Abstract
Accumulation of α1(IV) and α2(IV) collagen is one of the characteristic pathological changes in glomerulosclerosis. Although the Col4a2 gene is known to have a 0.3-kb critical enhancer element with the GAACAAT motif, which transcription factor binds and transactivates this motif has not been identified. In this study, we found that SRY-related HMG box 9 (SOX9) was bound to the GAACAAT motif in the Col4a2 enhancer in vitro and in vivo in mesangial cells. SOX9 strongly activated this enhancer when cotransfected with Col4a2 enhancer-promoter construct in mesangial cells and Swiss/3T3 cells. Mutation in the GAACAAT motif eliminated the activation by SOX9. Furthermore, transforming growth factor-β (TGF-β) treatment induced the expression of SOX9 and Col4a2, and a small interfering RNA against SOX9 reduced Col4a2 expression induced by TGF-β treatment in mesangial cells. In vivo, we found that the expression of SOX9 was dramatically increased along with the expression of TGF-β and Col4a2 in mouse nephrotoxic nephritis. These results indicate that SOX9 is essential for Col4a2 expression in mesangial cells and might be involved in the accumulation of α2(IV) collagen in experimental nephritis.
Glomerulosclerosis is an end stage of various forms of glomerulonephritis and is defined as the segmental or global collapse or closure of capillary loops with associated mesangial matrix expansion.1 α1 and α2 chains of type IV collagen are major components of this expanded extracellular matrix.1,2,3 α1 and α2(IV) collagen are encoded by Col4a1 and Col4a2 genes, respectively. Although decreasing the expressions of α1 and α2(IV) collagen in mesangial cells might lead to new approaches for the management of glomerulosclerosis, little is known about the regulation of Col4a1 and Col4a2 gene expression. α1 and α2(IV) collagen in glomeruli are up-regulated in response to cytokines, growth factors, and mechanical factors such as intraglomerular hypertension and hyperfiltration.4 Among them, transforming growth factor-β (TGF-β) has been implicated in glomerular extracellular matrix accumulation both in vitro and in vivo.5,6 It mediates matrix turnover by increasing synthesis of collagen rather than inhibiting production of matrix proteases.7 However, the molecular mechanism involved in this process is not fully understood yet.
There are six isoforms of collagen IV. In the mature glomerulus, heterotrimers composed of two α1 and one α2 collagen chains are present in the mesangium, whereas the α3, α4, and α5 chains are present in the glomerular basement membrane. The α6 chain is found only in Bowman’s capsule. The Col4a1 and Col4a2 genes, in human and mouse, are linked in a head-to-head arrangement and are transcribed by a bidirectional promoter in opposite orientations.8,9,10,11 Although the bidirectional promoter for the Col4a1 and Col4a2 genes is highly conserved between human and mouse, this region by itself has a very low transcriptional activity in transiently transfected cells.8,9 Subsequently, a 2.7-kb enhancer element 5.5 kb upstream from the first exon of Col4a1 was found to increase the transcription of Col4a2 gene.8,12,13 Within this 2.7-kb enhancer element, a 0.3-kb enhancer element 4.2 kb upstream from the transcription start site of Col4a2 is transcriptionally active in cells that produce α1 and α2(IV) collagen.14 This enhancer activity requires two identical elements (GAACAAT).14 However, which transcription factor transactivates this motif has not been identified. We hypothesized that TGF-β, one of the most potent factors contributing to accumulation of extracellular matrix and glomerulosclerosis, might induce Col4a2 expression through the critical GAACAAT motif. Therefore, we tried to find a transcription factor that is up-regulated by TGF-β treatment and can bind to the critical GAACAAT motif.
Recently, a family of transcription factors, SRY-related HMG box (SOX) protein family was identified. They are characterized by the presence of a DNA-binding domain, and their target sequence is (A/T)(A/T)CAA(A/T)G.15,16,17 Although each SOX protein binds to similar motifs in the minor groove of the DNA helix, they regulate a distinct set of target genes. They are expressed in a tissue-specific manner and have various functions in a number of developmental processes.18 Among SOX family members, SOX9 is highly expressed in chondrocytes and Sertoli cells of the testis.19,20 Mutations in and around the Sox9 gene in human result in campomelic dysplasia, severe skeletal malformation syndrome, and XY sex reversal in male patients.21,22 SOX9 is a critical regulator of many chondrocyte-specific proteins, such as type II collagen, type XI collagen, and aggrecan.23,24,25
We examined the expression of SOX proteins in mesangial cells and found that SOX9 was markedly up-regulated by TGF-β treatment before induction of Col4a2. Therefore, we assumed that SOX9 might be involved in the regulation of Col4a2 in the kidney. In this study, we show that SOX9 binds to the critical element in Col4a2 gene enhancer and that SOX9 transactivates this enhancer element. To determine the potential role of SOX9 in glomerulosclerosis in vivo, we studied its expression in accelerated nephrotoxic nephritis (NTN) in mice, a model of postinflammatory fibrogenesis associated with mesangial matrix expansion.26,27 Our results show that expression of SOX9 was dramatically increased along with the expression of TGF-β and Col4a2 in this model.
Materials and Methods
Cell Cultures
Mouse kidney mesangial cells were characterized and maintained in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum and glutamine as previously described.28 The cultured cells fulfilled the criteria generally accepted for glomerular mesangial cells.29 Swiss/3T3 cell lines were obtained from the American Type Cell Culture collection (Manassas, VA).
Cell Treatment
Mesangial cells were plated in six-well plates. Twenty-four hours later, cells were treated with 1 ng/ml human recombinant TGF-β1 (R&D Systems, Minneapolis, MN) or control vehicle for the indicated time. Total RNA was isolated by TRIzol reagent (Invitrogen, Carlsbad, CA). Mesangial cells were lysed in RIPA buffer (50 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1% Nonidet P-40, 0.25% sodium dodecyl sulfate, 1 mmol/L Na3VO4, 2 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 μg/ml aprotinin). Samples were rotated and centrifugated for 30 minutes at 4°C, and the supernatants were used for immunoblotting.
Preparation of Total RNA and Quantitative Reverse Transcription-Polymerase Chain Reaction
One microgram of total RNA was used to prepare complementary DNA with Superscript III reverse transcriptase (Invitrogen). Amplification was conducted in an ABI Prism 7900 Sequence Detection System (Roche, Basel, Switzerland) using the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Primers specific for glyceraldehyde-3-phosphate dehydrogenase are 5′-GGCAAATTCAACGGCACAG-3′ (forward) and 5′-GCCTCACCCCATTTGATGTTA-3′ (reverse); SOX1, 5′-CCAAGATGCACAACTCGGAGA-3′ (forward) and 5′-TCTTGAGCAGCGTCTTGGTCT-3′ (reverse); SOX2, 5′-TTCGGTGATGCCGACTAGAAA-3′ (forward) and 5′-AGACTTTTGCGAACTCCCTGC-3′ (reverse); SOX4, 5′-AAAATCCAGCGTGCCCCAT-3′ (forward) and 5′-GCTCAACACAAATGCCAAACG-3′ (reverse); SOX9, 5′-TCCAGCAAGAACAAGCCACAC-3′ (forward) and 5′-CAGCGCCTTGAAGATAGCATT-3′ (reverse); SOX10, 5′-TACCCTCACCTCCACAATGCT-3′ (forward) and 5′-CCTTTTTGTGCTGCATCCG-3′ (reverse); SOX11, 5′-CTCATCGCTGTGATGTGTGGA-3′ (forward) and 5′-AGTGCATTGAGTCTGCTTCGC-3′ (reverse); SOX15, 5′-AACGCCTTCATGGTGTGGA-3′ (forward) and 5′-CGAGTTTTTGCTCTTACGCCG-3′ (reverse); SOX17, 5′-CTACCCCGACATTTGACGGTT-3′ (forward) and 5′-TCGTGTAGCCCCTCAACTGTT-3′ (reverse); SOX18, 5′-TCGCCTCCTCATTTACACACC-3′ (forward) and 5′-TTCACCACCAATCCTGGCA-3′ (reverse); Col4a1, 5′-TTCAGATTCCGCAGTGCCCTA-3′ (forward) and 5′-TTCTCATGCACACTTGGCAGC-3′ (reverse); and Col4a2, 5′-TGGCTGAGGAGGAAATCAAGC-3′ (forward) and 5′-AATGGCGTTGCACGGAAGT-3′ (reverse). The cycling parameters were 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C, and 60 seconds at 60°C. Glyceraldehyde-3-phosphate dehydrogenase, a common housekeeping gene, was used as an internal control for an equal amount of starting material.
Immunoblotting Analysis
Twenty micrograms of each sample was applied to sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and immunoblotted. Rabbit anti-SOX9 antibody was from Chemicon International (Temecula, CA), and rabbit anti-type IV collagen antibody was from Biodesign International (Kennebunk, ME). Equal loading of proteins was verified by blotting for actin. The immunoreactive bands were visualized and quantificated by imaging densitometer, Science Lab 99 Image Gauge (Fujifilm, Tokyo, Japan). The values of SOX9 and α2(IV) collagen were normalized to that of actin and compared with the values of 0 hours.
Plasmid Constructs
The β-gal Reporter Vector pNASSβ (Clontech, Mountain View, CA) was used as negative control plasmid (no promoter). Col4a2 promoter reporter plasmid (no enhancer) was constructed as follows. The 0.8-kb NcoI-XbaI fragment of the 5′-flanking region of Col4a2 gene was subcloned into the pNASSβ. An enhancer fragment for Col4a2 gene14 4.2 kb upstream of transcription start site of the Col4a2 gene was prepared by polymerase chain reaction (PCR) using the sequence-specific oligodeoxynucleotide primers with Eco RI site at the 5′ end and NcoI site at the 3′ end. The fragment was then inserted into the unique EcoRI and NcoI site of pNASSβ, in front of the promoter. Series of 5′ deletion fragments were constructed by PCR using the oligodeoxynucleotide primers. Among them, the 4174-3972 fragment was constructed by restriction digestion at EcoRI site made by site-directed mutagenesis kit (Stratagene, La Jolla, CA). Point mutations in the 0.3-kb enhancer fragment were made by site-directed mutagenesis kit. Primers for 4290-3972 are 5′-GAAGACATTCCCAATGACCCG-3′ (forward) and CCCCAGCACGAATCTCTGTTAG (reverse); 4192-3972, 5′-TGCTGGCTGGGAAGAACAATG-3′ (forward) and reverse is the same as 4290-3972; 4174-3972, 5′-GCTGGGAAGAAGAATTCCCCGAGATAATGAGTTAGG-3′ (sense) and 5′-CCTAACTCAGGATCTCGGGGAATTCTTCTTCCCAGC-3′ (antisense); mutant type 1, 5′-CTGGGAAGAACGCTGCCCCGAGATCCTGAGTTAG-3′ (sense) and 5′-CTAACTCAGGATCTGGGGGCAGCGTTCTTCCCAG-3′ (anti-sense); and mutant type 2, 5′-CCATGTTTGGGAACGCTCGCTGTTCAATTCAAGGCTG-3′ (sense) and 5′-CAGCCTTGAATTGAACAGCGAGCGTTCCCAAACATGG-3′ (anti-sense). All constructions made with mutagenesis kit and products of PCR were confirmed by DNA sequencing. N-terminally flagged SOX9 expression constructs were used for transient transfection experiments in Swiss/3T3 cells.23
Reporter Assay
Transfection was performed using FuGENE6 transfection reagent (Roche) according to the manufacturer’s instructions. Mouse mesangial cells (1.0 × 105) were seeded onto six-well plates. Six hours later, cells were transfected with 1 μg of reporter construct along with 100 ng of firefly luciferase under the control of cytomegalovirus promoter as an internal control. For overexpression experiments, mesangial cells or Swiss/3T3 cells were transfected with 500 ng of reporter construct with 500 ng of either the vector encoding SOX9 or the mock vector in the presence of 100 ng of firefly luciferase under the control of cytomegalovirus promoter. Forty-eight hours later, the cells were harvested in reporter lysis buffer, and β-galactosidase and luciferase activities were measured using the Luminescent β-galactosidase reporter system (BD Biosciences, San Jose, CA) and the luciferase reporter assay system (Promega, Madison, WI). β-Galactosidase results were normalized for luciferase activity. Transfection experiments were repeated at least three times. β-Galactosidase per luciferase activity data were expressed as means ± SD.
Elecrophoretic Mobility Shift Assays
SOX9 was synthesized by a transcription/translation-coupled reticulocyte lysate system (Promega) with an N-terminally flagged SOX9 expression vector. Electrophoretic mobility shift assays were carried out using 1 μl of in vitro synthesized SOX9 with 0.1 μg of poly(dG-dC)·poly(dG-dC) as described previously.30 The sequence of the sense strands of the double-stranded oligonucleotides were as follows: wild-type Col4a2 enhancer, 5′-GGAAGAACAATGCCCCGAGATCCTG-3′, and mutant type Col4a2 enhancer, 5′-GGAAGAACGCTGCCCCGAGATCCTG-3′ (mutated sequence is underlined). The wild-type and mutant probes were made by annealing. For supershift assays, lysates were incubated with anti-Flag antibody (Sigma-Aldrich, St. Louis, MO) for 20 minutes before protein-DNA binding reactions.
Chromatin Immunoprecipitation Assay
Mesangial cells were seeded in 10-cm dishes (1 × 106 cells/dish) and treated with 1 ng/ml TGF-β1 or control vehicle for 3 hours. Chromatin immunoprecipitation (ChIP) assay was performed using ChIP assay kit (Active Motif, Carlsbad, CA) according to manufacturer’s protocol with SOX9 antibody (Chemicon) or control IgG. Input DNA (2.5%) or 5 μl of immunoprecipitated DNA was used as a template in the PCR reaction. PCR amplification was performed using Taq polymerase with primers to amplify the region containing the GAACAAT motif in Col4a2 gene enhancer. The primers are 5′-TGACCTTTCATTGTGTGCTGGAC-3′ (forward) and 5′-GGTTTGGCATTTGGAACTCCG-3′ (reverse). For nonspecific genomic DNA contaminations, we used an element from the mouse genomes located at −7252 and at −7058 upstream of the Col4a2 gene transcription start site as negative control. The primers are 5′-TCCACAGGTCAAACTCCTCTAGTCC-3′ (forward) and 5′-ACAGTTGGTGAAGGATTGGGC-3′ (reverse). PCR was performed under the following conditions: 95°C for 10 minutes followed by 40 cycles at 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, ending with a final extension at 72°C for 5 minutes. The PCR products were run on 2% agarose gels and visualized by ethidium bromide staining.
RNA Interference
Stealth small interference RNA (siRNA) against SOX9 (5′-UGACGUCGAAGGUCUCAAUGUUGGA-3′) and Stealth RNA Interference (RNAi) negative control duplex were provided by Invitrogen. Mesangial cells were used for the knockdown experiment with siRNA. Cells were transfected with the siRNA against SOX9 or control RNA (50 pmol/ml) using Lipofectamine 2000 according to the manufacturer’s instructions. Then the cells were starved for 48 hours and treated with TGF-β or vehicle for 6 hours. Knockdown of SOX9 and Col4a2 were confirmed by reverse transcriptase (RT)-PCR analysis and immunoblotting.
Animals and Induction of Accelerated NTN
CD1ICR mice were obtained from Shimizu Laboratory Animal Center (Hamamatsu, Japan). All mice were housed under specific pathogen-free conditions. All animal experiments were performed in accordance with institutional guidelines, and the Review Board of Kyoto University granted ethical permission to perform this study. Nephrotoxic serum was prepared as described previously. Male CD1ICR mice (6 to 7 weeks old) weighing 20 to 30 g were sensitized by subcutaneous injection of 1 mg of normal sheep IgG in Freund’s complete adjuvant in divided doses. Five days later, mice were injected with 0.05 ml of nephrotoxic serum daily for 3 days. At intervals 7 and 14 days after the first dose of nephrotoxic serum, groups of mice (10 to 12 per group) were sacrificed, and the kidneys were removed for the experiments. Whole-kidney RNA was isolated using RNeasy (Qiagen Inc., Valencia, CA). Preparation of total RNA and quantitative RT-PCR were conducted as described before. Primers specific for TGF-β1 were 5′-GCAACAATTCCTGGCGTTACC-3′ (forward) and 5′-CGCTGAATCGAAAGCCCTGTA-3′ (reverse).
Histological Study
Kidney halves were fixed in methyl Carnoy’s solution and embedded in paraffin. Sections (2 μm) were stained with periodic acid-Schiff for routine histology. For the immunohistochemistry, remaining kidneys were fixed in 4% paraformaldehyde and snap-frozen, and 5-μm-thick cryostat sections were prepared. Sections were rehydrated in phosphate-buffered saline and incubated with 10% normal donkey serum for 20 minutes and then incubated with rabbit anti-SOX9 antibody (Chemicon) (1:300 dilution), rabbit anti-TGF-β antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (1:50 dilution), or rabbit anti-type IV collagen antibody (Progen, Heidelberg, Germany) (1:200 dilution). The sections were washed in phosphate-buffered saline and incubated with fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG antibody (Chemicon) for 60 minutes.
Statistical Analysis
The data are expressed as the means ± SD. Comparison among more than two groups was performed by one-way analysis of variance followed by the post hoc analysis (Bonferroni/Dunn test) to evaluate statistical significance. All analyses were performed using StatView (SAS Institute, Cary, NC). Statistical significance was defined as P < 0.05.
Results
TGF-β Increases SOX9 in Mesangial Cells
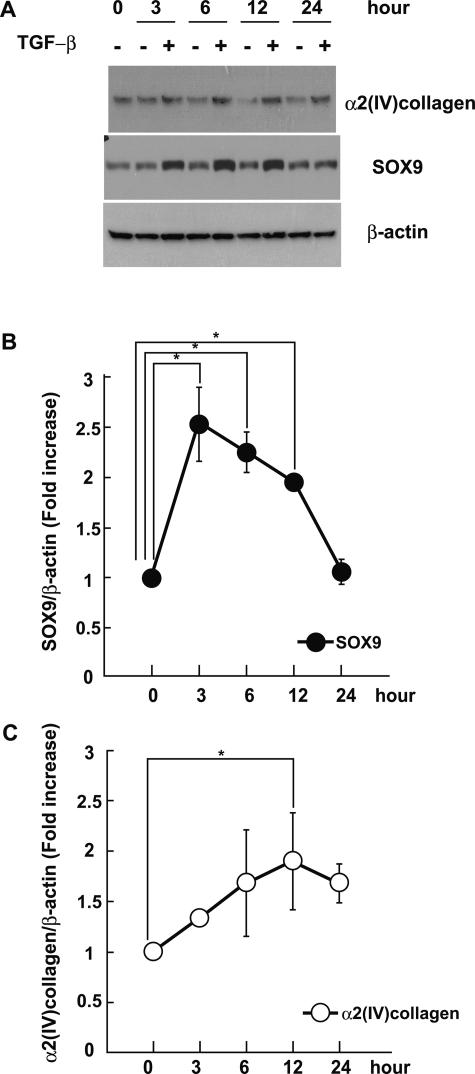
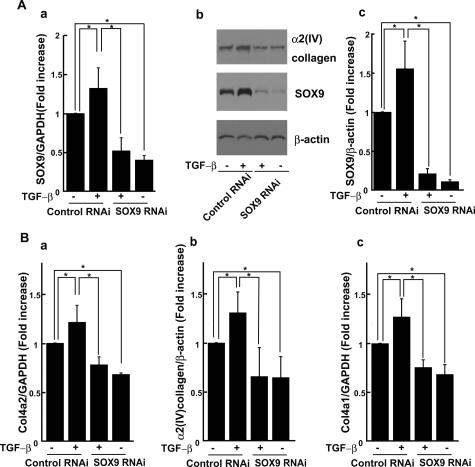
We first examined the effects of TGF-β on mRNA levels of SOX protein family in mesangial cells. SOX protein family was selected and examined according to the review by Bowles et al.18 Total RNA from mesangial cells treated with 1 ng/ml TGF-β were analyzed by RT-PCR, and we found that the levels of SOX9 mRNA were increased 3 and 6 hours after administration of TGF-β (Table 1). SOX4 and -15 were detected but not increased by TGF-β treatment, whereas SOX1, -2, -10, -11, -17, and -18 were not detectable in mesangial cells. TGF-β also increased SOX9 protein levels in mesangial cells at 3 hours, and the increase persisted for 12 hours (Figure 1, A and B). Up-regulation of α2(IV) collagen was found at 12 hours, followed by the increase in SOX9 expression (Figure 1, A and C).
Table 1.
Expression of SOX4, -9, and -15 mRNA in Mesangial Cells Treated with 1 ng/ml TGF-β for 3 or 6 Hours
| 3 hours | P | 6 hours | P | |
|---|---|---|---|---|
| SOX4 | 1.07 (0.08) | n.s. | 1.09 (0.09) | n.s. |
| SOX9 | 1.50 (0.15) | <0.05 | 1.92 (0.33) | <0.05 |
| SOX15 | 0.86 (0.13) | n.s. | 1.02 (0.32) | n.s. |
The means and SD of three independent experiments examined by quantitative RT-PCR are shown. The results are presented as fold increase compared with that of control. n.s., not significant.
Figure 1.
A: TGF-β increased SOX9 and α2(IV) collagen proteins in mesangial cells. Mouse mesangial cells were cultured for the indicated periods of time in the presence or absence of 1 ng/ml TGF-β. Total cell lysates were examined by immunoblotting analysis using anti-SOX9 and anti-type IV collagen antibodies. An actin immunoblot was used to control for equal loading. The figure presents data from one of three experiments that produced similar results. B and C: Optical densitometry of SOX9 and α2(IV) collagen in immunoblotting. The values of SOX9 and α2(IV) collagen were normalized for that of actin and compared with the values of 0 hours. The average value of three independent experiments is shown. *P < 0.05 compared with control.
Activities of Col4a2 Enhancer in Mesangial Cells
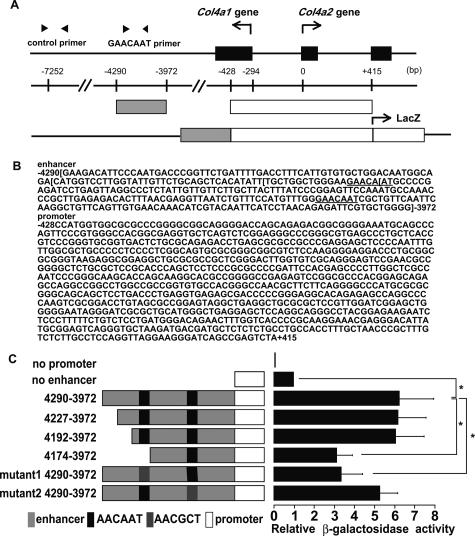
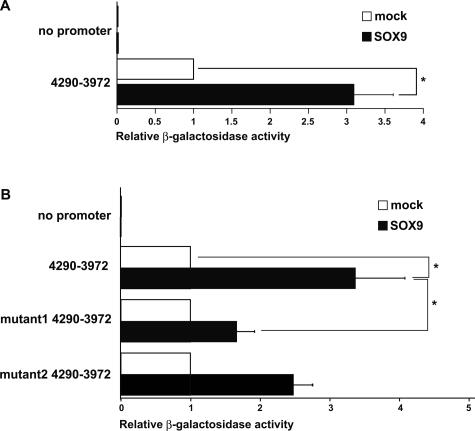
The arrangement of exons of Col4a1 and Col4a2 genes and the orientation of transcription initiation sites and map of promoter and enhancer constructs are shown in Figure 2A. The sequences of these elements were shown in Figure 2B. We tested the activities of Col4a2 enhancer elements in mouse mesangial cells (Figure 2C). In this promoter and enhancer orientation, β-galactosidase activity measures Col4a2 gene activity. The negative control plasmid was not active at all, and basal transcriptional activity of the 0.8-kb promoter fragment was low, but enhancer-promoter plasmid (4290-3972) showed a 6.2-fold increase in activity compared with promoter plasmid. To determine the site responsible for the up-regulation, we prepared various deletion mutants of this 4290-3972 enhancer fragment. Their transcriptional activities were then determined in transiently transfected cells. Although deletion mutant 4192-3972 did not affect the enhancer activity, transfection of deletion mutant 4174-3972, which lacks GAACAAT, resulted in a decrease in the enhancer activity to about one-half of the original construct. Mutation of the nucleotide sequence (mutant 1), GAACAAT to GAACGCT, also resulted in a decrease of the enhancer activity (Figure 2C). The 0.3-kb Col4a2 enhancer fragment, 125 bp downstream of the former GAACAAT, has another GAACAAT sequence (Figure 2B), which is also critical for transactivation in PYS-2 cells.14 Unexpectedly, mutation in the latter one (mutant 2) slightly, but not significantly, decreased the enhancer activity. Therefore, the former GAACAAT sequence is responsible for effective transactivation of Col4a2 in mesangial cells.
Figure 2.
AACAAT of the 0.3-kb enhancer element is critical for activation in mesangial cells. A: Genomic map of the mouse Col4a1 and Col4a2 genes with exons of Col4a1 and Col4a2 genes (black boxes) and the orientation of transcription initiation sites are shown. Scale bar indicates the distance (bp) of fragments relative to Col4a1 gene transcription start site. Enhancer and promoter fragments used during construction of plasmids are indicated as gray box and open box, respectively. Arrowheads show primers used in ChIP assay. B: The sequences of these promoter and enhancer elements were shown. The number indicates the distance (bp) of fragments relative to Col4a1 gene transcription start site. The left and right brackets in the sequence indicate the end points of deletions. The underlined sequences are former and latter GAACAAT motif. C: Schematic diagram of promoter and wild-type, deleted, or mutated enhancer elements indicated by the positions of the first and last nucleotides relative to the transcription start site of Col4a1 gene (left). Col4a2 promoter is displayed as a white box, and enhancer is displayed as a gray box. The wild-type critical element, AACAAT, is displayed as a black box, and the mutated one, AACGCT, is displayed as a dark gray box. Activities of the enhancer-promoter reporter constructs in mesangial cells are shown. Mesangial cells were transfected with 1 μg of reporter construct with 100 ng of firefly luciferase under the control of cytomegalovirus promoter as an internal control. The average values of three independent experiments in triplicate are shown as fold increase compared with that of no enhancer. *P < 0.05 compared with control.
SOX9 Bound to the Col4a2 Enhancer Element
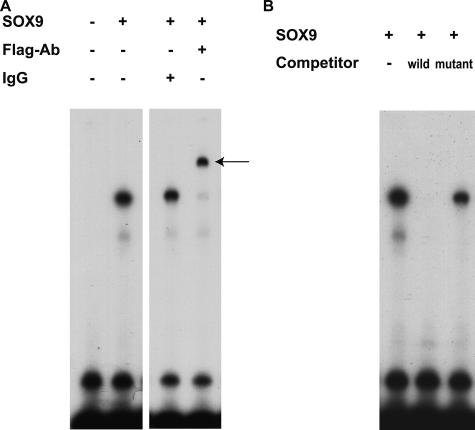
To determine whether the critical GAACAAT motif was bound by SOX9, the radiolabeled fragment derived from Col4a2 enhancer element was tested in electrophoretic mobility shift assays with in vitro synthesized flagged SOX9 protein. SOX9 formed complex with a probe containing the Col4a2 enhancer element. Furthermore, the DNA-protein complex was supershifted by adding antibody against Flag, indicating that SOX9 directly bound to the Col4a2 enhancer element (Figure 3A). The DNA-SOX9 complex could not be formed with an excess amount of unlabeled probe but formed with the same amount of unlabeled mutated probe in the GAACAAT motif (Figure 3B). These observations indicate that the Col4a2 enhancer element interacts with SOX9 through the consensus SOX9 binding motif GAACAAT.
Figure 3.
SOX9 bound to the Col4a2 enhancer element through GAACAAT motif in vitro. A: Electrophoretic mobility shift assay was performed using the Col4a2 enhancer probe with in vitro synthesized flagged SOX9. SOX9 bound to the Col4a2 enhancer probe (lanes 1 and 2), and the DNA-protein complex was supershifted by adding antibodies against Flag (lane 4). An arrow indicates supershifted SOX9-DNA complexes. B: Electrophoretic mobility shift assay was performed using in vitro synthesized SOX9 with unlabeled wild-type or mutated probes as competitors. SOX9-DNA complex was competed out by an excess amount of wild-type probes but not by mutated probes in the GAACAAT motif.
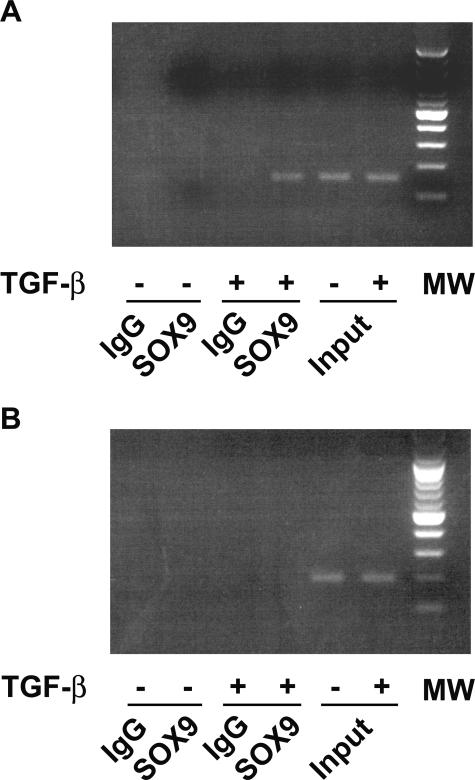
Next, we used ChIP assay to demonstrate that endogenous SOX9 binds to the Col4a2 enhancer in vivo. The anti-SOX9 antibody precipitated chromatin containing GAACAAT motif in Col4a2 enhancer in mesangial cells treated with TGF-β (Figure 4A). However, we could not find immunoprecipitated chromatin containing GAACAAT motif without TGF-β treatment. In contrast, upstream chromatin amplified with control primers were not seen in the same samples (Figure 4B), suggesting that chromatin amplified with GAACAAT primers was not the result of genomic DNA contamination.
Figure 4.
SOX9 interacts with the Col4a2 gene enhancer in vivo. A and B: Chromatin prepared from mesangial cells treated with TGF-β or vehicle was immunoprecipitated with IgG or SOX9 antibody as indicated. PCR was done with oligonucleotide pairs for two regions of the Col4a1 gene, one corresponding to the enhancer region (−4259 to −4100) containing the GAACAAT (enhancer with GAACAAT; A) and the other one corresponding to an upstream region (−7252 to −7058) (negative control; B). Ten microliters of sonicated and precleared chromatin was used as input DNA. MW, molecular weight.
SOX9 Activates Col4a2 Enhancer-Promoter Construct
Then we examined whether overexpression of SOX9 could induce transcriptional activation of the Col4a2 enhancer-promoter construct in mesangial cells and in Swiss/3T3 cells. The transcriptional activity of the Col4a2 enhancer-promoter construct was up-regulated when the cells were cotransfected with the SOX9 expression vector both in mesangial cells and Swiss/3T3 cells (Figure 5, A and B). Cotransfection of SOX9 resulted in a threefold increase in a relative β-galactosidase activity compared with that of mock vector. SOX9 overexpression increased the transcriptional activity of the mutant enhancer fragment 2 (mutation in the latter GAACAAT), although the increase was slightly smaller than that of wild type. However, SOX9 overexpression was unable to increase transcriptional activity of the mutant enhancer fragment1 (mutation in the former GAACAAT). Thus, transactivation by SOX9 would be mainly through the former GAACAAT fragment.
Figure 5.
SOX9 activated the enhancer-promoter reporter constructs. A: Activation of the enhancer-promoter reporter construct by SOX9 in mesangial cells. Mesangial cells were transfected with 1 μg of reporter construct with 100 ng of firefly luciferase under the control of cytomegalovirus promoter as an internal control. The average values of three independent experiments in triplicate are shown as fold increase compared with that of no enhancer. B: Activation of wild-type or mutant type enhancer-promoter reporter constructs by SOX9 in Swiss/3T3 cells. *P < 0.05 compared with control.
Involvement of SOX9 in Up-Regulation of Col4a2
To investigate the role of SOX9 in the up-regulation of Col4a2, we used stealth siRNA to reduce the expression of SOX9. Mesangial cells treated with 1 ng/ml TGF-β or control vehicle were harvested and analyzed by RT-PCR at 48 hours after transfection. SOX9 mRNA expression was induced by TGF-β treatment in cells transfected with control RNAi, and it was suppressed by transfection with siRNA against SOX9 (Figure 6A, a). In immunoblot analyses, SOX9 was markedly reduced in cells transfected with siRNA compared with that transfected with control RNAi in both TGF-β treated or nontreated cells (Figure 6A, b and c). As for Col4a2, TGF-β treatment increased the Col4a2 mRNA expression, and the expression was suppressed by knockdown of SOX9 in both TGF-β-treated and nontreated cells (Figure 6B, a). In immunoblot analyses, TGF-β treatment induced α2(IV) collagen expression in cells transfected with control RNAi, and the up-regulation was suppressed by knockdown of SOX9 (Figure 6, A, b, and B, b). These results indicate that endogeneous SOX9 is involved in the regulation of Col4a2 expression via both up-regulation by TGF-β and basal expression in mesangial cells. In addition, Col4a1 mRNA expression was also increased by TGF-β treatment, and the expression was suppressed by knockdown of SOX9 in both TGF-β-treated or nontreated cells in a similar way as Col4a2 (Figure 6B, c).
Figure 6.
Involvement of SOX9 in Col4a2 up-regulation in mesangial cells. A: SOX9 mRNA and protein was reduced by transfection of siRNA against SOX9 in cells treated or untreated with TGF-β. Gene expression of SOX9 was examined by quantitative RT-PCR using mRNA of mesangial cells transfected with siRNA against SOX9 or control RNAi (a). An immunoblot analysis of mesangial cells transfected with siRNA against SOX9 or control RNAi, treated with TGF-β or nontreated (b). Optical densitometry of SOX9 in immunoblotting (c). The average value of three independent experiments was shown. The results were presented as the fold increase or decrease compared with the values of cells transfected with control RNAi and nontreated cells. B: Col4a2 mRNA expression was suppressed by transfection of siRNA against SOX9 (a). Optical densitometry of α2(IV) collagen in immunoblotting (b). Col4a1 mRNA expression was also suppressed by transfection of siRNA against SOX9 (c). The average value of three independent experiments was shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05 compared with control.
SOX9 Expression in Mouse NTN
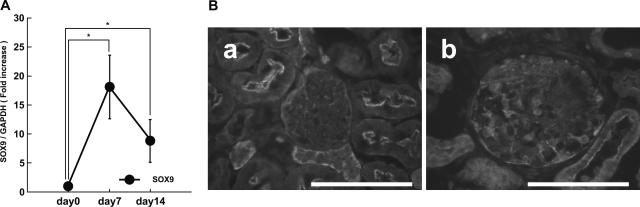
In the normal mouse kidney, the expression of SOX9 was quite low. We induced NTN to examine whether SOX9 is up-regulated along with Col4a1 and Col4a2 in mice. Renal expression of SOX9 was examined by quantitative RT-PCR and immunohistological analysis. Total RNA was prepared from the whole kidneys, and the expression of SOX9 mRNA 7 and 14 days after the induction of nephritis was examined. Figure 7A shows that SOX9 mRNA was significantly increased at days 7 and 14. Immunohistological analysis confirmed that SOX9 was expressed at a low level in the glomerulus before induction, and its expression was dramatically induced at day 7 (Figure 7B, a and b).
Figure 7.
Up-regulation of SOX9 in NTN. A: Mice were sacrificed before induction of NTN and at days 7 and 14 after induction of NTN. Gene expression of SOX9 was examined by quantitative RT-PCR using whole-kidney mRNA. The results were presented as the fold increase compared with the values obtained before the induction of NTN (day 0). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. B: Immunohistological analysis of SOX9 in NTN. Note that nuclear staining of SOX9 was seen in the glomeruli at day 7 (b), although SOX9 was hardly expressed in the glomeruli before induction (a). *P < 0.05 compared with control.
Expression of TGF-β, Col4a1, and Col4a2
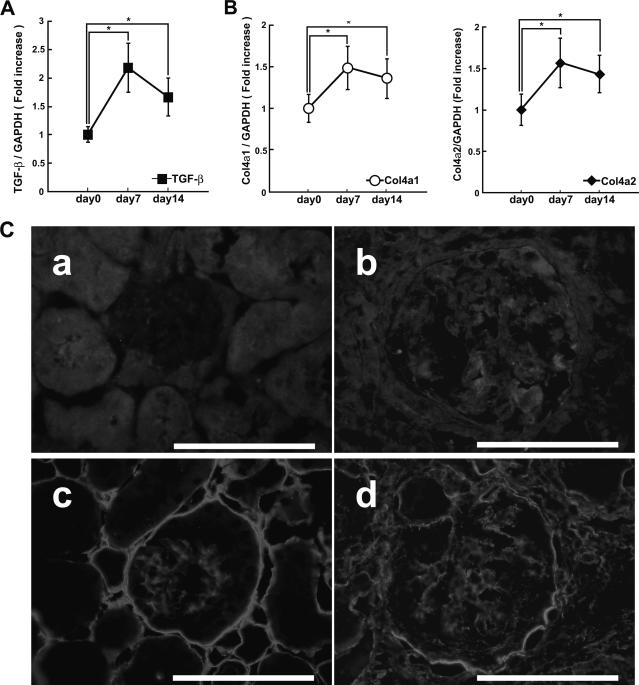
To analyze the development of glomerulosclerosis, we examined gene expression of TGF-β, Col4a1, and Col4a2 by quantitative RT-PCR. Quantitative RT-PCR showed that TGF-β, Col4a1, and Col4a2 mRNA were increased at days 7 and 14 (Figure 8, A and B). We noticed that SOX9, TGF-β, Col4a1, and Col4a2 were increased at days 7 and 14 in a parallel fashion. Immunohistological analysis revealed that TGF-β was hardly seen in the glomerulus before induction of NTN but was highly expressed in the glomerulus at day 7 (Figure 8C, a and b). α1 and α2(IV) Collagen expression was induced in the glomerulus at day 7 (Figure 8C, c and d). We also found extensive mesangial cell proliferation and accumulation of extracellular matrix at day 7, in addition to prominent epithelial crescent formation (Figure 9, a and b).
Figure 8.
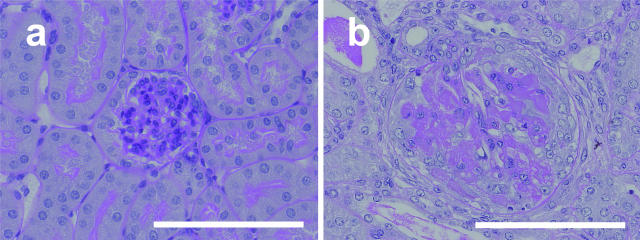
Expression of TGF-β, Col4a1, and Col4a2 in NTN. Gene expression of TGF-β (A) and Col4a1 and Col4a2 (B) was examined by quantitative RT-PCR using whole-kidney mRNA. The results were presented as the fold increase compared with the values obtained before the induction of NTN (day 0). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05 compared with control. C: Immunohistochemical findings of TGF-β (a and b) and α1 and α2(IV) collagen (c and d) at control (a and c) and day 7 (b and d). TGF-β was up-regulated in the glomeruli at day 7 (b), although it was hardly seen before induction (a). Accumulation of α1 and α2(IV) collagen was also observed at day 7 (d) compared with before induction (c). White scale bar = 100 μm. Original magnifications, ×400.
Figure 9.
Light microscopy. Periodic acid-Schiff methenamine-stained paraffin-embedded sections of mouse kidney before induction of NTN (a) and at day 7 (b) are shown. Increased mesangial cell number and extracellular matrix were seen in large glomeruli at day 7. White scale bar = 100 μm. Original magnifications, ×400.
Discussion
In this study, we demonstrate the role of SOX9 as a critical transcriptional regulator of Col4a2 in mesangial cells by showing that SOX9 bound to and activated the enhancer element with the GAACAAT motif in the Col4a2 gene and that SOX9 was involved in the Col4a2 expression induced by TGF-β treatment. Furthermore, we show that the expression of SOX9 was markedly increased in mouse NTN, suggesting that SOX9 is involved in the increase of Col4a2 in mesangial matrix hypertrophy rather than expression of Col4a2 in normal mouse glomerular basement membrane. Moreover, our study expands the list of collagen genes likely to be regulated by SOX9 other than cartilage collagen genes.
In the present study, Col4a1 and Col4a2 genes are regulated by SOX9 in a similar way, suggesting that SOX9 functions as a common transcription factor to regulate both genes. Moreover, SOX9 interacts with the critical GAACAAT motif in Col4a2 gene enhancer. It is likely that this motif is also critical for Col4a1 gene because Col4a1 and Col4a2 genes share enhancer and bidirectional promoter,8 although the orientation of transcription of these genes is opposite. Therefore, SOX9 would be involved in the coordinated expression of these genes through interacting with the GAACAAT motif.
The 0.3-kb enhancer element used in our study has two identical sequences of seven nucleotides, GAACAAT, and both elements are critical for active transcription in PYS-2 cells.14 Mutational analysis revealed that the former GAACAAT motif is critical in mesangial cells, but the latter is not. This difference could be explained by the difference of cell type. SOX9 activates the 0.3-kb element mainly through the former GAACAAT motif because SOX9 could not activate the enhancer element with mutations in the former GAACAAT motif. Although both motifs are in agreement with the SOX9-binding sequence, SOX9 activates transcription through the former motif preferably. SOX9 transactivates type XI collagen gene Col11α2 at chondrocyte-specific enhancer elements, but mutations in adjacent DNA sequences can eliminate the enhancer activity.31 SOX proteins need the associated factors to bind the sites adjacent to the SOX sites for stable complex formation with the target DNA and transactivation.31,32 Therefore, it seems that associated molecules of SOX9 can bind to the nearby sequences of the former but not the latter GAACAAT site. Moreover, we found that overexpression of SOX9 increased the Col4a2 enhancer-promoter activity in mesangial cells, Swiss/3T3 cells, and COS-7 cells but not in HEK-293T cells (data not shown). This result also suggests that SOX9 might activate the Col4a2 enhancer-promoter activity in concert with particular associated factors and that it may be present in myofibroblasts and fibroblasts, but not in epithelial cells. SOX9 functions as a dimer when activating cartilage collagen genes, Col2a1, Col11a2, Col9a2, and Col27a1, through paired SOX-binding sites arranged in opposite orientation facing each other.24,31,33,34,35 However, Col4a2 enhancer element does not contain SOX-binding site around GAACAAT motif, suggesting that SOX9 transactivates the Col4a2 gene as a monomer, as CD-RAP36 or anti-Müllerian hormone promoter.37
Apart from the 0.3-kb element located 4.2 kb upstream of Col4a2 gene,14 which we found to be target of SOX9, another 0.3-kb element located 3.3 kb upstream of Col4a2 gene is reported.12 We first prepared both 0.3-kb enhancer elements and examined the transcriptional activities in mesangial cells by transient transfection and found that the element located 3.3 kb upstream was only one-half as active as the element located 4.2 kb upstream (data not shown). Moreover, we demonstrated that the element located 4.2 kb upstream was responsible for the up-regulation of Col4a2 by TGF-β administration. Considering that TGF-β is a potent fibrogenic cytokine and is involved in glomerulosclerosis, the element located 4.2 kb upstream is more critical for Col4a2 accumulation.
Our results from knockdown of SOX9 suggest that SOX9 is involved in basal Col4a2 expression as well as TGF-β-induced expression in mesangial cells. However, ChIP assay shows that endogenous SOX9 is associated with the enhancer only when mesangial cells are treated with TGF-β. TGF-β is known to regulate transcription through recruitment of histone deacetylases.38 A possible explanation is that chromatin structure is modified by administration of TGF-β.
Although mRNA expression of SOX9 markedly increased at day 7, mRNA expression of Col4a2 at day 7 was less than twofold compared with that at day 0 in nephrotoxic nephritis. This could be because Col4a2 is already present in normal mouse mesangium. On the other hand, because SOX9 mRNA expression in normal mouse kidney was quite low, only a small induction leads to a marked increase of SOX9 mRNA at day 7 in RT-PCR analysis.
In summary, we show that the critical enhancer element of the Col4a2 gene is a target for SOX9. Our results indicate that TGF-β up-regulates SOX9 expression, which enhances transcription of the Col4a2 gene.
Acknowledgments
We thank Hideo Uchiyama (Taigenkai Hospital, Gifu, Japan), Ayumi Hosotani, and Maki Watanabe (Kyoto University) for excellent technical assistance.
Footnotes
Address reprint requests to Hidenori Arai, M.D., Ph.D., Department of Geriatric Medicine, Kyoto University Graduate School of Medicine, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan. E-mail: harai@kuhp.kyoto-u.ac.jp.
Supported by the grants-in-aid from the Ministry of Education, Culture, Science, Sports, and Technology of Japan; by grants from the Japanese Ministry of Health, Labor, and Welfare; by a grant from Takeda Science Medical Research Foundation; and by a research grant from the National Institutes of Health (DK30932 to D.J.S.).
References
- Peten EP, Striker LJ, Carome MA, Elliott SJ, Yang CW, Striker GE. The contribution of increased collagen synthesis to human glomerulosclerosis: a quantitative analysis of alpha 2IV collagen mRNA expression by competitive polymerase chain reaction. J Exp Med. 1992;176:1571–1576. doi: 10.1084/jem.176.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer G, Phan SH, Wiggins RC. Analysis of renal fibrosis in a rabbit model of crescentic nephritis. J Clin Invest. 1988;82:998–1006. doi: 10.1172/JCI113710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J, Johnson RJ, Gordon K, Iida H, Pritzl P, Yoshimura A, Campbell C, Alpers CE, Couser WG. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int. 1991;40:477–488. doi: 10.1038/ki.1991.235. [DOI] [PubMed] [Google Scholar]
- Striker GE, He CJ, Liu ZH, Yang DC, Zalups RK, Esposito C, Striker LJ. Pathogenesis of nonimmune glomerulosclerosis: studies in animals and potential applications to humans. Lab Invest. 1995;73:596–605. [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Poncelet AC, Schnaper HW. Regulation of human mesangial cell collagen expression by transforming growth factor-beta1. Am J Physiol. 1998;275:F458–F466. doi: 10.1152/ajprenal.1998.275.3.F458. [DOI] [PubMed] [Google Scholar]
- Burbelo PD, Martin GR, Yamada Y. Alpha 1(IV) and alpha 2(IV) collagen genes are regulated by a bidirectional promoter and a shared enhancer. Proc Natl Acad Sci USA. 1988;85:9679–9682. doi: 10.1073/pnas.85.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöschl E, Pollner R, Kuhn K. The genes for the alpha 1(IV) and alpha 2(IV) chains of human basement membrane collagen type IV are arranged head-to-head and separated by a bidirectional promoter of unique structure. EMBO J. 1988;7:2687–2695. doi: 10.1002/j.1460-2075.1988.tb03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen R, Huotari M, Hostikka SL, Prockop DJ, Tryggvason K. The structural genes for alpha 1 and alpha 2 chains of human type IV collagen are divergently encoded on opposite DNA strands and have an overlapping promoter region. J Biol Chem. 1988;263:17217–17220. [PubMed] [Google Scholar]
- Kaytes P, Wood L, Theriault N, Kurkinen M, Vogeli G. Head-to-head arrangement of murine type IV collagen genes. J Biol Chem. 1988;263:19274–19277. [PubMed] [Google Scholar]
- Burbelo PD, Bruggeman LA, Gabriel GC, Klotman PE, Yamada Y. Characterization of a cis-acting element required for efficient transcriptional activation of the collagen IV enhancer. J Biol Chem. 1991;266:22297–22302. [PubMed] [Google Scholar]
- Killen PD, Burbelo PD, Martin GR, Yamada Y. Characterization of the promoter for the alpha 1 (IV) collagen gene. DNA sequences within the first intron enhance transcription. J Biol Chem. 1988;263:12310–12314. [PubMed] [Google Scholar]
- Tanaka S, Kaytes P, Kurkinen M. An enhancer for transcription of collagen IV genes is activated by F9 cell differentiation. J Biol Chem. 1993;268:8862–8870. [PubMed] [Google Scholar]
- Laudet V, Stehelin D, Clevers H. Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res. 1993;21:2493–2501. doi: 10.1093/nar/21.10.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking BM, Muscat GE, Koopman PA, Dowhan DH, Dunn TL. Trans-activation and DNA-binding properties of the transcription factor, Sox-18. Nucleic Acids Res. 1995;23:2626–2628. doi: 10.1093/nar/23.14.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny P, Swift S, Connor F, Ashworth A. An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein. EMBO J. 1992;11:3705–3712. doi: 10.1002/j.1460-2075.1992.tb05455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227:239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- Wright E, Hargrave MR, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater LC, Lefebvre V, de Crombrugghe B. Chondrocyte-specific enhancer elements in the Col11a2 gene resemble the Col2a1 tissue-specific enhancer. J Biol Chem. 1998;273:14998–15006. doi: 10.1074/jbc.273.24.14998. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, Nifuji A, Noda M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem. 2000;275:10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham PS, Csizmadia V, Soler D, Hines D, Gerard CJ, Salant DJ, Hancock WW. Lack of chemokine receptor CCR1 enhances Th1 responses and glomerular injury during nephrotoxic nephritis. J Clin Invest. 1999;104:1549–1557. doi: 10.1172/JCI7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Arai H, Yanagita M, Matsubara T, Kanamori H, Nakano T, Iehara N, Fukatsu A, Kita T, Doi T. Growth arrest-specific gene 6 is involved in glomerular hypertrophy in the early stage of diabetic nephropathy. J Biol Chem. 2003;278:18229–18234. doi: 10.1074/jbc.M213266200. [DOI] [PubMed] [Google Scholar]
- Davies M. The mesangial cell: a tissue culture view. Kidney Int. 1994;45:320–327. doi: 10.1038/ki.1994.41. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Ono K, Morimoto T, Wada H, Hirai M, Hidaka K, Morisaki T, Heike T, Nakahata T, Kita T, Hasegawa K. Acetylation of GATA-4 is involved in the differentiation of embryonic stem cells into cardiac myocytes. J Biol Chem. 2005;280:19682–19688. doi: 10.1074/jbc.M412428200. [DOI] [PubMed] [Google Scholar]
- Bridgewater LC, Walker MD, Miller GC, Ellison TA, Holsinger LD, Potter JL, Jackson TL, Chen RK, Winkel VL, Zhang Z, McKinney S, de Crombrugghe B. Adjacent DNA sequences modulate Sox9 transcriptional activation at paired Sox sites in three chondrocyte-specific enhancer elements. Nucleic Acids Res. 2003;31:1541–1553. doi: 10.1093/nar/gkg230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Cheah KS, Kondoh H. Mechanism of regulatory target selection by the SOX high-mobility-group domain proteins as revealed by comparison of SOX1/2/3 and SOX9. Mol Cell Biol. 1999;19:107–120. doi: 10.1128/mcb.19.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P, Tang P, Liu S, Dewing P, Harley VR, Vilain E. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum Mol Genet. 2003;12:1755–1765. doi: 10.1093/hmg/ddg182. [DOI] [PubMed] [Google Scholar]
- Zhou G, Lefebvre V, Zhang Z, Eberspaecher H, de Crombrugghe B. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J Biol Chem. 1998;273:14989–14997. doi: 10.1074/jbc.273.24.14989. [DOI] [PubMed] [Google Scholar]
- Jenkins E, Moss JB, Pace JM, Bridgewater LC. The new collagen gene COL27A1 contains SOX9-responsive enhancer elements. Matrix Biol. 2005;24:177–184. doi: 10.1016/j.matbio.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WF, Zhang X, Sakano S, Lefebvre V, Sandell LJ. Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by Sox9. J Bone Miner Res. 1999;14:757–763. doi: 10.1359/jbmr.1999.14.5.757. [DOI] [PubMed] [Google Scholar]
- De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol Cell Biol. 1998;18:6653–6665. doi: 10.1128/mcb.18.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;24:2543–2555. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]