Abstract
A low-sulfated chondroitin sulfate proteoglycan (CSPG) has been shown to be the receptor for the adherence of Plasmodium falciparum-infected red blood cells (IRBCs) in human placenta. Recently, hyaluronic acid (HA) has been suggested as an additional receptor even though IRBC binding to HA and the presence of HA at locations where IRBCs adhere in the placenta have not been established. In this study, we investigated whether HA is also a receptor for IRBC binding. IRBCs from infected placentas as well as those from different laboratory strains could bind to CSPG but not to HA. In a cell depletion assay, IRBCs from infected placentas could bind quantitatively to CSPG. Although CSPG is present both in the intervillous space and on the syncytiotrophoblast surface, HA is absent in these locations. These data conclusively demonstrate that CSPG, but not HA, is a receptor for IRBC adherence in the placenta. Our data also show, for the first time, that the IRBC-binding CSPG in the placenta is of fetal origin and that, in P. falciparum-infected placentas, the CSPG level is significantly increased, which could exacerbate IRBC adherence and placental pathogenesis. These results have important implications for the development of anti-IRBC adhesion-based vaccine for pregnancy-associated malaria.
Of the estimated 2 to 3 million annual fatalities attributable to malaria, >90% of death is caused by Plasmodium falciparum, the most virulent among the four protozoan parasites that cause malaria in humans.1,2,3 Although several factors are likely to contribute to the virulence of P. falciparum, it is widely thought that sequestration of parasite-infected red blood cells (IRBCs) in the microvascular capillaries of vital organs plays a central role.3,4,5,6,7,8,9,10,11,12 The IRBC sequestration has been reported to be mediated by endothelial cell adhesion molecules, such as thrombospondin, CD36, intercellular adhesion molecule 1, vascular cell adhesion molecule 1, E-selectin, P-selectin, and platelet endothelial cell adhesion molecule/CD31.3,4,5,6,7,8,9,10,11,12 The array of different adhesive mechanisms used by the parasite seems to confer a selective advantage for its efficient survival in the host by switching from one adherent type to another as the host develops adhesion inhibitory antibodies and other phenotype-specific immunity. Thus, in malaria endemic areas, almost all individuals by adulthood develop immunity that effectively controls infection and avoid pathogenesis. However, in the case of women during pregnancy, placenta expresses a new receptor that was previously unavailable for IRBC adherence.13 In the absence of antibodies that block the sequestration of parasites in the placenta, parasites efficiently multiply, and IRBCs extensively accumulate in the placenta. Consequently, monocytes/macrophages infiltrate into the placenta and produce proinflammatory mediators, which probably affect placental function and contribute to clinical conditions of placental malaria, including low birth weight of babies, premature delivery, abortion, stillbirth, maternal anemia, and infant and maternal morbidity and mortality.2,13,14,15,16,17,18
In 1996, Fried and Duffy13 reported that IRBC adherence in the placenta is mediated by chondroitin 4-sulfate (C4S). Since then, a number of investigators have demonstrated the specificity of IRBC binding to C4S.19,20,21 A previous study from our laboratory has shown that a uniquely low-sulfated chondroitin sulfate proteoglycan (CSPG) mediates IRBC adherence, and that the CSPG is localized predominantly in the intervillous space and to a lesser extent on the surface of the syncytiotrophoblast cells.22 We and others have also demonstrated that a dodecasaccharide motif of C4S consisting of two four-sulfated and four-nonsulfated disaccharide moieties comprise the minimum motif for optimal IRBC binding.23,24,25,26 Thus, C4S has been shown to be the receptor for placental IRBC adherence.
It has been reported that hyaluronic acid (HA) is an additional receptor for IRBC adherence in human placenta and that some IRBC populations can bind to both C4S and HA, exhibiting dual adherent specificities.27,28,29,30 However, the presence of HA in human placenta at locations where IRBCs are known to adhere has not been demonstrated, and thus, it remains unclear whether HA is a receptor for placental IRBC adherence or not. If HA is indeed an additional receptor in the placenta for IRBC adherence, then this phenomenon has profound implications for the rational design of vaccine for women of child-bearing age, because a vaccine that specifically blocks the adherence of IRBC to C4S would not be sufficient to control placental infections. Therefore, we undertook a comprehensive study to determine whether HA is also a receptor for IRBC adherence in the placenta. Our data show that IRBCs do not bind to HA and that HA is not present at detectable levels in the intervillous space or on the syncytiotrophoblast surface, the locations where IRBCs adhere in the infected placentas. Thus, our results support the view that vaccines based on preventing adhesion of IRBC in the placenta should focus primarily on the C4S-adherent parasite proteins.
Although the low-sulfated CSPG has been shown to be the receptor for P. falciparum IRBC adherence in the placenta, it is not known whether the CSPG is produced by the mother or the fetus and whether its expression is altered on P. falciparum infection. Because aggrecan family CSPGs are known to be involved in cytokine regulation,31 and one of the suggested functions of CSPGs is to mobilize cytokines, hormones, and growth factors in tissues,26 it is possible that low-sulfated CSPG plays an important role in the function of placenta. Moreover, altered expression of the CSPG is likely to affect the placental function, contributing to placental pathology. Therefore, an additional objective of this study was to determine the origin of CSPG and its level of expression in the malaria-infected placentas. Our data indicate that the low-sulfated CSPG is secreted to the intervillous space by the fetal placental tissues and that the level of CSPG is substantially elevated in P. falciparum-infected placentas.
Materials and Methods
Reagents
Streptococcus species HA and biotinylated HA-binding protein (HABP) were obtained from EMD Biosciences, La Jolla, CA. Human umbilical cord HA, bovine vitreous humor HA (bvhHA), and bovine chondroitin sulfate A (CSA) were purchased from Sigma, St. Louis, MO. Horseradish peroxidase-conjugated streptavidin and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfuric acid) substrates were obtained from KPL, Gaithersburg, MD. Protease-free Proteus vulgaris chondroitinase ABC (120 U/mg), Streptomyces hyalurolyticus hyaluronidase (2000 turbidity-reducing U/mg), and C6S were purchased from Seikagaku America, Falmouth, MA. Recombinant human intercellular adhesion molecule-1 (ICAM-1)/Fc chimera was from R&D Systems, Minneapolis, MN. Vectastain Elite ABC kit (containing biotinylated goat anti-rabbit IgG, horseradish peroxidase-conjugated avidin, and diaminobenzidine tetrahydrochloride color developing substrate, hematoxylin, and methyl green) was from Vector Laboratories, Burlingame, CA. P. falciparum CS2 parasite strain was provided by MR4/American Type Culture Collection, Manassas, VA. Human blood and serum for parasite culturing were obtained from Hershey Medical Center, Pennsylvania State University, Hershey, PA. Partially sulfated C4S with 40% 4-sulfate, prepared by regioselective 6-O-desulfation of bovine tracheal CSA followed by fractionation of the product by diethylaminoethyl (DEAE)-Sepharose chromatography, was from a previous study.32
Tissue and Blood Samples
P. falciparum-infected blood and tissue samples were obtained from the term placentas of Cameroonian women, who were admitted for delivery at the Central Hospital, Yaounde, Cameroon. Before the collection of placentas, the nature of the project was explained and informed consents were obtained from the patients. The project was approved by the Ethical Committee, Ministry of Health, Cameroon, and by the institutional review board at Georgetown University, Washington, DC. Blood samples of P. falciparum-infected placentas were obtained and cryopreserved in glycerolyte solution as reported previously,33 transported, and stored at −80°C until used. The tissues (1 × 1 cm) from the infected placentas were fixed with 10% neutral buffered formalin in phosphate-buffered saline (PBS), pH 7.2. Tissues were cut into 5-μm-thick sections, mounted on glass slides, stained with hematoxylin and eosin, and examined by light microscopy. Normal placentas at term and at different gestational stages were obtained from healthy women who had normal or premature delivery at the Hershey Medical Center. The tissue collection was approved by the Institutional Review Board, Pennsylvania State University College of Medicine, Hershey, PA.
Purification of Glycosaminoglycans (GAGs)/Proteoglycans of Human Placenta
All GAG/proteoglycan isolation was performed on ice-bath or in cold room at 4°C as previously reported.21,26 Human placentas were collected immediately after delivery, the umbilical cord and fetal membranes completely removed, and the tissue was cut into pieces, suspended in ice-cold PBS, pH 7.2, containing a mixture of protease inhibitors, and stirred gently with a magnetic stirrer for 2 to 3 hours. The extracts were centrifuged at 10,000 rpm for 30 minutes in a Sorvall centrifuge. The clear supernatants were diluted with 10 mmol/L sodium phosphate, pH 7.2, to a final NaCl concentration of 50 mmol/L and loaded onto DEAE-Sepharose columns (2.5 × 18 cm) equilibrated with 20 mmol/L Tris-HCl, 50 mmol/L NaCl, and 10 mmol/L ethylenediaminetetraacetic acid, pH 8.0. The columns were washed with the same buffer and then eluted with gradients of 50 to 600 mmol/L NaCl in 20 mmol/L Tris-HCl and 10 mmol/L ethylenediaminetetraacetic acid, pH 8.0. The column effluents were collected in fractions (10 ml), and absorption at 280 and 260 nm was measured. Aliquots of fractions were analyzed for uronic acid and fractions representing uronic acid peaks were pooled, dialyzed, and lyophilized.
The GAGs/proteoglycans isolated from DEAE-Sepharose chromatography were dissolved (2 mg/ml) in 25 mmol/L sodium phosphate, pH 7.2, containing 50 mmol/L NaCl, 0.02% NaN3, 4 mol/L guanidine hydrochloride, and 42% (w/w) CsBr.21 The solutions were centrifuged in a Beckman 50 Ti rotor (Fullerton, CA) at 44,000 rpm for 65 hours at 14°C. Gradients were collected from the bottom of the tubes into 15 equal fractions, absorption at 260 and 280 nm was measured, and the uronic acid contents determined. The GAGs/proteoglycans-containing fractions, indicated by the presence of uronic acid, were pooled, dialyzed, and lyophilized. The GAGs/proteoglycans were purified further by gel filtration on Sepharose CL-6B columns (1.5 × 83 cm) as reported previously.33
Compositional Analysis of Placental GAGs/Proteoglycans
The disaccharide compositional analysis of the GAGs/proteoglycans was performed according to Sugahara and colleagues.34 The purified GAGs/proteoglycans (∼50 μg) were digested with chondroitinase ABC (20 mU) in 50 μl of 100 mmol/L Tris-HCl, pH 8.0, containing 30 mmol/L NaOAc and 0.01% bovine serum albumin (BSA) at 37°C for 5 hours as described previously.21 The GAGs/proteoglycans (∼50 μg) were also digested with S. hyalurolyticus hyaluronidase (100 turbidity reducing U/ml) in 20 mmol/L NaOAc and 150 mmol/L NaCl, pH 6.0, at 60°C for 4 hours.35 The samples were dried in a Speed-Vac and analyzed by high-performance liquid chromatography (Waters, Milford, MA) on a Microsorb-MV 100-5 amino-bonded column (4.6 × 250 mm; Varian, Lake Forest, CA) using a linear gradient of 16 to 530 mmol/L NaH2PO4 at a flow rate of 1 ml/minute.
Preparation of HA-BSA Conjugate
Streptococcus species HA (10 mg) was dissolved in 5 ml of 0.2 mol/L NaCl, pH 4.7, and mixed with a solution of BSA (10 mg) in 1.0 ml of 0.2 mol/L NaCl. 1-Ethyl-2-(2-dimethylaminopropyl)carbodiimide (2.5 mg) was added and stirred for 1 hour while maintaining the pH at 4.7 by the addition of 0.1 mol/L HCl. The solution containing HA-BSA was purified on columns of Sepharose CL-4B (1.5 × 70 cm) in 20 mmol/L Tris-HCl, pH 7.6, with 150 mmol/L NaCl and 4 mol/L guanidine-HCl. Fractions (2 ml) were collected and monitored for uronic acid and protein by measuring absorbance at 530 and 280 nm, and the fractions containing the HA-BSA conjugate were pooled, dialyzed, and lyophilized.
Analysis of HA Coated onto Plastic Plates
The 96-well microtiter plates were coated with 50-μl solutions of 0.01 to 40 μg/ml HA-BSA conjugate, HABP-HA complex, bvhHA, and Streptococcus species HA, in PBS, pH 7.2, at room temperature for 3 hours and blocked with 200 μl of 2% BSA for 2 hours. The wells were incubated with 50 μl of biotinylated HABP (0.6 μg/ml in PBS) at room temperature for 1 hour. The wells were washed, and the bound HABP was detected with 1:100 diluted horseradish peroxidase-conjugated streptavidin using 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfuric acid color developing reagent. The color development was stopped by the addition of 2 mol/L HCl, and the absorbance was measured at 405 nm.
The level of HA coated on plastic plates was also assessed by inhibition of HABP binding with soluble HA. Ninety-six-well microtiter plates were coated with HA or HA-BSA conjugate and blocked with BSA as outlined above. Solutions of Streptococcus species HA at various concentrations in PBS were mixed with equal volumes of biotinylated-HABP (0.6 μg/ml in PBS). After incubating the solutions at room temperature for 1 hour, 50-μl aliquots were transferred to the microtiter plate wells. The plates were allowed to stand at room temperature for 2 hours and were washed, and the HABP bound to the wells was detected as above. Note that the commercially available HABP is derived from the N-terminal region of bovine nasal cartilage CSPG and link proteins. The HABPs thus obtained contain covalently linked C4S chains, and as such, it is unsuitable for immobilizing HA for IRBC binding analysis.
Histochemical and Immunohistochemical Analysis of Placental Tissues
Pieces (∼1 cm2) of placental tissues were fixed in PBS, pH 7.2, containing 2% formalin and 0.5% glutaraldehyde for 10 minutes and then heated in a microwave oven for 60 seconds at a precalibrated power setting so that the solution attained 45°C.22 The fixed tissues were transferred to ice-cold PBS and stored at 4°C. The tissues were paraffin-embedded, cut into 5-μm-thick sections onto glass slides, deparaffinized with xylene, and rehydrated by sequential immersion in 100, 95, and 70% ethanol and then in Tris-buffered saline, pH 8.0 (TBS). For immunohistochemical analysis using antibodies against the core proteins of placental CSPG receptor, the tissue sections were treated with protease-free chondroitinase ABC (50 mU/ml) in 100 mmol/L Tris-HCl and 30 mmol/L NaOAc, pH 8.0, at 37°C for 3 hours and washed with TBS.22 Before staining, all tissue sections were pretreated with 3% H2O2 for 4 minutes and washed with TBS. The tissue sections were blocked with 1:60 diluted normal goat serum in TBS for 3 hours followed by incubation with 10 μg/ml of the purified rabbit antibodies against the core proteins of low-sulfated placental CSPG.22 The slides were washed and incubated for 30 minutes with 1:60 diluted biotinylated goat anti-rabbit IgG (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA). For analysis of HA, the sections were incubated with 4-μg/ml solutions of HABP. Tissue sections treated overnight with S. hyalurolyticus hyaluronidase (100 turbidity reducing U/ml) in 20 mmol/L NaOAc and 150 mmol/L NaCl, pH 6.0, at 37°C, were used as control to determine the specificity of HABP staining. After washing, HABP-treated slides were incubated with horseradish peroxidase-conjugated avidin for 20 minutes, washed, and then incubated with 0.1% diaminobenzidine tetrahydrochloride and 0.02% H2O2 for 5 minutes according to the Vectastain Elite ABC kit procedure. The slides were washed with water, counterstained with methyl green, dehydrated, mounted using Permount, and examined by light microscopy.22
Parasite Culturing and Selection of C4S-Adherent Phenotypes
C4S-adherent IRBCs were selected by panning of 3D7 clone derived from NF54, parent NF54, FCR3, and CS2 P. falciparum parasites on plastic plates coated with the human placental low-sulfated CSPG as reported previously.23 The parasites were cultured in RPMI 1640 medium using 10% O+ human serum and type O+ human red blood cells and synchronized as reported previously.23,36
IRBC Adherence and Inhibition Assays
Solutions (15 μl/spot) of placental low-sulfated CSPG receptor (0.2 μg/ml), HA-BSA (10 μg/ml), bvhHA (40 μg/ml), Streptococcus species HA (40 μg/ml), or ICAM-1 (5 μg/ml) were spotted (∼3.5 mm diameter) on plastic Petri dishes and allowed to coat overnight at 4°C. The spots were blocked with 2% BSA in PBS, pH 7.2, for 2 hours at room temperature and overlaid with 2% erythrocytes from P. falciparum-infected placental blood (∼10% parasitemia) or parasite culture suspension (20 to 30% parasitemia). After 30 minutes at room temperature, the unbound cells were washed, and the bound cells were fixed with 2% glutaraldehyde in PBS, pH 7.2, stained with Giemsa, and counted by light microscopy. For inhibition analysis, 4% erythrocytes from parasite-infected placental blood or parasite culture suspensions mixed with an equal volume of C4S (80 μg/ml) or Streptococcus species HA (200 μg/ml) in PBS, pH 7.2. The solutions were incubated at room temperature for 30 minutes with occasional mixing and then overlaid onto CSPG-coated spots. The bound IRBCs were measured as above.
Depletion of IRBCs from Blood Samples of P. falciparum-Infected Placentas
The whole bottom surface of a series of plastic Petri dishes (35-mm-diameter plates) was coated overnight at 4°C with 1.2 ml of 0.2 μg/ml solution of placental CSPG in PBS, pH 7.2. The plates were blocked with 2% BSA at room temperature for 2 hours. The erythrocytes from the blood samples of the parasite-infected placentas (∼10% parasitemia) were stained with SYBR Green, and 2% suspensions of stained cell pellet in PBS were overlaid onto a CSPG-coated plate so as to cover the entire bottom surface of the plates. After 30 minutes at room temperature, the unbound cells were completely removed and transferred to a second CSPG-coated plate. After 30 minutes, the unbound cells from the second plate were transferred to a third CSPG-coated plate, and the procedure was continued up to a total of 12 CSPG-coated plates. The bound IRBCs in the plates were fixed with 1% glutaraldehyde, and the plates were photographed under a fluorescence microscope as well as under a bright field.
Results
Preparation and Immobilization of HA-BSA Conjugates
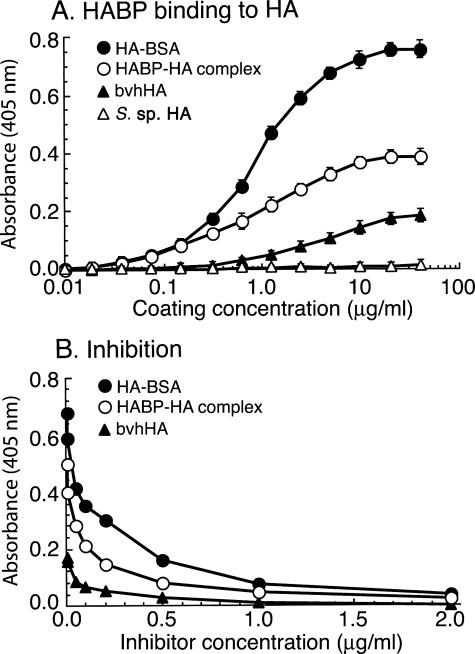
The commercial HA preparations from animal sources contain low to moderate levels of C4S.37 Therefore, to avoid the possibility that low levels of C4S in HA from animal sources complicating the analysis, IRBC binding assays were performed using HA from Streptococcus species known to be absolutely free from C4S because bacteria do not synthesize C4S. HA is a negatively charged and an extremely hydrophilic polysaccharide, and pure HA cannot be coated directly onto plastic Petri dishes for IRBC adherence analysis. Therefore, to immobilize HA on the plastic surface for the adherence assay, we conjugated Streptococcus species HA to BSA and coated the plastic Petri dishes with the HA-BSA conjugate. To ascertain that HA-BSA, compared with pure HA, efficiently coated the plastic surface, we measured the extent of immobilized HA and HA-BSA conjugate by enzyme-linked immunosorbent assay by direct binding of the HABP to the coated plates as well as by dose-dependent inhibition of HABP binding by soluble HA. The results indicated that the HA-BSA conjugate was efficiently coated onto the surface in a dose-dependent manner with a saturated level of coating attained at 10 μg/ml (Figure 1A). The binding of HABP was specifically inhibited by soluble HA in a dose-dependent manner (Figure 1B); BSA, CSA, and C6S could not inhibit HABP binding to HA-BSA (not shown). The data also indicated that HA conjugated to BSA is readily accessible for the ligand binding. Compared with HA-BSA conjugate and HA-HABP complex, the unconjugated bacterial HA was unable to coat plastic plates at measurable levels even at a coating concentration of 40 μg/ml (Figure 1A). These data together demonstrate that pure HA is not directly adsorbed onto the plastic surface. HA can also be immobilized to plastic plates at substantial levels when the bacterial HA was preincubated with HABP and the HA-HABP complex formed is allowed to coat plastic plates (Figure 1A). In this case, the immobilization of HA is because of the adsorption of HA-HABP complex onto plastic surface through HABP. However, it should be noted that HABP contains C4S chains, and therefore, HA immobilized using HABP is unsuitable for IRBC binding analysis unless C4S chains are removed by chondroitinase ABC treatment. In previous studies that reported IRBC binding to HA, bvhHA was used for the analysis of IRBC binding.27,28,29,30 Therefore, we also assessed the coating of bvhHA to plastic plates. As shown in Figure 1A, a low but significant amount of HA was coated onto the plastic surface; the immobilization of HA when coated with bvhHA is likely through HABPs present in samples that are adsorbed onto the plastic surface. However, bvhHA is also unsuitable for testing the binding of IRBCs to HA because bvhHA contains a low level of CSPG that efficiently coats plastic plates37 and binds IRBCs.
Figure 1.
Analysis of HA and HA-BSA conjugate coating efficiency to plastic surface by enzyme-linked immunosorbent assay. The samples were coated to 96-well microtiter plates, blocked with BSA, and then incubated with biotin-conjugated HABP. For inhibition of HABP binding to the coated plates, HABP was preincubated with various concentrations of Streptococcus species HA. In both cases, the wells were washed, and the bound HABP was measured using streptavidin-horseradish peroxidase conjugate and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfuric acid color developing substrate. The assays were performed three times, each in duplicate, and average absorbance values plotted. A: Binding of HABP to spots coated with HA-BSA (•), HABP-HA complex (○), bvhHA (▴), and Streptococcus species HA (▵). B: Inhibition of HABP binding to HA-BSA (•), HABP-HA complex (○), and bvhHA (▴) by soluble Streptococcus species HA. HA-BSA was efficiently coated, whereas Streptococcus species HA was not coated at a measurable level. The level of HA coating with HABP-HA complex was ∼50% that of HA-BSA complex. bvhHA was coated at moderate level, and the coating was presumably attributable to the presence of HABPs.
Analysis of P. falciparum IRBC Binding to Immobilized HA
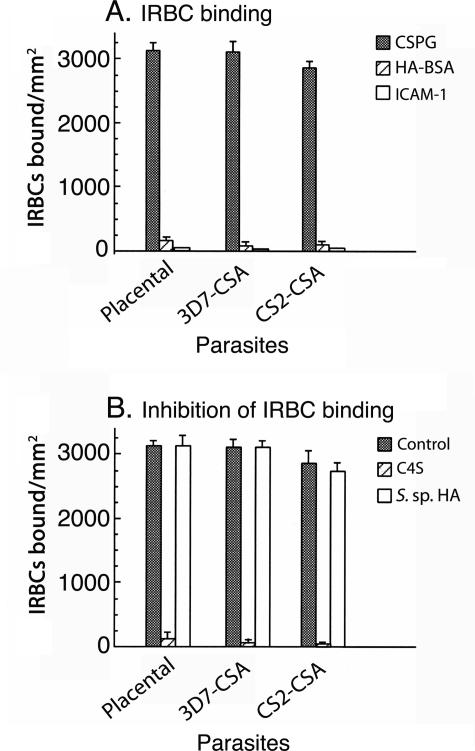
We studied the binding specificities of the parasites from two infected human placentas and four different laboratory parasite strains by an in vitro binding assay, and the data showed that IRBCs could bind to the human placental low-sulfated CSPG but not to HA-BSA conjugate (Figure 2A and data not shown). As reported previously,23 the binding of the placental IRBCs to the placental CSPG could be inhibited by C4S but not by HA, chondroitin, or C6S (Figure 2B and data not shown). It has been previously reported that the laboratory parasite strain named CS2, when selected for C4S binding, can adhere to both HA and C4S, exhibiting dual-receptor binding specificity.28 However, in our study, CS2-CSA IRBCs could bind only to the placental CSPG but not to the HA-BSA conjugate (Figure 2A). As in the case of the placental parasite isolates, the binding of CS2-CSA IRBCs to the placental CSPG was inhibited by soluble C4S but not by HA or C6S (Figure 2B and data not shown). When CS2-CSA IRBCs were tested for binding to bvhHA, as reported previously,27,28,29 a low level of IRBC binding was observed; 8 to 10% compared with that of placental CSPG (data not shown). However, pure HA was unable to inhibit the binding, whereas C4S could efficiently inhibit (data not shown), indicating that the observed IRBC binding to bvhHA was attributable to a low level of C4S present in bvhHA preparation. We also tested whether CSPG-adherent IRBCs can also bind ICAM-1; both placental isolates and CS2-CSA IRBCs were unable to bind to ICAM-1 (Figure 2A). Similar results were also obtained with C4S-adherent IRBCs from FCR3 and NF54 parasite strains (data not shown). We also tried to select potentially HA-adherent parasites from four different laboratory strains, namely FCR3, CS2, NF54, and 3D7, by panning on plastic plates coated with HA-BSA conjugate using procedures similar to those used for the selection of C4S- adherent parasites. In all four cases, despite repeated panning, HA-adherent parasites could not be selected, whereas in parallel, panning using placental CSPG readily selected C4S-adherent parasites. Together, the above data unambiguously show that the placental adherent P. falciparum IRBCs can bind to C4S but not to HA or ICAM-1.
Figure 2.
P. falciparum IRBC binding and inhibition analyses. A: For IRBC binding assays, suspensions of erythrocytes from P. falciparum-infected placental blood samples and C4S-adherent 3D7 and CS2 parasite cultures were overlaid onto spots coated with CSPG (gray bars), HA-BSA (hatched bars), and ICAM-1 (open bars) in plastic Petri dishes. After 30 minutes, the unbound cells were washed off, and the bound cells were fixed with glutaraldehyde, stained with Giemsa, and counted under a light microscope. B: For inhibition analysis, erythrocytes from placental blood and parasite cultures were preincubated separately with 80 μg/ml C4S (hatched bars) or 200 μg/ml of Streptococcus species HA (open bars), and the bound cells were assessed as above. Gray bars show IRBC binding controls (IRBC binding to placental CSPG in the absence of inhibitors). Both assays were performed three times, each time in duplicates. Error bars are indicated.
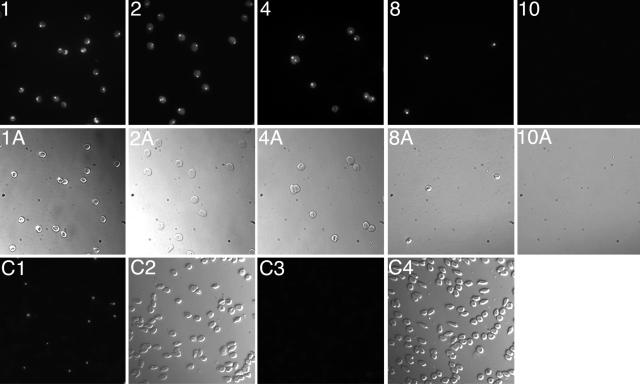
To determine whether all IRBCs in the placental isolates are C4S adherent or have other adherent characteristics as well, we developed a cell depletion assay using a series of CSPG-coated plates. IRBCs from placental blood samples were allowed to bind to a CSPG-coated plate, and the unbound IRBCs from this plate were overlaid onto a second CSPG-coated plate. The unbound IRBCs from the second plate were overlaid onto a third CSPG-coated plate, and this process was continued through a total of 12 CSPG-coated plates. The idea was that if all IRBCs are C4S-adherent phenotype, after overlaying the unbound cells from one plate to the next, eventually all IRBCs should be completely depleted from the blood samples. In this assay, we found that the number of IRBCs bound per unit area to the CSPG-coated plates was gradually decreased from plate 1 through successive plates, and in plate 9, only a few IRBCs were bound; no IRBC binding was evident in plate 10 and in subsequent plates (Figure 3). In parallel, when the unbound cells from various plates were tested for parasitemia, the levels of IRBCs compared with those in the cell suspension used for overlaying onto plate 1 were also decreased gradually from plates 1 through 9, and IRBCs were completely absent in the unbound fraction from plate 9 (Figure 3, C3). Thus, IRBCs from the placental isolates could bind quantitatively to the placental CSPG. These data together with the observation that placental IRBCs do not bind to HA or ICAM-1 (Figure 2A)38 demonstrate that the C4S is the primary receptor that mediates the binding of P. falciparum IRBCs in human placenta. However, because of the limited number of placental parasite isolates used, the above results do not definitively eliminate the possible presence of a minor population of placental IRBCs binding to HA. A critical requirement for IRBCs to adhere in placenta is that HA must be present either in the intervillous space or on the syncytiotrophoblast surface. Therefore, we analyzed placentas for the presence of HA by biochemical and immunohistochemical studies (see below).
Figure 3.
Assessment of P. falciparum placental IRBC binding to CSPG. Suspensions of washed cells from the blood of P. falciparum-infected placentas with ∼10% parasitemia, stained with SYBR Green, were overlaid onto CSPG-coated Petri dishes. The unbound cells from the first plate were allowed to bind to a second CSPG-coated plate, and so on. The bound IRBCs in each plastic plate were fixed and photographed under a fluorescence microscope. 1, 2, 4, 8, and 10: Fluorescent micrographs showing the binding of IRBCs to the corresponding CSPG-coated plates; 1A, 2A, 4A, 8A, and 10A: corresponding light micrographs. Binding of IRBCs to plates 3, 5, 7, and 9 are not shown; IRBCs were absent in unbound cell suspension from plate 9, and therefore, no IRBC binding was expected in plates 11 and 12. C1 and C2, respectively, are the fluorescent and light micrographs of the smears of cells, on glass slides, overlaid onto the first plate. C3 and C4, respectively, are the fluorescent and light micrographs of the smear of the cells overlaid onto the 10th CSPG-coated plastic plate used for IRBC binding. As shown, IRBC binding to the plates was gradually decreased from the first plate to the subsequent plates, and the unbound cells from the ninth plate had no IRBCs, and thus IRBC binding was not observed in the 10th plate. Erythrocytes from the infected placentas were also tested for binding to ICAM-1; no IRBC binding was evident (see Figure 2).
Biochemical Characterization of GAGs/CSPGs Present in the Intervillous Space of Human Placenta
We have previously reported that IRBC-adherent CSPG is present in the intervillous space and that the purified proteoglycan fraction is associated with a low amount of HA, ∼1% compared with total GAG chains.21 However, it is possible that this HA was attributable to the residual umbilical cord tissues remained in the placentas. Therefore, in the present study, we purified buffer-extractable GAGs/CSPGs from placentas in which the tissues around the umbilical cord attachment sites were completely removed to avoid contamination of placentas with HA from umbilical cord. Placentas were extracted with ice-cold isotonic buffer containing a mixture of protease inhibitors to avoid any tissue damage and leak of GAGs from stromal tissue matrix; because isotonic buffer without detergent was used, membrane-bound and/or tissue matrix GAGs/proteoglycans were not extracted. The total uronic acid-containing GAGs/CSPGs in the placental extracts were isolated by DEAE-Sepharose ion-exchange chromatography as reported previously.21 The placental tissue extracts were applied to the columns at 50 mmol/L NaCl concentration to ascertain that HA, if present, quantitatively binds to the columns. The bound GAGs/CSPGs were isolated by elution with 50 to 600 mmol/L NaCl gradient and purified by CsBr density gradient centrifugation followed by gel filtration on Sepharose CL-4B. The purified GAGs/CSPGs were characterized by the disaccharide compositional analysis after treatment separately with chondroitinase ABC and S. hyalurolyticus hyaluronidase; the latter enzyme specifically degrades HA but not chondroitin sulfates.39 We found that chondroitinase ABC could quantitatively degrade the purified GAGs/CSPGs to form disaccharides (Table 1), whereas no detectable disaccharides were formed when GAGs/CSPGs were treated with S. hyalurolyticus hyaluronidase. These results demonstrated that HA is not present in the intervillous space of human placenta.
Table 1.
Biochemical Composition of GAGs/CSPGs Isolated from Human Placenta
| Individual placentas (serial number) | Disaccharide composition (% molar proportion)*
|
||
|---|---|---|---|
| Chondroitinase ABC†
|
S. hyalurolyticus hyaluronidase‡ | ||
| Δdi-0S | Δdi-4S | Δdi-6S | |
| Normal-term placentas§ | |||
| 1 | 92 | 8 | ND |
| 2 | 95.5 | 4.5 | ND |
| 3 | 90.5 | 9.5 | ND |
| 4 | 89 | 11 | ND |
| 5 | 92 | 8 | ND |
| 6 | 96 | 4 | ND |
| 7 | 93 | 7 | ND |
| 8 | 97 | 3 | ND |
| 9 | 96.5 | 3.5 | ND |
| 10 | 95 | 5 | ND |
| Preterm placentas¶ | |||
| 16 weeks | 96 | 4 | ND |
| 24 weeks | 93 | 7 | ND |
| 28 weeks | 90 | 10 | ND |
| 33 weeks | 91 | 9 | ND |
Calculated from the areas of the disaccharide peaks by assuming that the different disaccharides have similar molar response factors.
Disaccharides released by treatment of the purified human placental GAGs/CSPGs with chondroitinase ABC.
Disaccharides released by incubation of total placental GAGs with S. hyalurolyticus hyaluronidase.
Normal-term placentas of the US women.
Placentas from P. falciparum-infected Cameroonian women who had aborted pregnancies. The GAGs/CSPGs purified in the previously published study40 were analyzed for the presence of HA; in all four placentas, HA was not detectable (ND).
Histochemical and Immunohistochemical Analyses of HA and CSPGs in Human Placenta of Different Gestational Stages and in Infected Placentas
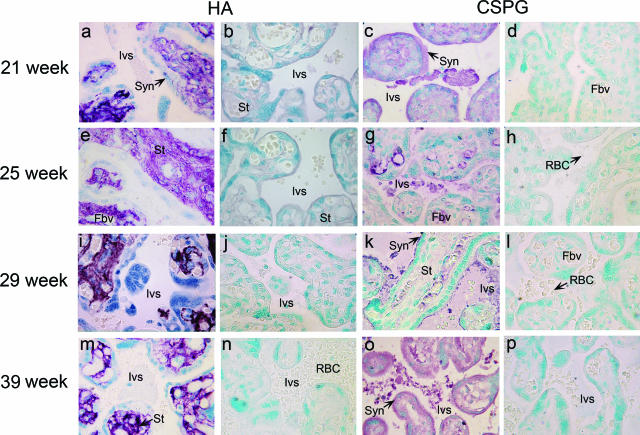
Although the above biochemical analysis showed the absence of HA in the intervillous space, its presence on the syncytiotrophoblast surface was not excluded. We have previously shown that IRBC-adherent CSPG in the intervillous space is expressed throughout the second and third trimester of the pregnancy.40 To determine whether HA is present in the placental intervillous space and whether it is expressed in a pregnancy stage-specific manner, we performed histochemical analysis of tissue sections of human placentas at different gestational stages (uninfected US individuals) using HABP. In parallel, we also performed immunohistochemical analysis of tissue sections of placentas collected using antibodies raised against the core proteins of the low-sulfated placental CSPG.22 In all placentas examined, the tissue sections probed with HABP showed strong staining only in the stroma of the villi and tissue matrices; neither intervillous space nor the syncytiotrophoblast layer were stained (Figure 4, a, e, i, and m). The specificity of the HABP staining was confirmed by preincubation of the tissue sections with S. hyalurolyticus hyaluronidase, which completely abolished the staining in the tissue stroma (Figure 4, b, f, j, and n). Probing of the chondroitinase ABC-treated placental tissue sections with antibodies specific to the core proteins of the placental CSPG receptor strongly stained the fibrous material in the intervillous space and fibrous filament-like structures on the syncytiotrophoblast layer in the placentas from different developmental stages (Figure 4, c, g, k, and o). No staining was observed with control preimmune sera from the same rabbit used for producing antibodies against placental CSPG (Figure 4, d, h, l, and p). Thus, the above data indicate that while CSPG is present in the intervillous space and on the syncytiotrophoblast lining, HA is not present at detectable levels at either locations in the placentas of the second and third trimesters, the period during which the pregnant women experience the peak prevalence of parasite infection.
Figure 4.
Histochemical and immunohistochemical analyses of HA and CSPG in human placentas of different gestational stages. The gestational stages of the placentas are indicated in the left margin. HA: Untreated (a, e, i, and m) and S. hyalurolyticus hyaluronidase-treated (b, f, j, and n) placental tissue sections were histostained with HABP. Only the tissue stroma was intensely stained with HABP, and staining was not observed either in the intervillous space or on the syncytiotrophoblast surface in placentas of all gestational stages (a, e, i, and m). In all cases, no staining was observed after treatment with S. hyalurolyticus hyaluronidase (b, f, j, and n). CSPG: The placental tissue sections were treated with chondroitinase ABC to remove GAG chains and then immunostained using either rabbit polyclonal antibodies against the core proteins of the placental low-sulfated CSPG (c, g, k, and o) or preimmune serum from the rabbit used for raising antibodies against the placental CSPG (d, h, l, and p). The anti-CSPG antibodies stained fibrous, filamentous materials in the intervillous space and the fibrous projections of the syncytiotrophoblast lining. Preimmune serum-treated tissue sections showed no staining. All tissue sections were photographed under light microscopy. Ivs, intervillous space; Syn, syncytiotrophoblasts; Fbv, fetal blood vessels; St, stromal tissue; RBC, red blood cells. Original magnifications, ×100.
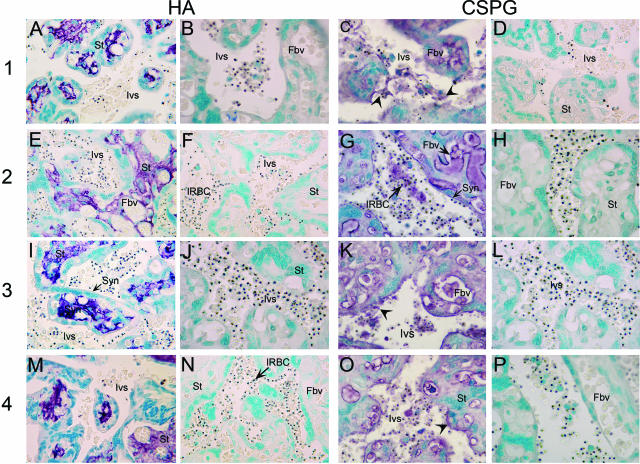
We also performed immunohistochemical analysis of the P. falciparum-infected term placentas (from Cameroonian women) using HABP and antibodies against the core proteins of the placental CSPG.22 As in the case of uninfected placentas, HABP strongly stained the stroma of the villi and tissue matrices in the tissue sections of parasite-infected placentas (Figure 5, A, E, I, and M). No staining was observed in the intervillous space, on the syncytiotrophoblast surface, or in the fetal blood vessels. The strong staining of tissue stroma with HABP was completely abolished when tissue sections were preincubated with S. hyalurolyticus hyaluronidase (Figure 4, b, f, j, and n), confirming that the observed staining in the stromal tissue is HA-specific. The tissue sections of parasite-infected placentas were also strongly stained with antibodies against the core proteins of the low-sulfated placental CSPG (Figure 5, C, G, K, and O); no staining was evident when probed with preimmune serum (Figure 5, D, H, L, and P). Interestingly, the staining of the tissue sections of infected placentas in the blood space and villous tissues was considerably stronger than uninfected placentas (Figures 4 and 5, compare c, g, k, and o with C, G, K, and O). These anti-CSPG antibodies also strongly stained the sections of parasite-infected placentas in and around the syncytiotrophoblast cell layer of both infected and uninfected placentas. In addition, relatively more intensely stained linings of CSPG along the syncytiotrophoblast cell layer were clearly evident in parasite-infected placentas compared with normal placentas. Examination of tissue sections also revealed that, in both normal and parasite-infected placentas, the fetal villous tissue and blood space of the fetal vessels were consistently stained with anti-CSPG antibodies; the staining at these locations was more intense than that in the intervillous space (Figure 4, c, g, k, and o; and Figure 5, and C, G, K, and O). Because infected placentas were not available for biochemical studies, the level of CSPG receptor in these placentas was assessed relative to normal placentas based on the intensity of staining with anti-placental CSPG antibodies. The staining intensity of the infected placental tissue sections was consistently higher than that of normal placentas (Table 2). These results strongly suggest that the low-sulfated CSPG is produced by the fetus and secreted to the maternal blood space. Furthermore, the relatively more intense staining of the infected placenta compared with noninfected placentas strongly suggests that the CSPG is synthesized at increased levels by the fetal tissue in response to inflammatory cytokines produced in the infected placentas.
Figure 5.
Histochemical and immunohistochemical analyses of HA and CSPG in P. falciparum-infected human placentas. The number at the left side of the figure refers to term placentas of four different infected individuals (1 to 4) with parasitemia of 3, 8, 21, and 31%, respectively. HA: Untreated (A, E, I, and M) or S. hyalurolyticus hyaluronidase-treated (B, F, J, and N) placental tissue sections were histostained with HABP. CSPG: Placental tissue sections treated with chondroitinase ABC were immunostained with either the rabbit polyclonal antibodies against the core proteins of the placental low-sulfated CSPG (C, G, K, and O) or preimmune serum from the same rabbit (D, H, L, and P). The stained tissue sections were photographed under a light microscope. In both panels, the staining pattern was similar to that shown in the corresponding panel in Figure 4, but the staining with antibodies against the core proteins of placental CSPG is much more pronounced in the tissue sections of infected placentas compared with normal placentas. Ivs, intervillous space; Syn, syncytiotrophoblasts; Fbv, fetal blood vessels; St, stromal tissue. Arrows indicate the CSPG staining in the intervillous space; arrowheads indicate diffused layers of CSPG attached to the syncytiotrophoblast surface. Original magnifications, ×100.
Table 2.
Immunohistochemical Analysis of Normal and P. falciparum-Infected Human Term Placentas Using Rabbit Polyclonal Antibodies Against the Core Proteins of Placental Low-Sulfated CSPG
| Placenta | % Parasitemia | Staining intensity*
|
||
|---|---|---|---|---|
| Intervillous space | Syncytiotrophoblast | Fetal blood vessel space | ||
| Normal† | − | ++ | ++ | + |
| Infected-1‡ | 3 | +++ | +++ | ++ |
| Infected-2 | 8 | +++ | ++ | +++ |
| Infected-3 | 21 | ++ | +++ | +++ |
| Infected-4 | 31 | +++ | ++ | +++ |
Relative intensity of staining of the placental tissue sections (Figures 4 and 5) by visual score on an arbitrary scale of ++++ (high intensity) to − (background level staining similar to that with preimmune serum).
Normal placenta from US women; four different placentas were analyzed, and results from a representative placenta are given.
P. falciparum-infected placentas from Cameroonian women.
Discussion
The objective of this study was twofold: to determine whether HA is an additional receptor for P. falciparum IRBC adherence in the human placenta and to establish whether the low-sulfated CSPG is produced by the fetus or by the mother and whether the IRBC adherence in the placenta alters the level of CSPG expression. The data presented here show that HA is not a placental receptor for IRBC adherence and that CSPG is the predominant and, most likely, the exclusive receptor. The following evidence supports this assertion. First, IRBCs from the P. falciparum-infected placentas as well as IRBCs from several laboratory strains, including the parasite that was previously reported to bind to both HA and C4S, could bind specifically and efficiently only to CSPG but not to HA. Second, in an IRBC depletion assay, using a series of CSPG-coated plates (Figure 3), all IRBCs from the infected placentas bound quantitatively to the placental CSPG receptor, and nonbinding parasites were not detected. Third, although C4S-adherent IRBCs could be selected readily from four different laboratory parasite strains, FCR3, 3D7, NF54, and CS2, by panning on immobilized CSPG, in all four cases repeated panning on HA-BSA-immobilized plates was unable to select IRBCs with adherent specificity to HA. Because P. falciparum, during the erythrocytic life cycle, is known to undergo clonal switching to produce 1 to 2% parasites per generation with adherent specificity to a distinct receptor,41 HA-binding IRBCs should be readily selected if indeed the parasite switches to the HA-adherent phenotype. Fourth, although adherent IRBCs could be selected, at low levels, by panning of laboratory parasite strains on bvhHA-coated plates, the selected parasites did not bind to HA-BSA but instead bound to the placental CSPG; the IRBCs bound to bvhHA was efficiently inhibited by C4S but not by pure HA. Previously, the binding of IRBCs to bvhHA was considered indicative of HA binding specificity.27,28,29,30 Our conclusion that HA is not a receptor for P. falciparum IRBC is consistent with the recent observation by Fried and colleagues38 that IRBCs in the blood samples from 46 infected placentas could bind only to C4S but not to HA. Of note, although Fried and colleagues38 found that IRBCs from 2 of 46 placentas showed significant binding to HA-BSA conjugate, the binding was inhibited by C4S but not by HA.
More importantly, a requirement for HA to be a physiologically relevant receptor for placental IRBC adherence is that HA must be present in the intervillous space and/or on the syncytiotrophoblast surface, where IRBCs adhere in the parasite-infected placentas. The biochemical and histochemical data described in this study clearly demonstrate that HA is not expressed in the placenta at locations where IRBCs sequester during the second and third trimesters, the gestational stages at which the placental infection occurs. Thus, digestion of total GAGs/CSPGs, purified from the isotonic buffer-soluble extracts of the placentas, with S. hyalurolyticus hyaluronidase was unable to release detectable levels of HA disaccharides (the enzyme readily degraded standard HA used as a control), whereas treatment with chondroitinase ABC quantitatively degraded the GAGs into disaccharides. Histochemical analysis also showed the absence of HA both in the intervillous space and on the syncytiotrophoblast surface in placentas at different stages of the second and third trimesters of pregnancy. The observed absence of HA at these locations was not attributable to the inability of the HABP to bind HA given that the analysis revealed the expected presence of HA in the matrix of the stromal tissue that served as a reliable internal standard. The intense staining of stromal tissue matrix with HABP and complete abolition of staining when placental tissue sections were pretreated with HA-specific S. hyalurolyticus hyaluronidase is consistent with the expected abundant presence of HA in the tissue matrices, where maternal blood has no direct contact. Collectively, our data unequivocally demonstrate that HA is not present either in the intervillous space or on the syncytiotrophoblast surface and that IRBCs do not bind to HA. Of note, we have previously reported that the human placenta contains very low levels of HA; 1 to 2% of the total GAGs in the intervillous space.21 However, in the present study, when precautions were taken to completely remove the umbilical cord stem region of the placenta and used mild extraction procedures, only intervillous space GAGs but not those in the tissue matrix were extracted with the plain isotonic buffer.
The results of this study also provide evidence regarding the origin of the unusually low-sulfated aggrecan-type CSPG that mediates P. falciparum IRBC adherence in the intervillous space and explain the pattern of the observed IRBC adherence in infected placentas. The immunohistochemical analysis consistently indicated the presence of significantly higher levels of the CSPG in the fetal blood vessels and in the fetal villi. Thus, our data suggest that the proteoglycan produced by the fetus diffuses from the fetal vessels to the villi and then secreted through syncytiotrophoblast lining to the placental intervillous space. During this process, the CSPG accumulates as dense layers near the syncytiotrophoblast lining and diffuses to the intervillous space. Previous studies have observed, in P. falciparum-infected placentas, layers of densely immobilized IRBCs along the syncytiotrophoblast lining without physical attachment to the cell surface.42 Based on this IRBC binding pattern, it was thought that IRBCs that are bound to the syncytiotrophoblast surface got detached during fixation of the placental tissue sections. However, considering the CSPG distribution pattern observed here, it is more likely that dense adherence of IRBCs along the syncytiotrophoblast lining actually correspond to the accumulation of the low-sulfated CSPG secreted from the fetal villi in those areas.
Acknowledgments
We thank Clement Seudieu for providing P. falciparum-infected placental blood samples.
Footnotes
Address reprint requests to D. Channe Gowda, Department of Biochemistry and Molecular Biology, Pennsylvania State University College of Medicine, 500 University Dr., Hershey, PA 17033. E-mail: gowda@psu.edu.
See related commentary on page 1817
Supported by the US Public Health Service (National Institute of Allergy and Infectious Diseases grants AI45086 to D.C.G. and AI43888 to D.W.T.), and the Pennsylvania State University College of Medicine (Dean’s Feasibility Grant to D.C.G.).
References
- Sachs J, Malany P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- Snow RW, Craig M, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant population. Bull World Health Organ. 1999;77:624–640. [PMC free article] [PubMed] [Google Scholar]
- Weatherall DJ, Miller LH, Baruch DI, Marsh K, Doumbo OK, Casals-Pascual C, Roberts DJ. Malaria and the red cell. Hematology Am Soc Hematol Educ Program. 2002:35–57. doi: 10.1182/asheducation-2002.1.35. [DOI] [PubMed] [Google Scholar]
- Pasloske BL, Howard RJ. Malaria, the red cell, and the endothelium. Annu Rev Med. 1994;45:283–295. doi: 10.1146/annurev.med.45.1.283. [DOI] [PubMed] [Google Scholar]
- Mackintosh CL, Beeson JG, Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol. 2004;20:597–603. doi: 10.1016/j.pt.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cooke B, Coppel R, Wahlgren M. Falciparum malaria: sticking up, standing out and out-standing. Parasitol Today. 2000;16:416–420. doi: 10.1016/s0169-4758(00)01753-1. [DOI] [PubMed] [Google Scholar]
- Sherman IW, Eda S, Winograd E. Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect. 2003;5:897–909. doi: 10.1016/s1286-4579(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Heddini A, Pettersson F, Kai O, Shafi J, Obiero J, Chen Q, Barragan A, Wahlgren M, Marsh K. Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect Immun. 2001;69:5849–5856. doi: 10.1128/IAI.69.9.5849-5856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse CF, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan KE, Thway Y, Win K, Aikawa M, Lobb RR. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CJ, Craig A, Roberts D, Newbold CI, Berendt AR. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J Clin Invest. 1997;100:2521–2529. doi: 10.1172/JCI119794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutiger CJ, Heddini A, Fernadez V, Muller WA, Wahlgren M. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- Xiao L, Yang C, Dorovini-Zis K, Tandon NN, Ades EW, Lal AA, Udhayakumar V. Plasmodium falciparum: involvement of additional receptors in the cytoadherence of infected erythrocytes to microvascular endothelial cells. Exp Parasitol. 1996;84:42–55. doi: 10.1006/expr.1996.0088. [DOI] [PubMed] [Google Scholar]
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- Brabin BJ, Romagosa C, Abdelgalil S, Menendez C, Verhoeff FH, McGready R, Fletcher KA, Owens S, D’Alessandro U, Nosten F, Fischer PR, Ordi J. The sick placenta—the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- McGregor IA, Wilson ME, Billewicz WZ. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birth weight and placental weight. Trans R Soc Trop Med Hyg. 1983;77:232–244. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, Font F, Alonso PL. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- Duffy PE, Fried M. Plasmodium falciparum adhesion in the placenta. Curr Opin Microbiol. 2003;6:371–376. doi: 10.1016/s1369-5274(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Beeson JG, Duffy PE. The immunology and pathogenesis of malaria during pregnancy. Curr Top Microbiol Immunol. 2005;297:187–227. doi: 10.1007/3-540-29967-x_6. [DOI] [PubMed] [Google Scholar]
- Rogerson SJ, Brown GV. Chondroitin sulphate A as an adherence receptor for Plasmodium falciparum-infected erythrocytes. Parasitol Today. 1997;13:70–75. doi: 10.1016/s0169-4758(96)10081-8. [DOI] [PubMed] [Google Scholar]
- Pouvelle B, Fusai T, Lepolard C, Gysin J. Biological and biochemical characteristics of cytoadhesion of Plasmodium falciparum-infected erythrocytes to chondroitin-4-sulfate. Infect Immun. 1998;66:4950–4956. doi: 10.1128/iai.66.10.4950-4956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achur RN, Valiyaveettil M, Alkhalil A, Ockenhouse CF, Gowda DC. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J Biol Chem. 2000;275:40344–40356. doi: 10.1074/jbc.M006398200. [DOI] [PubMed] [Google Scholar]
- Muthusamy A, Achur RN, Bhavanandan VP, Fouda GG, Taylor DW, Gowda DC. Plasmodium falciparum-infected erythrocytes adhere both in the intervillous space and on the villous surface of the human placenta by binding to the low-sulfated chondroitin sulfate proteoglycan receptor. Am J Pathol. 2004;164:2013–2025. doi: 10.1016/S0002-9440(10)63761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhalil A, Achur RN, Valiyaveettil M, Ockenhouse CF, Gowda DC. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J Biol Chem. 2000;275:40357–40364. doi: 10.1074/jbc.M006399200. [DOI] [PubMed] [Google Scholar]
- Fried M, Lauder RM, Duffy PE. Plasmodium falciparum: adhesion of placental isolates modulated by the sulfation characteristics of the glycosaminoglycan receptor. Exp Parasitol. 2000;95:75–78. doi: 10.1006/expr.2000.4510. [DOI] [PubMed] [Google Scholar]
- Chai W, Beeson JG, Lawson AM. The structural motif in chondroitin sulfate for adhesion of Plasmodium falciparum-infected erythrocytes comprises disaccharide units of 4-O-sulfated and non-sulfated N-acetylgalactosamine linked to glucuronic acid. J Biol Chem. 2002;277:22438–22446. doi: 10.1074/jbc.M111401200. [DOI] [PubMed] [Google Scholar]
- Achur RN, Valiyaveettil M, Gowda DC. The low sulfated chondroitin sulfate proteoglycans of human placenta have sulfate group-clustered domains that can efficiently bind Plasmodium falciparum-infected erythrocytes. J Biol Chem. 2003;278:11705–11713. doi: 10.1074/jbc.M211015200. [DOI] [PubMed] [Google Scholar]
- Beeson JG, Rogerson SJ, Cooke BM, Reeder JC, Chai W, Lawson AM, Molyneux ME, Brown GV. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat Med. 2000;6:86–90. doi: 10.1038/71582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Beeson JG, Kogelberg H, Brown GV, Lawson AM. Inhibition of adhesion of Plasmodium falciparum-infected erythrocytes by structurally defined hyaluronic acid dodecasaccharides. Infect Immun. 2001;69:420–425. doi: 10.1128/IAI.69.1.420-425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson JG, Brown GV. Plasmodium falciparum-infected erythrocytes demonstrate dual specificity for adhesion to hyaluronic acid and chondroitin sulfate A and have distinct adhesive properties. J Infect Dis. 2004;189:169–179. doi: 10.1086/380975. [DOI] [PubMed] [Google Scholar]
- Duffy MF, Byrne TJ, Elliott SR, Wilson DW, Rogerson SJ, Beeson JG, Noviyanti R, Brown GV. Broad analysis reveals a consistent pattern of var gene transcription in Plasmodium falciparum repeatedly selected for a defined adhesion phenotype. Mol Microbiol. 2005;56:774–788. doi: 10.1111/j.1365-2958.2005.04577.x. [DOI] [PubMed] [Google Scholar]
- Nietfeld JJ, Huber-Bruning O, Bylsma JW. Cytokines and proteoglycans. EXS. 1994;70:215–242. doi: 10.1007/978-3-0348-7545-5_13. [DOI] [PubMed] [Google Scholar]
- Muthusamy A, Achur RN, Valiyaveettil M, Madhunapantula SV, Kakizaki I, Bhavanandan VP, Gowda DC. Structural characterization of the bovine tracheal chondroitin sulfate chains and binding of Plasmodium falciparum-infected erythrocytes. Glycobiology. 2004;14:635–645. doi: 10.1093/glycob/cwh077. [DOI] [PubMed] [Google Scholar]
- Achur RN, Muthusamy A, Madhunapantula SV, Bhavanandan VP, Seudieu C, Gowda DC. Chondroitin sulfate proteoglycans of bovine cornea: structural characterization and assessment for the adherence of Plasmodium falciparum-infected erythrocytes. Biochim Biophys Acta. 2004;1701:109–119. doi: 10.1016/j.bbapap.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Shigeno K, Masuda M, Fujii N, Kurosaka A, Takeda K. Structural studies on the chondroitinase ABC-resistant sulfated tetrasaccharides isolated from various chondroitin sulfate isomers. Carbohydr Res. 1994;255:145–163. doi: 10.1016/s0008-6215(00)90976-5. [DOI] [PubMed] [Google Scholar]
- Ohya T, Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970;198:607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Valiyaveettil M, Achur RN, Alkhalil A, Ockenhouse CF, Gowda DC. Plasmodium falciparum cytoadherence to human placenta: evaluation of hyaluronic acid and chondroitin 4-sulfate for binding of infected erythrocytes. Exp Parasitol. 2001;99:57–65. doi: 10.1006/expr.2001.4642. [DOI] [PubMed] [Google Scholar]
- Fried M, Domingo GJ, Gowda CD, Mutabingwa TK, Duffy PE. Plasmodium falciparum: chondroitin sulfate A is the major receptor for adhesion of parasitized erythrocytes in the placenta. Exp Parasitol. 2006;113:36–42. doi: 10.1016/j.exppara.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hatae Y, Makita A. Colorimetric determination of hyaluronate degraded by Streptomyces hyaluronidase. Anal Biochem. 1975;64:30–36. doi: 10.1016/0003-2697(75)90400-5. [DOI] [PubMed] [Google Scholar]
- Agbor-Enoh ST, Achur RN, Valiyaveettil M, Leke R, Taylor DW, Gowda DC. Chondroitin sulfate proteoglycan expression and binding of Plasmodium falciparum-infected erythrocytes in the human placenta during pregnancy. Infect Immun. 2003;71:2455–2461. doi: 10.1128/IAI.71.5.2455-2461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyes S, Horrocks P, Newbold C. Antigenic variation at infected red blood surface in malaria. Annu Rev Microbiol. 2001;55:673–707. doi: 10.1146/annurev.micro.55.1.673. [DOI] [PubMed] [Google Scholar]
- Yamada M, Steketee R, Abramowsky C, Kida M, Wirima J, Heyman D, Rabbbeage J, Breman J, Aikawa M. Plasmodium falciparum associated placental pathology: a light and electron microscopic and immunohistologic study. Am J Trop Med Hyg. 1989;41:161–168. doi: 10.4269/ajtmh.1989.41.161. [DOI] [PubMed] [Google Scholar]