Abstract
Angiogenesis, or neovascularization, is tightly controlled by positive and negative regulators, many of which reside in the extracellular matrix. We have now identified eight novel 19- to 20-residue peptides derived from the α4, α5 and α6 fibrils of type IV collagen, which we have designated tetrastatins, pentastatins, and hexastatins, respectively. We have shown that these endogenous peptides suppress the proliferation and migration of HUVECs in vitro. By performing clustering analyses of the sequences using sequence similarity criteria and of the experimental results using a hierarchical algorithm, we report that the clusters identified by the experimental results coincide with the sequence-based clusters, indicating a tight relationship between peptide sequence and anti-angiogenic potency. These peptides may have potential as anti-angiogenic therapeutic agents.
Keywords: Angiogenesis, inhibitor, endogenous, endothelial cell, tetrastatin, pentastatin, hexastatin
Introduction
Angiogenesis, the process of developing a novel vascular network from a pre-existing one, is tightly controlled by various endogenous regulators [1]. These regulatory elements include pro- and anti-angiogenic proteins or their fragments [2]. Many of the regulators of angiogenesis have been determined to be fragments of extracellular matrix proteins or of circulating factors [3; 4]. The basement membrane, the thin membrane that envelops the endothelium, contains a variety of these angiogenesis regulators.
Basement membranes are composed of several major and minor glycoprotein constituents [5]. Type IV collagen is ubiquitously present in these membranes, where it is the major collagenous component. Type IV collagen molecules secreted by endothelial and epithelial cells self-associate into polygonal networks that interact with laminin, as well as with proteoglycans and other glycoproteins, to form basement membranes.
Each type IV collagen molecule is a trimer composed of three α chains. Six genetically distinct type IV collagen α chains (α(IV)) have been identified: the α1, α2, α3, α4, α5 and α6 fibrils [5]. These chains share basic structural features and show extensive sequence homology. The major structural features of the α chains include a collagenous domain of about 1,400 amino acids containing the repetitive triplet sequence glycine (Gly)-X-Y, in which X and Y represent a variety of other amino acids; a non-collagenous carboxy-terminal (NC1) domain of about 230 residues; and a non-collagenous amino-terminal sequence of 15–20 residues.
Various NC1 domains of the α(IV) fibrils have been shown to exert anti-angiogenic effects that are mediated mainly by inhibiting the proliferation and migration of endothelial cells. Arresten is a 26-kDa molecule derived from the NC1 domain of the α1 chain of type IV collagen [6; 7]. This molecule inhibits endothelial cell proliferation and migration, and there is evidence that it may act as a decoy, blocking the binding of α1β1 integrin to type I collagen [7]. It is interesting that in another study the total NC1 domain of the α1(IV) fibril was shown to lack any anti-angiogenic activity [8]. Canstatin is a 24-kDa fragment of the α2 chain of type IV collagen [9]. Like arresten, canstatin inhibits endothelial cell proliferation, migration and tube formation in a dose-dependent manner. Tumstatin is a fragment of the NC1 domain of the α3 chain of type IV collagen [10]. Synthetic peptides derived from tumstatin have been shown to inhibit the proliferation of endothelial cells as well as melanoma and other epithelial tumor cell lines [11]. This inhibitory activity of tumstatin is thought to be mediated through αvβ3 integrin binding [12]. The remaining three NC1 domains from type IV collagen, α4, α5 and α6, have also been screened for anti-angiogenic activity [8], and the full NC1 domains from the α4 and α5 fibrils were found to lack any such activity. The α6 domain has been shown to regulate endothelial cell adhesion and migration, but with significantly lower potency than the homologous domains from the α2 and α3 fibrils.
Using a bioinformatics analysis we have now identified a set of eight novel short peptides of 19 or 20 amino acid residues in length, derived from the α4, α5 and α6 fibrils of type IV collagen, as putative angiogenesis inhibitors. We have performed in vitro endothelial cell proliferation and migration assays using these peptides and demonstrated their potency. We have also used the information from their sequence homologies and their activity profiles from the in vitro assays to cluster the peptides and provide evidence that there is an underlying amino acid sequence-function relationship.
Materials and Methods
Cell Culture
Primary human umbilical vein endothelial cells (HUVECs) from a single donor were purchased from Cambrex (Walkersville, MD). The cells were propagated in EGM-2 medium, consisting of a basal cell medium with 2% FBS, growth factors (hbFGF and VEGF) and antibiotics (gentamicin/amphotericin B). All the cells used were from passage 3 to passage 6.
Peptide Synthesis and Handling
The peptides used were produced by the custom peptide synthesis facility in the Department of Oncology, Johns Hopkins University, and a commercial provider (Abgent, San Diego, CA) using a solid-phase synthesis technique. HPLC and mass spectroscopy analyses of each peptide were performed. In each case, the synthetic procedure yielded 10 mg of >95% pure peptide. The peptides were solubilized in water before use. In the cases of highly hydrophobic peptides, dimethyl sulfoxide (DMSO) at a maximum concentration of 0.1% (v/v) was used as a solvent; we verified experimentally that at this concentration the solvent had no effect on the experimental results.
In Vitro Cell Viability Assay
The effects of our anti-angiogenic agents on the proliferation of endothelial were assessed by measuring the metabolic activity of the live cells using the colorimetric cell proliferation reagent WST-1 (Roche, Indianapolis, IN). WST-1 is a substrate in a colorimetric assay that measures the metabolic activity of viable cells [13].
Approximately 2x103/well were seeded in a 96-well microplate. The response of endothelial cells to anti-angiogenic molecules has been shown to be substrate dependent [18]. In order to minimize interference with substrate-dependent signaling pathways and delineate the pure effect of the predicted fragments on the endothelial cell proliferation we chose to seed them without any matrix component present. Furthermore, additional interference can arise from possible fragments of the extracellular matrix components, different from those studied, that can be produced by matrix metalloproteinases expressed by the endothelial cells and that can be anti-angiogenic.
The viability of the cells was determined after a 3-day exposure to the peptide solution. Each peptide was tested at seven different concentrations: 0.01, 0.1, 1 and 10 μg/ml and 20, 30 and 40 μg/ml. The molecular size of each of the peptides is approximately 2.5 kDa; thus, these concentrations translate to 4, 40, 400, and 4,000 nM and 8, 12 and 16 μM, respectively. Each of the concentrations was tested simultaneously in quadruplicate, and each of the experiments was repeated three times. As a positive control (i.e., decreasing viability) we applied 100 ng/ml (0.22 μM) TNP-470 (a synthetic analogue of fumagillin) along with the full medium. As a negative control (equivalent to normal viability) the cells were cultured without any agent in full medium, containing growth factors and serum. The samples were read at a wavelength of 570 nm in an ELISA plate reader Victor3 V (Perkin Elmer). The amount of color produced was directly proportional to the number of viable cells. A more detailed description of the Materials and Methods is provided in the Online Supplement.
In-vitro Cell Migration Assay
A modified Boyden chamber migration assay (BD Biosciences, San Jose, CA) was used to examine endothelial cell migration in the presence of an activator. The lower compartment of the Boyden chamber was separated from the upper (containing the endothelial cells) by a laminin-coated polycarbonate filter with pores small enough to allow only the active passage of the cells (3-μm pore size).
The cells were applied to the upper compartment of the chamber. Typically, 20x103 cells in a volume of 50 μl were added to each well. The growth factor VEGF (Invitrogen, Carlsbad, California) was used as the activator. It was added to serum-free medium in the lower chamber to give a final concentration of 20 ng/ml in a total volume of 225 μl. VEGF in serum-free medium was also used alone as a positive control. The anti-angiogenic peptides to be tested were individually added to the lower chamber at 30 μg/ml, together with 20 ng/ml VEGF in serum-free medium. The serum- and growth factor-free medium was used alone as a negative control. The chambers were then incubated for 20 h at 37°C. The cells that had migrated into the lower chamber were stained with calcein (Invitrogen, Molecular Probes, Carlsbad, CA) 90 min prior to termination of the experiment. They were counted by measuring the fluorescence at 485 nm excitation and 510 nm emission in a fluorescence plate reader (Victor 3V, Perkin Elmer). The results were scaled so that 100% represented the positive control, and 0% represented the number of migrating cells in the negative control.
Computational Analysis
Statistical significance was assessed using Student’s t-test, with p-values < 0.001 defined as significant. The multiple sequence similarity dendrogram was calculated using the Clustalw algorithm [14]. This tree was visualized using Jalview [15]. The clustering and statistical analysis was performed using Matlab. We implemented a hierarchical clustering analysis using the Euclidean distances as a distance metric [16]. Once the distance was calculated, the experimental observations were grouped into a binary, hierarchical cluster tree by linking the pairs of observations that were in closer proximity according to the metric used.
Results and Discussion
By using a bioinformatics approach, we have identified a set of eight novel short peptides, 19–20 amino acids in length, that are derived from the non-collagenous domains of the α4, α5 and α6 fibrils of type IV collagen: three derived from α4, three from α5 and two from α6 fibrils. Technical details of this bioinformatics analysis are not essential for the present study and are the subject of a separate report. These peptides display sequence similarities to known anti-angiogenic fragments derived from the non-collagenous domains of the α1 (arresten), α2 (canstatin) and α3 (tumstatins) fibrils of collagen IV. We introduce the term tetrastatin to characterize the peptides derived from the α4 fibril of type IV collagen, pentastatin for the peptides derived from the α5 fibril and hexastatin for the peptides derived from the α6 fibril. The amino acid sequences of these peptides are shown in Table 1. Having hypothesized that the predicted fragments possess anti-angiogenic properties, we assessed their anti-angiogenic potency in established in vitro assays.
Table 1.
Amino acid sequences of the tested peptides
| Peptide Name | Accession Number | Peptide Sequence |
|---|---|---|
| Tetrasratin-1 | CAA56943(1514–1533) | LPVFSTLPFAYCNIHQVCHY |
| Tetrasratin-2 | CAA56943(1524–1543) | YCNIHQVCHYAQRNDRSYWL |
| Tetrasratin-3 | CAA56943(1628–1646) | AAPFLECQGRQGTCHFFAN |
| Pentastatin-1 | AAF66217(1516–1535) | LRRFSTMPFMFCNINNVCNF |
| Pentastatin-2 | AAF66217(1526–1545) | FCNINNVCNFASRNDYSYWL |
| Pentastatin-3 | AAF66217(1632–1650) | SAPFIECHGRGTCNYYANS |
| Hexastatin-1 | AAB19039(1629–1647) | ATPFIECSGARGTCHYFAN |
| Hexastatin-2 | AAB19039(1526–1545) | YCNINEVCHYARRNDKSYWL |
Two of the most prominent characteristics of the angiogenic process are the proliferation of endothelial cells near a maternal vessel, where the novel sprouting bud emerges, and their coordinated migration along a chemotactic gradient. To evaluate the anti-proliferative and anti-migratory potency of our candidate peptides, we used in vitro proliferation and migration assays and measured their potency as compared to positive and negative controls.
The short peptides inhibit the proliferation of HUVECs
We first tested the ability of each of the eight short peptides to inhibit the proliferation of HUVECs in an in vitro assay (Fig. 1). As a negative control, we added only the full medium to the cells; as a positive control, we added the full medium with 100 ng/ml of TNP-470 (fumagillin). TNP-470 is a microtubule-stabilizing agent that has the ability to induce endothelial cell apoptosis [17]. The optical signal from the proliferation assay was scaled so that 0% represented the signal from the negative control and 100% represented the signal from the wells to which TNP-470 was added. We expressed this scaled result as peptide activity relative to the activity of TNP-470.
Figure 1.

Effect of the peptides from α4, α5 or α6 fibrils of type IV collagen on the proliferation of HUVECs. After 3 days of incubation with the test peptides, the endothelial cells were stained and counted using a colorimetric assay. A–C, activity of the peptides derived from the α4 fibril. D–F, activity of the peptides derived from the α5 fibril and G, H, activity of the peptides derived from the α6 fibril. The results are scaled so that 0% represents the optical signal from the negative control (endothelial cells incubated with medium containing growth factor and serum, data not shown) and 100% represents the signal from the positive control (cells incubated with 100 ng/ml TNP-470, data not shown). Vertical bars indicate the standard error. All values are significantly different from 0% at p<0.001 except those marked by an NS sign (non-significant). In all cases the standard error for the controls was less than 2% (n=8).
The tetrastatins (Fig. 1A–C) reached a maximal activity level of ~20–25% when compared to TNP-470, and their EC50 values in the in vitro assay ranged from 0.01 to 0.1 μg/ml. The pentastatins (Fig. 1D–F) were the most potent of the peptides tested in the proliferation experiments. Pentastatin-1 reached 100% of the TNP-470 activity at a concentration of 16 μ and the pentastatin-2 activity peaked at 70% at that same concentration. For both of these peptides, the EC50 was <10 μg/ml, or 4 μM. The maximal activity of pentastatin-3 reached 15%. The maximal activity of the two hexastatins was 25%, and their activities exhibited a biphasic pattern, reaching a maximum at an intermediate concentration (~1μg/ml) and declining with increasing concentration. At concentrations above 1μg/ml, the activity of hexastatin-1 declined to minimal levels and became insignificant at the maximum tested concentration of 40 μg/ml. In contrast, hexastatin-2 reached a nadir at 10 μg/ml but then gradually regained its activity at higher concentrations.
The short peptides suppress the ability of HUVECs to migrate in the presence of VEGF
We also assessed the anti-angiogenic effect of the eight peptides by measuring their ability to inhibit the migration of endothelial cells in a Boyden chamber assay. We express the migration results as migration inhibition relative to the used controls (Fig. 2).
Figure 2.
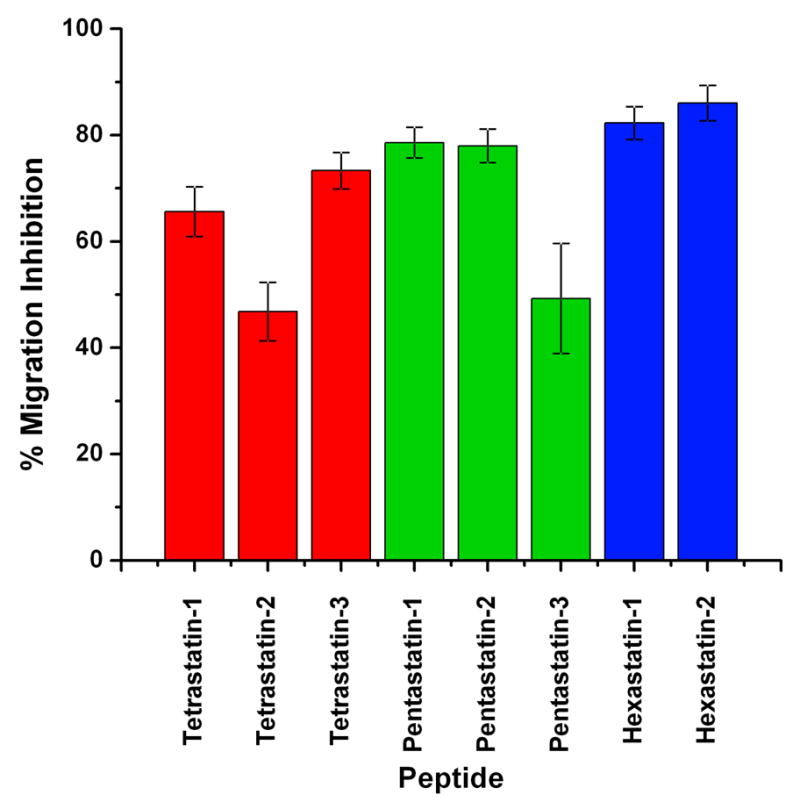
Effect of the peptides on the migration of HUVECs in a modified Boyden chamber migration assay. Endothelial cells were allowed to migrate for 20 h in the presence of 20 ng/ml VEGF and 30 μg/ml peptide solution, then stained with calcein and counted. The fluorescent signal was scaled so that the figure represents the percentage of migration inhibition. Here 100% represents the negative control (endothelial cells in serum- and growth factor-free medium, data not shown) or the total inhibition of the cell migration and 0% the positive control (migration in the presence of 20 ng/ml VEGF, data not shown) or zero inhibition of cell migration. Vertical bars indicate the standard error. All values are significantly different from 0% at p<0.001. In all cases the standard error for the controls was less than 4% (n=8).
Of the eight tested peptides, the two hexastatins (derived from the α6 fibril of type IV collagen) were the most potent in suppressing endothelial cell migration. In the presence of either hexastatin-1 or hexastatin-2, the migration of the HUVECs was inhibited to 90% levels of that seen for the positive control (VEGF alone). Pentastatin-1 and pentastatin-2, derived from the α5 fibrils, suppressed the migration of the endothelial cells to a similar extent (75% inhibition), whereas pentastatin-3 inhibited the endothelial cell migration by 45%. Tetrastatins, derived from the α4 fibrils, also significantly decreased the migration of the endothelial cells. The migration observed in the presence of tetrastatin-1 was inhibited by 65% of the control level; for tetrastatin-2 the inhibition reached 45%, and for tetrastatin-3 75%. For all of the cases the results were statistically significant with p<0.001.
Combinatorial analysis of the sequence-function relationship of the peptides
The next question to be addressed was whether there is a relationship between the sequence characteristics of the peptides and their potency in the in vitro assays. In particular, we were interested in ascertaining whether the common amino acid sequences behave similarly in the experimental screening.
In order to answer this question, we treated the sequence similarities of the peptides and their potencies in the proliferation assay as independent data sets. We performed a clustering analysis to search for inter-relationships within each of the two independent data sets. We then matched the two sets of results from the clustering analyses. If there was an underlying relationship between the sequence characteristics and the potency profiles, we would expect that the sequences that cluster only because of sequence similarities would behave similarly and that their potency profiles would also cluster. If there were no matches, and even though the sequences clustered because of similarities but their potency profiles did not match, we would be able to identify which elements of the sequences might be responsible for the lack of convergence.
In order to perform a clustering analysis of the amino acid sequences of the peptides, we used the Jalview software to analyze the information from the multiple sequence alignments and build a dendogram using as a metric their average distance, calculated on the basis of the percent sequence identity (Fig. 3A). This average distance algorithm is a bottom-up clustering method; it is a greedy algorithm that constructs the tree in a stepwise fashion. From this analysis we identified a set of three distinct clusters: the first was composed of tetrastatin-3 and hexastatin-1; the second, pentastatin-1 and pentastatin-2; and the third, tetrastatin-1, tetrastatin-2 and hexastatin-2. Pentastatin-3 did not precisely fit any of these three clusters but was most closely associated with the cluster containing pentastatin-1 and pentastatin-2.
Figure 3.

Clustering analysis of the peptide sequences and of the activities of the individual peptides in proliferation assays. A. The multiple sequence similarity dendrogram calculated using as a metric the average length of the peptides, based on the percentage sequence identity. The peptides were clustered on the basis of their sequence identity. B. The heatmap represents the scaled activities of the peptides in the in vitro proliferation assay. The peptides were aligned according to their similarities in terms of activity profile, and the associated hierarchical tree was calculated.
The second step in our analysis involved searching for possible clustering of the proliferation results, i.e., identifying groups of peptides with similar potency profiles. For each of the peptides we used the scaled potency in the proliferation assay as an input metric and performed hierarchical clustering, using the Euclidian distance of the results as a distance metric. The Euclidian distance measures the absolute distance between two points in the results space, which in this case is defined by the eight vectors that include the scaled proliferation results. We then we used the average linkage to generate a hierarchical tree [20].
The results from the clustering analysis of the proliferation results are shown in Figure 3B. The scaled proliferation results are represented as a heatmap and aligned according to the hierarchical clustering algorithm [20]. From the associated hierarchical tree we were able to identify three distinct clusters: the first consisting of pentastatin-1 and pentastatin-2, the second, hexastatin-1, tetrastatin-3 and pentastatin-3; and the third, terastatin-1, tetrastatin-2 and hexastatin-2.
When we compared the sequence identity clusters produced using the multiple sequence alignment algorithm to the peptide activity clusters calculated using the hierarchical clustering, we found a complete overlapping of the two clusters. These results are indicative of a sequence-activity correlation; the activities of each of the peptides are inherent in their sequence characteristics. This result also indicates that one can use sequence similarity approaches to search for similar peptide sequences as a means of locating fragments that might exhibit a specific activity, in this case putative anti-angiogenic fragments: peptide fragments that are responsible for the anti-angiogenic activities of the longer peptides or whole proteins of which they are a part.
Conclusions
Using a bioinformatics approach, we have identified a set of short peptides derived from the α4, α5 and α6 fibrils of type IV collagen that have putative anti-angiogenic properties. We named these peptides tetrastatins, pentastatins and hexastatins, respectively. Using HUVECs, we have evaluated the potency of these peptides as anti-angiogenic agents in proliferation and migration assays. Endothelial cell proliferation and migration are vital for the initiation and growth of a vascular sprout from a maternal vessel. The ability of the peptides to interfere with these processes in vitro is indicative of their ability to inhibit angiogenesis. Once the in vitro activity of these peptides has been conclusively established, their anti-angiogenic properties need to be tested further in vivo in healthy animals and in animal models of various diseases.
In the proliferation assay, all of the peptides are active, but with different potencies. The EC50 of the peptides was on the order of 1–10 μg/ml, or 400–4,000 nM. The most active peptides were those derived from the α5 fibril of type IV collagen, pentastatin-1 and pentastatin-2 in particular. Interestingly, the set of peptides derived from the α6 fibril showed a biphasic response, with their activity reaching a maximum at an intermediate concentration and then declining with increasing peptide concentration.
In the migration assay, the peptides significantly inhibited the ability of the endothelial cells to migrate in the presence of VEGF. Of the eight tested peptides, those derived from the α6 fibril were the most potent. This result is in agreement with previous studies in which the NC1 domain of the α6 fibrils of type IV collagen was shown to severely decrease endothelial cell adhesion [8].
From the set of the tested peptides those derived form the α5 fibrils of type IV collagen pentastatin-1 and pentastatin-2 provided cumulatively the strongest inhibition in both the proliferation and migration experiments.
In order to demonstrate a sequence-activity correlation, we correlated the peptide sequences with their potencies in the proliferation assay. The peptide sequences were clustered in a dendogram calculated based on the average distance of their identities, and the results from the proliferation experiments were also clustered using the Euclidian distances of their scaled activity. The sets of clusters that were calculated independently were found to coincide, demonstrating a strong peptide sequence-activity correlation. This study suggests that the activity of longer anti-angiogenic peptides may be localized within shorter peptide sequences, or active domains, and that one can use computational approaches in order to identify such active domains.
Supplementary Material
Acknowledgments
The authors thank Zaver Bhujwalla and Roberto Pili for useful discussions; David Noren, Venu Raman, Kristine Glunde, Noriko Mori, Paul Winnard and David Qian for their valuable advice on the experimental assays and Deborah McClellan for editorial assistance. The work was supported in part by NIH grants NHLBI R01 HL079653 and NCI P50 CA103175.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 3.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–79. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y. Endogenous angiogenesis inhibitors and their therapeutic implications. Int J Biochem Cell Biol. 2001;33:357–69. doi: 10.1016/s1357-2725(01)00023-1. [DOI] [PubMed] [Google Scholar]
- 5.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–24. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 6.Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–6. [PubMed] [Google Scholar]
- 7.Sudhakar A, Nyberg P, Keshamouni VG, Mannam AP, Li J, Sugimoto H, Cosgrove D, Kalluri R. Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J Clin Invest. 2005;115:2801–10. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Jr, Hudson BG, Brooks PC. New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem. 2000;275:8051–61. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 9.Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209–15. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 10.Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem. 2000;275:21340–8. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 11.Maeshima Y, Manfredi M, Reimer C, Holthaus KA, Hopfer H, Chandamuri BR, Kharbanda S, Kalluri R. Identification of the anti-angiogenic site within vascular basement membrane-derived tumstatin. J Biol Chem. 2001;276:15240–8. doi: 10.1074/jbc.M007764200. [DOI] [PubMed] [Google Scholar]
- 12.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci U S A. 2003;100:4766–71. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 13.Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518–20. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clamp M, Cuff J, Searle SM, Barton GJ. The Jalview Java alignment editor. Bioinformatics. 2004;20:426–7. doi: 10.1093/bioinformatics/btg430. [DOI] [PubMed] [Google Scholar]
- 16.Romesburg HC. Cluster analysis for researchers. Learning Publications Lifetime; Belmont, Calif: 1984. [Google Scholar]
- 17.Farinelle S, Malonne H, Chaboteaux C, Decaestecker C, Dedecker R, Gras T, Darro F, Fontaine J, Atassi G, Kiss R. Characterization of TNP-470-induced modifications to cell functions in HUVEC and cancer cells. J Pharmacol Toxicol Methods. 2000;43:15–24. doi: 10.1016/s1056-8719(00)00080-0. [DOI] [PubMed] [Google Scholar]
- 18.Addison CL, Nor JE, Zhao H, Linn SA, Polverini PJ, Delaney CE. The response of VEGF-stimulated endothelial cells to angiostatic molecules is substrate-dependent. BMC Cell Biol. 2005;6:38. doi: 10.1186/1471-2121-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Castro E, Sigrist CJ, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–5. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLachlan GJ. Cluster analysis and related techniques in medical research. Stat Methods Med Res. 1992;1:27–48. doi: 10.1177/096228029200100103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


