Abstract
Pathological angiogenesis is associated with the fibrogenic progression of chronic liver diseases. Experimental data suggest that hypoxia and vascular endothelial growth factor (VEGF) may stimulate proliferation and synthesis of type I collagen in activated, myofibroblast-like rat hepatic stellate cells (HSC/MFs). In this study, we investigated whether hypoxia, recombinant VEGF, or angiopoietin 1 (Ang-1) may affect other crucial profibrogenic features. In human HSC/MFs, which constitutively express VEGF receptor-1 and -2 (VEGFR-1, VEGFR-2) and the Ang-1 receptor Tie-2, exposure to hypoxia, VEGF, or Ang-1 resulted in a Ras/Erk-dependent stimulation of chemokinesis and chemotaxis. Migration of human HSC/MFs under hypoxic conditions involved up-regulation of VEGF-A, Ang-1, and related receptors and was mainly dependent on VEGFR-2 (Flk-1). In specimens from either cirrhotic rat livers or from patients with hepatitis C virus-related cirrhosis, HSC/MFs expressed proangiogenic factors and related receptors in areas of active fibrogenesis (ie, at the leading or lateral edge of developing incomplete fibrotic septa). Data presented herein suggest that VEGF and Ang-1 may contribute to fibrogenesis by acting as hypoxia-inducible, autocrine, and paracrine factors able to recruit myofibroblast-like cells. Moreover, HSC/MFs, in addition to their established profibrogenic role, may also contribute to neoangiogenesis during chronic hepatic wound healing.
Angiogenesis is a hypoxia-stimulated and growth factor-dependent process consisting in the formation of new vascular structures from pre-existing blood vessels. Formation of new vessels is known to occur in several organs and to be critical for both growth and repair of tissues in several pathophysiological conditions.1,2,3,4,5,6 However, it has become increasingly clear that angiogenesis occurring during chronic wound healing and fibrogenesis provides a key contribution to disease progression. Pathological angiogenesis, as recently reviewed,7 has indeed been described in chronic inflammatory/fibrotic liver diseases of different etiology.
Hepatic angiogenesis differs from homologous processes in other tissues for a number of reasons, including the existence of two different types of microvascular structures in the liver (ie, large vessels lined by a continuous endothelium versus sinusoids lined by a fenestrated endothelium),8 the apparent production of the liver-specific angiogenic factor AN-GPTL3,9 and the unique but not homogenous phenotypic profile and functional role of hepatic stellate cells (HSCs).10,11,12,13 HSCs are also regarded as liver-specific pericytes, but their role in modulating angiogenesis, particularly in pathological conditions, may substantially differ from the role attributed to microcapillary pericytes.7 During the fibrotic progression of chronic liver diseases (CLDs), activated and myofibroblast-like HSCs (HSC/MFs) play a major profibrogenic role together with portal (myo)fibroblast and, possibly, bone marrow-derived stem cells, giving rise to hepatic populations of highly proliferative, profibrogenic, and contractile myofibroblast-like cells (MFs).10,11,12,13,14,15,16
Possible interplay and/or association between fibrogenesis and angiogenesis in CLDs is now suggested and supported by several findings: 1) angiogenesis and up-regulation of vascular endothelial growth factor (VEGF) expression has been documented in different models of acute and chronic liver injury7,17,18,19,20,21 as well as in specimens from human fibrotic/cirrhotic liver and hepatocellular carcinoma7,22,23,24; 2) in HSCs, hypoxia has been shown to up-regulate expression of VEGF,20,25,26,27 VEGF receptor type I (fms-like tyrosine kinase receptor or Flt-1),20,25 and collagen type I20; 3) VEGF has been proposed to directly stimulate proliferation and expression of α1(I)-procollagen mRNA in activated rat HSCs21; and 4) paracrine expression of VEGF by rat HSCs as well as by hepatocytes has been shown to regulate the phenotype (ie, fenestration and CD-31 expression) of liver sinusoidal endothelial cells,28 a feature of possible relevance in CLDs. Data concerning expression of angiopoietins are, at present, much more limited.7,22 Recent work has demonstrated expression of angiopoietin 1 (Ang-1) in human activated HSC/MFs and its up-regulation by hypoxia.27
In the present study, we report that VEGF-A and Ang-1 can stimulate migration and chemotaxis of human HSC/MFs and that, in liver tissue obtained either from cirrhotic rats or from patients with hepatitis C virus (HCV)-related cirrhosis, α-smooth muscle actin (α-SMA)-positive cells in areas of active fibrogenesis express VEGF-A and Ang-1 and their related receptors. These novel data suggest that hypoxia-dependent synthesis and release of VEGF and Ang-1 by activated HSC/MFs may contribute to both fibrogenesis and neovascularization by their actions on MF-like cells and sinusoidal endothelial cells.
Materials and Methods
Materials
Enhanced chemiluminescence reagents, nitrocellulose membranes (Hybond-C extra), and secondary Cy3-conjugated antibodies were from Amersham Pharmacia Biotech (Cologno Monzese, Milano, Italy). Human recombinant growth factors and cytokines, including VEGF and Angiopoietin-1, were from PeproTech Inc. (Rocky Hill, NJ). Antibodies against phosphorylated and unphosphorylated Erk1/2 were from Upstate Biotechnology (Lake Placid, NY). Monoclonal and polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), except those against α-SMA and fluorescein isothiocyanate-conjugated antibodies (obtained from Sigma Aldrich Spa, Milano, Italy) and against CD-31 (BD Pharmingen, Erembodegem, Belgium). The monoclonal neutralizing antibody against Flk-1 was obtained from ImClone (New York, NY); although originally raised against mouse epitope, this antibody was found to also cross-react with human Flk-1, as confirmed by the block of Erk1/2 phosphorylation in human HSC/MFs treated with VEGF (data not shown). All of the other reagents were of analytical grade and obtained from Sigma Chemical Co. (Sigma Aldrich Spa).
Cell Isolation and Culture
The use of human material was approved by Human Research Review Committee of the Università di Firenze, where cells were isolated and characterized from surgical wedge sections of human livers not suitable for transplantation, as extensively described elsewhere.29 Cells obtained by at least three different human livers were cultured in Iscove’s medium supplemented with 20% fetal bovine serum and, unless otherwise stated, used between passages 4 and 7 as fully activated HSC/MFs with a marker profile identical to that of interface myofibroblasts described in fibrotic and cirrhotic human livers.13,30 HSC/MFs were plated in normoxic conditions to obtain the desired subconfluence level (65 to 70%) in a relatively short time (ie, to prevent significant synthesis of extracellular matrix components) and then left for 24 hours in serum-free Iscove’s medium to obtain cells at the lowest level of spontaneous proliferation before the addition of the different stimuli. This procedure minimizes differences between cells coming from different individuals, as detailed in previous studies.31,32,33 Mean distribution for the cell cycle in cells serum-starved for 24 hours and then cultured for an additional 20 hours is ∼50% in G0/G1 phase, 35% in S phase, and the remaining in G2/M phase.31 Moreover, 24-hour serum-deprived cells continue to increase in cell number in serum-free medium for at least an additional 96 hours, at which time the number of cells is doubled.32
In experiments designed to evaluate the role of hypoxia, as previously detailed,27 serum-deprived and subconfluent human HSC/MFs (65 to 70%) were incubated in strictly controlled hypoxic conditions (3% O2) for up to 24 hours. In these studies cells were tested for nonoriented migration in the wound-healing assay (WHA) (see later), and the culture medium of cells exposed to hypoxia was collected at different time points (hypoxia-conditioned medium).
Western Blot
Cell lysates obtained by HSC/MFs were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% or 7.5% acrylamide gels. The blots were incubated with desired primary antibodies and then incubated with peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulins in Tris-buffered saline-Tween containing 2% (w/v) nonfat dry milk as previously described33,34,35,36 and developed with the enhanced chemiluminescence reagents according to the manufacturer’s instructions. To evaluate signaling of VEGF receptors (Flt-1 and Flk-1) and Ang-1 receptor (Tie-2), the state of phosphorylation of Erk1/2 and c-Akt (to evaluate involvement of Ras/Erk signaling and of PI 3-K activity, respectively), was analyzed as reported elsewhere.33,34,35,36,37
Cell Migration and Chemotaxis
Nonoriented migration (chemokinesis) and chemotaxis of human HSC/MFs were evaluated as previously described34,37 by using the WHA (incubation time, 18 hours) and the modified Boyden’s chamber assay (incubation time, 6 hours), respectively.
Analysis of Other Phenotypic Responses of Human HSC/MFs
Proliferation was evaluated by means of incorporation of radiolabeled [3H]thymidine and by cell counting using platelet-derived growth factor (PDGF)-BB as a positive control, as previously described.34,35,36 Synthesis and release in the culture medium of MCP-1 (as a parameter of proinflammatory responses) and of procollagen type I (as a parameter of synthesis of extracellular matrix components) was evaluated by enzyme-linked immunosorbent assay as previously described27,34 and using interleukin-1 and transforming growth factor-β1, respectively, as positive controls.
Animal Experiments
All animals received humane care, and experimental protocols were conducted according to national and local guidelines. Male adult Wistar rats (Harlan-Nossan, Correnzana, Italy), initial weight 200 to 220 g, were fed with a standard pelleted diet and water ad libitum. Advanced fibrosis was induced by chronic treatment with CCl4 administered by gavage twice a week for 9 weeks.38,39 Control animals received an equal volume of vehicle.
Human Tissues and Immunofluorescence Analysis
Normal human liver tissue was obtained from two surgical liver biopsies from patients undergoing uncomplicated cholecystectomy. Liver tissue characterized by evident cirrhosis (METAVIR F4) was obtained from five patients with HCV-related liver cirrhosis undergoing orthotopic liver transplantation. The use of this material conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the University of Florence Human Research Review Committee.
Indirect immunofluorescence was performed on liver cryostat sections from frozen surgical sections of control and cirrhotic human liver as well as from control and cirrhotic rat liver frozen samples (6 to 8 μm thick) essentially as previously described.27,38 For both rat and human liver, cryostatic sections and primary antibodies against α-SMA (1:250 dilution) or against VEGF, Ang-1, Flt-1, Flk-1, and Tie-2 (1:100 dilution) were used. Immunopositivity was revealed by the appropriate Cy3-conjugated (1:1000 dilution) or fluorescein isothiocyanate-conjugated antibodies (1:200 dilution). Nuclear staining (blue fluorescence) was obtained by treating liver sections with 4,6-diamidino-2-phenylindole (DAPI), as previously described.27,34
Statistical Analysis
Data in bar graphs represent means ± SEM, and means were obtained from average data of at least three independent experiments. Luminograms and morphological images are representative of at least three experiments with similar results. Statistical analysis was performed by Student’s t-test or with analysis of variance for analysis of variance when appropriate (P < 0.05 was considered significant).
Results
Human HSC/MFs Express TyrK Receptors for VEGF and Ang-1: A Constitutive and Hypoxia-Inducible Event
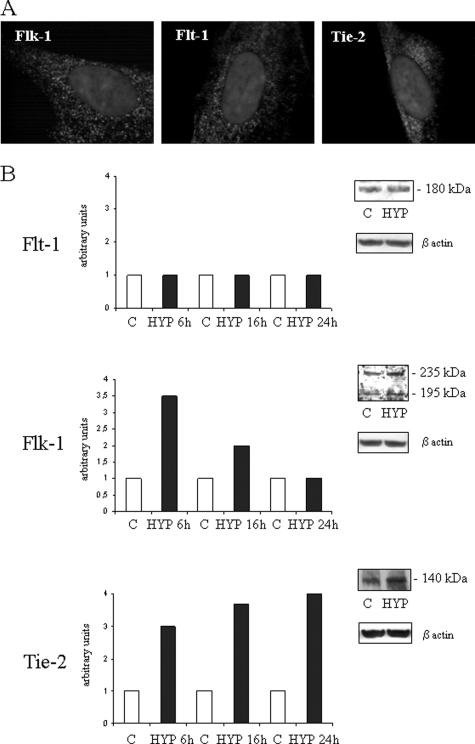
In a previous study, we have shown that in human HSC/MFs both hypoxic conditions and the profibrogenic adipokine leptin significantly up-regulate expression of VEGF (VEGF-A) and that these cells express Ang-1.27 We first evaluated whether human HSC/MFs express receptors for these two proangiogenic factors. HSC/MF constitutively express both Flt-1 (VEGFR-1) and Flk-1 (VEGFR-2) as well as Tie-2, the receptor for Ang-1 (Figure 1, A and B). Exposure of human HSC/MFs to controlled hypoxic conditions27 resulted in a selective up-regulation of the synthesis of both 235- and 195-kd isoforms of Flk-1 as well as Tie-2 but apparently not of Flt-1 (Figure 1B). In particular, increased synthesis of Flk-1 isoforms was a rather early event, with a peak detected after 6 hours of hypoxia, whereas increased synthesis of Tie-2 was a more progressive and time-dependent event reaching an apparent maximum after 24 hours of hypoxia.
Figure 1.
Expression of VEGF and Ang-1 receptors in human HSC/MFs under normoxic or hypoxic conditions. A: Constitutive expression of Flt-1, Flk-1, and Tie-2 as shown by indirect immunofluorescence staining. B: Western blot analysis of kinetics of expression of the three receptors under normoxia (C) or under hypoxic conditions (HYP). Data from a typical experiment (of three performed) are expressed in arbitrary units as densitometric analysis of bands observed at the different time points as compared with respective bands observed in control (normoxic) cell lysates at time 0. Insets provide a representative image of blots comparing bands for the three antigens obtained in control or hypoxic condition at 24 hours (Flt-1), 6 hours (Flk-1), or 16 hours (Tie-2). Molecular masses of receptors are provided in the insets. Sample loading was evaluated by reblotting the same membranes with antibodies raised against β-actin. Original magnifications, ×1000.
VEGF and Ang-1 Stimulated Nonoriented Migration and Chemotaxis of HSC/MFs in a Ras/Erk-Dependent Way
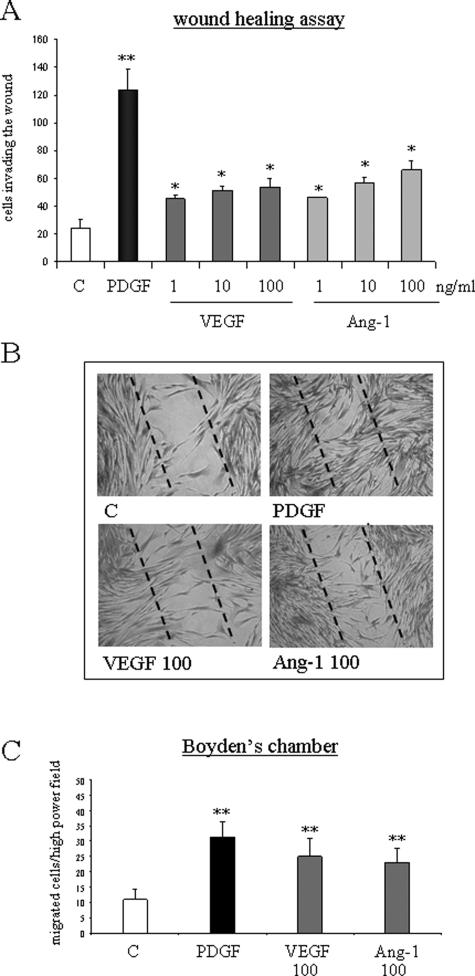
We next explored whether recombinant human VEGF and Ang-1 were able to stimulate nonoriented migration (chemokinesis) and chemotaxis of human HSC/MFs. In a WHA, to evaluate nonoriented migration,33,37 exposure of human HSC/MFs to angiogenic cytokines for 18 hours was followed by a significant and dose-dependent increase in cell invasion of the artificial wound (Figure 2, A and B). These data were associated with a significant stimulation of chemotaxis by both recombinant VEGF and Ang-1, measured in Boyden’s chambers, comparable with the one induced by PDGF-BB, used as positive control at the end of 6 hours of incubation (Figure 2C).
Figure 2.
VEGF and Ang-1 stimulate nonoriented migration and chemotaxis of human HSC/MFs. Nonoriented migration was assessed by means of WHA (A, B), whereas chemotaxis was assessed by means of Boyden’s chamber (C). WHA was performed on cells seeded on 24-well plates coated with collagen type I, grown to confluence in complete medium, and then incubated for an additional 24 hours in serum-free medium. An artificial lesion was generated in the cell layer to remove a linear area of cells, and then cultured cells were allowed 18 hours to migrate in the absence (control) or in the presence of increasing concentrations of human recombinant VEGF or Ang-1 or in the presence of human recombinant PDGF-BB (10 ng/ml) used as a positive control. Chemotaxis assay (C) was performed in trypsinized 24-hour-starved cells placed in Boyden’s chambers and then exposed to VEGF or Ang-1 (both used at 100 ng/ml) or to PDGF-BB (10 ng/ml) used as positive control. Data in bar graphs (A, C) represent mean ± SEM (n = 5, in triplicate) and are expressed as number of cells migrated in the artificial lesion (WHA, A) or in the filter (C), respectively. *P < 0.05 and **P < 0.01 versus control values. Representative images of nonoriented migration of human HSC/MFs under the different experimental conditions indicated are provided in B. Original magnifications, ×100.
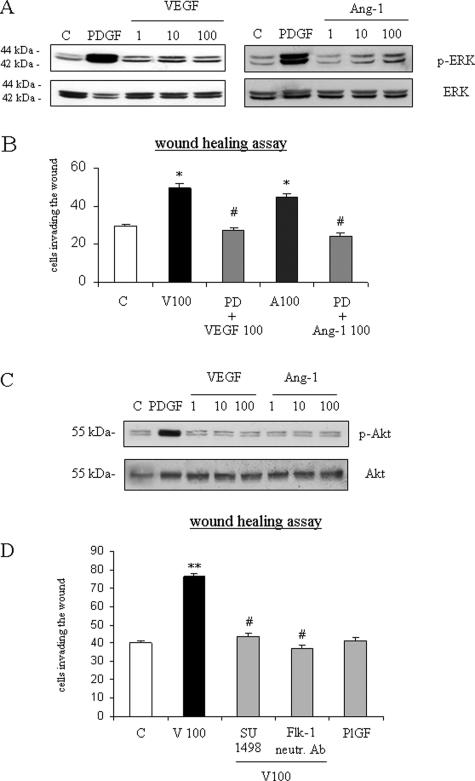
Nonoriented migration in response to VEGF or Ang-1 was abolished in the WHA by pretreating human HSC/MFs with the pharmacological MEK inhibitor PD98095 (Figure 3B). Accordingly, we found that both VEGF and Ang-1 increased phosphorylation of Erk1/2 (Figure 3A), a key signaling event for human HSC/MF migration and chemotaxis.40 However, differently from what has been reported for PDGF-BB but similarly to what has been recently described for human HSC/MF migration induced by superoxide anion,40 neither VEGF nor Ang-1 were able to affect the degree of phosphorylation of c-Akt, a measure of involvement of phosphatidylinosil 3-kinase (Figure 3C). Migration in response to VEGF was also inhibited by pretreatment with SU1498, a specific pharmacological inhibitor of Flk-1 activity (Figure 3D). The action of VEGF-A on Flk-1 receptor was confirmed also by pretreating cells with a monoclonal anti-Flk-1 neutralizing antibody. The use of the neutralizing antibody almost completely blocked VEGF-dependent migration. Moreover, placenta growth factor, or PlGF, a member of the VEGF family that is independent of hypoxia and known to interact only with Flt-1, was ineffective on HSC/MF migration (Figure 3C).
Figure 3.
VEGF- and Ang-1-dependent migration of human HSC/MFs involves activation of Ras/Erk signaling and, for VEGF action, Flk-1 receptor. For evaluation of Ras/Erk signaling (A), confluent and 24-hour-starved HSC/MFs were incubated for 15 minutes in the presence of increasing concentrations of human recombinant VEGF or Ang-1 (range, 1 to 100 ng/ml) or in the presence of human recombinant PDGF-BB (10 ng/ml) used as positive control. Cell lysates, processed as described in Materials and Methods, were used in Western blot analysis to detect the state of phosphorylation of extracellular regulated kinase (Erk1/2, p42, and p44; A) or c-Akt (C) by using specific antibodies directed against the phosphorylated form of Erk. Sample loading was evaluated by reblotting the same membranes with antibodies raised against Erk or c-Akt. Representative blots are shown of three independent experiments. To characterize nonoriented migration, WHA (B, D) was performed as described in the legend of Figure 2. Basal conditions were cells not exposed (C) or exposed to human recombinant VEGF (100 ng/ml), Ang-1 (100 ng/ml), PlGF (100 ng/ml), or PDGF-BB (10 ng/ml). When required, cells to be treated with VEGF or Ang-1 were pretreated for 30 minutes with the following agents: 30 μmol/L PD98095 (PD); 2 μmol/L of the TyrK inhibitor SU1498; 0.045 mg/ml (final dilution) of monoclonal neutralizing antibodies against Flk-1 that, although originally raised against mouse epitope were found to also cross-react with human Flk-1, as confirmed by the block of Erk1/2 phosphorylation in human HSC/MFs treated with VEGF (data not shown). Data in bar graphs (B, D) represent mean ± SEM (n = 4, in triplicate) and are expressed as number of cells migrated in the artificial lesion. *P < 0.05 and **P < 0.01 versus control values. #P < 0.01 versus values in cells stimulated with VEGF or Ang-1.
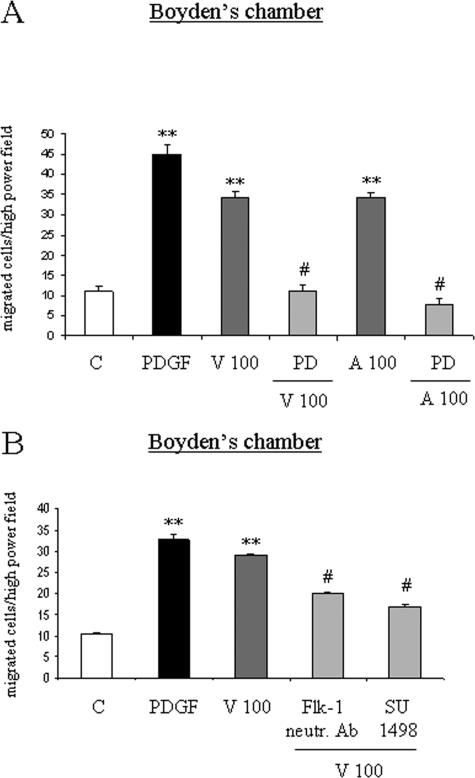
When VEGF- and Ang-1-dependent chemotaxis were investigated, we found that oriented migration stimulated by the two angiogenic factors was also prevented by the MEK inhibitor PD98095 (Figure 4A). Similarly to what has been reported for nonoriented migration, VEGF-dependent chemotaxis was significantly inhibited by the Flk-1 pharmacological inhibitor SU1498 (Figure 4B) and the anti-Flk-1 neutralizing antibody. Once again, PlGF was ineffective on HSC/MF chemotaxis (data not shown).
Figure 4.
VEGF- and Ang-1-dependent chemotaxis of human HSC/MFs is prevented by inhibitors of TyrKs and of Ras/Erk signaling and, for VEGF action, involves Flk-1 receptor. Chemotaxis experiments were performed as described in the legend of Figure 2. Basal and experimental conditions were identical to those described in the legend of Figure 3. Data in bar graphs (A, B) represent mean ± SEM (n = 4, in triplicate) and are expressed as number of cells migrated in the artificial lesion. **P < 0.01 versus control values. #P < 0.01 versus values in cells stimulated with VEGF or Ang-1.
In a series of parallel experiments (data not shown), we also evaluated in our standard culture conditions the action of VEGF and Ang-1 on other selected phenotypic responses of human HSC/MFs. By using recombinant VEGF or Ang-1, we could not detect any significant increase in cell proliferation or procollagen type I synthesis in human HSC/MFs that were normally responsive to PDGF-BB or transforming growth factor-β1, respectively, used as positive control. Finally, neither VEGF nor Ang-1 significantly affected synthesis of MCP-1 in HSC/MF preparations normally responding to interleukin-1, used as a positive control.
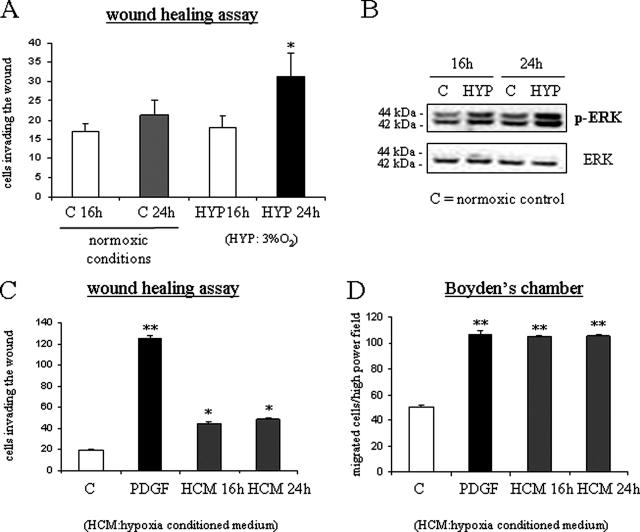
Hypoxia and Hypoxic Medium Stimulate Nonoriented Migration and Chemotaxis of HSC/MFs
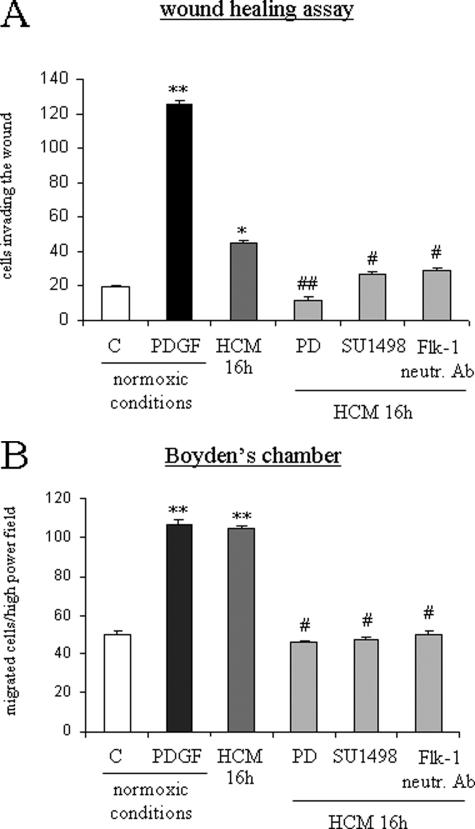
To define the possible influence of hypoxia, a WHA in normoxic and hypoxic conditions was first performed. When serum-deprived cells were incubated in hypoxic conditions, in the absence of any additional stimulus, invasion of the artificial wound was detected starting at 16 hours with a further increase at 24 hours (Figure 5A). Increased migration of human HSC/MFs was paralleled by a significant increase in the degree of Erk1/2 phosphorylation in lysates of cells obtained from hypoxic cells (Figure 5B). To establish whether cell migration was dependent on autocrine factors produced by human HSC/MFs during incubation under hypoxic conditions, media conditioned by cells exposed to hypoxia were collected after 16 and 24 hours and then used in wound healing and chemotaxis assays performed in normoxic conditions. Hypoxia-conditioned medium induced a significant stimulation of both nonoriented migration (Figure 5C) and of chemotaxis (Figure 5D). Moreover, both nonoriented migration (Figure 6A) and chemotaxis (Figure 6B) were significantly inhibited by pretreating cells with PD98095, SU-1498, or the anti-Flk1 neutralizing antibody.
Figure 5.
Hypoxia as well as hypoxic-conditioned medium stimulate nonoriented migration and chemotaxis of human HSC/MFs. WHA (A) was performed as reported in the legends of Figures 2 and 4 in both normoxic (control and PDGF, 24 hours) and hypoxic conditions (16 hours or 24 hours, HYP16 and HYP24). Human HSC/MFs in normoxic conditions behaved as usual: a low number of control cells was found to invade the artificial lesion after a 24-hour incubation, whereas a massive invasion was detected after exposure to PDGF-BB. Western blot analysis of activation of Ras/Erk signaling (B) was performed as described in the legend of Figure 2 using cell lysates obtained after 16 or 24 hours from either cells incubated in normoxic (control) or hypoxic conditions. Wound healing (C) and chemotaxis (D) assays were also performed using normoxic cells exposed to hypoxic-conditioned medium of human HSC/MFs exposed to hypoxia for 16 or 24 hours as well as to PDGF-BB (10 ng/ml) used as positive control. Data in bar graphs (A, C, and D) represent mean ± SEM (n = 4, in triplicate) and are expressed as number of cells migrated in the artificial lesion or in the filter of Boyden’s chambers. *P < 0.05 and **P < 0.01 versus control values.
Figure 6.
Migration in hypoxic conditions or stimulated by hypoxic conditioned medium involves Flk-1 receptor. Wound healing (A) and chemotaxis (B) assays were performed as described in the legend of Figure 2. When required, cells designed to be exposed at experimental time 0 to hypoxic medium collected after 16 hours were pretreated for 30 minutes with the following agents: 30 μmol/L PD98095 (PD); 2 μmol/L of the TyrK inhibitor SU1498; 0.045 mg/ml (final dilution) of monoclonal neutralizing antibodies against Flk-1. Data in bar graphs represent mean ± SEM (n = 4, in triplicate) and are expressed as number of cells migrated in the artificial lesion or found in the filter of Boyden’s chambers. *P < 0.05 and **P < 0.01 versus control values; #P < 0.05 and ##P < 0.01 versus values obtained in the presence of hypoxia-conditioned medium.
HSC/MFs Express Angiogenic Factors in Vivo in Areas of Active Fibrogenesis in a Rat Model of Chronic Wound Healing
This part of the study was specifically designed to evaluate the spatial and cellular distribution of proangiogenic factors in an in vivo model of hepatic chronic wound healing and fibrogenesis. To this aim, the model of chronic intoxication with CCl4 for 9 weeks in the rat was selected. A preliminary evaluation of the progression of fibrosis and angiogenesis in this model had revealed that at 9 weeks an ideal balance exists between active fibrogenesis (the presence of both large bridging septa and developing incomplete septa) and neoangiogenesis (the presence of new vascular structures within fibrous bridging septa). In our morphological analysis, we detected three well-defined in vivo patterns of expression of angiogenic factors, one being characteristic of normal liver and two described in cirrhotic liver for large bridging fibrotic septa and incomplete and/or developing septa.
Normal Rat Liver
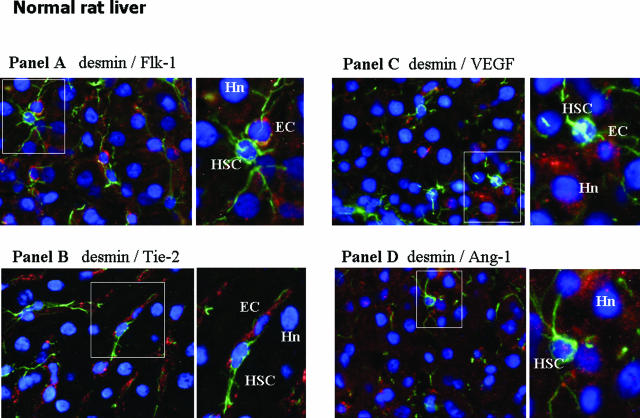
In normal rat liver (Figure 7, A–D), double immunostaining for the HSC marker desmin indicated that quiescent HSCs do not express Flk-1, Tie-2, Flt-1, VEGF, or Ang-1 (Figure 7 and data not shown), because no sign of co-localization of these antigens was found in desmin-positive cells. Flk-1 and Tie-2 (Figure 7, A and B) seemed to be expressed almost exclusively in cells with topographical and morphological features of sinusoidal endothelial cells. As already reported,27 a faint immunopositivity for VEGF was detected in some hepatocytes. As reported by others, Ang-1 expression was barely detectable in normal liver,24 and images did not allow a definitive assignment to any specific cell population.
Figure 7.
In vivo localization of HSC, proangiogenic cytokines, and related receptors in normal liver (A–D). Immunofluorescence was performed on liver cryostat sections from the liver of control (ie, untreated) rats at 9 weeks in the CCl4-dependent chronic protocol for fibrosis induction. All panels include the following: 1) a larger image on the left side offering electronic merging of images acquired for single fluorescence (blue fluorescence, DAPI staining; green fluorescence, desmin staining; red fluorescence, growth factor or receptor under analysis: A: Flk-1; B: Tie-2; C: VEGF; D: Ang-1); and 2) a higher digital magnification (right) of the boxed area. Hn, hepatocyte nucleus; EC, endothelial cell. Original magnifications, ×400.
Cirrhotic Rat Liver
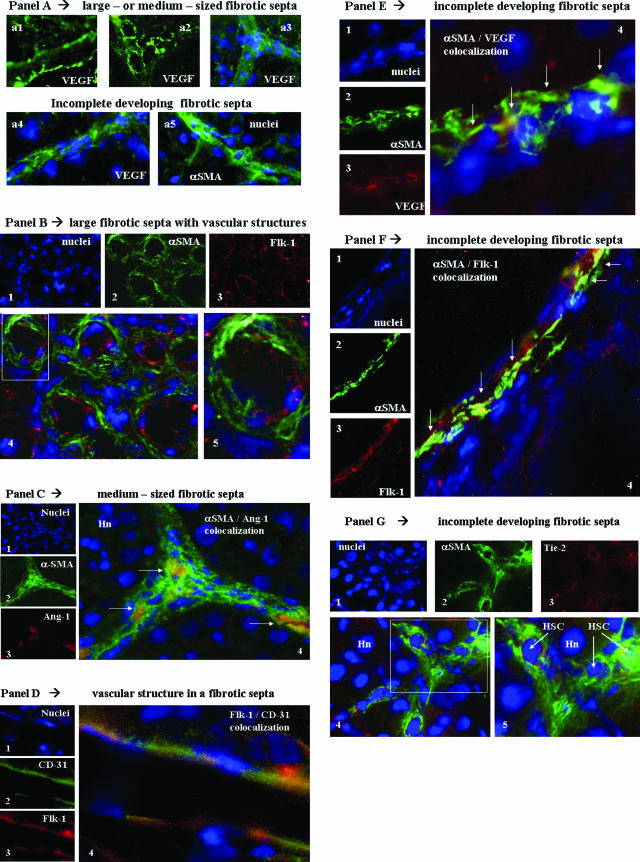
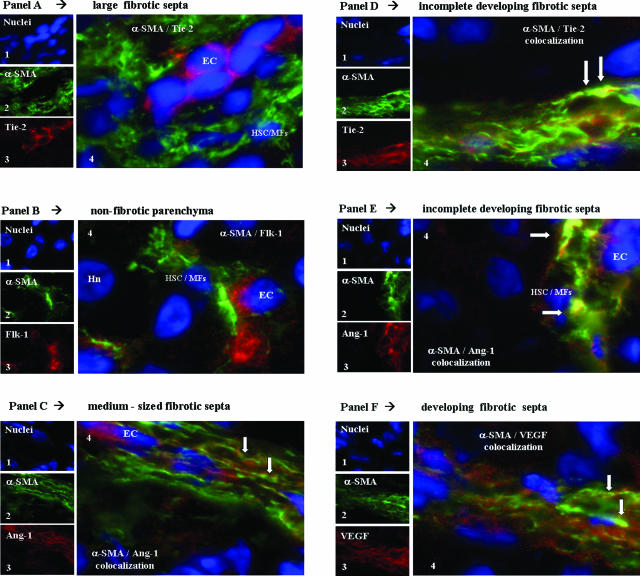
In chronically injured rat livers (9 weeks of intoxication with CCl4), first morphological observations (Figure 8A) concerning the expression of the proangiogenic factor VEGF suggested the existence of two distinct patterns of distribution of immunopositivity for this antigen. In large- as well as medium-sized bridging septa, the presence of single or multiple well-defined vascular structures lined by cells positive for VEGF (Figure 8A, a1 to a3), likely representing endothelial cells, were clearly evident. However, by observing development of incomplete septa (ie, tiny fibrotic septa in the parenchyma), it was realized that cells positive for VEGF (Figure 8Aa4) were presenting a morphology closely similar to that of α-SMA-positive cells (Figure 8Aa5). We next started to perform double-indirect immunofluorescence staining designed to analyze in these two areas the distribution of positivity for α-SMA (used as a standard marker of activated myofibroblast-like cells) and major angiogenesis-related factors including VEGF, Ang-1, Flk-1, and Tie-2. To clarify results, data are presented by summarizing observations related to large- and medium-sized septa as compared with those obtained for incomplete developing septa, respectively.
Figure 8.
In vivo localization of HSC/MFs, proangiogenic cytokines, and related receptors in fibrotic rat livers. Immunofluorescence was performed on liver cryostat sections from the liver of chronically injured rats obtained at 9 weeks in the CCl4-dependent chronic protocol for fibrosis induction. A: Reported images (green fluorescence) of immunostaining for VEGF (a1 to a4) or α-SMA (a5) in the presence or absence of concomitant nuclear stain (blue fluorescence, DAPI staining). In B, C, E, F, and G are reported the following: 1) tiny images representing image acquisition of single fluorescence always identifying in the same section nuclei (1, blue fluorescence, DAPI staining), α-SMA (2, green fluorescence), or the growth factor or receptor under analysis (3, red fluorescence; B: Flk-1; C: Ang-1; E: VEGF; F: Flk-1; G: Tie-2); 2) a larger image offering combined immunofluorescence (4, electronic merging of images); and 3) a higher digital magnification of the boxed area (5, when present). In D are reported the following: tiny images representing (see before) in the same section nuclei (1), CD-31 (2, green fluorescence), and Flk-1 (3, red fluorescence); and a larger image (4, electronic merging of images) offering combined immunofluorescence. Areas of co-localization between α-SMA and the antigen under analysis (yellow/orange fluorescence) are indicated by arrows. Hn, hepatocyte nucleus; EC, endothelial cell. Original magnifications: ×200 (Aa1–Aa3); ×400 (Aa4, Aa5, B, C, F); ×1000 (D, E, G).
Large- and Medium-Sized Bridging Fibrotic Septa: In these septa, always containing one or more easily recognizable vascular structures [Figure 8, A (a1 to a3) and B], immunopositivity for α-SMA was usually independent from immunopositivity for angiogenic factors. Figure 8B offers a representative example of a section of a large fibrotic septa in which immunopositivity for Flk-1 was evident in α-SMA-negative cells lining the vascular lumen (likely representing endothelial cells); α-SMA-positive/Flk-1-negative cells were invariably surrounding Flk-1-positive cells (Figure 8B). Indeed, this scenario of angiogenic factor-positive/α-SMA-negative cells lining a vascular lumen was observed in large septa whenever immunopositivity for VEGF or Tie-2 versus α-SMA was found in the same section (data not shown). The only apparent exception to this rule was found when double staining for α-SMA/Ang-1 was performed: with this procedure, we found some α-SMA-positive cells coexpressing Ang-1 in medium-sized fibrotic septa (Figure 8C) but not in larger septa. To establish whether Flk-1 and VEGF were correctly identifying endothelial cells, particularly in cirrhotic livers, we also performed double-staining immunofluorescence analysis for these antigens and for the endothelial marker CD-31. In cirrhotic rat livers a clear co-localization of both Flk-1 (Figure 8D) and VEGF (data not shown) with CD-31 in cells lining a vascular structure was observed.
Incomplete Developing Fibrotic Septa: When double immunostaining for α-SMA and the other angiogenesis-related factors was focused on tiny incomplete developing septa, a different scenario was detected. Morphological analysis clearly suggested that α-SMA-positive cells (ie, HSC/MFs), particularly those localized at the leading or lateral edges of these septa, coexpressed major proangiogenic factors, including VEGF (Figure 8E), Flk-1 (Figure 8F), or Tie-2 (Figure 8G).
Expression of Angiogenic Factors by HSC/MFs in Vivo in Areas of Active Fibrogenesis in Human Cirrhotic Livers
On the basis of the morphological findings described for cirrhotic rat liver, we next performed immunofluorescence studies designed to investigate the expression of the same factors in cryostat sections obtained from patients with HCV-related cirrhosis (score Metavir F4). Similarly to what was observed in cirrhotic rat livers, once again two distinct patterns of distribution involving large bridging septa and developing incomplete septa (Figure 9, A–F) were observed.
Figure 9.
In vivo localization of HSC/MFs, proangiogenic cytokines and related receptors in cirrhotic human livers from chronic HCV patients (A–F). Immunofluorescence was performed on cryostat sections from the liver of HCV patients scored METAVIR F4. In A–F are reported the following: tiny images representing image acquisition of single fluorescence always identifying in the same section nuclei (1, blue fluorescence, DAPI staining), α-SMA (2, green fluorescence), or the growth factor or receptor under analysis (3, red fluorescence; proangiogenic factors identified: A, D: Tie-2; B: Flk-1; C, E: Ang-1; F: VEGF); and a larger image offering combined immunofluorescence (4, electronic merging of images). Areas of co-localization between α-SMA and the antigen under analysis (yellow/orange fluorescence) are indicated by arrows. HSC/MFs, activated hepatic stellate cells; Hn, hepatocyte nucleus; EC, endothelial cell. Original magnifications: ×400 (C, F); ×1000 (A, B, D, E).
Large Bridging Septa
In large bridging septa as well as in the nonfibrotic parenchyma, immunopositivity for VEGF, Flk-1, Ang-1, and Tie-2 was almost always clearly distinct from that of α-SMA. Figure 9A offers a representative section of a large septa in which α-SMA-negative but Tie-2-positive cells (ie, endothelial cells) are entirely surrounded by α-SMA-positive MF-like cells that do not express Tie-2. Similarly, Figure 9B indicates that in the nonfibrotic parenchyma positivity for Flk-1 is again clearly independent from positivity for α-SMA. Similarly to what was found in cirrhotic rat livers, the only exception was appreciated in medium-sized fibrotic septa where some cells at the surface/border of the septa (Figure 9C) showed co-localization of α-SMA and Ang-1; in the same image other Ang-1-positive cells were not expressing α-SMA.
Incomplete Developing Fibrotic Septa
When the analysis was focused on these septa, we found a scenario closely resembling that described for corresponding structures in cirrhotic rat livers. Indeed, we detected evident co-localization of α-SMA with the individual receptors or angiogenic factors that was mostly evident in cells at the leading or lateral edge of incomplete developing fibrotic septa in human HCV cirrhotic livers, as it can be appreciated for cells coexpressing α-SMA and Tie-2 (Figure 9D), α-SMA, and Ang-1 (Figure 9E), or α-SMA and VEGF (Figure 9F). Thus, a similar scenario may be observed in human cirrhotic livers and in experimental cirrhosis in the rat.
Discussion
The available experimental data suggest that angiogenesis and related growth factors may significantly contribute to fibrosclerotic development in conditions of CLD whatever the etiology.7 In particular, experimental studies have pointed out that VEGF may play a major role by directly affecting mainly rat HSC/MFs by stimulating the synthesis of collagen type I and, to a lesser extent, in particular conditions of culture, inducing cellular proliferation.17,18,20 The main novel finding provided by the present study is that VEGF and, to a lesser extent, Ang-1 can operate as hypoxia-dependent, autocrine and paracrine factors able to stimulate nonoriented migration and chemotaxis of human HSC/MFs through the activation of Ras/Erk signaling. These observations suggest that VEGF and Ang-1 may significantly contribute to fibrogenesis also by recruiting profibrogenic cells into areas of active chronic injury, inflammation, and tissue remodeling. The relevance of this concept for CLDs is strengthened by the fact that human HSC/MFs do not respond significantly to VEGF or Ang-1 in terms of proliferation or procollagen type I and MCP-1 synthesis.
It is well established that during the fibrogenic progression of CLDs toward cirrhosis excess deposition of extracellular matrix and capillarization of sinusoids increases the resistance to blood flow and lowers oxygen delivery, thus rendering the tissue hypoxic.7 The in vitro experiments reported herein outline a direct link between exposure to hypoxic conditions of HSC/MFs and release of proangiogenic cytokines able to stimulate their migration and chemotaxis. In this context, VEGF seems to play a major role by interacting with Flk-1. The involvement of Flk-1 is in complete agreement with several reports indicating that VEGFR-2 is the receptor able to mediate the most relevant biological effects of VEGF, whereas the role of Flt-1, likely a decoy receptor, is still rather undefined.1,2,3,4,5,6 Along these lines, previous studies have shown that HSC/MFs are able to express VEGF receptors Flt-1 and Flk-1 and that this feature is acquired during the process of HSC activation. Moreover, under hypoxic conditions, HSC/MFs also respond by up-regulating their synthesis of VEGF as well as of Ang-1 (this study).17,18,20,27 These in vitro observations suggest that, after their activation and phenotypical modulation, HSCs tend to acquire a generic proangiogenic phenotype. This feature seems to be confirmed by evidence obtained in animal models of hepatic fibrogenesis.18,19,20,21 However, the relative in vivo relevance of the two VEGF receptors, Flt-1 (VEGFR-1) and Flk-1 (VEGFR-2), remains controversial. Corpechot and colleagues20 reported a predominant up-regulation of Flt-1 under conditions of chronic injury induced by DEN, whereas other studies have suggested a predominant up-regulation of Flk-1 in the model of chronic CCl4-induced liver injury.17,18 Regardless, a more relevant role of Flk-1 is clearly suggested by the work of Yoshiji and colleagues.21 These authors showed that after the in vivo administration of neutralizing antibodies raised against the two main VEGF-A receptors in the chronic model of CCl4-induced fibrosis, the anti-Flk-1 antibody was by far the most effective in reducing neovascularization as well as crucial parameters of fibrosis, including the number of α-SMA-positive cells present in liver tissue. On the basis of our in vitro results, this latter finding, originally interpreted as a consequence of the impairment of VEGF-dependent stimulation of proliferation and collagen synthesis, could now be attributed also to a specific inhibition of HSC/MF recruitment.
This interpretation is also supported by the morphological analysis performed in fibrotic/cirrhotic livers obtained at 9 weeks in the chronic CCl4 experimental models, a time point characterized by established fibrosis and portal hypertension,38,39 as well as in livers obtained from patients with HCV-related cirrhosis. As previously reported, the expression of VEGF and Ang-1 as well as of Flt-1, Flk-1, and Tie-2 become clearly evident in liver tissue in the presence of a chronic wound-healing reaction.7,18,19,20,24,27 In this context, co-localization data provided by the present study highlight that the concomitant expression of VEGF, Flk-1, and Tie-2 is restricted to activated α-SMA-positive cells localized at the leading edges of developing incomplete fibrotic septa, whereas in large bridging septa the expression of this proangiogenic panel is limited to endothelial cells. This distribution likely reflects two different phases of angiogenic process occurring in the hepatic chronic wound-healing reaction: an earlier phase, occurring in developing incomplete septa in which fibrogenesis and angiogenesis may be driven by activated HSC/MFs, and a later phase, occurring in large bridging septa where the chronic wound-healing reaction is less active and the fibrogenic transformation is more established. In this latter setting, the almost exclusive expression of proangiogenic factors in endothelial cells is likely to be aimed at the stabilization of the newly formed vessels.
Together, the data of the present study suggest that interface HSC/MFs may represent a cellular phenotype potentially able to modulate multiple and concomitant processes such as neoangiogenesis, inflammation, and fibrogenesis. Indeed, these cells represent a target for the multiple actions of VEGF and Ang-1, including stimulation of collagen type I synthesis and recruitment of HSCs (this study).20,21 At the same time, HSC/MFs are a significant source of these angiogenic cytokines under conditions of hypoxia7,25,27 and acute and chronic liver injury,7,17,18,19,20,21,23,24 possibly through the contribution of a number of growth factors, proinflammatory cytokines, and conditions of altered metabolic control, as recently suggested by data indicating that leptin is able to up-regulate VEGF.27
In conclusion, the novel observations provided by the present study contribute to the understanding of the complex series of events connecting fibrogenesis and angiogenesis in the progression of CLDs. Although further in vivo studies are needed for a more detailed characterization of this scenario, the present data suggest that HSC/MFs, in addition to their already established profibrogenic properties, may be involved in the modulation of the relationships between hypoxia, inflammatory response, angiogenesis, and fibrogenesis.
Footnotes
Address reprint requests to Prof. Maurizio Parola, Ph.D., Università degli Studi di Torino, Dipartimento Medicina e Oncologia Sperimentale, C.so Raffaello 30, 10125 Torino, Italy. E-mail: maurizio.parola@unito.it.
Supported by the Italian Ministero dell’Università e della Ricerca Fondo per gli Investimenti della Ricerca di Base (FIRB) protocol RBAU01SHY4 to M.Pinzani and M.Parola; Programmi di Ricerca di Interesse Nazionale (PRIN) project 2004061213 to F.M.; PRIN project 2006067527 to M.Parola), the Regione Piemonte (to M.Parola), the Fondazione Cassa di Risparmio di Torino (CRT) (to M.Parola), and the Italian Liver Foundation (to M.Pinzani and F.M.).
E.N. and S.C. contributed equally to the study.
References
- Yancopoulos GD, Davis S, Gale N, Rudge J, Wiegand S, Holash J. Vascular specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber H, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe P. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–1195. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- McCuskey RS, Reilly F. Hepatic microvasculature: dynamic structure and its regulation. Semin Liver Dis. 1993;13:1–12. doi: 10.1055/s-2007-1007333. [DOI] [PubMed] [Google Scholar]
- Camenisch G, Pisabarro M, Sherman D, Kowalski J, Nagel M, Hass P, Xie MH, Gurney A, Bodary S, Liang XH, Clark K, Beresini M, Ferrara N, Gerber HP. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin αvβ3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277:17281–17290. doi: 10.1074/jbc.M109768200. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Liver fibrosis: from bench to bedside. J Hepatol. 2003;38:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Friedman SL. A moving target in hepatic fibrogenesis. Hepatology. 2004;40:1041–1043. doi: 10.1002/hep.20476. [DOI] [PubMed] [Google Scholar]
- Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Saile B. Mesenchymal cells in the liver—one cell type or two? Liver. 2002;22:283–294. doi: 10.1034/j.1600-0676.2002.01726.x. [DOI] [PubMed] [Google Scholar]
- Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Ankoma-Sey V, Matli M, Chang KB, Lalazar A, Donner DB, Wong L, Warren RS, Friedman SL. Coordinated induction of VEGF receptors in mesenchymal cell types during rat hepatic wound healing. Oncogene. 1998;17:115–121. doi: 10.1038/sj.onc.1201912. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Mochida S, Mashiba S, Inao M, Matsui A, Ikeda H, Ohno A, Shibuya M, Fujiwara K. Expression of vascular endothelial growth factor in nonparenchymal as well as parenchymal cells in rat liver after necrosis. Biochem Biophys Res Commun. 1999;254:587–593. doi: 10.1006/bbrc.1998.9984. [DOI] [PubMed] [Google Scholar]
- Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, Raleigh J, Housset C, Poupon R. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary fibrosis. Am J Pathol. 1999;155:1065–1073. doi: 10.1016/S0002-9440(10)65209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Yamazaki M, Tsujinoue H, Imazu H, Masaki T, Fukui H. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52:1347–1354. doi: 10.1136/gut.52.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Caveda L, Sanz-Cameno P, Arroyo A, Martin-Vilchez S, Majano P, Garcia-Buey L, Sanchez-Madrid F, Moreno-Otero R. Hepatocyte growth factor activates endothelial pro-angiogenic mechanisms relevant in chronic hepatitis C-associated neoangiogenesis. J Hepatol. 2003;38:660–667. doi: 10.1016/s0168-8278(03)00053-9. [DOI] [PubMed] [Google Scholar]
- Shimoda K, Mori M, Shibuta K, Banner B, Barnard G. Vascular endothelial growth factor/vascular permeability factor mRNA expression in patients with chronic hepatitis C and hepatocellular carcinoma. Int J Oncol. 1999;14:353–359. doi: 10.3892/ijo.14.2.353. [DOI] [PubMed] [Google Scholar]
- Medina J, Sanz-Cameno P, Garcia-Buey L, Martin-Vilchez S, Lopez-Cabrera M, Moreno-Otero R. Evidence of angiogenesis in primary biliary cirrhosis: an immunohistochemical descriptive study. J Hepatol. 2005;42:124–131. doi: 10.1016/j.jhep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology. 2000;31:141–148. doi: 10.1002/hep.510310122. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Luk JM, Ikeda K, Man K, Chu AC, Kaneda K, Fan ST. Regulatory role of vHL/HIF-1α in hypoxia-induced VEGF production in hepatic stellate cells. Biochem Biophys Res Commun. 2004;317:358–362. doi: 10.1016/j.bbrc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, Vizzutti F, Anania FA, Milani S, Rombouts K, Laffi G, Pinzani M, Marra F. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–1348. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol. 2004;287:G757–G763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- Casini A, Pinzani M, Milani S, Grappone C, Galli G, Jezequel AM, Schuppan D, Rotella CM, Surrenti C. Regulation of extracellular matrix synthesis by transforming growth factor β1 in human fat-storing cells. Gastroenterology. 1993;105:245–253. doi: 10.1016/0016-5085(93)90033-9. [DOI] [PubMed] [Google Scholar]
- Cassiman D, Roskams T. Beauty is in the eye of the beholder: emerging concepts and pitfalls in hepatic stellate cell research. J Hepatol. 2002;37:527–535. doi: 10.1016/s0168-8278(02)00263-5. [DOI] [PubMed] [Google Scholar]
- Failli P, De Franco RM, Caligiuri A, Gentilini A, Romanelli RG, Marra F, Batignani G, Guerra CT, Laffi G, Gentilini P, Pinzani M. Nitrovasodilators inhibit platelet-derived growth factor-induced proliferation and migration of activated human hepatic stellate cells. Gastroenterology. 2000;119:479–492. doi: 10.1053/gast.2000.9354. [DOI] [PubMed] [Google Scholar]
- Caligiuri A, De Franco RM, Romanelli RG, Gentilini A, Meucci M, Failli P, Mazzetti L, Rombouts K, Geerts A, Vanesia M, Gentilini P, Marra F, Pinzani M. Antifibrogenic effects of canrenone, an antialdosteronic drug, on human hepatic stellate cells. Gastroenterology. 2003;124:504–520. doi: 10.1053/gast.2003.50058. [DOI] [PubMed] [Google Scholar]
- Novo E, Marra F, Zamara E, Valfrè di Bonzo L, Monitillo L, Cannito S, Petrai I, Mazzocca A, Bonacchi A, De Franco RSM, Colombatto S, Autelli R, Pinzani, Parola M. Overexpression of Bcl-2 by activated human hepatic stellate cells: resistance to apoptosis as a mechanism of progressive hepatic fibrogenesis in humans. Gut. 2006;55:1174–1182. doi: 10.1136/gut.2005.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamara E, Novo E, Marra F, Gentilini A, Romanelli RG, Caligiuri A, Robino G, Tamagno E, Aragno M, Danni O, Autelli R, Colombatto S, Dianzani MU, Pinzani M, Parola M. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60–68. doi: 10.1016/s0168-8278(03)00480-x. [DOI] [PubMed] [Google Scholar]
- Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Gentilini P, Dianzani MU. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robino G, Parola M, Marra F, Caligiuri A, De Franco RM, Zamara E, Bellomo G, Gentilini P, Pinzani M, Dianzani MU. Interaction between 4-hydroxy-2,3-alkenals and the platelet-derived growth factor-beta receptor. Reduced tyrosine phosphorylation and downstream signaling in hepatic stellate cells. J Biol Chem. 2000;275:40561–40567. doi: 10.1074/jbc.M007694200. [DOI] [PubMed] [Google Scholar]
- Marra F, Gentilini A, Pinzani M, Choudury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor’s actions on hepatic stellate cells. Gastroenterology. 1997;112:1297–1306. doi: 10.1016/s0016-5085(97)70144-6. [DOI] [PubMed] [Google Scholar]
- Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83:1183–1190. [PubMed] [Google Scholar]
- Sansoè G, Aragno M, Mastrocola R, Restivo F, Mengozzi G, Smedile A, Rosina F, Danni O, Parola M, Rizzetto M. Neutral endopeptidase (EC 3.4.24.11) in cirrhotic liver: a new target to treat portal hypertension? J Hepatol. 2005;43:791–798. doi: 10.1016/j.jhep.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Novo E, Marra F, Zamara E, Valfrè di Bonzo L, Caligiuri A, Cannito S, Antonaci C, Colombatto S, Pinzani M, Parola M. Dose dependent and divergent effects of superoxide anion on cell death, proliferation and migration of activated human hepatic stellate cells. Gut. 2006;55:90–97. doi: 10.1136/gut.2005.069633. [DOI] [PMC free article] [PubMed] [Google Scholar]