the Mann-Whitney test. Original magnifications, ×1000 (insets).
To examine a possible role of L-selectin-binding HSPGs in microvascular BM in monocyte/macrophage recruitment, we studied rolling and adhesion of monocytic U937 cells (expressing L-selectin; not shown) over an artificial BM consisting of laminin (Lam; a major component of all vascular BMs29), in combination with either albumin (Alb) as a control, or heparin-albumin (HepAlb). The latter consists of multiple chains of heparin (a highly sulfated HS GAG chain known to bind L-selectin15,16) covalently linked to an albumin backbone, used here as a model for L-selectin-binding HSPG. First, we examined rolling and adhesion under a continuous shear stress of 1.0 dyne/cm2, considered to be within the physiological flow range in the microvasculature.27,30 Under these conditions, no rolling of the cells was observed (not shown), and only a minimal amount of cells adhered to Lam + Alb or Lam + HepAlb (Figure 3E). However, following I/R blood flow in renal capillaries is significantly reduced for a prolonged period of time, being approximately one-quarter of normal levels at 24 hours after reperfusion.31 We therefore performed the same experiment under a reduced continuous shear stress of 0.25 dyne/cm2. Under these conditions adhesion of U937 cells to Lam + HepAlb was significantly increased compared with Lam + Alb (Figure 3E). Preincubation of Lam + HepAlb-coated coverslips with an excess of the L-selectin-IgM chimeric protein reduced adhesion to control levels, showing that adhesion is dependent on L-selectin-binding sites (Figure 3E). No significant differences in cell rolling were observed between the different coatings or different shear stress conditions used in these experiments (not shown). Together, these data indicate that L-selectin-binding HSPGs induced in microvascular BM may contribute to monocyte recruitment on I/R by enhancing monocyte adhesion.
Early Monocyte/Macrophage Influx Is Impaired in Hspg2Δ3/Δ3×Col18a1−/− Mice
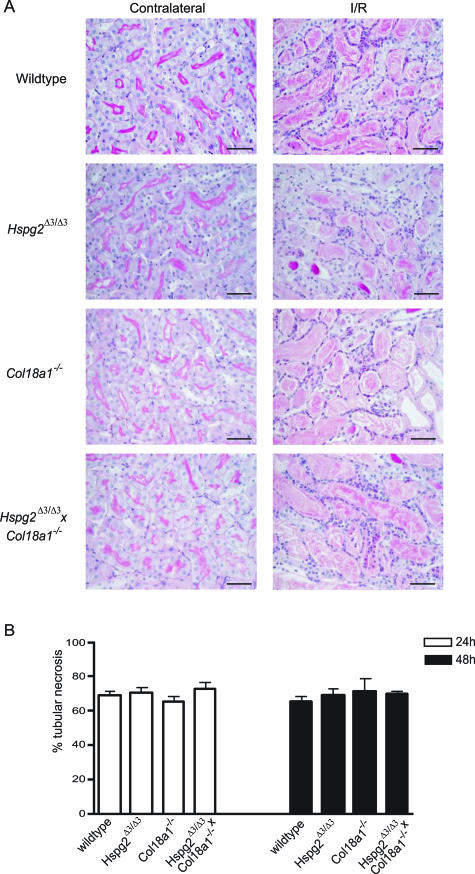
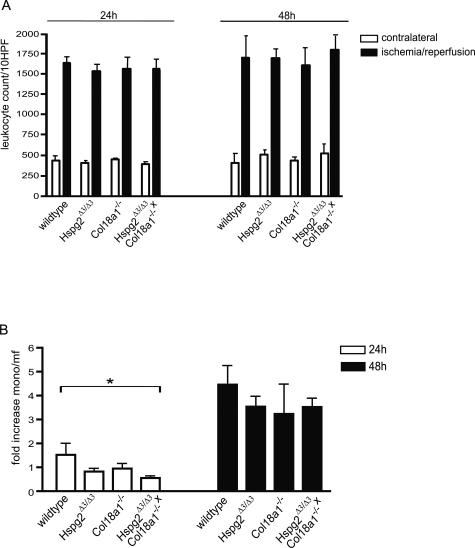
We next determined the role of BM HSPGs in leukocyte influx upon renal I/R in vivo using mice that lack functional BM HSPGs. Agrin- and perlecan-deficient mice have been generated; however, these mice die at birth because of different developmental defects and could therefore not be used.32,33 Instead, we decided to use perlecan mutant mice (Hspg2Δ3/Δ3), which lack three of four potential HS attachment sites on the perlecan protein,21 as well as collagen type XVIII knockout mice (Col18a1−/−),22 and crossbred Hspg2Δ3/Δ3×Col18a1−/− double mutants.21 In these mice, unilateral renal warm I/R was performed, and the kidneys were analyzed at 24 and 48 hours after reperfusion. As shown in Figure 4A, renal I/R injury resulted in a similar degree of damage based on histology in the different groups, showing severe tubular necrosis and influx of leukocytes. Indeed, quantification of renal I/R induced damage as the percentage of tubular necrosis was similar in wild-type versus mutant mice (Figure 4B). Total interstitial leukocyte counts showed a marked increase in leukocyte influx in I/R compared with contralateral kidneys, and no differences were observed between the different groups (Figure 5A). However, staining for the monocyte/macrophage-specific marker F4/80 revealed a significantly reduced influx of these cells in the Hspg2Δ3/Δ3×Col18a1−/− double mutants compared with wild-type animals at 24 hours after I/R (Figure 5B). Monocyte/macrophage influx was not statistically different in either Hspg2Δ3/Δ3 or Col18a1−/− single mutant mice compared with wild type at 24 hours after I/R. At 48 hours after I/R, no statistical difference was observed in monocyte/macrophage influx in the kidneys of the various mutant/knockout mice compared with wild type (Figure 5B). These data indicate that early monocyte/macrophage influx on renal I/R is impaired in mice mutated for two of three BM HSPGs (Hspg2Δ3/Δ3×Col18a1−/− double mutants); however, this impairment is only observed at 24 hours after I/R.
Figure 4.
Renal histology and the percentage of tubular necrosis upon renal I/R is similar in mice lacking functional BM HSPGs compared with wild type. A: Periodic acid-Schiff/hematoxylin-stained regions of the outer medulla of contralateral and I/R (t = 24 hours) kidney of wild-type mice, Hspg2Δ3/Δ3 mutants, Col18a1−/−, and crossbred Hspg2Δ3/Δ3×Col18a1−/− mutant mice. B: Percentage of tubular necrosis was determined (see Materials and Methods) in I/R kidneys (t = 24 hours and t = 48 hours) of wild-type mice, Hspg2Δ3/Δ3 mutants, Col18a1−/−, and crossbred Hspg2Δ3/Δ3×Col18a1−/− mutant mice. Bars represent mean ± SEM of four mice per group. Scale bars = 50 μm.
Figure 5.
Early monocyte/macrophage influx is specifically impaired upon renal I/R in mice lacking functional BM HSPGs. A: Total interstitial leukocyte count was determined in contralateral kidneys and I/R kidneys of wild-type mice, Hspg2Δ3/Δ3 mutants, Col18a1−/−, and crossbred Hspg2Δ3/Δ3×Col18a1−/− mutant mice at 24 and 48 hours after I/R. Values represent mean ± SEM of 10 fields per kidney using four mice per group. B: Influx of monocytes/macrophages (mono/mf) was determined using the specific marker F4/80 in kidneys of wild-type mice, Hspg2Δ3/Δ3 mutants, Col18a1−/−, and crossbred Hspg2Δ3/Δ3×Col18a1−/− mutant mice at 24 and 48 hours after I/R and is expressed as fold induction compared with the values in contralateral kidneys. Values represent mean ± SEM obtained from five fields per kidney using four mice per group. *P = 0.005 using the Kruskal-Wallis test.
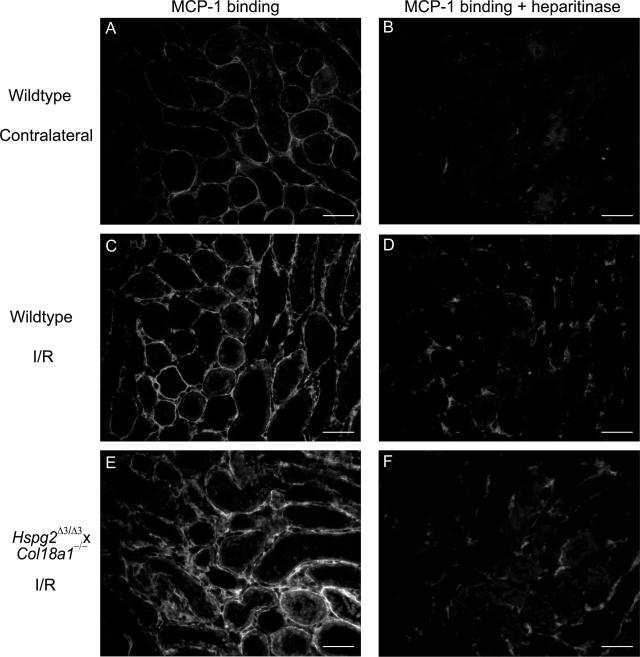
Next, we studied L-selectin binding upon renal I/R in the kidneys of Hspg2Δ3/Δ3, Col18a1−/−, and crossbred double mutants compared with wild-type mice. Strikingly, no differences were found in L-selectin binding to microvascular BM in the mutant mice compared with wild type (not shown). Apart from L-selectin, HSPGs are also able to bind and present chemokines. To investigate whether the impaired early monocyte/macrophage influx, we observed in the crossbred Hspg2Δ3/Δ3×Col18a1−/− mice could result from an altered chemokine binding, we examined MCP-1 binding in the kidneys of wild type compared with Hspg2Δ3/Δ3×Col18a1−/− mice. MCP-1 is a dominant chemokine involved in monocyte/macrophage recruitment on renal I/R, and binding properties of MCP-1 to proteoglycans are well defined.34,35,36 In wild-type contralateral kidneys, MCP-1 binds to TBMs, and binding is completely abrogated by heparitinase I pretreatment, showing that the MCP-1-binding structures are HSPGs (Figure 6, A and B). However, at 24 hours after I/R, MCP-1 binding was also observed in interstitial matrix, including the microvascular BM (Figure 6C). A significant part of this MCP-1 binding was sensitive to heparitinase I and therefore mediated by HSPGs, although some residual interstitial MCP-1 binding remained (Figure 6D). In the I/R kidneys of Hspg2Δ3/Δ3×Col18a1−/− mice MCP-1 binding was found to be similar to that in wild-type mice (Figure 6, E and F). Together, these data indicate that although L-selectin and MCP-1 binding to microvascular BM HSPGs is induced on renal I/R, this binding is not (exclusively) mediated by either perlecan-HS and/or collagen type XVIII. However, early monocyte/macrophage influx was impaired in the kidneys of mice mutated for both of these BM HSPGs.
Figure 6.
Interstitial MCP-1 binding is increased upon renal I/R and is unaltered in Hspg2Δ3/Δ3×Col18a1−/− mutant mice compared with wild type. MCP-1 binding was determined (see Materials and Methods) in tissue sections of contralateral kidneys (A, B) and in I/R kidneys (t = 24 hours) (C, D) of wild-type mice. Heparitinase I pretreatment of sequential tissue sections was performed to identify HSPG-dependent binding (B, D). Similar MCP-1 binding was observed in I/R kidneys (t = 24 hours) of Hspg2Δ3/Δ3×Col18a1−/− crossbred mutant mice (E, F) and after heparitinase I pretreatment (F). Result shown is representative of four mice per group. Scale bars = 50 μm.
Microvascular BM HSPGs Bind L-Selectin and MCP-1 in Human Renal Transplant Biopsies
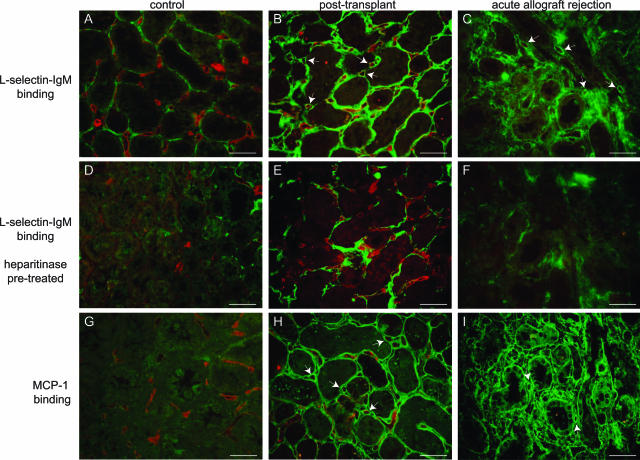
To examine whether our results in rodents can be extrapolated to the clinically relevant situation, we studied L-selectin and MCP-1 binding, as well as interstitial leukocyte influx in human control kidneys, renal allograft biopsies taken shortly after transplantation (representing I/R; post-transplant), and acute allograft rejection biopsies (Table 1). In human control kidney, binding of L-selectin to TBM was observed, as well as some minor binding associated with peritubular capillaries (Figure 7A). However, in post-transplant biopsies prominently induced L-selectin binding to peritubular capillary microvascular BM was observed, in addition to apparently increased binding to TBMs and interstitial matrix (Figure 7B). Binding to microvascular BM and TBM was sensitive to heparitinase I pretreatment (Figure 7, D and E), showing that these ligands are HSPGs. The remaining L-selectin binding in interstitial matrix was sensitive to chondroitinase ABC pretreatment, which specifically degrades chondroitin/dermatan sulfate proteoglycans, indicating that L-selectin also binds chondroitin/dermatan sulfate proteoglycans present in these tissue sections (not shown). No L-selectin binding was observed in the glomeruli of either control or post-transplant biopsies (not shown). MCP-1 binding in post-transplant biopsies was very similar to L-selectin binding (Figure 7H), although no significant MCP-1 binding was observed in human control kidney (Figure 7G). Heparitinase I pretreatment completely abolished microvascular BM MCP-1 binding in post-transplant biopsies, indicating that this binding is exclusively mediated by HSPGs (not shown).
Table 1.
Quantification of L-Selectin and MCP-1 Binding Associated with Peritubular Capillaries, HSulf-1 and HSulf-2 Expression, and Interstitial Leukocyte Counts in Human Renal Biopsies
| Case no. | Diagnosis (Banff score) | L-selectin binding HSPGs* | MCP-1 binding HSPGs* | HSulf-1 expression (number;intensity)† | HSulf-2 expression (number;intensity)† | Interstitial leukocyte count/field |
|---|---|---|---|---|---|---|
| 1 | Control | 0 | nd‡ | 1;1 | 0 | 20 |
| 2 | Control | 0 | 0 | 2;1 | 0 | 22 |
| 3 | Control | 0 | 1 | 3;3 | 0 | 30 |
| 4 | Control | 0 | 0 | 1;1 | 0 | 23 |
| 5 | Post-transplant | 4 | 4 | nd | nd | 30 |
| 6 | Post-transplant | 2 | 3 | nd | nd | 26 |
| 7 | Post-transplant | 3 | 4 | nd | nd | 39 |
| 8 | Acute rejection (IA) | 0 | 0 | 1;2 | nd | 122 |
| 9 | Acute rejection (IA) | 0 | 0 | 1;2 | 0;0 | 91 |
| 10 | Acute rejection (IB) | 0 | 0 | 0;0§ | 0;0§ | 247 |
| 11 | Acute rejection (IB) | 0 | 0 | 0;0§ | 0;0§ | 398 |
| 12 | Acute rejection (IIA) | 3 | 3 | 0;0 | 0;0 | 120 |
| 13 | Acute rejection (IIA) | 2 | 2 | 0;0 | 0;0 | 82 |
| 14 | Acute rejection (IIA) | 1 | 1 | 0;0 | 0;0 | 180 |
| 15 | Acute rejection (IIB) | 0 | 0 | 1;1 | 0;0 | 78 |
| 16 | Acute rejection (IIB) | 3 | 4 | 0;0 | nd | 140 |
| 17 | Acute rejection (III) | 0 | 0 | nd | nd | 103 |
L-selectin-IgM and MCP-1 binding to HSPGs associated with peritubular capillaries was scored as follows: 0, <10%; 1, 10 to 25%; 2, 25 to 50%; 3, 50 to 75%; 4, >75% of peritubular capillaries involved.
HSulf-1 and HSulf-2 expression were detected by in situ hybridization and scored both as numbers of peritubular capillaries involved (0, no expression detected; 1, <25%; 2, 25 to 50%; 3, 50 to 75%; 4, >75% of peritubular capillaries involved) and estimated intensity of staining on a scale ranging from 0 (no staining) to 4 (very intense).
nd, not determined.
Peritubular capillaries could not be identified because of disruption of tissue morphology.
Figure 7.
L-selectin and MCP-1 binding HSPGs are induced in post-transplant and acute allograft rejection biopsies. L-selectin binding (A–C, green) was determined in tissue sections of human control kidney (A, case 4), post-transplant (B, case 7), and acute allograft rejection biopsies (C, case 16). Sequential sections were pretreated with heparitinase I to identify HSPG-dependent binding (D–F). In parallel, MCP-1 binding (G–I, green) was detected in human control kidney (G, case 4), post-transplant (H, case 7), and acute allograft rejection biopsies (I, case 16). In all cases, double staining using Ulex europaeus agglutinin I for endothelium was performed (A–I, red). Arrows indicate the binding of L-selectin or MCP-1 associated with peritubular capillaries. Scale bars = 50 μm.
In a number of biopsies from patients diagnosed with acute allograft rejection (all defined as type II acute vascular rejection according to Banff 97 classification), L-selectin binding associated with peritubular capillaries was detected, which was sensitive to heparitinase I pretreatment (Figure 7, C and F; Table 1). MCP-1 binding in these biopsies was clearly more extensive than L-selectin binding (Figure 7I) but was also completely sensitive to heparitinase I pretreatment (not shown). In addition, to control for the possibility that endogenous MCP-1 was detected instead of binding of MCP-1, we performed stainings on tissue sections that had not been preincubated with recombinant MCP-1. Endogenous MCP-1 was not detected in the interstitium of either control (case 3) or acute vascular rejection (case 12), although some staining was observed in the cytoplasm of tubular epithelial cells in the acute vascular rejection biopsy (not shown). Taken together, these data show an enhanced MCP-1 binding to HSPGs associated with peritubular capillaries and TBM in post-transplant biopsies and a subset of acute allograft rejection biopsies. Although peritubular capillaries could generally be identified based on morphology, in the biopsies showing L-selectin and MCP-1 binding, endothelial staining using Ulex europaeus agglutinin I was diminished (Figure 7, C and I), as was staining for CD31, CD34, and von Willebrand factor (not shown). This suggests that peritubular capillaries showing L-selectin and MCP-1 binding HSPGs in their BM were affected by some degree of endothelial damage, which is supported by the fact that these biopsies were classified as type II acute vascular rejection.
Interstitial leukocyte influx is clearly a prominent feature of acute allograft rejection (Table 1). However, no direct correlation was found between the interstitial leukocyte count and the presence or absence of microvascular BM L-selectin and MCP-1-binding HSPGs (Table 1). We thus conclude that our observations regarding induction of L-selectin and MCP-1 binding to microvascular BM HSPGs in experimental renal I/R can be extrapolated to the clinically relevant setting of kidney transplantation, although no clear relation was found between the presence of L-selectin- and MCP-1-binding HSPGs on one hand and interstitial leukocyte influx on the other hand.
Loss of HSulf-1 Expression in Peritubular Capillaries in Acute Allograft Rejection Biopsies
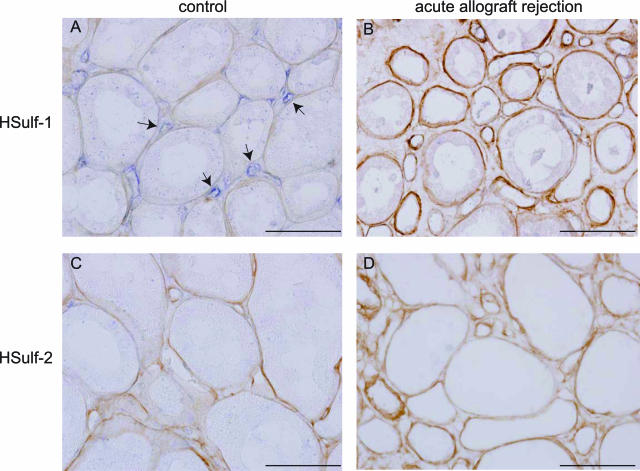
Recently, the extracellular endosulfatases HSulf-1 and HSulf-2 have been described to specifically cleave 6-O-sulfate residues of HS GAG chains, known to be critical for L-selectin binding.16,17 To investigate whether the variation in binding of L-selectin and MCP-1 to HSPGs associated with peritubular capillaries we observed in the different acute allograft rejection biopsies could be correlated to changes in HSulf-1 and/or HSulf-2 expression, we performed in situ hybridization assays for these molecules in the acute renal allograft rejection biopsies as well as in control kidneys. In control kidneys, HSulf-1 was expressed by renal peritubular endothelial cells, whereas expression of HSulf-2 was not detected (Figure 8, A and C). In the glomerulus, expression of both HSulf-1 and HSulf-2 was detected, whereas sense controls did not show any staining, demonstrating specificity of the probes (not shown). Interestingly, in acute allograft rejection biopsies showing microvascular L-selectin and MCP-1-binding HSPGs, expression of HSulf-1 in peritubular endothelial cells could not be detected (Figure 8B; Table 1). In biopsies in which no microvascular L-selectin- and MCP-1-binding HSPGs could be detected, HSulf-1 expression was detected in peritubular capillaries, with exception of two biopsies in which peritubular capillaries could not be identified because of disruption of tissue morphology as a result of the inflammatory response (Table 1). No expression of HSulf-2 was detected in interstitial microvascular endothelial cells upon acute allograft rejection (Figure 8D). These data suggest that loss of HSulf-1 expression may be involved in the induction of L-selectin binding to microvascular BM HSPGs.
Figure 8.
Endothelial HSulf-1 expression is down-regulated in acute allograft rejection biopsies. Expression of HSulf-1 (A, B) and HSulf-2 (C, D) was detected using in situ hybridization (blue staining) in human control kidney (A, C; case 3) and acute allograft rejection biopsies (B, D; case 12). Sections were counterstained for collagen type IV to identify renal BM structures (brown). Arrows indicate endothelial HSulf-1 expression in the microvasculature. Scale bars = 50 μm.
Discussion
In this study, we provide evidence that HSPG alterations can influence the I/R-induced inflammatory response upon kidney transplantation, by the induction of L-selectin binding as well as chemokine (MCP-1) binding to these molecules. Based on these properties, we refer to this type of HSPGs as proinflammatory HSPGs. Interestingly, these HSPGs are not expressed on the endothelial cell surface, but in underlying microvascular BMs. In fact, we did not observe L-selectin ligands expressed on the luminal side of endothelial cells in any of the human biopsies tested or in the animal models. This contradicts a previous study using antibodies to detect endothelial L-selectin binding glycoproteins in post-transplant and acute rejection biopsies.37 Although we used the L-selectin-IgM chimeric protein instead, this probe is able to detect L-selectin-binding glycoproteins.20,23,38 To control for our findings, we stained biopsies included in our study using MECA-79 and HECA-452, antibodies directed against glycoprotein L-selectin ligands that are described to be up-regulated upon kidney transplantation.37 Both antibodies, as well as the L-selectin-IgM chimeric protein, intensely stain high endothelium in human tonsil as a positive control (not shown). However, we did not observe endothelial staining in either post-transplant or acute rejection biopsies using either MECA-79 or HECA-452 antibody, although HECA-452 did stain tubular epithelial cells in all biopsies and control kidney (Supplementary Figure 1, see http://ajp.amjpathol.org).
A previous study by Ali and colleagues39 reported an increased expression of HS in peritubular capillary blood vessel walls upon acute renal transplant rejection. Although we detect a modification rather than increased expression of microvascular BM HSPGs, especially at the early time points investigated here (rat kidneys 24 hours after I/R and human posttransplant biopsies), both these studies emphasize that extracellular matrix HSPGs are subject to regulation in the transplantation setting. We hypothesized that this alteration can affect leukocyte extravasation because of an altered binding of the leukocyte adhesion molecule L-selectin as well as chemokines. Indeed, we showed that monocytic cell adhesion is increased in the presence of L-selectin-binding HSPG in artificial BM. In addition, early monocyte/macrophage influx is impaired in mice lacking both perlecan-HS and collagen type XVIII BM HSPGs. Despite this functional effect, strikingly, no differences in L-selectin or MCP-1 binding were observed in the kidneys of these mice upon I/R. This may be explained by the fact that a third BM HSPG, agrin, is still present in these mice, and we are detecting binding of L-selectin and MCP-1 to agrin HS GAG-chains. The possibility that L-selectin and MCP-1 binding is exclusively mediated by agrin is contradicted by the observation that early monocyte/macrophage influx was significantly impaired upon renal I/R in Hspg2Δ3/Δ3×Col18a1−/− double-mutant mice because these mice lack functional perlecan HS and collagen type XVIII, but agrin is not targeted. Indeed, we previously showed that in the kidneys of these double-mutant mice the distribution of HS chains in BMs was similar to that in wild-type mice,15 which can only be explained by the presence of agrin. In our opinion, the possibility that agrin, perlecan, and collagen type XVIII HS GAG chains are modified to bind L-selectin and chemokine is more likely, as the cellular machinery to make and modify HS GAG chains is likely to be shared between the three. If so, this would result in some level of redundancy in the system. Indeed, this would explain why we still observe L-selectin and MCP-1 binding to microvascular BM upon I/R in the Hspg2Δ3/Δ3×Col18a1−/− double-mutant mice (binding to agrin HS GAG-chains), whereas early monocyte/macrophage influx was impaired because of the reduction of available binding sites for L-selectin and chemokines. The apparent additive effect of mutation in perlecan-HS and collagen type XVIII supports this option, and it may explain why we did not find a significant difference between the groups at a later time point (extravasation is impaired but not impossible because of the presence of agrin). Future experiments using conditional agrin-deficient mice, possibly crossbred with mice lacking perlecan-HS and/or collagen type XVIII, may provide ultimate proof regarding this concept. Another possibility may be that upon renal I/R, HS GAG chains are added onto regions of the perlecan molecule that are not targeted in the Hspg2Δ3/Δ3 mutant mice, or that L-selectin binding is mediated by cell-surface HSPGs (syndecans or glypicans) shed abluminally by the microvascular endothelial cells. This latter possibility is contradicted by preliminary data from our group showing that syndecan-1, -4, or glypican-1 could not be detected on, or associated with, peritubular capillaries in either human control kidney, upon renal inflammation, or proteinuric kidney diseases (unpublished observation). It should also be noted that although we focus on the importance of L-selectin and chemokine binding to BM HSPGs, we cannot formally exclude the possibility that our findings in the different mutant mice are a result of altered physical properties of their BMs. We and others have not found clear BM alterations (apart from the targeted mutation) at the light microscopy level in the different mice used in this study15,21,40; however, it has been described that Col18a1−/− mice have structurally altered epithelial BMs at the level of electron microscopy.41 Whether these ultrastructural alterations also occur in the (micro)vascular BM in the kidney remains unclear at this point.
Biosynthesis of HSPGs is a complex process involving many different enzymes that act in concert to produce HS domain patterning.11,13 Careful analysis of alterations in biosynthetic enzyme expression and/or activity is necessary to study whether the induction of proinflammatory BM HSPGs is regulated at the level of biosynthesis. The cells actually producing microvascular BM HSPGs could be the endothelial cells themselves (shown to express collagen type XVIII42), although pericytes are also known to produce substantial amounts of HSPGs, including agrin and collagen type XVIII (Dr. J. van Horssen, Department of Molecular Cell Biology and Immunology, Vrije Universiteit University Medical Center; personal communication). Because renal BM HSPGs are reported to have a turn-over rate (t½) of ∼3 to 4 hours, alterations in GAG composition can take effect within the time span studied here.43 However, in addition to possible alterations in biosynthetic enzyme activity, GAG-modifying enzymes acting extracellularly could also be involved in the induction of proinflammatory HSPGs. We show that loss of HSulf-1 expression is observed in peritubular capillaries of acute allograft rejection biopsies in which microvascular proinflammatory BM HSPGs are present. HSulf-1 is an extracellular enzyme that specifically acts on 6-O-sulfate residues known to be critical for L-selectin binding to HSPG/heparin.16,17,44,45 A possible role for HSulf-1 in the induction of proinflammatory BM HSPGs could be the following. Under normal conditions, HSulf-1 is expressed by peritubular endothelial cells, resulting in continuous 6-O-desulfation of locally produced HS GAG chains, which prevents the HSPGs from binding to L-selectin. Indeed, preliminary in vitro data indicate that L-selectin binding to heparin-albumin is dose dependently inhibited by preincubation of the latter with medium conditioned by peritubular capillary endothelial cells (which contains sulfatase activity), whereas this effect is not observed using control medium (unpublished observation). In a subset of acute allograft rejection biopsies, loss of peritubular HSulf-1 expression was observed. In this case, locally produced HSPG GAG chains (eg, by pericytes) could retain 6-O-sulfation, enabling interaction with L-selectin and possibly chemokines, presuming that no other specific endosulfatases are present. This mechanism could actually bypass the need for endothelial cells to capture leukocytes from the bloodstream, which is compatible with the fact that endothelial damage is a prominent feature of I/R damage and type II allograft rejection.
To which extent our data can be extrapolated to other types of renal inflammation, including primary kidney disease and chronic allograft nephropathy, remains to be determined. However, evidence for the importance of HS GAG chains in other types of inflammation is provided by an interesting study by Wang and colleagues,46 showing that endothelial HS deficiency impairs L-selectin- and chemokine-mediated neutrophil migration in thioglycollate-induced peritonitis, contact dermatitis, and local lipopolysaccharide-induced inflammation. On the other hand, the proinflammatory alterations of vascular BM HSPGs, as described in our study, may actually also have beneficial effects. For example, the protective role of endothelial progenitor cells is being increasingly recognized in renal disease and transplantation.5,47 There is some evidence that endothelial progenitor cells home in an L-selectin-dependent manner,48 and we have previously shown that CD34+ progenitor cells can adhere to HSPG/heparin at reduced shear stress.27 Modification of vascular BM HSPGs may therefore act both to enhance inflammation, as demonstrated in this study, and restore endothelial damage by providing attachment sites for homing of endothelial progenitor cells. Future studies may establish whether this is indeed the case.
Supplementary Material
Acknowledgments
We thank Dr. S. D. Rosen and Dr. A. Bistrup for kindly providing the L-selectin-IgM-encoding plasmid, and undergraduate students Ineke Gerritsen and Suzanne Spliethoff for technical assistance.
Footnotes
Address reprint requests to Johanna W.A.M. Celie, Ph.D., Department of Molecular Cell Biology and Immunology, VU University Medical Center, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands. E-mail: p.celie@vumc.nl.
Supported by the Academy of Finland (grant 44843); the Sigrid Jusélius Foundation; and the Van Walree Fund, Royal Netherlands Academy of Arts and Sciences (grant to J.W.A.M.C.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Demers P, Elkouri S, Sirois MG, Cartier R. Coronary artery endothelial dysfunction after ischemia-reperfusion and acute untreated rejection in a canine heterotopic heart transplantation model. Transplantation. 2001;71:26–32. doi: 10.1097/00007890-200101150-00005. [DOI] [PubMed] [Google Scholar]
- Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol. 2003;285:F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol. 2002;282:F1140–F1149. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Woywodt A, Schroeder M, Gwinner W, Mengel M, Jaeger M, Schwarz A, Haller H, Haubitz M. Elevated numbers of circulating endothelial cells in renal transplant recipients. Transplantation. 2003;76:1–4. doi: 10.1097/01.TP.0000074569.65127.26. [DOI] [PubMed] [Google Scholar]
- Erickson AC, Couchman JR. Still more complexity in mammalian basement membranes. J Histochem Cytochem. 2000;48:1291–1306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- Prydz K, Dalen KT. Synthesis and sorting of proteoglycans. J Cell Science. 2000;113:193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Kreuger J, Spillmann D, Li JP, Lindahl U. Interactions between heparan sulfate and proteins: the concept of specificity. J Cell Biol. 2006;174:323–327. doi: 10.1083/jcb.200604035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celie JW, Keuning ED, Beelen RH, Drager AM, Zweegman S, Kessler FL, Soininen R, van den Born J. Identification of L-selectin binding heparan sulfates attached to collagen type XVIII. J Biol Chem. 2005;280:26965–26973. doi: 10.1074/jbc.M502188200. [DOI] [PubMed] [Google Scholar]
- Wang L, Brown JR, Varki A, Esko JD. Heparin’s anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J Clin Invest. 2002;110:127–136. doi: 10.1172/JCI14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Chien J, Staub J, Avula R, Greene EL, Matthews TA, Smith DI, Kaufmann SH, Roberts LR, Shridhar V. Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. J Biol Chem. 2003;278:23107–23117. doi: 10.1074/jbc.M302203200. [DOI] [PubMed] [Google Scholar]
- Li J, Kleeff J, Abiatari I, Kayed H, Giese NA, Felix K, Giese T, Buchler MW, Friess H. Enhanced levels of Hsulf-1 interfere with heparin-binding growth factor signaling in pancreatic cancer. Mol Cancer. 2005;4:14. doi: 10.1186/1476-4598-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celie JWAM, Beelen RHJ, van den Born J. Effect of fixation protocols on in situ detection of L-selectin ligands. J Immunol Methods. 2005;298:155–159. doi: 10.1016/j.jim.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai N, Eklund L, Marneros AG, Oh SP, Keene DR, Tamarkin L, Niemela M, Ilves M, Li E, Pihlajaniemi T, Olsen BR. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002;21:1535–1544. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistrup A, Bhakta S, Lee JK, Belov YY, Gunn MD, Zuo F, Huang C, Kannagi R, Rosen SD, Hemmerich S. Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raats CJ, Bakker MA, Hoch W, Tamboer WP, Groffen AJ, van den Heuvel LP, Berden JH, van den Born J. Differential expression of agrin in renal basement membranes as revealed by domain-specific antibodies. J Biol Chem. 1998;273:17832–17838. doi: 10.1074/jbc.273.28.17832. [DOI] [PubMed] [Google Scholar]
- Peri G, Milanese C, Matteucci C, Ruco L, Zhou D, Sozzani S, Coletta I, Mantovani A. A new monoclonal antibody (5D3–F7) which recognizes human monocyte-chemotactic protein-1 but not related chemokines. Development of a sandwich ELISA and in situ detection of producing cells. J Immunol Methods. 1994;174:249–257. doi: 10.1016/0022-1759(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Shively JE, Conrad HE. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976;15:3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]
- Netelenbos T, van den Born J, Kessler FL, Zweegman S, Huijgens PC, Drager AM. In vitro model for hematopoietic progenitor cell homing reveals endothelial heparan sulfate proteoglycans as direct adhesive ligands. J Leukoc Biol. 2003;74:1035–1044. doi: 10.1189/jlb.1202593. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- Wagner MC, Eckman JR, Wick TM. Sickle cell adhesion depends on hemodynamics and endothelial activation. J Lab Clin Med. 2004;144:260–267. doi: 10.1016/j.lab.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Tada T, Brodsky SV, Tanaka H, Noiri E, Kajiya F, Goligorsky MS. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol. 2002;282:F1150–F1155. doi: 10.1152/ajprenal.00310.2001. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziel WA, Mukaida N, Yokoyama H. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol. 2003;14:2503–2515. doi: 10.1097/01.asn.0000089563.63641.a8. [DOI] [PubMed] [Google Scholar]
- Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Tomosugi N, Mukaida N, Matsushima K, Egashira K, Yokoyama H. Gene therapy expressing amino-terminal truncated monocyte chemoattractant protein-1 prevents renal ischemia-reperfusion injury. J Am Soc Nephrol. 2003;14:1066–1071. doi: 10.1097/01.asn.0000059339.14780.e4. [DOI] [PubMed] [Google Scholar]
- Hoogewerf AJ, Kuschert GS, Proudfoot AE, Borlat F, Clark-Lewis I, Power CA, Wells TN. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36:13570–13578. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- Kirveskari J, Paavonen T, Häyry P, Renkonen R. De novo induction of endothelial L-selectin ligands during kidney allograft rejection. J Am Soc Nephrol. 2000;11:2358–2365. doi: 10.1681/ASN.V11122358. [DOI] [PubMed] [Google Scholar]
- Smith PL, Gersten KM, Petryniak B, Kelly RJ, Rogers C, Natsuka Y, Alford JA, III, Scheidegger EP, Natsuka S, Lowe JB. Expression of the α(1,3) fucosyltransferase Fuc-TVII in lymphoid aggregate high endothelial venules correlates with expression of L-selectin ligands. J Biol Chem. 1996;271:8250–8259. doi: 10.1074/jbc.271.14.8250. [DOI] [PubMed] [Google Scholar]
- Ali S, Malik G, Burns A, Robertson H, Kirby JA. Renal transplantation: examination of the regulation of chemokine binding during acute rejection. Transplantation. 2005;79:672–679. doi: 10.1097/01.tp.0000155961.57664.db. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, Liu B, Cao Y, Tryggvason K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- Utriainen A, Sormunen R, Kettunen M, Carvalhaes LS, Sajanti E, Eklund L, Kauppinen R, Kitten GT, Pihlajaniemi T. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet. 2004;13:2089–2099. doi: 10.1093/hmg/ddh213. [DOI] [PubMed] [Google Scholar]
- Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol. 1998;153:611–626. doi: 10.1016/S0002-9440(10)65603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuffo EL, Hunt JR, Moss J, Woodrow D, Davies M, Mason RM. A steady-state labelling approach to the measurement of proteoglycan turnover in vivo and its application to glomerular proteoglycans. Biochem J. 1996;320:301–308. doi: 10.1042/bj3200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto T, Uchida H, Yamazaki H, Keino-Masu K, Matsui A, Masu M. Identification of a novel nonlysosomal sulphatase expressed in the floor plate, choroid plexus and cartilage. Genes Cells. 2002;7:173–185. doi: 10.1046/j.1356-9597.2001.00502.x. [DOI] [PubMed] [Google Scholar]
- Nagamine S, Koike S, Keino-Masu K, Masu M. Expression of a heparan sulfate remodeling enzyme, heparan sulfate 6-O-endosulfatase sulfatase FP2, in the rat nervous system. Brain Res Dev Brain Res. 2005;159:135–143. doi: 10.1016/j.devbrainres.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006;17:932–942. doi: 10.1681/ASN.2005121250. [DOI] [PubMed] [Google Scholar]
- Biancone L, Cantaluppi V, Duo D, Deregibus MC, Torre C, Camussi G. Role of L-selectin in the vascular homing of peripheral blood-derived endothelial progenitor cells. J Immunol. 2004;173:5268–5274. doi: 10.4049/jimmunol.173.8.5268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.