Abstract
Although angiotensin II (Ang II) type 1 receptor antagonist ameliorates proteinuria, its pharmacological mechanism and the differential roles of Ang II type 1 receptor (AT1R) and type 2 receptor (AT2R) are not well understood. We analyzed the effect of Ang II type 1 receptor antagonist on proteinuria caused by antibody against nephrin, a functional molecule of glomerular slit diaphragm and dysfunction of which is involved in the development of proteinuria in several glomerular diseases. We show here that AT1R antagonist ameliorated proteinuria by preventing a reduction in the functional molecules of the slit diaphragm. We also analyzed the role of AT1R- or AT2R-mediated actions on the expression of the slit diaphragm molecules in an in vivo study of normal rat and in an in vitro study of cultured podocytes. AT1R-mediated action hampered the mRNA expression of the slit diaphragm molecules, whereas AT2R-mediated action enhanced it. These findings indicate that Ang II receptor subtypes play opposite roles in regulating the barrier function of glomerular capillary wall and that the enhancement of AT2R stimulation may serve as a potential therapeutic strategy for proteinuria.
Angiotensin II (Ang II) plays an essential role in maintenance of the vascular homeostasis and of several cellular and tissue functions in the physiological state. It is also understood that an excessive Ang II action contributes to the pathogenesis of hypertension, heart failure, renal diseases, and several other diseases. Ang II action is explained as being based on blood pressure-dependent and -independent mechanisms. Some recent studies focused on the significance of the pressure-independent mechanism of Ang II action.1,2 However, the tissue-specific function of local Ang II is not well understood. Although some reports3,4,5,6 have suggested that Ang II type 1 receptor (AT1R) and type 2 receptor (AT2R) exhibit opposite functions, this is still controversial.
A number of clinical7,8,9,10 and experimental11,12,13,14,15 studies have demonstrated that angiotensin-converting enzyme inhibitor and AT1R antagonist reduce renal tissue damage and proteinuria. However, their pharmacological mechanism is not well understood. In the nephrology field, clarification of the mechanism of proteinuria is one of the most important themes. Filtration barrier of the kidney glomerulus preventing the leak of plasma proteins into primary urine comprises three layers16: the endothelial cells, the glomerular basement membrane, and the visceral epithelial cells (podocytes). Although the function of the glomerular basement membrane has been emphasized during the past 3 decades, it is recently becoming accepted that slit diaphragm located between adjacent foot processes of podocytes functions as the final barrier of the glomerular capillary wall.17 Recent studies show that the dysfunction of the slit diaphragm is involved in the development of proteinuria in several common diseases such as minimal change type nephrotic syndrome and membranous nephropathy.18 Investigations of the role of Ang II action in regulating the barrier function of the slit diaphragm will surely lead to understanding better the mechanism of proteinuria.
In the present study, we first analyzed the expression of AT1R and AT2R in anti-nephrin antibody (ANA)-induced nephropathy of which proteinuria was caused by down-regulation of the slit diaphragm functional molecules. Then, we investigated the effect of the AT1R antagonist on proteinuria in this model. We demonstrate that both AT1R and AT2R were expressed on the podocyte and that their expressions were clearly elevated in the proteinuric state. We show that the AT1R antagonist ameliorates the peak level of proteinuria by preventing a reduction in the expression of slit diaphragm functional molecules. In this study, we also investigated the selective functions of AT1R- and AT2R-mediated actions in vivo and in vitro. We show that AT1R-mediated action reduces the mRNA expression of the slit diaphragm-associated molecules and that AT2R-mediated action enhances that of these molecules. This is the first report directly demonstrating that AT1R action hampers and AT2R action enhances the expression of the functional molecules that are directly involved in the development of proteinuria, a major clinical symptom.
Materials and Methods
Animals and Cultured Podocytes
All in vivo experiments were performed using specific pathogen-free female Brown Norway rats weighing 100 to 110 g at the age of 7 to 8 weeks. All rats used in this study were purchased from Charles River Japan (Atsugi, Japan) and fed with normal salt diet. All animal experiments conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Cultivation of conditionally immortalized mouse podocytes kindly donated by Dr. P. Mundel (Albert Einstein College of Medicine, Bronx, NY) was conducted as reported previously.19 The differentiated cells showing the prominent processes and mRNA expression of the differentiated molecules nephrin and podocin were used for the experiments.
Experimental Protocols
Experimental protocols for in vivo studies are shown in Figure 1.
Figure 1.
Experimental protocols of in vivo studies. In experiment 1, rats were intravenously injected with ANA, and the kidneys were removed just before the injection and 1 hour and 24 hours and 3, 5, 8, 11, and 15 days after the injection. In experiment 2, rats were injected with ANA and treated with AT1R antagonist, hydralazine, or AT2R antagonist, and the kidneys were assessed on days 5 and 15. In experiment 3, rats were continuously injected with Ang II or AT2R agonist by osmotic pump, and kidneys were assessed at 24 and 72 hours.
Experiment 1
To analyze the kinetics of podocyte-associated molecules and Ang II receptors, a total of 40 rats were intravenously injected with 10 mg of anti-nephrin monoclonal antibody prepared as described previously,20 and five rats each were sacrificed just before the injection and at 1 and 24 hours, and 3, 5, 8, 11, and 15 days after the injection. The control group consisted of five rats, which were injected with 10 mg of irrelevant IgG1 (RVG1; mouse anti-rotavirus IgG1) and were sacrificed 15 days after the injection. After blood sampling, the kidneys were removed, weighed, cut into portions, and used in immunofluorescence studies. Glomerular RNA was prepared from the remaining kidney tissues pooled from five rats in each group for reverse transcriptase-polymerase chain reaction (RT-PCR). Twenty-four-hour urine samples were collected, and their protein concentrations were measured as described previously.21 Plasma Ang II concentration, total protein, total serum cholesterol, triglyceride, serum creatinine (SCr), blood urea nitrogen, and urinary creatinine (UCr) levels were measured. Plasma Ang II concentration was determined with radioimmunoassay. The 24-hour endogenous creatinine clearance (CCr) was calculated using the following formula: CCr [ml/minute per 100 g body weight (BW)] = UCr (mg/dl) × 24-hour urine volume (ml) × 1/SCr (mg/dl) × 1/1440 (minute) × 1/BW (g) × 100. The blood pressure (BP) was measured using a tail-cuff pressure analysis system (BP-98A; Softron, Tokyo, Japan). Mean BP (MBP) was calculated using the following formula using the data of diastolic BP (DBP) and systolic BP (SBP): MBP = DBP + (SBP-DBP)/3.
Five rats each were injected with ANA or phosphate-buffered saline, and the kidneys were removed on day 8 after the injection. Glomeruli isolated from the kidneys were solubilized, and the glomerular lysate was used for Western blot analysis. Western blot analysis was performed basically according to the method previously reported.21 In brief, glomeruli were solubilized with radioimmunoprecipitation assay buffer (0.1% sodium dodecyl sulfate, 1% sodium deoxycholate, 1% Triton X-100, 150 mmol/L NaCl, and 10 mmol/L ethylenediaminetetraacetic acid in 25 mmol/L Tris-HCl, pH 7.2) with protease inhibitors. The solubilized material was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 7.5% acrylamide gel and transferred to a polyvinylidene fluoride transfer membrane (Pall Corp., Pensacola, FL). After blocking with bovine skim milk, the strips of membrane were exposed to rabbit anti-rat nephrin, rabbit anti-rat podocin, or anti-rat β-actin antibodies. They were then washed and incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG. The reaction was developed with an alkaline phosphatase chromogen kit (Biomedica, Foster City, CA). The intensity of the corresponding band was determined by image analysis using the Bio Doc-It System (UVP, Inc., Upland, CA). All results were corrected for the amount of protein in the sample by dividing by the intensity of the internal control β-actin. This procedure was performed three times. The percentage of the total amount of each molecule in ANA nephropathy compared with the control group is shown in bar graphs as mean ± SD.
Experiment 2
To analyze the effect of AT1R antagonist on proteinuria and on the expression of slit diaphragm-associated molecules in ANA-induced nephropathy, a total of 28 rats were injected with ANA. AT1R antagonist (candesartan, CV11974, 5 mg/kg per day, n = 14; Takeda Chemical Industries, Osaka, Japan) was orally administered every day by a gavage from day 0 just after the induction of disease. For the control group, a vehicle (distilled water) (n = 14) was administered. The BP was measured as described above. Seven rats from each group were sacrificed on days 5 and 15. Their blood was sampled, and cryostat sections for the immunofluorescence study and total RNA for RT-PCR were prepared as described previously.22,23 The amount of urinary protein excretion/day was determined as described above. Glomerular immunofluorescent staining for nephrin was semiquantitatively graded on the kidney sections of each rat on day 15 in ANA-induced nephropathy using the following scale: grade 0: markedly discontinuous distribution of nephrin, no linear staining; grade 1: heterogeneous distribution of nephrin along the glomerular capillary loop, linear staining on less than 50% of the total glomerular capillary loop; grade 2: heterogeneous distribution of nephrin along the glomerular capillary loop, linear staining on 50 to 90% of the total glomerular capillary loop; grade 3: almost normal, continuous distribution along the glomerular capillary loop. Next, to analyze the effect of hydralazine on proteinuria and on the expression of slit diaphragm-associated molecules in ANA-induced nephropathy, a total of 20 rats were injected with ANA, and hydralazine (5 mg/kg per day, n = 10; Nihon Ciba-Geigy, Hyogo, Japan) or a vehicle (distilled water) (n = 10) was orally administered every day. Five rats from each group were sacrificed on days 5 and 15. Next, to analyze the effect of AT2 receptor antagonist on proteinuria in ANA-induced nephropathy, a total of 10 rats were injected with ANA, and the rats were administered with AT2R antagonist (PD123319, 30 mg/kg per day; Sigma Aldrich Chemie, Steinheim, Germany) or vehicle (distilled water) with the Alzet osmotic pump (Durect Corp., Cupertino, CA) for 15 days. Then, the kinetics of proteinuria were analyzed.
Experiment 3
To analyze the effect of Ang II on the mRNA expression of slit diaphragm-associated molecules in rats, the Ang II (200 ng/kg per minute; Sigma Aldrich Chemie) was administered to normal rats for 24 or 72 hours with the Alzet osmotic pump. As control, vehicle (distilled water) was administered. Then, five rats for each group were sacrificed at 24 hours and 72 hours, and the kidneys were used for RT-PCR and morphological analyses. Urine samples were collected for 24 hours just after the start of the continuous injection, and protein concentrations were measured as described previously,21 and albumin concentration was measured by immunological method. To analyze the effect of Ang II on the expression of nephrin and podocin in protein level, five rats each were continuously injected with Ang II or distilled water for 24 hours as described above, and kidneys were removed. Glomeruli isolated from kidneys were solubilized, and the glomerular lysates were used for Western blot analysis. Procedures for Western blot analysis were described above. Next, to analyze the effect of AT2R agonist on the mRNA expression of slit diaphragm-associated molecules in rats, AT2R agonist (CGP42112A, 0.55 ng/kg per minute; Sigma Aldrich Chemie) or vehicle (distilled water) was administered to normal rats for 24 hours and 72 hours as described above. Then, five rats for each group were sacrificed at 24 hours and 72 hours, and the kidneys were used for RT-PCR and morphological analyses.
Experimental 4
To examine the expression of the AT1 and AT2 receptors in the cultured podocytes, we first performed immunofluorescence studies with specific antibodies. To analyze the effect of Ang II on the expression of nephrin in the cultured podocytes, the cells were treated with 1 × 10−8 mol/L Ang II for 1 hour in the presence or absence of AT1R antagonist (CV11974, 1 × 10−6 mol/L) or AT2R antagonist (PD123319, 1 × 10−6 mol/L). Then, to analyze the effect of the AT2R agonist, the cultured podocytes were treated with CGP42112A (1 × 10−9 mol/L or 1 × 10−8 mol/L) for 1 hour in the presence or absence of AT2R antagonist (PD123319, 1 × 10−6 mol/L or 1 × 10−5 mol/L). The cells were harvested for RT-PCR study. The experiments were repeated at least three times. To analyze further the effect of Ang II and AT2R agonist, the cultured podocytes were treated with Ang II or CGP42112A as described above and were harvested for real-time RT-PCR analyses. The experiments for real-time RT-PCR were repeated three times.
RT-PCR
Semiquantitative RT-PCR with RNA of glomeruli and cultured podocytes were performed basically according to the method described previously.22,23 In brief, glomeruli were isolated from pooled kidneys by differential sieving through mesh brass sieves (nos. 60, 150, and 200). The total RNA was extracted from isolated glomeruli by TRIzol (Life Technologies, Inc., Gaithersburg, MD). For the quantification of PCR product, all samples including control were run on the same gel, and the band intensity was determined by image analysis using BioDoc-It System (UVP Inc.). The ratio of the densitometric signal of the molecules examined to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was determined. The data are shown as ratios relative to control findings and expressed as means ± SD of the results of three independent experiments. Real-time RT-PCR was performed with a Smart Cycler System (Takara, Otsu, Japan) basically according to the manufacturer’s manual. The sequences of the primers are described in Table 1. The template cDNAs were amplified in a 25-μl total volume of PCR buffer containing 2.5 μl of 10× SPL buffer; 0.00084 μl of SYBR Green I; 3 mmol/L MgCl2; 0.3 mmol/L each dATP, dCTP, dGTP, and dTTP; 0.3 μmol/L of each of the 5′ and 3′ primers; and 1.25 U of Takara Ex Taq for SC. Two-step PCRs were run on a Smart Cycler System I (Takara) of denaturation at 95°C for 3 seconds, annealing at individual temperatures for 30 seconds. PCR products of AT1R, AT2R, nephrin, and GAPDH were subcloned into the plasmid vector pCR II-TOPO (TOPO TA cloning kit dual promoter; Invitrogen, Carlsbad, CA). The plasmid DNAs subcloned with the PCR products were isolated and purified with a plasmid midi kit (Qiagen, Hilden, Germany). The concentration of each plasmid was determined by measurements of the absorbance at 260 nm, and its copy number was calculated. To generate the calibration curves, the stock solutions were diluted from 104 to 109 copies/μl. The fluorescence intensity of 400 nmol/L 5-carboxyfluorescein (carboxyfluorescein) was defined as 1000 U, and the threshold was set at 30 U of fluorescence intensity. The Ct value was defined as the cycle number at which the fluorescence intensity curve crosses this threshold. The calibration curve was plotted as Ct (x axis) versus the logarithm of the starting copy number (y axis). The copy numbers are shown as means ± SD of the results of three independent experiments.
Table 1.
PCR Primers Used in This Study
| Probe | Sense primer, 5′ to 3′ | Anti-sense primer, 5′ to 3′ | Temperature (°C) | Size (bp) | Reference or accession no. |
|---|---|---|---|---|---|
| Nephrin* | CTG ACT GGG CTG AAG CCT TCT | AAG AGC ACA GGC AGC AGG GG | 58 | 203 | Ref. 23 |
| Podocin* | CCT GTG AGT GGC TTC TTG TCC TC | GGA GAC GCT TCA TAG TGG TTT GCA | 58 | 378 | Ref. 23 |
| ZO-1 | TTC CAA AGA CAG CAG GTG GTG AT | CCA GTT CCA GCA TCT CGT GGT T | 58 | 277 | D14340 |
| Podocalyxin* | AGC GAC AAA CCA GCC AAG CAA TG | TGG TGA GGG CTT GCT GTG CTA T | 58 | 351 | AF109393 |
| AT1 receptor | GGA AAC AGC TTG GTG GTG ATT G | CCA ATG GGG AGT GTT GAG TTC | 58 | 450 | M87003 |
| AT2 receptor | CCG GCA GAT AAG CAT TTG GAA G | GAA TGC CAA CAC AAC AGC AGC | 58 | 680 | D16840 |
| Nephrin† | AGC TGT GGA ATG TAA CCC GAG C | TGG GGG GCA AAT CGG ACG ACA AG | 61 | 404 | AY183460 |
| Podocin† | CCT GCG AGT GGC TTC TTG TCC TC | AGA GGC GCT TCA TGG TGG TTT GCA | 58 | 378 | AY050309 |
| GAPDH | CTC TAC CCA CGG CAA GTT CAA | GGA TGA CCT TGC CCA CAG C | 60 | 515 | Ref. 48 |
Primer for rat.
Primer for mouse, others; for rat and mouse.
Immunofluorescence Microscopy
Immunofluorescence studies were performed basically according to the method previously reported.21 Antibodies used in this study are as follows: mouse anti-rat nephrin monoclonal antibody,20 rabbit anti-rat nephrin antibody,21 rabbit anti-rat podocin antibody,23 rabbit anti-AT1R antibody (sc-1173; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit anti-AT2R antibody (sc-9040; Santa Cruz Biotechnology, Inc.), mouse anti-podocalyxin antibody (4D5),24 mouse anti-synaptopodin antibody (Progen, Heidelberg, Germany), goat anti-WT1 antibody (sc-15421; Santa Cruz Biotechnology), mouse anti-RECA1 antibody (Serotec, Oxford, UK), and mouse anti-rat Thy1.1 antibody.25,26
Statistical Analysis
Statistical significance was evaluated using the unpaired t-test, the Mann-Whitney U-test, or the one-way analysis of variance. Values were expressed as the mean ± SD. Differences at P < 0.05 were considered significant. Data were analyzed using StatView for Windows (Abacus Concepts, Berkeley, CA).
Results
Experiment 1: The Analyses of Slit Diaphragm-Associated Molecules and the RAS Parameters in ANA-Induced Nephropathy
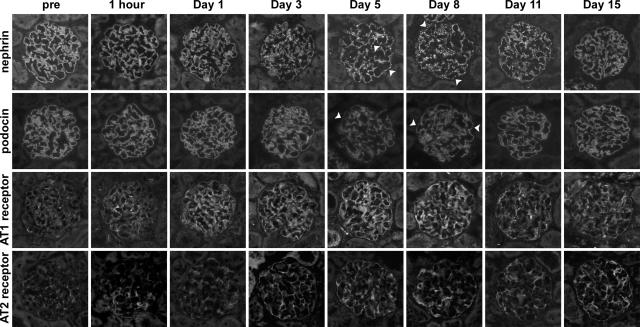
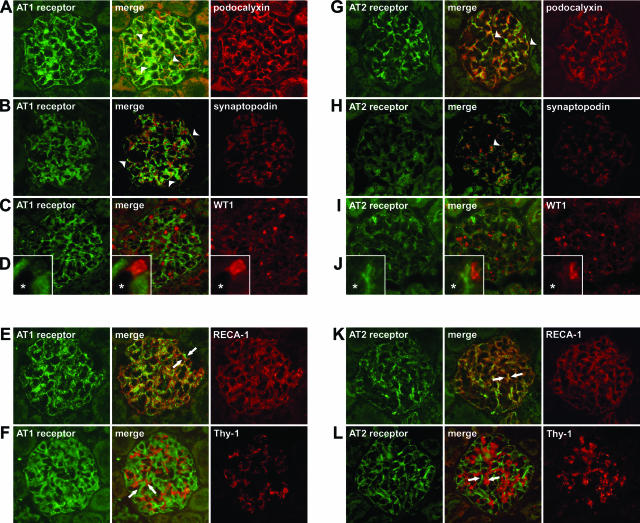
The kinetics of proteinuria caused by anti-nephrin monoclonal antibody is shown in Figure 2A. Figure 2, B and C, shows the kinetics of glomerular mRNA. The expression of the slit diaphragm-associated molecules, nephrin, podocin, and ZO-1 was altered in ANA-induced nephropathy, whereas that of podocalyxin was not. The glomerular expression of AT1R was already increased at 1 hour, and the increased level was maintained throughout the experimental period. AT2R expression also increased during the experimental period. Real-time RT-PCR techniques also demonstrated that the expression of AT1R and AT2R on day 8 had increased (Figure 2D). The copy number of the mRNA for AT1R increased to 276%, and that of the mRNA for AT2R increased to 1147%. The proportion of the copy number of AT2R to that of AT1R was 8.9% in normal glomeruli, and it increased to 37.2% on day 8 of ANA-induced nephropathy. The amount of immunoreactive nephrin and podocin was clearly reduced in the proteinuric state (Figure 2E). The stainings of nephrin and podocin shifted from a linear-like pattern to a discontinuous pattern, and the intensity of staining decreased in the proteinuric state (Figure 3). The staining of AT1R and AT2R were slight in normal glomeruli, in the form of a dot-like pattern along the glomerular capillary loops. The intensity of the stainings of AT1R and AT2R gradually increased with increasing proteinuria. Double-labeling studies with podocyte markers, podocalyxin, synaptopodin, and WT1 demonstrated that the glomerular AT1R and AT2R were expressed mainly in the podocytes (Figure 4). The laboratory findings are summarized in Table 2. The systolic BP increased on day 5 in ANA-induced nephropathy, if compared with the age-matched RVG1 control group. The data of BP levels are summarized in Table 3.
Figure 2.
The kinetics of proteinuria and the glomerular mRNA expression of slit diaphragm-associated molecules and Ang II receptors in ANA-induced nephropathy. A: The kinetics of proteinuria in ANA-induced nephropathy. B: Characteristic agarose-gel electrophoretic patterns of semiquantitative RT-PCR. C: The ratios of the densitometric signal of slit-diaphragm-associated molecules and Ang II receptors to that of GAPDH. The data are shown as ratios relative to the prefindings. Decreased mRNA expression of nephrin, podocin, and ZO-1 was observed. Both AT1R and AT2R expression increased. D: Real-time RT-PCR findings of AT1R and AT2R on day 8. The copy number of the mRNA for AT1R increased to 276% and that of AT2R increased to 1147%. The ratio of the copy number of AT2R to that of AT1R was 8.9% in normal glomeruli, whereas it increased to 37.2% on day 8 of ANA-induced nephropathy. E: Western blot finding of nephrin and podocin on day 8. The amounts of immunoreactive nephrin and podocin were clearly reduced at the peak of proteinuria.
Figure 3.
The kinetics of nephrin, podocin, AT1R, and AT2R in ANA-induced nephropathy. The stainings of nephrin and podocin in normal glomeruli presented a linear pattern, with continuous fine granules (prefindings). However, the stainings shifted to a discontinuous pattern in the proteinuric state (arrowheads). AT1R and AT2R are scantly expressed along the capillary loop in normal glomeruli, and its expression increased with increasing proteinuria.
Figure 4.
The localization of AT1R and AT2R in the glomeruli. Dual-labeling immunofluorescence studies with glomerular cell markers were performed on kidney sections on day 5 of ANA-induced nephropathy. Both AT1R and AT2R were localized in the podocytes of proteinuric-state kidneys, as shown by double-labeling with podocyte cell surface marker podocalyxin (A, G), podocyte cytoplasmic marker synaptopodin (B, H), and podocyte nuclear marker WT1 [C, I, (D, J, enlargement of the capillary area; *lumen)]. Staining of AT1R and AT2R were detected very closely to podocalyxin staining (A, G; arrowheads). AT1R and AT2R stained closely to synaptopodin, a podocyte cytoplasmic marker, but staining was detected slightly offset from the synaptopodin staining (B, H; arrowheads). AT1R and AT2R were detected at the cells whose nuclei were positive for anti-WT1 staining (C, D, I, J). Both AT1R and AT2R were observed on the outside of endothelial cells, as shown by double-labeling with RECA-1 (E, K; arrows). Neither AT1R nor AT2R localized in the mesangial area, as shown by double-labeling with Thy-1.1 (F, L; arrows).
Table 2.
The Laboratory Findings in Anti-Nephrin Antibody-Induced Nephropathy
| Pre | 1 Hour | Day 1 | Day 3 | |
|---|---|---|---|---|
| Angiotensin II* (pg/ml) | 43.0 ± 5.9 | 32.4 ± 12.6 | 69.2 ± 22.5 | 57.8 ± 21.2 |
| Total protein (g/dl) | 5.4 ± 0.1 | 5.2 ± 0.4 | 5.6 ± 0.2 | 4.7 ± 0.2§ |
| Total cholesterol (mg/dl) | 55.8 ± 4.5 | 56.2 ± 2.3 | 64.4 ± 3.1† | 81.8 ± 3.7¶ |
| Triglyceride (mg/dl) | 30.3 ± 5.5 | 28.8 ± 1.9 | 32.4 ± 8.6 | 48.6 ± 17.3 |
| Serum creatinine (mg/dl) | 0.33 ± 0.02 | 0.26 ± 0.02‡ | 0.25 ± 0.03‡ | 0.27 ± 0.02‡ |
| BUN (mg/dl) | 34.2 ± 2.8 | 31.0 ± 2.6 | 45.6 ± 5.2‡ | 38.2 ± 2.8 |
| Ccr (ml/minute/g BW) | 0.51 ± 0.06 | 0.52 ± 0.08 | 0.64 ± 0.08† | 0.49 ± 0.11 |
Plasma angiotensin II concentration.
Mean ± SD (†P < 0.05, ‡P < 0.01, §P < 0.001, ¶P < 0.0001, versus pre).
(table continues)
Table 2.
Continued
| Day 5 | Day 8 | Day 11 | Day 15 |
|---|---|---|---|
| 58.0 ± 19.7 | 40.8 ± 11.1 | 37.0 ± 8.9 | 26.4 ± 4.2‡ |
| 5.1 ± 0.1‡ | 4.6 ± 0.4‡ | 5.6 ± 0.3 | 5.6 ± 0.2 |
| 97.4 ± 5.7¶ | 105.2 ± 16.9§ | 152.8 ± 25.6§ | 77.4 ± 9.5‡ |
| 53.8 ± 16.4† | 56.4 ± 18.9† | 103.0 ± 43.2† | 37.2 ± 11.0 |
| 0.27 ± 0.01§ | 0.29 ± 0.03 | 0.27 ± 0.04† | 0.29 ± 0.02† |
| 43.0 ± 5.6† | 36.5 ± 4.1 | 39.5 ± 4.3 | 27.8 ± 4.4† |
| 0.60 ± 0.12 | 0.54 ± 0.06 | 0.59 ± 0.20 | 0.53 ± 0.05 |
Table 3.
The Kinetics of Blood Pressure in Anti-Nephrin Antibody (ANA)-Induced Nephropathy
| Pre | Day 3 | Day 5 | Day 13 | |
|---|---|---|---|---|
| Systolic blood pressure | ||||
| ANA nephropathy (n = 7) (mm Hg) | 115.8 ± 5.7 | 116.3 ± 8.0 | 126.9 ± 8.7* | 118.3 ± 7.8 |
| RVG1 control (n = 7) (mm Hg) | 116.9 ± 8.3 | 117.0 ± 12.7 | 112.2 ± 6.0 | 115.1 ± 14.0 |
| Mean blood pressure | ||||
| ANA nephropathy (n = 7) (mm Hg) | 82.9 ± 3.9 | 86.1 ± 6.1 | 96.8 ± 7.7 | 90.3 ± 11.1 |
| RVG1 control (n = 7) (mm Hg) | 86.0 ± 7.7 | 87.5 ± 9.4 | 85.5 ± 9.4 | 90.8 ± 13.3 |
| Diastolic blood pressure | ||||
| ANA nephropathy (n = 7) (mm Hg) | 66.7 ± 5.4 | 71.1 ± 5.8 | 81.8 ± 9.3 | 76.5 ± 13.2 |
| RVG1 control (n = 7) (mm Hg) | 70.6 ± 7.4 | 72.7 ± 8.1 | 72.5 ± 11.5 | 78.8 ± 13.3 |
Mean ± SD,
P < 0.05 versus RVG1 control at the same time point.
Experiment 2: AT1R Antagonist Prevented a Reduction in the Expression of Nephrin and Other Slit Diaphragm-Associated Molecules and Attenuated Proteinuria in ANA-Induced Nephropathy
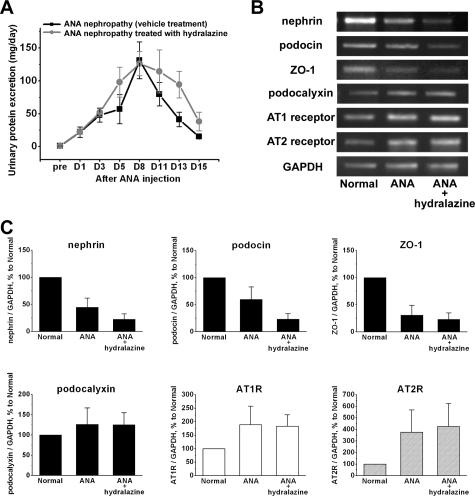
AT1R antagonist treatment significantly reduced proteinuria on day 8 (67.5 ± 23.7 versus 111.3 ± 29.9 mg/day, P < 0.05) and day 11 (37.9 ± 9.0 versus 79.3 ± 23.8 mg/day, P < 0.005), although it did not reduce proteinuria in the early phase of the disease (Figure 5A). Although the AT1R antagonist treatment slightly reduced blood pressure (Table 4), the treatment did not affect the value of CCr (ml/minute per 100 g BW: AT1R antagonist versus vehicle; 0.44 ± 0.10 versus 0.44 ± 0.06, not significant). In the vehicle group, decreased glomerular mRNA expression of nephrin (41.2% to normal control), podocin (40.2%), and ZO-1 (53.7%) was detected on day 5, but no decreased expression of these molecules was detected in the AT1R antagonist group (nephrin, 127.9%; podocin, 197.0%; ZO-1, 149.1% of normal control) (Figure 5, B and C). Real-time PCR techniques also demonstrated that decreased expression of nephrin and podocin on day 5 in ANA-induced nephropathy was prevented by AT1R antagonist (Figure 5D). The glomerular expression of the Ang II receptors increased in the proteinuric state. No effect of AT1R antagonist treatment on the expression of the Ang II receptors was observed. The staining of nephrin on day 15 in the vehicle group displayed various patterns, from a linear pattern (grade 3) to a markedly disrupted pattern (grade 0) (Figure 6). No grade 0 glomeruli were observed in the AT1R antagonist group. The number of grade 1 glomeruli was lower in the AT1R antagonist group than in the vehicle group.
Figure 5.
The effect of AT1R antagonist on proteinuria and on the expression (on day 5) of slit-diaphragm-associated molecules and Ang II receptors in ANA-induced nephropathy. A: AT1R antagonist treatment significantly reduced the peak value of proteinuria, although it did not reduce proteinuria in the early phase of the disease. B: Representative agarose-gel electrophoretic patterns on semiquantitative RT-PCR. C: The ratios of the densitometric signals of slit-diaphragm-associated molecules and Ang II receptors to that of GAPDH. The data are shown as ratios relative to normal findings. A decrease in the expression of nephrin, podocin, and ZO-1 was prevented by AT1R antagonist. AT1R antagonist treatment did not affect the expression of the Ang II receptors. D: Real-time RT-PCR findings of nephrin and podocin. Real-time RT-PCR also demonstrated that a decrease in the expression of nephrin and podocin on day 5 was prevented by AT1R antagonist.
Table 4.
Effect of AT1R Antagonist (AT1RA) or Hydralazine on Blood Pressure in Anti-Nephrin Antibody-Induced Nephropathy
| Treatment | Pre | Day 3 | Day 5 | Day 13 |
|---|---|---|---|---|
| Systolic blood pressure | ||||
| AT1RA (n = 7) (mmHg) | 122.8 ± 7.8 | 108.5 ± 8.1† | 114.9 ± 7.9* | 113.8 ± 9.8* |
| Vehicle (n = 7) (mm Hg) | 125.1 ± 9.7 | 124.0 ± 6.1 | 124.1 ± 4.1 | 124.8 ± 6.9 |
| Mean blood pressure | ||||
| AT1RA (n = 7) (mm Hg) | 95.8 ± 8.4 | 81.3 ± 7.0† | 88.4 ± 7.2* | 87.2 ± 8.2 |
| Vehicle (n = 7) (mm Hg) | 97.3 ± 12.3 | 94.6 ± 4.9 | 96.8 ± 4.4 | 95.2 ± 6.0 |
| Diastolic blood pressure | ||||
| AT1RA (n = 7) (mm Hg) | 82.5 ± 10.7 | 67.8 ± 7.6† | 75.3 ± 7.4 | 74.0 ± 7.8 |
| Vehicle (n = 7) (mm Hg) | 82.5 ± 10.7 | 79.8 ± 5.3 | 83.2 ± 6.3 | 80.5 ± 6.4 |
| Systolic blood pressure | ||||
| Hydralazine (n = 5) (mm Hg) | 110.9 ± 5.8 | 73.7 ± 4.0§ | 103.9 ± 8.9† | 105.1 ± 4.4† |
| Vehicle (n = 5) (mm Hg) | 113.3 ± 2.8 | 119.7 ± 10.5 | 128.1 ± 7.7 | 119.3 ± 7.7 |
| Mean blood pressure | ||||
| Hydralazine (n = 5) (mm Hg) | 81.7 ± 5.7 | 55.7 ± 3.0§ | 81.9 ± 10.9* | 81.8 ± 4.3 |
| Vehicle (n = 5) (mm Hg) | 83.1 ± 3.5 | 89.8 ± 9.2 | 99.4 ± 5.6 | 92.8 ± 10.1 |
| Diastolic blood pressure | ||||
| Hydralazine (n = 5) (mm Hg) | 67.1 ± 5.9 | 46.9 ± 4.8‡ | 68.3 ± 9.3* | 70.1 ± 4.6 |
| Vehicle (n = 5) (mm Hg) | 68.0 ± 5.1 | 74.9 ± 8.8 | 85.1 ± 6.9 | 79.6 ± 11.7 |
Mean ± SD,
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.0001 versus vehicle group at the same time point.
Figure 6.
The effects of AT1R antagonist on the staining of nephrin on day 15 after ANA injection. A: The glomerular immunofluorescence staining for nephrin was semiquantitatively graded using the scale described in Materials and Methods, and the representative staining pattern for each grade is shown. The staining of nephrin on day 15 in the vehicle group displayed various patterns, from a linear pattern (grade 3) to a markedly disrupted pattern (grade 0). No grade 0 glomeruli were observed in the AT1R antagonist group. B: The number of grade 1 glomeruli was less in the AT1R antagonist group than in the vehicle group. (The total number of glomeruli of grade 0 and 1 is 20.6 ± 10.0 in vehicle treatment, and is 11.3 ± 3.6 in AT1RA treatment group; P < 0.05).
Hydralazine treatment did not reduce proteinuria (Figure 7A), although it clearly lowered the BP on day 3, day 5, and day 13, if compared with the vehicle group (Table 4). The effect of hydralazine in lowering blood pressure was more evident than that of AT1R antagonist (Table 4). The hydralazine treatment did not affect the value of CCr (ml/minute per 100 g BW: hydralazine versus vehicle; 0.53 ± 0.07 versus 0.49 ± 0.07, not significant). The treatment did not prevent the reduction of mRNA expression for nephrin, podocin, and ZO-1 (Figure 7, B and C). AT2R antagonist (PD123319) treatment did not reduce proteinuria (mg/day, control versus AT2R antagonist treatment: pre, 0.97 ± 0.42 versus 0.74 ± 0.09; day 3, 35.8 ± 11.0 versus 40.0 ± 9.0; day 8, 44.8 ± 21.5 versus 44.7 ± 11.0; day 13, 34.5 ± 8.6 versus 35.1 ± 13.2; day 15, 12.4 ± 6.2 versus 15.1 ± 8.4).
Figure 7.
The effect of hydralazine on proteinuria and on the expression (on day 5) of slit-diaphragm-associated molecules and Ang II receptors in ANA-induced nephropathy. A: The kinetics of proteinuria. Hydralazine failed to reduce proteinuria during the entire experimental period. B: Representative agarose-gel electrophoretic patterns on semiquantitative RT-PCR. C: The ratios of the densitometric signals of slit-diaphragm-associated molecules and Ang II receptors to that of GAPDH. The data are shown as ratios relative to normal findings and expressed as means ± SD of the results of three independent experiments. Decreased glomerular mRNA expression of nephrin, podocin, and ZO-1 was observed on day 5 in the vehicle group. The decrease in the expression of these molecules was not prevented by hydralazine treatment.
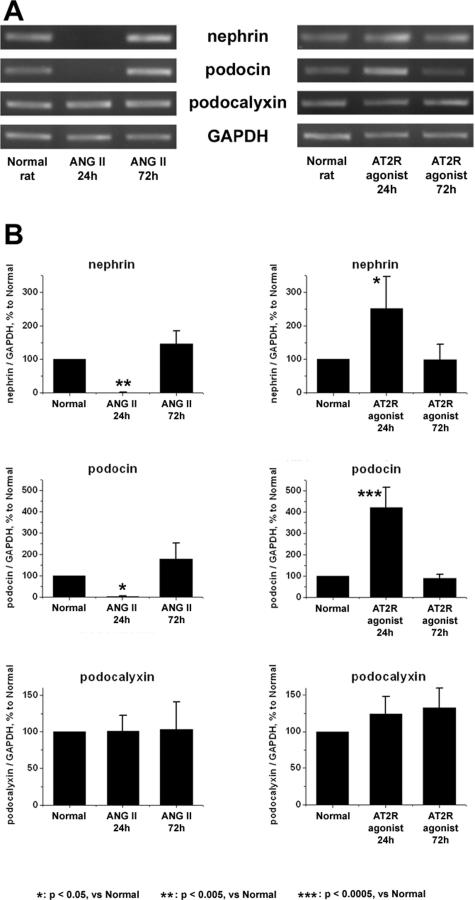
Experiment 3: mRNA Expression of Nephrin and Podocin Was Lowered by Ang II and Was Enhanced by AT2R Agonist in the Normal Rats
Continuous administration of Ang II for 24 hours clearly suppressed the mRNA expression of nephrin and podocin (Figure 8), although it did not reduce the expression of these molecules in protein level (nephrin, 76.5 ± 23.8% of normal control; podocin, 122.2 ± 49.6% of normal control). The continuous administration of the AT2R agonist CGP42112A boosted the mRNA expression of nephrin (250.7 ± 96.1% of normal control) and podocin (421.8 ± 92.6% of normal control). These effects of Ang II and AT2R agonist on mRNA expression of nephrin and podocin were not detected in rats treated with continuous administration for 72 hours (Figure 8). Any morphological alterations were not observed in glomeruli of rats treated with Ang II or AT2R agonist. Neither abnormal proteinuria nor abnormal albuminuria were detected in rats treated with Ang II [proteinuria, mg/day; Ang II treatment versus vehicle, 1.41 ± 1.06 versus 1.06 ± 0.22, not significant: urinary albumin concentration; Ang II treatment, negative (<0.4 mg/L), vehicle, negative (<0.4 mg/L)]. No altered level of glomerular mRNA expression of AT1R and AT2R was detected in rats treated with Ang II or AT2R agonist.
Figure 8.
The glomerular mRNA expression of nephrin, podocin, and podocalyxin in the rats treated with Ang II or AT2R agonist. Normal rats were subcutaneously injected with Ang II or AT2R agonist (CGP42112A) with an osmotic pump. The glomerular mRNA expression of nephrin, podocin, and podocalyxin was analyzed by RT-PCR. A: Characteristic agarose-gel electrophoretic patterns. B: The ratios of the densitometric signals of nephrin, podocin, and podocalyxin to those of GAPDH. The data are shown as ratios relative to findings of normal rat and expressed as means ± SD of the results of three independent experiments. The continuous administration of Ang II for 24 hours suppressed the glomerular expression of nephrin and podocin. In contrast, the continuous administration of CGP42112A for 24 hours increased the glomerular expression of nephrin (250.7 ± 96.1% of normal control) and podocin (421.8 ± 92.6% of normal control).
Experiment 4: Ang II Lowered the Expression of Nephrin Through the Type 1 Receptor, and AT2R Agonist Enhanced It in Cultured Podocytes
The expression of AT1R and AT2R was clearly detected in the cultured podocytes (Figure 9A). Stimulation by Ang II (1 × 10−8 mol/L) lowered nephrin mRNA expression (P < 0.005, Figure 9B). AT1R antagonist (CV11974, 1 × 10−6 mol/L) prevented the decrease in nephrin by Ang II. However, AT2R antagonist (PD123319, 1 × 10−6 mol/L) did not prevent the decrease in nephrin mRNA. Stimulation by the AT2R agonist (CGP42112A, 1 × 10−8 mol/L) increased nephrin mRNA expression (P < 0.05, Figure 9C). This effect of CGP42112A was reversed by PD123319. Real-time RT-PCR analyses also showed that Ang II (1 × 10−8 mol/L) treatment lowered mRNA expression of nephrin (P < 0.0005, Figure 9D) and that AT2R agonist (CGP42112A, 1 × 10−8 mol/L) increased nephrin mRNA expression (P < 0.05, Figure 9E).
Figure 9.
Immunofluorescence findings of Ang II receptors (A) and the mRNA expression of nephrin treated with Ang II or AT2R agonist in mouse cultured podocytes (B–E). Both AT1R and AT2R were expressed in cultured podocyte (A). Findings of semiquantitative RT-PCR analyses of nephrin in cultured podocyte treated with Ang II (B) and with AT2R agonist (CGP42112A) (C). Representative agarose gel electrophoretic patterns are shown at the top. The ratio of the densitometric signal of nephrin to that of GAPDH was determined. Stimulation with a dose of 1 × 10−8 mol/L Ang II decreased mRNA expression of nephrin (***P < 0.0005). A decrease in nephrin expression by Ang II was prevented by AT1R antagonist (CV11974). B: AT2R antagonist (PD123319) did not prevent the decrease in nephrin mRNA. AT2R agonist (CGP42112A) increased nephrin mRNA expression (*P < 0.05). C: This effect of CGP42112A was reversed by PD123319. Findings of real-time RT-PCR analyses of nephrin in cultured podocyte treated with Ang II (D) and with AT2R agonist (CGP42112A) (E). Real-time RT-PCR analyses also showed that Ang II treatment lowered mRNA expression of nephrin (***P < 0.0005) (D) and that AT2R agonist (CGP42112A) increased nephrin mRNA expression (*P < 0.05) (E).
Discussion
In the present study, we elucidated whether and how AT1R- and AT2R-mediated actions regulated the function of the slit diaphragm with in vivo and in vitro studies. For in vivo study, we adopted the experimental proteinuric state caused by an injection of anti-nephrin monoclonal antibody. Nephrin constitutes the extracellular site of the slit diaphragm and is accepted to be a key molecule in maintaining its barrier function.21,27 Although nephrin was originally identified as a product of a mutated gene in the congenital nephrotic syndrome,28 several studies have indicated that nephrin alterations contribute to the development of proteinuria in a variety of kidney diseases.21,29,30,31,32,33,34 As previously reported,21,27,35 the ANA causes massive proteinuria if injected into rats, independently of complement fixation or inflammatory cell recruitment. In this proteinuric model, no severe morphological alterations were detected, and the effacement of foot processes was observed at the very limited area.35 The antibody binding to nephrin causes down-regulation and the altered localization of the slit diaphragm-associated molecules, ZO-135 and podocin,23 as well as nephrin. Because podocin is understood to be one of the critical molecules of the slit diaphragm and ZO-1 was originally identified as a tight junction component and is accepted to be a functional molecule of the slit diaphragm, we concluded that the molecular rearrangement of these slit diaphragm molecules causes proteinuria.35 Thus, it is conceivable that this proteinuric state is the best model for analyzing the relation between Ang II action and the slit diaphragm function.
To begin this study, first, we analyzed the kinetics of slit diaphragm-associated molecules, plasma Ang II level, and the expression of Ang II receptors in ANA-induced nephropathy. We observed that the mRNA expression of nephrin already decreased at 1 hour after the ANA injection, and the consequent alteration in mRNA expression of podocin and ZO-1 was also detected. Slight increase of plasma Ang II level was observed already on day 1, the dual-labeling immunofluorescence studies showed that both AT1R and AT2R were expressed in podocytes, and their expression clearly elevated in the proteinuric state. It should also be noted that mRNA expression of AT2R was elevated more evidently than AT1R, although the significance of the result is not clear.
Next, we analyzed the effect of the AT1R antagonist on proteinuria and on the expression of slit diaphragm-associated molecules in ANA-induced nephropathy. AT1R antagonist treatment attenuated proteinuria on days 8 and 11 (Figure 5A). We also observed that AT1R antagonist treatment prevented a reduction in the expression of slit diaphragm-associated molecules on day 5, indicating that the AT1R antagonist treatment ameliorated proteinuria by preventing a reduction in the functional molecules of the slit diaphragm. We confirmed that this AT1R antagonist treatment did not affect the value of CCr. Although AT1R antagonist slightly lowered BP, its effect was milder than hydralazine (Table 4). A non-Ang II action-mediated antihypertensive drug hydralazine did not attenuate the proteinuria (Figure 7A) and did not prevent the reduction in the expression of slit diaphragm-associated molecules (Figure 7, B and C), although its effect in lowering blood pressure was more evident than AT1R antagonist. We also confirmed that AT2R antagonist treatment did not ameliorate the proteinuria. These results also support our understanding that AT1R antagonist directly acts on podocytes and ameliorates proteinuria. It should be noted that the treatment of AT1R antagonist ameliorated the peak value of proteinuria at the late phase of the disease, although it did not reduce proteinuria at the early phase. In this study, we used Brown-Norway rats, because the individual difference in the amount of proteinuria in this strain is smaller than in others.36 The time point when proteinuria peaked in Brown Norway rats is day 8 after the disease induction, which is later than that in other strains. We show here that the expression of the slit diaphragm-associated molecules was already reduced at the early phase of disease, and temporally recovered, and the reduced expression was observed again on day 5 in Brown Norway rats injected with ANA (Figure 2). AT1R antagonist treatment prevented the second phase reduction of the slit diaphragm molecules and ameliorated proteinuria at late phase. We have previously reported that the ANA binding caused down-regulation of not only its target molecule nephrin but also other slit diaphragm molecules23,35; however, the question how the consequent down-regulation of these molecules resulted remains unclear. Overexpression of AT1R and the increase in Ang II preceded the decreased expression of nephrin and podocin. These observations may suggest that the increased AT1R-mediated action contributed to the development of proteinuria from the early phase. However, it seems that the direct effect of antibody binding and the consequent alterations of the slit diaphragm molecules mainly contribute to the initiation phase of proteinuria. It is considered that Ang II-associated actions play a major role in the development of proteinuria at the later phase of disease. It has been reported that AT1R antagonist ameliorates proteinuria in several glomerular diseases including minimal change-type nephrotic syndrome, membranous nephropathy, and diabetic nephropathy.8,10 It is also reported that the dysfunction of the slit diaphragm is involved in the development of proteinuria in some of these diseases.29,30,32 Together with the observations in this study and these reports, it is plausible that AT1R-mediated action was involved in the reduction of the slit diaphragm molecules in several kinds of glomerular disease, whatever the etiological episodes are.
Based on all of the findings in in vivo studies in ANA-induced nephropathy, we hypothesized that Ang II directly acts on podocyte and affects the expression of slit diaphragm-associated molecules. To test this hypothesis, we analyzed the direct function of Ang II in the regulation of the expression of the molecules in in vivo study with normal rat and in vitro study of cultured podocytes. We observed that mRNA expression of nephrin was clearly reduced in the cultured podocytes treated with Ang II (Figure 9, B and D). We also observed that continuous administration of Ang II into normal rats for 24 hours clearly suppressed the mRNA expression of nephrin and podocin (Figure 8). It should be mentioned that reduction of mRNA expression of nephrin and podocin was not detected in rats treated with Ang II for 72 hours. Neither reduced expression of these molecules in protein level nor abnormal proteinuria were detected in rats treated with Ang II. Although it remains unclear why the effect of Ang II on the mRNA expression of nephrin and podocin was temporary, it is plausible that some compensatory reaction inhibiting the effect of the injected Ang II occurred in rats. Whatever the mechanism is, we could demonstrate here that Ang II treatment temporarily but clearly reduced the mRNA expression of the critical molecules of the slit diaphragm in both in vivo and in vitro analyses. It is conceivable that the findings can support the conclusion that AT1R antagonist ameliorated proteinuria in ANA-induced nephropathy by preventing a reduction of the expression of the functional molecules of the slit diaphragm.
Next, we analyzed the selective function of AT1R- and AT2R-mediated actions on podocytes. Several studies have shown that AT2R-mediated action counteracts AT1R-mediated action.3,4,5,6 Recently, Hashimoto and colleagues37 reported that overexpression of AT2R ameliorated glomerular injury in a mouse remnant kidney model. On the other hand, some reports have shown that AT2R action colludes with AT1R action. Although it has been accepted that the specific blockade for AT1R is more effective in reducing proteinuria, Cao and colleagues38 have recently reported that a dual AT1R and AT2R blockade reduced proteinuria more effectively than the single AT1R blockade. Thus, the selective function of AT1R- and AT2R-mediated actions in glomeruli is still controversial. To clarify this question, we analyzed the effect of AT2R-specific agonist on the expression of the slit diaphragm-associated molecules. We demonstrated here that continuous administration of AT2R agonist into normal rat for 24 hours enhanced the glomerular mRNA expression of nephrin and podocin (Figure 8). In vitro study with cultured podocytes also showed that the treatment of AT2R agonist promoted the mRNA expression of nephrin. We also demonstrated that Ang II action lowering the mRNA expression of nephrin of cultured podocytes was clearly abolished by the pretreatment of AT1R antagonist but not by that of AT2R-specific blockade. These findings indicated that AT2R-mediated action contributes to the maintenance of the barrier function of the slit diaphragm and that AT1R-mediated action has an opposite function. In the beginning of this study, we observed that the expression of AT2R rose more evidently than AT1R in proteinuric state. The elevated AT2R in a proteinuric state may play a role in preventing the development of disease. Based on these observations, we propose that the enhancement of AT2R stimulation may serve as a potential therapeutic strategy for proteinuria.
Glomerular podocyte is a terminally differentiated cell with a highly differentiated structure characterized by the interdigitating foot processes and the slit diaphragm connecting them.39,40,41,42,43,44 Because the adjacent foot processes of podocytes arise from the cell bodies of the neighboring cells, the slit diaphragm is a highly differentiated intercellular junction.45 To better understand the mechanism that maintains this highly differentiated structure of podocytes must be an important theme not only in nephrology but also in cell biology. Although some studies showed that Ang II action is involved in the regulation of cell proliferation and differentiation,46 the role of Ang II action on maintaining the differentiated condition of cells has not been well understood. We have recently reported that the expression of the slit diaphragm molecules was associated with the balance of cell cycle protein.47 In this study, we showed that AT1R-mediated action lowered the mRNA expression of the slit diaphragm functional molecules and that AT2R-mediated action enhanced them. Together, these results suggest that AT2R-mediated action contributes to the maintenance of the differentiation of podocytes.
In conclusion, the blocking of AT1R-mediated action prevents the lowering of the expression of slit diaphragm-associated molecules and attenuates the proteinuria of ANA-induced nephropathy. Our results imply that AT1R action contributes to the down-regulation of the functional molecules of the slit diaphragm and that AT2R action has the reverse function. This is the first report demonstrating that AT1R and AT2R actions play opposite roles in regulating the expression of the functional molecules of which dysfunction results in major clinical symptoms.
Acknowledgments
We thank Dr. Peter Mundel for his willingness to provide valuable material for this study; Dr. Tamaki Karasawa, Dr. Yutaka Harita, and Dr. Akira Saito for their helpful discussions; Ms. Mutsumi Kayaba, Ms. Chiharu Nagasawa, and Ms. Matsu Honma for their excellent technical assistance; and Dr. Satoko Suzuki, Mr. Taisei Suzuki, Mr. Hiroshi Suzuki, and Ms. Takako Suzuki for their cordial cooperation.
Footnotes
Address reprint requests to Hiroshi Kawachi, Department of Cell Biology, Institute of Nephrology, Niigata University Graduate School of Medical and Dental Sciences, 1-757 Asahimachi-dori, Niigata, 951-8510, Japan. E-mail: kawachi@med.niigata-u.ac.jp.
Supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan [grant-in-aids for scientific research (B) 13557084 and 14370317 to H.K., 15390268 to F.S., and (C) 18590886 to H.K.].
References
- Goodfriend TL, Elliott ME, Catt KJ. Angiotensin receptors and their antagonists. N Engl J Med. 1996;334:1649–1654. doi: 10.1056/NEJM199606203342507. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, Horiuchi M, Pratt RE, Dzau VJ. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci USA. 1995;92:10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband CH, Zhu M, Lu D, Reagan LP, Fluharty SJ, Posner P, Raizada MK, Sumners C. Functional interactions between neuronal AT1 and AT2 receptors. Endocrinology. 1997;138:2195–2198. doi: 10.1210/endo.138.5.5236. [DOI] [PubMed] [Google Scholar]
- Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, Dzau VJ, Takahashi H, Iwasaka T, Inada M, Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurko R, Kimura B, Kurian P, Crews FT, Phillips MI. Angiotensin II receptor subtypes play opposite roles in regulating phosphatidylinositol hydrolysis in rat skin slices. Biochem Biophys Res Commun. 1992;186:285–292. doi: 10.1016/s0006-291x(05)80805-8. [DOI] [PubMed] [Google Scholar]
- Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Mosconi L, Matalone M, Garini G, Salvadori M, Zoccali C, Scolari F, Maggiore Q, Tognoni G, Remuzzi G, The GISEN group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) (Writing Committee Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- Lafayette RA, Mayer G, Park SK, Meyer TW. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1992;90:766–771. doi: 10.1172/JCI115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi A, Perico N, Amuchastegui CS, Malanchini B, Mazerska M, Battaglia C, Bertani T, Remuzzi G. Short- and long-term effect of angiotensin II receptor blockade in rats with experimental diabetes. J Am Soc Nephrol. 1993;4:40–49. doi: 10.1681/ASN.V4140. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Obata J, Kimura H, Ohno S, Yoshida Y, Kawachi H, Shimizu F. Blocking angiotensin II ameliorates proteinuria and glomerular lesions in progressive mesangioproliferative glomerulonephritis. Kidney Int. 1999;55:877–889. doi: 10.1046/j.1523-1755.1999.055003877.x. [DOI] [PubMed] [Google Scholar]
- Benigni A, Tomasoni S, Gagliardini E, Zoja C, Grunkemeyer JA, Kalluri R, Remuzzi G. Blocking angiotensin II synthesis/activity preserves glomerular nephrin in rats with severe nephrosis. J Am Soc Nephrol. 2001;12:941–948. doi: 10.1681/ASN.V125941. [DOI] [PubMed] [Google Scholar]
- Macconi D, Ghilardi M, Bonassi ME, Mohamed EI, Abbate M, Colombi F, Remuzzi G, Remuzzi A. Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol. 2000;11:477–489. doi: 10.1681/ASN.V113477. [DOI] [PubMed] [Google Scholar]
- Tryggvason K, Bettersson E. Causes and consequences of proteinuria: the kidney filtration barrier and progressive renal failure. J Intern Med. 2003;254:216–224. doi: 10.1046/j.1365-2796.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- Kawachi H, Shimizu F. Molecular composition and function of the slit diaphragm: nephrin, the molecular responsible for proteinuria. Clin Exp Nephrol. 2000;4:161–172. [Google Scholar]
- Pavenstädt H. Roles of the podocyte in glomerular function. Am J Physiol. 2000;278:F173–F179. doi: 10.1152/ajprenal.2000.278.2.F173. [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Zuniga Mejia, Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- Orikasa M, Matsui K, Oite T, Shimizu F. Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J Immunol. 1988;141:807–814. [PubMed] [Google Scholar]
- Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia A, Sakai T, Yamamoto T, Salant DJ, Shimizu F. Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int. 2000;57:1949–1961. doi: 10.1046/j.1523-1755.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kawachi H, Morioka Y, Nakatsue T, Koike H, Ikezumi Y, Oyanagai A, Natori Y, Natori Y, Nakamura T, Gejyo T, Shimizu F. Fractalkine expression and the recruitment of CX3CR1+ cells in the prolonged mesangial proliferative glomerulonephritis. Kidney Int. 2002;61:2044–2057. doi: 10.1046/j.1523-1755.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- Kawachi H, Koike H, Kurihara H, Sakai T, Shimizu F. Cloning of rat homologue of podocin: expression in proteinuric states and in developing glomeruli. J Am Soc Nephrol. 2003;14:46–56. doi: 10.1097/01.asn.0000037401.02391.76. [DOI] [PubMed] [Google Scholar]
- Han GD, Koike H, Nakatsue T, Suzuki K, Yoneyama H, Narumi S, Kobayashi N, Mundel P, Shimizu F, Kawachi H. IFN-inducible protein-10 has a differential role in podocyte during Thy1.1 glomerulonephritis. J Am Soc Nephrol. 2003;14:3111–3126. doi: 10.1097/01.asn.0000097371.64671.65. [DOI] [PubMed] [Google Scholar]
- Kawachi H, Orikasa M, Matsui K, Iwanaga T, Toyabe S, Oite T, Shimizu F. Epitope-specific induction of mesangial lesions with proteinuria by a MoAb against mesangial cell surface antigen. Clin Exp Immunol. 1992;88:399–404. doi: 10.1111/j.1365-2249.1992.tb06461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi H, Oite T, Shimizu F. Quantitative study of mesangial injury with proteinuria induced by monoclonal antibody 1-22-3. Clin Exp Immunol. 1993;92:342–346. doi: 10.1111/j.1365-2249.1993.tb03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham PS, Kawachi H, Haydar SA, Chugh S, Addona TA, Charron KB, Holzman LB, Shia M, Shimizu F, Salant DJ. Nephritogenic mAb 5-1-6 is directed at the extracellular domain of rat nephrin. J Clin Invest. 1999;104:1559–1566. doi: 10.1172/JCI7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestilä M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723–1731. doi: 10.1016/S0002-9440(10)64128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardini E, Benigni A, Tomasoni S, Abbate M, Kalluri R, Remuzzi G. Targeted downregulation of extracellular nephrin in human IgA nephropathy. Am J Nephrol. 2003;23:277–286. doi: 10.1159/000072281. [DOI] [PubMed] [Google Scholar]
- Ahola H, Wang SX, Luimula P, Solin ML, Holzman LB, Holthofer H. Cloning and expression of the rat nephrin homolog. Am J Pathol. 1999;155:907–913. doi: 10.1016/S0002-9440(10)65190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ME, Mundel P, Boner G. Role of nephrin in renal disease including diabetic nephropathy. Semin Nephrol. 2002;22:393–398. doi: 10.1053/snep.2002.34724. [DOI] [PubMed] [Google Scholar]
- Yuan H, Takeuchi E, Taylor GA, McLaughlin M, Brown D, Salant DJ. Nephrin dissociates from actin, and its expression is reduced in early experimental membranous nephropathy. J Am Soc Nephrol. 2002;13:946–956. doi: 10.1681/ASN.V134946. [DOI] [PubMed] [Google Scholar]
- Nakatsue T, Koike H, Han GD, Suzuki K, Miyauchi N, Yuan H, Salant DJ, Gejyo F, Shimizu F, Kawachi H. Nephrin and podocin dissociate at the onset of proteinuria in experimental membranous nephropathy. Kidney Int. 2005;67:2239–2253. doi: 10.1111/j.1523-1755.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- Kawachi H, Kurihara H, Topham PS, Brown D, Shia MA, Orikasa M, Shimizu F, Salant DJ. Slit diaphragm-reactive nephritogenic MAb 5-1-6 alters expression of ZO-1 in rat podocytes. Am J Physiol. 1997;273:F984–F993. doi: 10.1152/ajprenal.1997.273.6.F984. [DOI] [PubMed] [Google Scholar]
- Gollner D, Kawachi H, Oite T, Oka M, Nagase M, Shimizu F. Strain variation in susceptibility to the development of monoclonal antibody 5-1-6-induced proteinuria in rats. Clin Exp Immunol. 1995;101:341–345. doi: 10.1111/j.1365-2249.1995.tb08361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Maeshima Y, Satoh M, Odawara M, Sugiyama H, Kashihara N, Mastubara H, Yamasaki Y, Makino H. Overexpression of angiotensin type 2 receptor ameliorates glomerular injury in a mouse remnant kidney model. Am J Physiol. 2004;286:F516–F525. doi: 10.1152/ajprenal.00294.2003. [DOI] [PubMed] [Google Scholar]
- Cao Z, Bonnet F, Candido R, Nesteroff SP, Burns WC, Kawachi H, Shimizu F, Carey RM, De Gasparo M, Cooper ME. Angiotensin type 2 receptor antagonism confers renal protection in a rat model of progressive renal injury. J Am Soc Nephrol. 2002;13:1773–1787. doi: 10.1097/01.asn.0000019409.17099.33. [DOI] [PubMed] [Google Scholar]
- Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol. 1995;192:385–397. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D’Agati V, Alpers CE. Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: role in podocyte proliferation and maturation. Kidney Int. 2000;58:674–683. doi: 10.1046/j.1523-1755.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- Nagata M, Shu Y, Tomari S. Role of cell cycle molecules in the pathophysiology of glomerular epithelial cells. Microsc Res Tech. 2002;57:203–207. doi: 10.1002/jemt.10074. [DOI] [PubMed] [Google Scholar]
- Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert S, Stoll M, Steckelings UM, Bottari SP, Unger T. The angiotensin II AT2 receptor inhibits proliferation and promotes differentiation in PC12W cells. Mol Cell Endocrinol. 1996;122:59–67. doi: 10.1016/0303-7207(96)03873-7. [DOI] [PubMed] [Google Scholar]
- Han GD, Suzuki K, Koike H, Suzuki K, Yoneyama H, Narumi S, Shimizu F, Kawachi H. IFN-inducible protein-10 plays a pivotal role in maintaining slit-diaphragm function by regulating podocyte cell-cycle balance. J Am Soc Nephrol. 2006;17:442–453. doi: 10.1681/ASN.2004090755. [DOI] [PubMed] [Google Scholar]
- Siegling A, Lehmann M, Platzer C, Emmrich F, Volk HD. A novel multispecific competitor fragment for quantitative PCR analysis of cytokine gene expression in rats. J Immunol Methods. 1994;177:23–28. doi: 10.1016/0022-1759(94)90139-2. [DOI] [PubMed] [Google Scholar]