Abstract
Lipopolysaccharide (LPS) induces the stress-responsive gene heme oxygenase-1 (HO-1). The present study examined the significance of HO-1 in response to LPS. In HO-1−/− mice, as compared with HO-1+/+ mice, LPS provoked a greater reduction in glomerular filtration rate and renal blood flow, increased renal cytokine expression, and increased activation of nuclear factor (NF)-κB. Conversely, HO-1-overexpressing renal epithelial cells, exposed to LPS, exhibited a blunted activation of NF-κB and less phosphorylation of its inhibitor, IκB. In HO-1−/− mice, as compared with HO-1+/+ mice, LPS provoked markedly greater elevations in serum levels of Th1 cytokines, Th2 cytokines, chemokines, and cytokines that stimulate bone marrow progenitors. The liver, a major source of serum cytokines, showed an increased activation of NF-κB in LPS-treated HO-1−/− mice. In addition, LPS provoked widespread apoptosis of immune cells in the spleen and thymus in HO-1−/− mice but not in HO-1+/+ mice. We conclude that HO-1 deficiency exhibits a heightened and dysregulated inflammatory response to LPS accompanied by greater impairment in renal hemodynamic response and widespread apoptosis of immune cells. Because polymorphisms in the HO-1 gene with diminished HO activity predispose to human disease, we speculate that our findings may be relevant to the clinical outcome in patients with sepsis syndromes.
Rising in incidence, sepsis and its syndromes afflict 750,000 patients each year, ∼30% of whom die from complications of these syndromes.1,2 Such mortality reflects the failure of specific organ systems (such as acute renal failure) or the cumulative adverse effects of the multiple organ dysfunction syndrome. Sepsis syndromes include not only conditions in which there is documented infection, as occurs in sepsis, but also those mimetic conditions without demonstrable infection, and which are differentiated by the nature of the ongoing inflammatory response. These include the systemic inflammatory response syndrome, the compensatory anti-inflammatory response syndrome, and the mixed anti-inflammatory response syndrome.3,4,5,6
An experimental approach commonly used in examining the pathogenesis of these syndromes involves the administration of lipopolysaccharide (LPS).7,8,9,10,11,12,13,14 Acting through the TLR4 receptor, LPS instigates inflammatory cascades that alter regional and systemic hemodynamics, immune responses, tissue oxygenation, cell survival, and a host of other processes that, individually or cumulatively, impair organ function and vitality.7,8,9,10,11,12,13,14 In addition to examining the injurious pathways instigated by LPS, attention is increasingly directed to countervailing processes that may be induced by LPS, in the expectation that elucidating these adaptive pathways may offer therapeutic insights broadly relevant to sepsis syndromes.
One such countervailing system is heme oxygenase (HO). HO is the rate-limiting enzyme in the degradation of heme, converting heme to biliverdin and concomitantly enabling the release of iron and the generation of carbon monoxide.15,16,17,18,19,20,21,22 HO exists in two isoforms, HO-1 being the inducible isoform elicited by a wide array of stimuli including ischemia, hypoxia, oxidant stress, LPS, cytokines, and irradiation; HO-2 represents the constitutive isoform. In several of these conditions, induction of HO-1 confers protective effects by virtue of its vasorelaxant, anti-inflammatory, and anti-apoptotic actions.15,16,17,18,19,20,21,22
The present study evaluated the functional significance of HO-1 in determining the response to LPS in vivo by adopting an approach based on HO-1−/− mice. In this study, attention was focused on the kidney for the following reasons: this organ is commonly impaired in sepsis, exhibiting a well-defined alteration in its hemodynamic profile7; the occurrence of acute renal failure in patients with sepsis substantially increases mortality23; even relatively mild renal insufficiency can adversely affect mortality in critically ill patients24; and, as recently demonstrated in relevant disease models, renal insults of diverse origin, even when relatively mild, can markedly exaggerate the systemic inflammatory response elicited by LPS.10,11,12 In addition to examining the renal and systemic responses to LPS in HO-1−/− mice, this study analyzed organs enriched in immune cells, in view of the increasing recognition that critical participants in the sepsis syndrome include altered immunity, in general, and apoptosis of lymphocytes, in particular.25,26,27,28
Materials and Methods
Overview of LPS-Treated HO-1−/− Mice
Homozygous HO-1-null mutant mice, used in this and previous studies by our laboratory,29,30,31 were generated by targeted disruption of the HO-1 gene as described by Poss and Tonegawa.32 Colonies of mice were maintained by breeding HO-1−/− males with HO-1+/− females. Offspring were genotyped at the time of weaning by using polymerase chain reaction to amplify the wild-type and mutant alleles of genomic DNA from tail samples. HO-1+/+ mice were used as controls. For all experiments, HO-1+/+ and HO-1−/− mice were age-matched, and mice from 10 to 28 weeks were used. For studies of hemodynamic and other responses to LPS, LPS (Escherichia coli, serotype 0127:B8, 1 mg/kg, catalog no. L3129, lot 123K4143; Sigma/Aldrich, St. Louis, MO) was administered intravenously to HO-1+/+ and HO-1−/− mice. At 24 hours after administration of LPS, mice were sacrificed for the harvest of tissues and plasma or subjected to renal hemodynamic measurements as described below. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic.
Renal Hemodynamic Studies in Mice
These studies were performed as described in detail in our previous investigations.33 Mice were anesthetized with sodium pentobarbital (60 mg/kg body weight, i.p.) and placed on a temperature-regulated table to maintain body temperature at 37°C. During these studies, a solution of 0.9% saline containing 2.25% bovine serum albumin and 0.75% fluorescein isothiocyanate-inulin (FITC-I; Sigma-Aldrich) was infused, and glomerular filtration rate (GFR) was determined by the clearance of inulin.34 Mean arterial pressure was continuously monitored throughout the experiment. Renal blood flow (RBF) was determined by an electromagnetic flow probe and a small animal blood flow meter (T206; Transonic Systems Inc., Ithaca, NY).35 Renal vascular resistance (RVR) was determined as the ratio of mean arterial pressure:RBF. Renal plasma flow (RPF) was calculated by RBF × (100 − hematocrit)/100. Filtration fraction was calculated as GFR/RPF.
mRNA Expression by Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
For analysis of gene expression, total RNA was extracted from mouse tissues using the Trizol method (Invitrogen, Carlsbad, CA), further purified with the RNeasy mini kit (Qiagen, Valencia, CA) and subsequently used in reverse transcription reactions (Transcriptor First Strand cDNA synthesis kit; Roche Applied Science, Indianapolis, IN) according to each manufacturer’s protocol. The resulting cDNA was used in quantitative real-time PCR reactions performed as described in detail by our previous publications and analyzed on an ABI Prism 7900HT (Applied Biosystems, Foster City, CA).31,36 Probe and primer sets used for quantification were designed with Primer Express software (Applied Biosystems) and are detailed in Table 1. In addition, a probe and primer set for the analysis of endothelin-1 (ET-1) gene expression was purchased from Applied Biosystems (TaqMan gene expression assay, stock no. Mm00438656) and was used in our standard procedure.
Table 1.
Primers and Probes Used for Quantitative Real-Time RT-PCR
| Gene | Primer/probe | Sequence |
|---|---|---|
| IL-6 | Forward | 5′-CCAGAAACCGCTATGAAGTTCCT-3′ |
| Reverse | 5′-CACCAGCATCAGTCCCAAGA-3′ | |
| TaqMan | 5′-(FAM)TCTGCAAGAGACTTCCATCCAGTTGCC(TAMRA)-3′ | |
| IL-10 | Forward | 5′-AGCAGCCTTGCAGAAAAGAGA-3′ |
| Reverse | 5′-AGTAAGAGCAGGCAGCATAGCA-3′ | |
| TaqMan | 5′-(FAM)-CCATCATGCCTGGCTCAGCA(TAMRA)-3′ | |
| MCP-1 | Forward | 5′-GGCTCAGCCAGATGCAGTTAA-3′ |
| Reverse | 5′-CCTACTCATTGGGATCATCTTGCT-3′ | |
| TaqMan | 5′-(FAM)-CCCCACTCACCTGCTGCTACTCATTCAC(TAMRA)-3′ | |
| RANTES | Forward | 5′-GCAAGTGCTCCAATCTTGCA-3′ |
| Reverse | 5′-CTTCTCTGGGTTGGCACACA-3′ | |
| TaqMan | 5′-(FAM)-CGTGTTTGTCACTCGAAGGAACCGC(TAMRA)-3′ | |
| TNF | Forward | 5′-TCTCTTCAAGGGACAAGGCTG-3′ |
| Reverse | 5′-ATAGCAAATCGGCTGACGGT-3′ | |
| TaqMan | 5′-(FAM)-CCCGACTACGTGCTCCTCACCCA(TAMRA)-3′ | |
| 18S | Forward | 5′-TGTCTCAAAGATTAAGCCATGCAT-3′ |
| Reverse | 5′-AACCATAACTGATTTAATGAGCCATTC-3′ | |
| TaqMan | 5′-(FAM) TACGCACGGCCGGTACAGTGAAACT(TAMRA)-3′ |
Electrophoretic Mobility Shift Assay (EMSA) for Analysis of Nuclear Factor (NF)-κB
Nuclear extracts were prepared from cells in culture and mouse tissues, and EMSA was performed for the assessment of activation of NF-κB as described in our previous studies.30,31,37 In brief, 5.0 μg of each extract were added to a binding reaction containing the following components: 20 mmol/L HEPES, pH 8.0, 100 mmol/L NaCl, 1.0 mmol/L MgCl2, 0.05 mmol/L ethylenediaminetetraacetic acid, 0.5 mmol/L dithiothreitol, 3% glycerol, 0.2 μg/μl poly(dI+dC), and 50,000 cpm of [32P]γATP-labeled NF-κB probe. A consensus oligonucleotide containing DNA binding sites for NF-κB transcription factors (catalog no. E3291; Promega, Madison, WI) was used. Bound and free probe were separated on a 5% TBE nondenaturing polyacrylamide gel and visualized by autoradiography. A concentrated anti-p65 antibody (catalog no. sc-109; Santa Cruz Biotechnology, Santa Cruz, CA) was used for supershift analysis of selected extracts.
Multiplex Protein Array System
Serum samples were collected using BD Microtainer serum separators (BD Biosciences, Franklin Lakes, NJ) and analyzed for cytokine levels using a multiplex bead suspension array system (Bio-Plex Mouse Cytokine 23-Plex Panel, catalog no. 171-F11241; Bio-Rad Laboratories, Hercules, CA), according to the manufacturer’s protocol.
Western Analysis
Western analysis was performed on proteins extracted from mouse tissues as described previously.31 In brief, lysate proteins (20 to 100 μg) were separated on 10% Tris-HCl gels and transferred to polyvinylidene difluoride membranes. Blots were incubated with primary antibodies to caspase-3, pIκBα, or IκBα (catalog nos. 9665, 9246, and 9242, respectively; Cell Signaling Technology, Danvers, MA) overnight at 4°C. This was followed by incubation with goat anti-mouse or anti-rabbit IgG secondary antibodies and visualization using a chemiluminescence method (Amersham Pharmacia, Piscataway, NJ). Equal protein loading was confirmed by immunoblotting for β-actin (catalog no. 612657; BD Transduction Laboratories, San Diego, CA).
Studies Using Cell Culture
Rat proximal tubular epithelial cells, engineered to overexpress HO-1 by stable transfection of NRK-52E cells (American Type Culture Collection, Manassas, VA) as described in detail in our previous study,37 were used in the present study to test the effect of HO-1 overexpression on responses to LPS treatment. Control cells (containing an empty cassette) and HO-1-overexpressing cells were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated fetal bovine serum, 20 mmol/L HEPES, 1 mmol/L sodium pyruvate, 100 μg/ml G418 sulfate, and 100 U/ml penicillin/100 μg/ml streptomycin.37 For NF-κB EMSA studies, cells were washed twice with phosphate-buffered saline (PBS) and incubated for 2 hours with serum-free DMEM or serum-free DMEM containing various concentrations of LPS (1.0 to 0.01 μg/ml). EMSA was performed as described above. For the pIκBα/IκBα studies, cells were incubated in serum-free DMEM or serum-free DMEM containing 1.0 μg/ml LPS for 5 to 30 minutes. Cells were then washed once with cold PBS and lysed in complete, prechilled RIPA buffer for Western analysis.
Terminal dUTP Nick-End Labeling (TUNEL) Method for Detection of Apoptosis
As previously used by our laboratory, an Apoptag Plus peroxidase in situ apoptosis kit (Chemicon, Temecula, CA) was used to assess apoptosis in mouse tissues.36 This method detects apoptosis-associated DNA fragmentation by labeling of 3′-OH termini with digoxigenin nucleotides using terminal deoxynucleotidyl transferase.
Statistics
Data are expressed as mean ± SEM. Data for HO-1−/− and HO-1+/+ mice for a given condition were compared using Student’s t-test for parametric data and the Mann-Whitney test for nonparametric data. Results were considered significant for P < 0.05.
Results
Renal Response to LPS
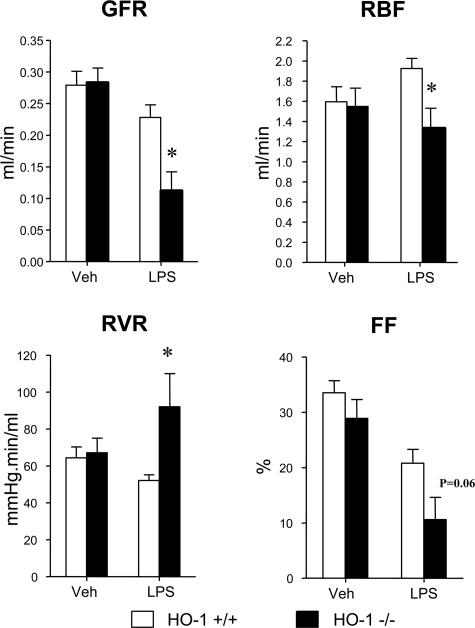
Renal hemodynamics in response to LPS and vehicle were assessed in HO-1+/+ and HO-1−/− mice and are shown in Figure 1. HO-1−/− mice, as compared with HO-1+/+ mice, subjected to LPS, demonstrated a significantly lower GFR and RBF, the latter attributable to increased RVR, and a tendency toward a lower filtration fraction (Figure 1). Mean arterial pressure was not significantly different between the HO-1+/+ and HO-1−/− mice treated with vehicle (100 ± 1 versus 100 ± 2 mm Hg, P = NS) or LPS (98 ± 1 versus 105 ± 6 mm Hg, P = NS).
Figure 1.
Renal hemodynamics in HO-1+/+ mice and HO-1−/− mice treated with vehicle (Veh) or LPS. The data demonstrate the following hemodynamic parameters: GFR, RBF, RVR, and filtration fraction (FF). n = 4 for all hemodynamic measurements in each of the vehicle-treated HO-1+/+ and HO-1−/− mice, n = 7 for measurements of GFR in the LPS-treated HO-1+/+ and HO-1−/− mice, and n = 5 for measurements of RBF, RVR, and filtration fraction in the LPS-treated HO-1+/+ and HO-1−/− mice. *P < 0.05 versus HO-1+/+ mice subjected to the same treatment.
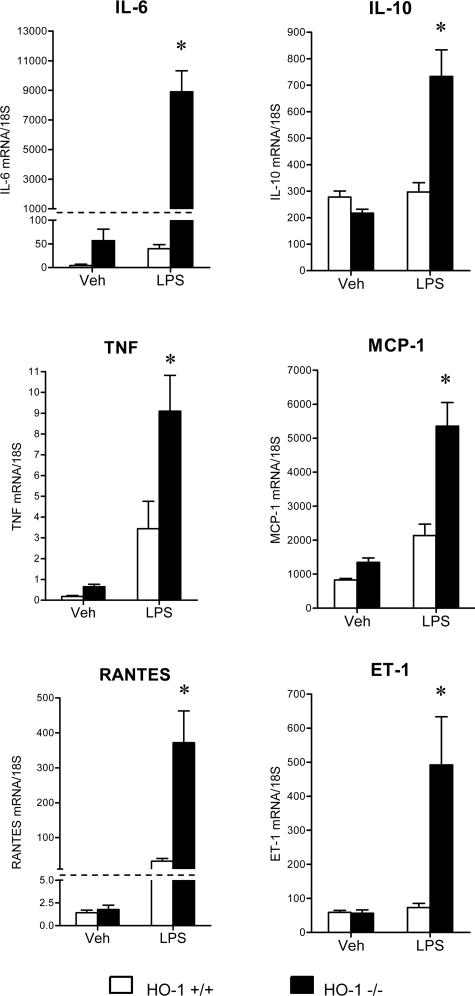
LPS induced minor histological changes in the kidney in both groups, with vascular congestion appearing somewhat more prominent in LPS-treated HO-1−/− mice (data not shown). Despite these minor differences in histological appearance, the kidneys in HO-1−/− mice exhibited a markedly exaggerated cytokine response to LPS. As shown in Figure 2, mRNA expression for interleukin (IL)-6, tumor necrosis factor (TNF), RANTES, IL-10, MCP-1, and ET-1 was markedly increased in HO-1−/− mice as compared with HO-1+/+ mice after the administration of LPS.
Figure 2.
Expression of cytokine mRNA in the kidney in HO-1+/+ and HO-1−/− mice treated with vehicle (Veh) or LPS. mRNA expression for IL-6, TNF, RANTES, IL-10, MCP-1, and ET-1 was determined by quantitative real-time RT-PCR. n = 6 in all groups. *P < 0.05 versus HO-1+/+ mice subjected to the same treatment.
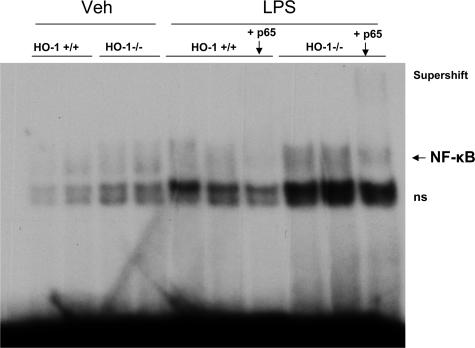
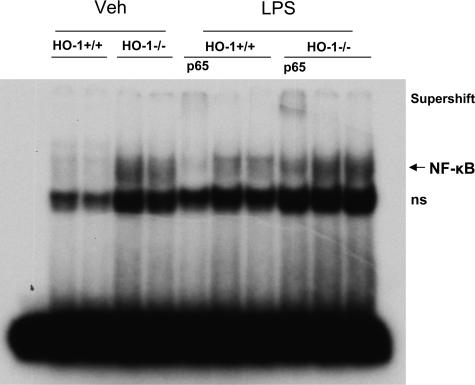
To determine the basis for such proinflammatory changes, studies were undertaken in the kidney to assess activation of the proinflammatory transcription factor NF-κB. NF-κB is involved in the expression of several of the cytokines exuberantly manifested in the kidney of LPS-treated HO-1−/− mice (eg, IL-6, TNF, RANTES, MCP-1). As shown by EMSA (Figure 3), activation of NF-κB in HO-1−/− mice still persisted 24 hours after the administration of LPS, whereas there was little evidence of such activation in HO-1+/+ mice after LPS.
Figure 3.
EMSA for NF-κB binding in the kidney in HO-1+/+ and HO-1−/− mice treated with vehicle (Veh) or LPS. Supershifting was conducted with an antibody to p65. ns, nonspecific binding.
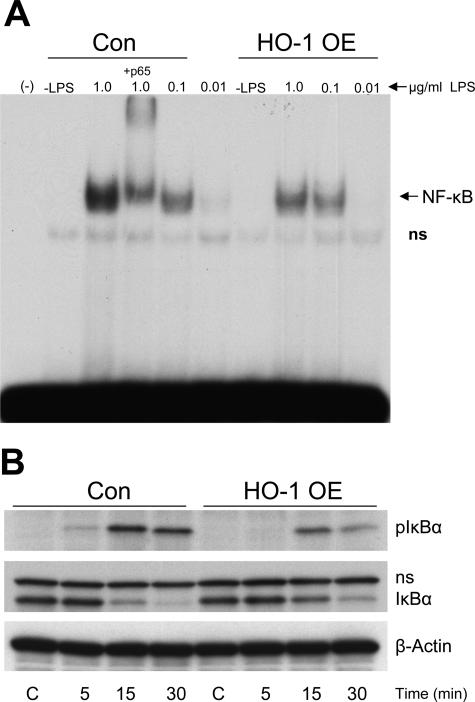
To determine a possible mechanism whereby activation of NF-κB can be influenced by HO-1, we used a complementary approach in which HO-1-overexpressing cells were exposed to LPS. As shown in Figure 4A, HO-1-overexpressing cells, as compared with control cells, exhibited a blunted activation of NF-κB in response to LPS. Because activation of NF-κB occurs after its inhibitory protein, IκB, undergoes phosphorylation and degradation, the expression of pIκB was evaluated by Western analysis. HO-1-overexpressing cells in response to LPS exhibit less expression of pIκB (Figure 4B), thereby providing a possible mechanism whereby HO-1 can suppress activation of NF-κB; namely, inhibition of phosphorylation of IκB.
Figure 4.
A: EMSA for NF-κB binding in HO-1-overexpressing cells (HO-1 OE) and control cells (Con) exposed to increasing concentrations of LPS. Supershifting was performed using a p65 antibody; (−) indicates a negative control that excludes nuclear extract; −LPS indicates cells not exposed to LPS. B: Expression of pIκB and IκB in HO-1-overexpressing cells (HO-1 OE) and control cells (Con) at 5, 15, and 30 minutes after exposure to LPS. Equivalency of protein loading was evaluated by immunoblotting for β-actin.
Concentration of Serum Cytokines after the Administration of LPS
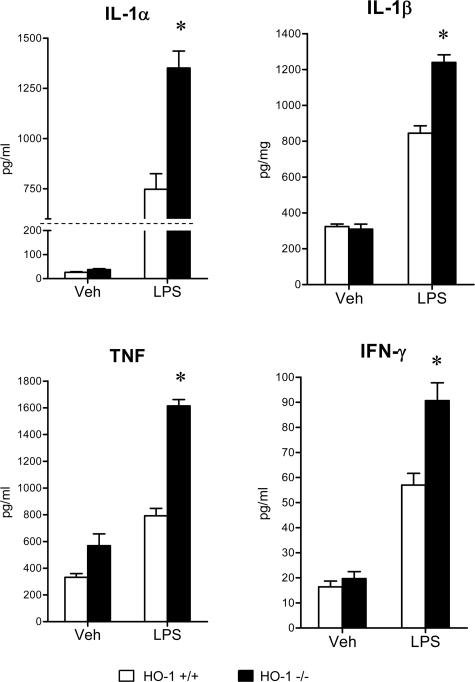
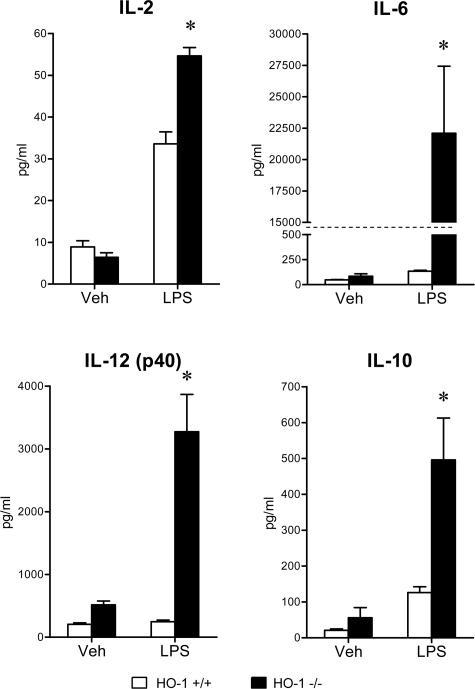
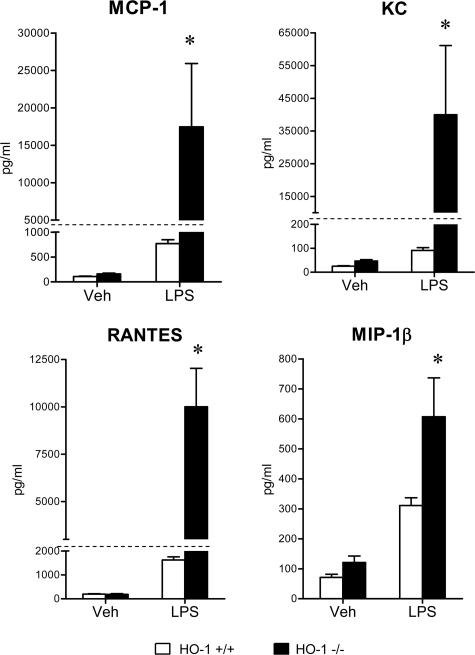
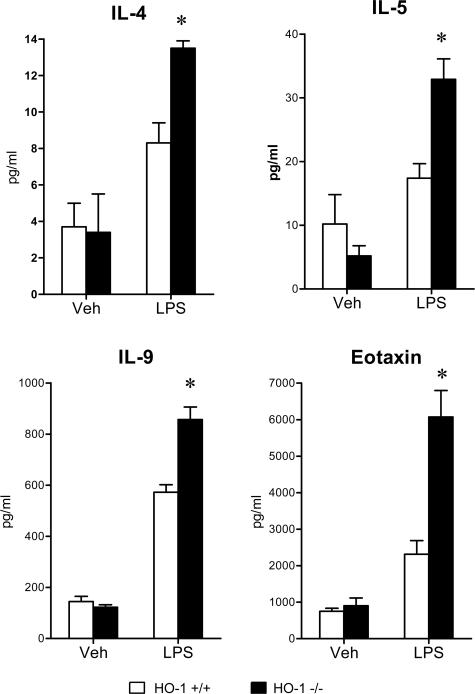
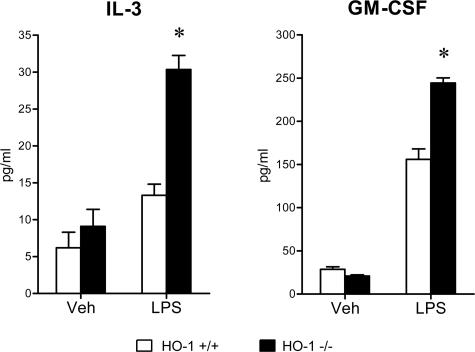
To evaluate the systemic inflammatory response to LPS, serum concentrations of a panel of cytokines were determined, and these data are summarized in Figures 5, 6, 7, 8, and 9. The concentrations of these serum cytokines were not significantly different in vehicle-treated HO-1+/+ and HO-1−/− mice. However, in response to LPS, HO-1−/− mice as compared with HO-1+/+ mice exhibited significantly greater amounts of Th1 cytokines (IL-1, IL-2, IL-6, IL-12, TNF, interferon-γ; Figures 5 and 6), chemokines (MCP-1, KC, RANTES, MIP-1β; Figure 7), Th2 cytokines and chemokines (IL-4, IL-5, IL-9, IL-10, eotaxin; Figures 6 and 8), and cytokine stimulators of bone marrow progenitor cells (IL-3 and GM-CSF; Figure 9).
Figure 5.
Serum Th1 cytokine levels (IL-1α, IL-1β, TNF, and interferon-γ) in HO-1+/+ and HO-1−/− mice treated with vehicle (Veh) or LPS. n = 6 in all groups except for vehicle-treated HO-1−/− group in which n = 5. *P < 0.05 versus HO-1+/+ mice subjected to the same treatment.
Figure 6.
Serum Th1 cytokine levels (IL-2, IL-6, and IL-12 p40), and Th2 cytokine level (IL-10) in HO-1+/+ and HO-1−/− mice treated with vehicle (Veh) or LPS. n = 6 in all groups except for vehicle-treated HO-1−/− mice in which n = 5. *P < 0.05 versus HO-1+/+ mice subjected to the same treatment.
Figure 7.
Serum chemokine levels (MCP-1, KC, RANTES, and MIP-1β) in HO-1+/+ and HO-1−/− mice treated with vehicle (Veh) or LPS. n = 6 in all groups except for vehicle-treated HO-1−/− mice in which n = 5. *P < 0.05 versus HO-1+/+ mice subjected to the same treatment.
Figure 8.
Serum Th2 cytokine/chemokine levels (IL-4, IL-5, IL-9, and eotaxin) in HO-1+/+ and HO-1−/− mice treated with vehicle (Veh) or LPS. n = 6 in all groups except for vehicle-treated HO-1−/− mice in which n = 5. *P < 0.05 versus HO-1+/+ mice subjected to the same treatment.
Figure 9.
Serum cytokine levels (stimuli for bone marrow progenitors, IL-3, and GM-CSF) in HO-1+/+ and HO-1−/− mice treated with vehicle (Veh) or LPS. n = 6 in all groups except for vehicle-treated HO-1−/− mice in which n = 5. *P < 0.05 versus HO-1+/+ mice subjected to the same treatment.
Because gene expression for many of the cytokines measured in serum (eg, IL-1, IL-6, IL-12, MCP-1, TNF, RANTES, MIP-1β, and so forth) is regulated by NF-κB, and because the liver is a significant source of cytokines appearing in serum, we assessed activation of NF-κB in the liver by EMSA. As demonstrated in Figure 10, NF-κB activation in response to LPS was greater in HO-1−/− mice, as compared with HO-1+/+ mice. Interestingly, even after the administration of vehicle (saline), the liver in HO-1−/− mice, but not HO-1+/+ mice, exhibited activation of NF-κB.
Figure 10.
EMSA for NF-κB binding in the liver in HO-1+/+ and HO-1−/− mice treated with vehicle (Veh) or LPS. Supershifting was performed using a p65 antibody. ns, nonspecific binding.
Alterations in the Spleen and Thymus
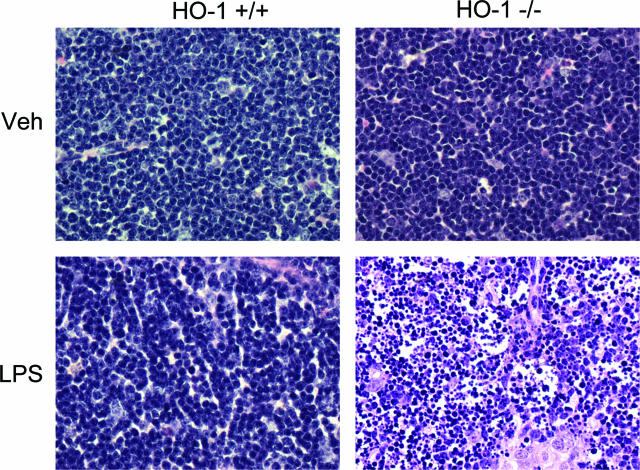
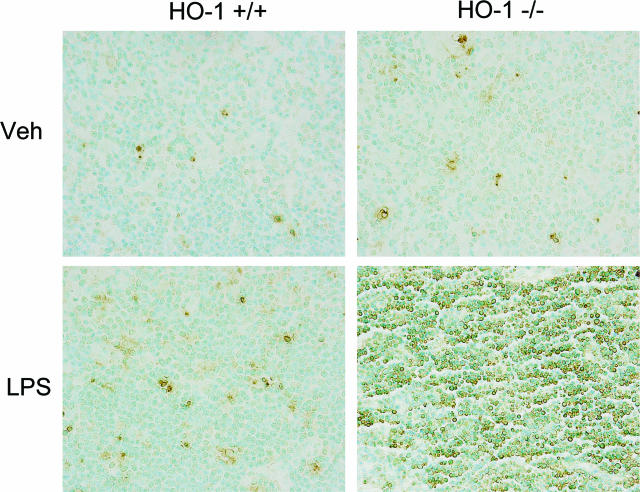
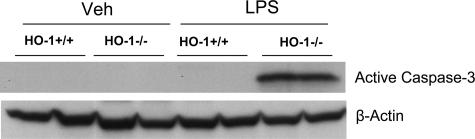
Because apoptosis of lymphocytes is well recognized in human and experimental sepsis,5,25,26,27,28 we evaluated the histological appearance of the spleen and thymus. After LPS, HO-1−/− mice but not HO-1+/+ mice exhibited marked evidence of apoptosis, the latter not observed in the spleen of vehicle-treated HO-1+/+ and HO-1−/− mice (data not shown). Apoptosis was fulminant in the thymus of LPS-treated HO-1−/− mice but not observed in the other three groups (Figures 11 and 12): the striking occurrence of apoptosis in the thymus in LPS-treated HO-1−/− mice, as observed on hematoxylin and eosin-stained sections (Figure 11), was confirmed by TUNEL staining (Figure 12), and was accompanied by marked activation of caspase-3 in this group but not in the other groups (Figure 13).
Figure 11.
Histological examination of the thymus stained with H&E. Representative sections of the thymus from vehicle-treated (Veh) HO-1+/+ and HO-1−/− mice, and from LPS-treated (LPS) HO-1+/+ and HO-1−/− mice. LPS-treated HO-1−/− mice (bottom right) showed evidence of extensive apoptosis, characterized by nuclear fragmentation and cytoplasmic condensation in the absence of an inflammatory response. Other groups, including LPS-treated HO-1+/+ mice, did not show significant apoptosis. Original magnifications, ×400.
Figure 12.
Histological examination of the thymus stained by the TUNEL method. Representative sections of the thymus from vehicle-treated (Veh) HO-1+/+ and HO-1−/− mice, and from LPS-treated (LPS) HO-1+/+ and HO-1−/− mice. Extensive apoptosis occurred in LPS-treated HO-1−/− mice (bottom right) but not in other groups. Original magnifications, ×400.
Figure 13.
Active caspase-3 expression in the thymus in HO-1+/+ and HO-1−/− mice treated with vehicle (Veh) or LPS. Equivalency of protein loading was evaluated by immunoblotting for β-actin.
Discussion
Our studies, providing the first analysis of the renal hemodynamic response to LPS in HO-1−/− mice, demonstrate that this genetic deficiency leads to more severely impaired renal hemodynamic function. In response to LPS, HO-1−/− mice, as compared with HO-1+/+ mice, exhibit reduced RBF (attributable to increased RVR), impaired GFR, and a tendency toward lower filtration fraction. A significant underpinning of this adverse hemodynamic response is thus heightened renal vasoconstriction in the kidney, and in this regard, it is notable that among the cytokines more markedly induced by LPS in the kidney in HO-1−/− mice is ET-1, which is recognized as a potent renal vasoconstrictor. Augmented induction of ET-1 in the kidney may account for this increased renal vasoconstriction in HO-1−/− mice subjected to LPS.
The greater impairment of renal hemodynamics in LPS-treated HO-1−/− mice observed in the current study contrasts with conclusions based on the use of metalloporphyrin inhibitors of HO activity to evaluate the role of the HO system in determining the renal hemodynamic response to LPS.38 In these latter studies, such inhibitors of HO activity, administered to LPS-treated wild-type mice, increased GFR and decreased RVR,38 findings that are not consistent with those derived in the present study when LPS was administered to HO-1−/− mice. Although the basis for these differences is uncertain at the present time, it is notable that metalloporphyrin inhibitors of HO activity may also affect other vasoactive systems besides HO, including nitric oxide synthase, guanylate cyclase, and atrial natriuretic peptides.39,40 Moreover, metalloporphyrin inhibitors can inhibit caspase activity,41 which has been incriminated as a mechanism of tissue injury in endotoxemia.8 The difficulty encountered in interpreting studies based on the use of metalloporphyrin HO inhibitors may be illustrated by studies using these inhibitors to evaluate the role of the HO system in renal ischemic injury: in this disease model, depending on the study, contrasting conclusions are reached in which the HO system is considered either a protectant against or a contributor to renal injury.42,43
In addition to greater impairment in renal hemodynamics, HO-1−/− mice also exhibited an exaggerated induction of cytokines in the kidney in response to LPS. Several of these genes are regulated by NF-κB.44,45 We thus determined whether there was a differential activation of NF-κB at this time point. Although HO-1+/+ mice exhibited little if any activation of NF-κB 24 hours after LPS, such activation was prominent in LPS-treated HO-1−/− mice. A complementary approach based on HO-1-overexpressing cells demonstrated a blunted activation of NF-κB in response to LPS in these cells. Phosphorylation of IκB was also diminished in LPS-treated HO-1-overexpressing cells as compared with LPS-treated control cells. Because less phosphorylation of IκB leads to less effective removal of this inhibitor,44,45 these findings in LPS-treated HO-1-overexpressing cells may account for the blunted activation of NF-κB by LPS in these cells. From these findings, we conclude that HO-1 deficiency exaggerates activation of NF-κB, and HO-1 overexpression reduces NF-κB activation.
That phosphorylation of IκB in response to LPS was less when HO-1 was overexpressed may offer insights into the mechanism whereby HO-1 inhibits activation of NF-κB and, in turn, NF-κB-dependent inflammatory responses. Phosphorylation of IκB enables nuclear translocation of NF-κB, and the attendant NF-κB-dependent transcription of proinflammatory genes. Bilirubin, a product of HO, can inhibit phosphorylation46,47; bile pigments may also reduce the inflammatory effects of LPS.48 It is thus possible that bile pigments, metabolites with recognized anti-inflammatory effects, may contribute to the suppressive effects of HO-1 on activation of NF-κB and NF-κB-dependent inflammatory processes. However, it must also be emphasized that other products of HO, such as carbon monoxide, exert potent anti-inflammatory effects. For example, carbon monoxide can suppress inflammatory responses via p38 MAPK and other signaling pathways49,50; carbon monoxide can inhibit production of proinflammatory cytokines that are also vasoconstrictive (such as ET-1) or proliferative (such as PDGF)51; carbon monoxide can also inhibit signaling processes induced by LPS and other ligands for Toll-like receptors.52
The exaggerated inflammatory response observed in the kidney in HO-1−/− mice after LPS was accompanied by an amplified cytokine response in the circulation. Such exuberance in cytokine production was quite comprehensive in that HO-1−/− mice exhibited an exaggerated response as assessed by Th1 cytokines, Th2 cytokines/chemokines, assorted chemokines, and cytokines that stimulate bone marrow progenitor cells. The present observations thus complement previous in vitro studies which demonstrate that splenocytes from HO-1−/− mice, exposed to LPS in vitro, exhibit a Th1 response.53 That both Th1 and Th2 cytokines, and cytokines with proinflammatory or anti-inflammatory properties, are all markedly up-regulated in a HO-1 deficiency state in vivo in response to LPS indicates that this deficiency leads to a generalized dysregulation in inflammation such that offsetting, countervailing, and mutually opposing species are concomitantly up-regulated. Although the full significance of these findings is uncertain at the present time, it is notable that the best predictors of mortality in critically ill patients with acute renal failure include not only IL-6 (a proinflammatory Th1 cytokine) and IL-8 (a chemokine for neutrophils) but also IL-10 (an anti-inflammatory Th2 cytokine).54
Previous studies have demonstrated increased mortality in HO-1−/− mice after the administration of LPS.55,56 In these studies, increased sensitivity to LPS in the setting of HO-1 deficiency is accompanied by increased oxidative stress,55,56 and the administration of N-acetylcysteine, an antioxidant, abrogates such sensitivity.56 That oxidative stress is heightened in response to LPS in an HO-1-deficient state may be relevant to the findings observed in the present studies. For example, oxidative stress can degrade IκB and thereby activate NF-κB and NF-κB-dependent pathways of inflammation; oxidative stress can also induce ET-1, a cytokine up-regulated in LPS-treated HO-1−/− mice, as shown in the present and previous studies56; oxidative stress may also contribute to apoptosis, the latter widespread in the thymus and spleen in LPS-treated HO-1−/− mice, as observed in the present studies. The capacity of HO-1 to restrain oxidative stress may emanate from the ability of this enzyme to degrade heme (a pro-oxidant metabolite that accumulates in injured tissue), to generate antioxidants such as bile pigments, and to regulate cellular levels of iron by inducing or recruiting iron-sequestering and iron-exporting proteins.
Studies of organs beside the kidney also demonstrated increased inflammatory responses in HO-1−/− mice after LPS. Of particular interest was the liver given the importance of this organ as a source for cytokines appearing in the serum in response to LPS. Interestingly, although activation of NF-κB was markedly greater in the liver in HO-1−/− mice after LPS, such differential response was also seen in the vehicle-treated mice. The increased propensity for NF-κB activation in the liver thus demonstrates the up-regulation of proinflammatory transcription factors even in the relatively unstressed HO-1−/− mouse.
A most striking finding in the present study was the severity of apoptosis observed in the thymus and spleen in LPS-treated HO-1−/− mice. Previous seminal studies demonstrate that T cells and B cells undergo apoptosis in human and experimental sepsis.5,26,27,28 Such apoptosis of lymphocytes likely represents a harbinger for a hypoimmune state and a hypoinflammatory condition as well as a mechanism for the acquired lymphopenia that is frequently observed in septic states.5,25,26,27,28 In addition, phagocytic uptake of apoptotic bodies may suppress monocytic/macrophage responses thereby accentuating the hypoinflammatory state. In this regard, it is notable that current paradigms for sepsis envision a phase of hypoimmunity as well as hyperimmunity, and the relative dominance of these contrasting states may vary.3,4,5,6 Systemic inflammatory response syndrome is described as a hyperinflammatory state, and compensatory anti-inflammatory response syndrome represents a hypoinflammatory condition. These conditions may occur in series as compensatory anti-inflammatory response syndrome emerges while systemic inflammatory response syndrome regresses; alternatively, these conditions may occur in parallel, and this co-existence of proinflammatory and anti-inflammatory changes is described as the mixed anti-inflammatory response syndrome. Our findings in the LPS-treated HO-1−/− mice thus encapsulate features that are reflective of both a hyperinflammatory state and an impending hypoimmune condition.
In summary, we demonstrate that HO-1 deficiency in the setting of endotoxemia as induced by LPS leads to impaired renal hemodynamics accompanied by an exaggerated inflammatory response and apoptosis in immune organs such as the thymus and spleen. We suggest that these observations may be relevant to sensitivity to endotoxemia in critically ill patients. Polymorphisms in the human HO-1 gene are well described and those that are associated with diminished HO activity predispose toward assorted diseases57; indeed, genetic deficiency of HO-1 leads to kidney and liver disease and early demise.58,59 We speculate that expression of HO-1 may influence the clinical outcome in sepsis syndromes.
Acknowledgments
We thank Mrs. Sharon Heppelmann for her secretarial expertise in the preparation of this work.
Footnotes
Address reprint requests to Dr. Karl A. Nath, Mayo Clinic, 200 First St., SW, Rochester, MN 55905. E-mail: nath.karl@mayo.edu.
Supported by the National Institutes of Health (grants DK47060 to K.A.N., DK68545 to M.D.G., DK16105 to J.P.G., and AI62261 and AI40384 to A.D.B.).
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med. 1996;125:680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125–1128. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Ronco C, Bonello M, Bordoni V, Ricci Z, D’Intini V, Bellomo R, Levin NW. Extracorporeal therapies in non-renal disease: treatment of sepsis and the peak concentration hypothesis. Blood Purif. 2004;22:164–174. doi: 10.1159/000074937. [DOI] [PubMed] [Google Scholar]
- Schor N. Acute renal failure and the sepsis syndrome. Kidney Int. 2002;61:764–776. doi: 10.1046/j.1523-1755.2002.00178.x. [DOI] [PubMed] [Google Scholar]
- Guo R, Wang Y, Minto AW, Quigg RJ, Cunningham PN. Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol. 2004;15:3093–3102. doi: 10.1097/01.ASN.0000145530.73247.F5. [DOI] [PubMed] [Google Scholar]
- Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol. 2005;289:F1324–F1332. doi: 10.1152/ajprenal.00124.2005. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Hanson SY, Lund S. Ischemic proximal tubular injury primes mice to endotoxin-induced TNF-alpha generation and systemic release. Am J Physiol. 2005;289:F289–F297. doi: 10.1152/ajprenal.00023.2005. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Hanson SY, Lund S. Acute nephrotoxic and obstructive injury primes the kidney to endotoxin-driven cytokine/chemokine production. Kidney Int. 2006;69:1181–1188. doi: 10.1038/sj.ki.5000022. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Lund S, Hanson S. Acute renal failure: determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol. 2006;291:F546–F556. doi: 10.1152/ajprenal.00072.2006. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Lund S, Hanson SY, Abrass CK. Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol. 2006;290:F1453–F1462. doi: 10.1152/ajprenal.00485.2005. [DOI] [PubMed] [Google Scholar]
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- Abraham N, Drummond G, Lutton J, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996;6:129–168. [Google Scholar]
- Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol. 2000;11:965–973. doi: 10.1681/ASN.V115965. [DOI] [PubMed] [Google Scholar]
- Dong Z, Lavrovsky Y, Venkatachalam MA, Roy AK. Heme oxygenase-1 in tissue pathology: the Yin and Yang. Am J Pathol. 2000;156:1485–1488. doi: 10.1016/S0002-9440(10)65019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar YS. Heme oxygenase-1 in renal injury: conclusions of studies in humans and animal models. Kidney Int. 2001;59:378–379. doi: 10.1046/j.1523-1755.2001.00505.x. [DOI] [PubMed] [Google Scholar]
- Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol. 2004;286:F425–F441. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Kanwar YS. A dynamic interplay between monocyte chemoattractant protein-1 and heme oxygenase-1: implications in renal injury. Kidney Int. 2005;68:896–897. doi: 10.1111/j.1523-1755.2005.00475.x. [DOI] [PubMed] [Google Scholar]
- Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. 2006;70:1120–1126. doi: 10.1038/sj.ki.5001579. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J. 2004;18:1185–1191. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Schmieg RE, Jr, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Karl IE, Buchman TG. Rapid onset of intestinal epithelial and lymphocyte apoptotic cell death in patients with trauma and shock. Crit Care Med. 2000;28:3207–3217. doi: 10.1097/00003246-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis—a potential treatment of sepsis? Clin Infect Dis. 2005;41(Suppl 7):S465–S469. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am J Pathol. 2000;156:1527–1535. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath KA, Vercellotti GM, Grande JP, Miyoshi H, Paya CV, Manivel JC, Haggard JJ, Croatt AJ, Payne WD, Alam J. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 2001;59:106–117. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int. 2005;68:611–622. doi: 10.1111/j.1523-1755.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncos JP, Grande JP, Murali N, Croatt AJ, Juncos LA, Hebbel RP, Katusic ZS, Nath KA. Anomalous renal effects of tin protoporphyrin in a murine model of sickle cell disease. Am J Pathol. 2006;169:21–31. doi: 10.2353/ajpath.2006.051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz JN, Gruenstein E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol. 1999;276:F172–F177. doi: 10.1152/ajprenal.1999.276.1.F172. [DOI] [PubMed] [Google Scholar]
- Traynor TR, Schnermann J. Renin-angiotensin system dependence of renal hemodynamics in mice. J Am Soc Nephrol. 1999;10(Suppl 11):S184–S188. [PubMed] [Google Scholar]
- Pedraza-Chaverri J, Murali NS, Croatt AJ, Alam J, Grande JP, Nath KA. Proteinuria as a determinant of renal expression of heme oxygenase-1: studies in models of glomerular and tubular proteinuria in the rat. Am J Physiol. 2006;290:F196–F204. doi: 10.1152/ajprenal.00230.2005. [DOI] [PubMed] [Google Scholar]
- Murali NS, Ackerman AW, Croatt AJ, Cheng J, Grande JP, Sutor SL, Bram RJ, Bren GD, Badley AD, Alam J, Nath KA. Renal upregulation of HO-1 reduces albumin-driven MCP-1 production: implications for chronic kidney disease. Am J Physiol. 2007;292:F837–F844. doi: 10.1152/ajprenal.00254.2006. [DOI] [PubMed] [Google Scholar]
- Poole B, Wang W, Chen YC, Zolty E, Falk S, Mitra A, Schrier R. Role of heme oxygenase-1 in endotoxemic acute renal failure. Am J Physiol. 2005;289:F1382–F1385. doi: 10.1152/ajprenal.00402.2004. [DOI] [PubMed] [Google Scholar]
- Grundemar L, Ny L. Pitfalls using metalloporphyrins in carbon monoxide research. Trends Pharmacol Sci. 1997;18:193–195. doi: 10.1016/s0165-6147(97)01065-1. [DOI] [PubMed] [Google Scholar]
- Serfass L, Burstyn JN. Effect of heme oxygenase inhibitors on soluble guanylyl cyclase activity. Arch Biochem Biophys. 1998;359:8–16. doi: 10.1006/abbi.1998.0887. [DOI] [PubMed] [Google Scholar]
- Blumenthal SB, Kiemer AK, Tiegs G, Seyfried S, Holtje M, Brandt B, Holtje HD, Zahler S, Vollmar AM. Metalloporphyrins inactivate caspase-3 and -8. FASEB J. 2005;19:1272–1279. doi: 10.1096/fj.04-3259com. [DOI] [PubMed] [Google Scholar]
- Kaizu T, Tamaki T, Tanaka M, Uchida Y, Tsuchihashi S, Kawamura A, Kakita A. Preconditioning with tin-protoporphyrin IX attenuates ischemia/reperfusion injury in the rat kidney. Kidney Int. 2003;63:1393–1403. doi: 10.1046/j.1523-1755.2003.00882.x. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Takahashi T, Suzuki T, Yamasaki A, Fujiwara T, Odaka Y, Hirakawa M, Fujita H, Akagi R. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med. 2000;28:809–817. doi: 10.1097/00003246-200003000-00033. [DOI] [PubMed] [Google Scholar]
- Jimi E, Ghosh S. Role of nuclear factor-kappaB in the immune system and bone. Immunol Rev. 2005;208:80–87. doi: 10.1111/j.0105-2896.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- Campbell KJ, Perkins ND. Regulation of NF-kappaB function. Biochem Soc Symp. 2006;(73):165–180. doi: 10.1042/bss0730165. [DOI] [PubMed] [Google Scholar]
- Hansen TW, Mathiesen SB, Walaas SI. Bilirubin has widespread inhibitory effects on protein phosphorylation. Pediatr Res. 1996;39:1072–1077. doi: 10.1203/00006450-199606000-00023. [DOI] [PubMed] [Google Scholar]
- Samb A, Taille C, Almolki A, Megret J, Staddon JM, Aubier M, Boczkowski J. Heme oxygenase modulates oxidant-signaled airway smooth muscle contractility: role of bilirubin. Am J Physiol. 2002;283:L596–L603. doi: 10.1152/ajplung.00446.2001. [DOI] [PubMed] [Google Scholar]
- Vachharajani TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different regional vascular beds. Am J Physiol. 2000;278:H1613–H1617. doi: 10.1152/ajpheart.2000.278.5.H1613. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995;96:2676–2682. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–1365. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet SF, Lee ME, Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation. 2000;102:3015–3022. doi: 10.1161/01.cir.102.24.3015. [DOI] [PubMed] [Google Scholar]
- Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Ohta K, Yachie A, Fujimoto K, Kaneda H, Wada T, Toma T, Seno A, Kasahara Y, Yokoyama H, Seki H, Koizumi S. Tubular injury as a cardinal pathologic feature in human heme oxygenase-1 deficiency. Am J Kidney Dis. 2000;35:863–870. doi: 10.1016/s0272-6386(00)70256-3. [DOI] [PubMed] [Google Scholar]
- Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]